Abstract
Both secretin and vasoactive intestinal polypeptide (VIP) receptors are responsible for the activation of adenylyl cyclases (ACs), which increase intracellular cyclic AMP (cAMP) levels in the exocrine pancreas. There are nine membrane-associated isoforms, each with its own pattern of expression and regulation. In this study we sought to establish which AC isoforms play a regulatory role in pancreatic exocrine cells. Using RT-PCR, AC3, AC4, AC6, AC7 and AC9 were found to be expressed in the pancreas. AC3, AC4, AC6 and AC9 were expressed in both pancreatic acini and ducts, whereas AC7 was expressed only in pancreatic ducts. Based on known regulation by intracellular signals, selective inhibitors and stimulators were used to suggest which isoforms play an important role in the induction of cAMP formation. AC6 appeared to be an important isoform because protein kinase A (PKA), PKC and calcium all inhibited VIP-induced cAMP formation, whereas calcineurin or calmodulin did not modify the response to VIP. Mice with genetically deleted AC6 were studied and showed reduced cAMP formation and PKA activation in both isolated pancreatic acini and duct fragments. The absence of AC6 reduced cAMP-dependent secretagogue-stimulated amylase secretion, and abolished fluid secretion in both in vivo and isolated duct fragments. In conclusion, several AC isoforms are expressed in pancreatic acini and ducts. AC6 mediates a significant part of pancreatic amylase and fluid secretion in response to secretin, VIP and forskolin through cAMP/PKA pathway activation.
Key points
Cyclic AMP (cAMP), produced from ATP and the enzyme adenylyl cyclase (AC), plays an important role in the regulation of pancreatic exocrine cells.
We identified five AC isoforms in pancreatic exocrine cells. AC3, AC4, AC6 and AC9 are expressed in both pancreatic acini and duct fragments, whereas AC7 is expressed only in duct fragments.
In mice deficient in AC6, cAMP formation and protein kinase A activation were impaired. As a consequence, a reduction in amylase secretion and pancreatic fluid production was observed.
These results indicate that AC6 plays a regulatory role in pancreatic exocrine cells.
Introduction
The exocrine pancreas is primarily composed of acini and ducts. Pancreatic acini release digestive enzymes into the duodenum to break down dietary constituents, whereas pancreatic ducts secrete a bicarbonate-rich fluid to neutralize the acidic chyme from the stomach. Gastrointestinal hormones and neurotransmitters act on specific receptors to activate several intracellular signals that regulate these physiological processes. Cyclic AMP (cAMP) is one of the intracellular signals and secretin and vasoactive intestinal polypeptide (VPAC) receptors are responsible for the increase in cAMP levels (Argent et al. 2012). Stimulation of cAMP-dependent protein kinase A (PKA) induces phosphorylation of several cellular targets, including activation of the cystic fibrosis transmembrane conductance regulator (CFTR) and 1,4,5-IP3 receptors (Williams & Yule, 2012).
Adenylyl cyclases (ACs) are enzymes which produce cAMP from ATP. To date, at least 10 different AC isoforms have been cloned and identified: nine ACs are hormonally regulated and associated with membranes and one, AC10, is cytosolic. Most tissues express several AC isoforms and, although all membrane-associated ACs are activated by Gαs, each isoform displays complex and distinctive regulatory properties. Based on that, ACs associated with membranes are classified into four groups: Group I consists of calcium/calmodulin-stimulated AC1, AC3 and AC8; Group II consists of Gβγ-stimulated AC2, AC4 and AC7; Group III consists of Giα/calcium/PKC/PKA-inhibited AC5 and AC6; and Group IV consists of calcium/calcineurin-inhibited AC9, which is forskolin-insensitive (Willoughby & Cooper, 2007; Sadana & Dessauer, 2009). Recently, AC9 activity has also been shown to be inhibited by Gαi/o proteins and PKC (Cumbay & Watts, 2004).
An increase in cAMP levels is involved in evoked pancreatic amylase and bicarbonate-rich fluid secretions; however, the expression profile of AC isoforms in each cell type and which isoforms mediate specific function is unknown. In fact, current understanding of the role of AC in pancreatic exocrine cells comes primarily from studies that use stimulators and inhibitors of intracellular signals (Collen et al. 1982; Gardner et al. 1983; Burnham et al. 1984; Burnham & Williams, 1984; O'Sullivan & Jamieson, 1992; Matsushita et al. 1993; Ohnishi et al. 1994; Stryjek-Kaminska et al. 1995; Akiyama et al. 1998).
In this study we establish (1) which membrane-associated AC isoforms are expressed in the intact pancreas, as well as in purified pancreatic acini and duct fragments, and (2) which AC isoform is necessary for the activation of the cAMP/PKA pathway and, as a consequence, for the secretory function in both pancreatic exocrine cells.
Methods
Ethical approval
Animals received care according to protocols approved by the University of Michigan Committee on Use and Care of Animals.
Materials
Collagenase (CLSPA) was purchased from Worthington Biochemical Co. (Lakewood, NJ, USA), collagenase P from Roche (Penzberg, Germany), bovine albumin fraction V (BSA) from MP Biomedicals (Solon, OH, USA), sulfated cholecystokinin octapeptide (CCK-8) from Research Plus (Bayonne, NJ, USA), H-89, forskolin, 3-isobutyl-1-methylxantine (IBMX), 8-bromoadenosine 3′,5′cyclic monophosphate sodium salt (8-Br-cAMP), 8-(4-chlorophenylthio)adenosine 3′,5′-cyclic monophosphate sodium salt (CPT-cAMP) and soybean trypsin inhibitor (SBTI) from Sigma Chemical (St Louis, MO, USA), 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate (8-pCPT-2′-O-Me-cAMP) from Biolog Life Science Institute (Bremen, Germany), DMEM and McCoy's 5A tissue culture medium from Invitrogen (Carlsbad, CA, USA), A23187, phorbol 12-myristate 13-acetate (PMA), W-7, FK-506, BAPTA-AM and GF-109203X from Calbiochem (La Jolla, CA, USA), and vasoactive intestinal polypeptide (VIP) and secretin from American Peptides (Sunnyvale, CA, USA). Other chemical reagents were obtained from Sigma Chemical.
Antibodies
Antibodies against the following proteins were used: goat polyclonal antibody to AC9 (sc-8576) from Santa Cruz Biotechnology (Santa Cruz, CA, USA); rabbit polyclonal antibody to α-amylase (A-8273) and mouse monoclonal antibody to β-tubulin Clone TUB 2.1 (T5201) from Sigma Chemical; mouse monoclonal antibody to Epac1 (#4155) and rabbit polyclonal antibody to PKA RI- α/β (#3927) from Cell Signaling Technology (Beverly, MA, USA); mouse monoclonal antibody to Rap1 (610195) from BD Transduction Laboratories (San Diego, CA, USA); and rat monoclonal antibody to keratin 19 (TROMA-III) from the Developmental Studies Hybridoma Bank, University of Iowa.
Animals
AC6-deficient mice were prepared as described (Tang et al. 2008). Male C57BL/6J AC6−/− mice were bred in our facilities from heterozygotes and maintained on a 12/12 h light–dark cycle with free access to water and food (5001 Rodent Diet; PMI Nutrition International, St Louis, MO, USA). Littermate WT mice were used as controls. Expression of AC6 was analysed by RT-PCR using the following primers: forward: 5′GCGGCCTGGGCCTCTCTACT3′ and reverse: 5′GCGGCCTGGGCCTCTCTACT3′ (336 bp) (Tang et al. 2008). When required, mice were killed in a CO2 chamber.
Histology
Small blocks of pancreas were fixed with 10% formalin, embedded in paraffin blocks, processed for haematoxylin and eosin staining and examined with a Nikon Eclipse TE200 inverted microscope equipped with a digital camera. For immunolocalization of amylase and keratin 19, tissues were fixed for 30 min with 1% formaldehyde (freshly prepared from paraformaldehyde), and cryostat sections were cut at 6 μm thickness as previously described (Sans et al. 2011) and mounted on SuperFrost Plus slices (Fischer, Houston, TX, USA) and incubated with PBS containing 2% normal goat serum and 0.3% Triton X-100 followed by a rabbit polyclonal antibody to α-amylase (diluted 1:5000) and a rat monoclonal anti-TROMA-III antibody (diluted 1:15). The sections were washed with PBS and incubated with 1:500 dilutions of goat anti-rabbit secondary antibody conjugated to Alexa 488 and goat anti-rat secondary antibody conjugated to Alexa 594. Fluorescence images were captured with an Olympus wide-field microscope and processed with Adobe Photoshop software.
Isolation of pancreatic acini
Pancreatic acini from WT or AC6−/− mice were prepared by enzymatic digestion with collagenase (CLSPA) followed by mechanical shearing as previously reported (Sabbatini et al. 2008).
Isolation and culture of duct fragments
Ducts were isolated from mouse pancreas and cultured overnight following methods previously described (Ishiguro et al. 1996). Mouse pancreas obtained from WT or AC6−/− mice was digested using 5 mg collagenase P (Roche) dissolved in 5 ml DMEM containing 0.1% BSA and 0.01% trypsin inhibitor (SBTI) and injected into the pancreas using a 27-gauge needle. Pancreases were incubated for 30 min at 37°C in a polypropylene Erlenmeyer flask with shaking at 140 r.p.m. This process was followed by vigorous hand shaking to disrupt the pancreas until the suspension became homogeneous. The suspension was then briefly centrifuged and the pellet resuspended with 20 ml DMEM containing 0.1% BSA, 0.01% SBTI and antibiotics and placed in a 150 mm Petri dish. Duct fragments were hand-picked using a 20 μl pipette and placed in a 35 mm Petri dish containing sterile McCoy's 5A tissue culture medium supplemented with 10% (v/v) fetal bovine serum, 2 mm l-glutamine, 0.15 mg ml−1 trypsin inhibitor, 0.1 IU ml−1 insulin and 4 μg ml−1 dexamethasone. Pancreatic acini and islets were isolated in the same way and placed in a 35 mm Petri dish with DMEM containing 0.1% BSA plus 0.01% SBTI. This step was repeated several times to remove loosely attached cells. For measurement of cAMP levels and PKA activity, overnight incubated duct fragments were hand-picked and placed in a 35 mm Petri dish containing DMEM without phenol red, 0.1% BSA and 0.01% SBTI. For measurement of ductal secretion in vitro, 24–30 h incubated duct fragments whose lumen was easy to see were hand-picked and placed in a tissue culture dish with a cover glass bottom coated with poly-d-lysine containing a bicarbonate-buffered solution (115 mm sodium chloride, 5 mm potassium chloride, 1 mm calcium chloride, 1 mm magnesium chloride, 10 mm d-glucose, 25 mm sodium bicarbonate equilibrated with 95% O2/5% CO2 and adjusted to pH 7.4).
Detection of the expression of AC isoforms
Expression of the nine different membrane-associated isoforms of AC in mouse intact pancreas, pancreatic acini, islets and duct fragments was assessed by RT-PCR. Total RNA was isolated from brain, kidney, parotid gland and pancreas using TRIzol reagent (Invitrogen), as well as from pancreatic acini, duct fragments and islets using an RNeasy® Mini kit (Qiagen, Valencia, CA, USA). First-strand cDNA was synthesized with a TaqMan RT-PCR kit (Applied Biosystems, Branchburg, NJ, USA). Intactness of RNA was assessed based on OD260/280 and agarose gel electrophoresis. One microgram of cDNA was used in each PCR reaction. Amplification with Taq DNA polymerase from Expand High Fidelity Enzyme Mix kit (Roche Diagnostics, IN, USA) was conducted using specific primers indicated in Supplemental Table S1. The relative expression of each isoform in pancreatic acini and ducts compared with the intact pancreas was evaluated by real-time quantitative PCR analysis using Absolute Blue SYBR Green ROX mix (Thermo Fisher Scientific, Waltham, MA, USA) and CFX96™ cycler (Bio-Rad laboratories, Hercules, CA). These PCR primers are listed in Table S2. To validate the amplification efficiency of each primer, RNA dilution series from mouse pancreas were made for each primer, and the slope of the calibration curve was analysed. Only primers showing efficiencies between 90 and 100% (−3.6 ≥ slope ≥−3.3) were used. β-Actin was used as a reference.
Measurement of cAMP levels
cAMP levels were measured as previously reported (Sabbatini et al. 2003). Pancreatic acini or overnight incubated duct fragments were preincubated for 30 min in DMEM without phenol red and then for 3 min in fresh DMEM without phenol red containing 1 mm IBMX. Acini or ducts were stimulated with either VIP (10 nm), secretin (100 nm) or forskolin (20 μm) for 12 min. cAMP was extracted in absolute ethanol and measured using a cAMP colorimetric enzyme immunoassay kit according to the instructions provided by the manufacturer (Cayman Chemical, Ann Arbor, MI, USA). Results are expressed as picomoles per milligram protein from acini lysate or picomoles per microgram protein from duct fragment lysate.
Measurement of PKA activity
PKA activity was measured using a non-radioactive colorimetric solid phase enzyme-linked immuno-absorbent assay kit (no. ADI-EKS-390A; Enzo Life Sciences, Plymouth Meeting, PA, USA). Results are expressed as nanograms active PKA per microgram protein.
Measurement of amylase secretion
Amylase secretion was measured as previously reported (Sabbatini et al. 2008). Acini from WT or AC6−/− mice were stimulated with VIP (0.1, 1, 10, 100 nm), secretin (100 nm), forskolin (20 μm) or CCK (10 pm, 30 pm, 100 pm, 1 nm), carbachol (10 μm), CPT-cAMP (100 μm) or 8-pCPT-2′-O-Me-cAMP (100 μm) for 30 min. Results are expressed as a percentage of initial acinar amylase content.
Measurement of ductal lumen expansion
Serial transmitted light images of pancreatic duct fragments were acquired by a Nikon A1 confocal microscope (20× objective lens) every 2 min for the indicated time. The Nikon TiE inverted microscope has a Tokai Hit (Shizuoka-ken, Japan) stage-top environmental chamber whose temperature is controlled at 37°C by a thermostat. The expansion of duct lumen was calculated in each recorded image using the image analysis feature of Nikon Image System (NIS) Elements version 4.0. After a 16 min basal period, duct fragments from WT or AC6−/− mice were stimulated with VIP (10 nm) or 8-Br-cAMP (500 μm) at 37°C and monitored for an additional period. The increase in the lumen is expressed as a fold increase over the period recording in respect to basal (time 0).
Measurement of pancreatic fluid in vivo
Isoflurane-anaesthetized WT or AC6−/− mice were prepared with a cannula into the bile duct near the liver and another one near the ampulla of Vater to collect pure pancreatic fluid as previously reported (Sans et al. 2011). Fluid was allowed to flow for 15 min after which basal secretion was collected for 30 min prior to intravenous stimulation with secretin (2 U kg−1 body weight) or VIP (0.5 μg kg−1 body weight). Results are expressed as nanolitres per 30 min per gram body weight.
Statistical analysis
Results are expressed as means ± SEM of 3–6 separate experiments. The statistical analysis was performed by analysis of variance (ANOVA) following by Student–Newman–Keuls test. A P value of 0.05 or less was considered statistically significant.
Results
Multiple AC isoforms are expressed in pancreatic exocrine cells
Using RT-PCR we found five different AC mRNAs in the intact pancreas: AC3, AC4, AC6, AC7 and AC9 (Fig. 1). AC1, AC2, AC5 and AC8 mRNAs were expressed in their respective positive control tissues, but not in the pancreas (Fig. 1). We then studied the expression of AC isoforms in pancreatic acini, ducts and islets. Expression of amylase, insulin and keratin 19 in pancreatic acini, islets and duct fragments, respectively, indicated the purity of the pancreatic cell preparation (Fig. 2A and B). In isolated pancreatic acini we found that AC3, AC4, AC6 and AC9 mRNAs are expressed, whereas in isolated pancreatic duct fragments and islets AC3, AC4, AC6, AC7 and AC9 mRNA are expressed (Fig. 2C). Using real-time quantitative PCR we evaluated the relative amounts of each isoform in pancreatic acini and ducts related to the intact pancreas. First, we confirmed the purity of the acini and duct preparation. Mean cycle threshold (CT) values are presented for transcripts corresponding to amylase, insulin, keratin 19, and AC isoforms AC3, AC4, AC6, AC7 and AC9 (Table 1). The results show that isolated pancreatic acini have statistically significantly higher transcript levels of AC6 and lower transcript levels of insulin compared with intact pancreas. Keratin 19 and AC7 were not detected in pancreatic acini. Isolated duct fragments showed statistically significantly higher transcript levels of keratin 19, AC4, AC6 and AC7 and lower transcript levels of amylase and insulin compared with the intact pancreas. Similar transcript levels of AC3 and AC9 were observed in pancreas, acini and ducts.
Figure 1. Five different AC isoforms are expressed in the pancreas.
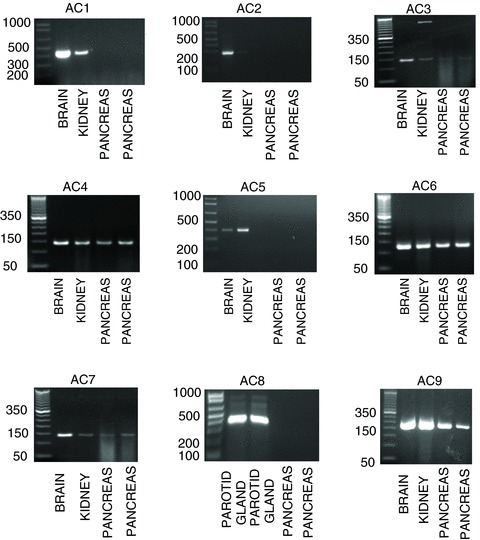
Expression of the nine membrane-associated AC isoforms was assessed in the intact pancreas by RT-PCR. The brain, kidney and parotid gland were used as a positive control. RT-PCR products yielded bands of the expected size (see Supplemental Table S1). Only AC3, AC4, AC6, AC7 and AC9 are expressed in the intact pancreas. Results shown are representative of four experiments.
Figure 2. Expression pattern of AC isoforms isolated pancreatic acini, duct fragments and islets.
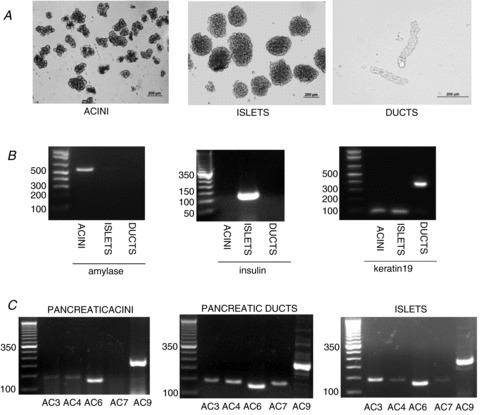
A and B, light micrographs images (A) and RT-PCR analysis of expression of amylase, insulin and keratin 19 (B) in isolated pancreatic acini, duct fragments and islets. Expression of amylase, insulin and keratin 19 in isolated pancreatic acini, islets and duct fragments, respectively, indicate the purity of the pancreatic cell preparations. RT-PCR yielded products of the expected size: amylase, 570 bp; insulin, 108 bp; keratin 19, 374 bp. C, RT-PCR analysis of expression of different AC isoforms in pancreatic acini, ducts and islets. Expression of AC3, AC4, AC6 and AC9 is found in pancreatic acini whereas expression of AC3, AC4, AC6, AC7 and AC9 is found in pancreatic ducts and islets. RT-PCR yielded products of the expected size: AC3, 141 bp; AC4, 136 bp; AC6, 123 bp; AC7, 354 bp; AC9, 227 bp.
Table 1.
AC expression in the whole pancreas, isolated acini and ducts
| Pancreas | Acini | Ducts | |
|---|---|---|---|
| Amylase | 13.78 ± 0.41 | 13.02 ± 0.45 | 24.3 ± 0.64** |
| Insulin | 23.04 ± 0.66 | 28.49 ± 0.53** | 32.79 ± 1.62** |
| Keratin 19 | 37.11 ± 0.79 | not detected | 33.86 ± 0.52* |
| AC3 | 31.38 ± 0.38 | 30.15 ± 0.02 | 31.30 ± 0.19 |
| AC4 | 31.41 ± 0.46 | 30.7 ± 1.00 | 26.63 ± 0.63* |
| AC6 | 29.39 ± 0.72 | 25.95 ± 0.02* | 26.33 ± 0.79* |
| AC7 | 34.57 ± 0.41 | not detected | 32.13 ± 0.38* |
| AC9 | 29.53 ± 0.75 | 30.02 ± 1.37 | 30.85 ± 0.95 |
| β-Actin | 26.28 ± 0.20 | 27.70 ± 0.62 | 25.06 ± 0.43 |
Real-time quantitative PCR for amylase, insulin, keratin 19 and AC isoform expression in the intact pancreas, isolated pancreatic acini and isolated duct fragments. Data are expressed as mean cycle threshold (CT) values ± SEM from 3–6 mice. CT values that are statistically significant vs. pancreas are highlighted in bold. *P < 0.05 and **P < 0.01 vs. pancreas.
VIP-stimulated cAMP formation is negatively regulated by PKA, PKC and calcium
To establish which AC isoform mediates cAMP generation by VIP we evaluated their regulation of cAMP formation by intracellular signals using a number of stimulators and inhibitors. VIP increased cAMP levels in pancreatic acini up to 17-fold. In the presence of the PKA inhibitor H-89 or the PKC inhibitor GF-109203X, an enhancement up to two-fold in VIP-stimulated cAMP generation was observed (Fig. 3A). In the presence of the calcium chelator BAPTA-AM, an enhancement in VIP-stimulated cAMP generation up to two-fold was also seen (Fig. 3B). To study further whether the effect of calcium is direct or indirectly mediated by either calcineurin or calmodulin, two inhibitors were used: W-7, which is a calmodulin inhibitor, and FK-506, which is a calcineurin inhibitor. Neither inhibitor modified the response to VIP (Fig. 3B), indicating that calcium inhibits the activity of AC in a direct manner. Several pathway stimulants including A23187, the phorbol ester PMA and the muscarinic agonist carbachol were tested, but none of them stimulated cAMP generation by themselves or modified VIP-stimulated cAMP generation (Fig. 3C).
Figure 3. VIP-stimulated cAMP formation is negatively regulated by PKA, PKC and calcium.
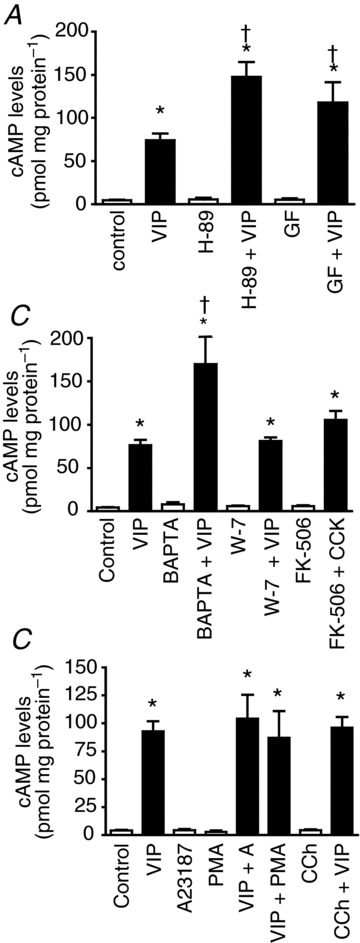
A and B, the PKA inhibitor H-89, the PKC inhibitor GF-109203X (A) and the calcium chelator BAPTA-AM (B) all enhance the response to VIP in pancreatic acini. W-7, which is a calmodulin inhibitor, and FK-506, which is a calcineurin inhibitor, do not modify the response to VIP (B). C, the calcium ionophore A23187, the phorbol ester PMA and the muscarinic agonist carbachol do not modify VIP-stimulated cAMP generation. GF, GF-109203X; CCh, carbachol. Values are means ± SEM; n = 5 mice. *P < 0.001 vs. control; †P < 0. 001 vs. VIP alone.
Given that PKA, PKC and calcium, in a direct manner, played a negative regulatory role in cAMP formation, and neither calcineurin nor calmodulin was involved in the regulation of cAMP generation, which excludes the participation of AC9 and AC3, respectively, we hypothesized that the calcium/PKC/PKA-inhibited AC6 plays an important role in the regulation of pancreatic exocrine cell activity. To test this hypothesis, mice with a deletion of AC6 were used.
Characterization of AC6−/− mice
We first performed RT-PCR analysis and confirmed that AC6 mRNA expression was absent from AC6−/− mouse pancreas (Fig. 4A). Using real-time quantitative PCR we found that the deletion of AC6 does not modify the expression of other pancreatic AC isoforms (Fig. 4B) as previously reported in heart (Tang et al. 2008). AC6−/− mice show no developmental, gross morphological (Fig. 4C) or pancreatic histological abnormalities as shown by haematoxylin and eosin staining and immunohistochemistry using anti-amylase antibody as a zymogen granule marker, and anti-keratin 19 antibody as a duct marker (Fig. 5A and B). These results indicate that AC6 is not required for pancreatic development and maintenance of cytoarchitecture, as well as showing that there are no gross adaptive changes in AC6−/− mice.
Figure 4. Characterization of AC6−/− mice.
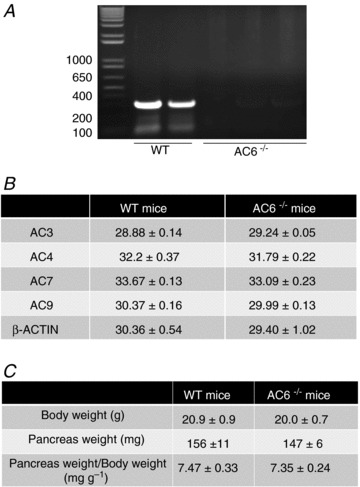
A, mRNA expression of AC6 was present in pancreas from WT mice (expected size: 350 bp) whereas it was undetectable in pancreas from AC6−/− mice. B, mRNA expression of AC3, AC4, AC7 and AC9 was analysed by real-time quantitative PCR analysis in both WT and AC6−/− mice. The deletion of AC6 does not affect the expression of other pancreatic AC isoforms. Data are expressed as mean cycle threshold (CT) values ± SEM from four mice. C, no developmental or gross morphological changes were observed in AC6−/− mice. Age: 45–51 days. Data are expressed as mean ± SEM from 10 mice.
Figure 5. AC6 deletion does not change pancreas morphology.
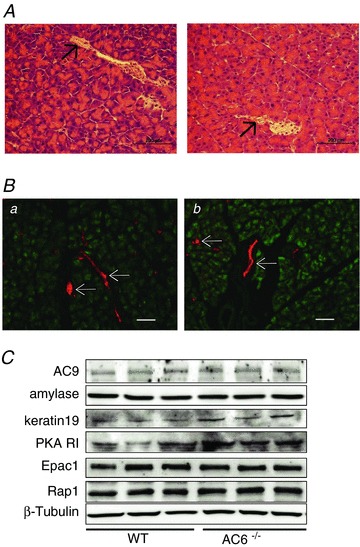
A, pancreas haematoxylin and eosin histological samples of WT (left panel) and AC6−/− mice (right panel). B, pancreatic acini and ducts were visualized by immunostaining with anti-amylase (green) and anti-keratin 19 (red), respectively. The pancreas looks normal; no morphological changes are observed in AC6−/− mice. Scale bar = 100 μm. Arrows indicate ducts. C, a representative immunoblot shows that AC6 deletion does not affect the protein content of AC9, amylase, keratin 19, Epac1 or Rap1 in total pancreas lysates. An increase in the protein content of the PKA regulatory subunit is observed in AC6−/− mice. Equivalent loading was confirmed using β-tubulin.
To determine whether AC6 deletion was associated with alterations of some molecular components of the pancreas, the protein content of AC9, amylase and keratin 19 was evaluated by Western blotting and no changes were observed (Fig. 5C). When cAMP is increased, two pathways are directly activated by cAMP in the exocrine pancreas: the PKA and Epac1/Rap1 pathways (Chaudhuri et al. 2007; Sabbatini et al. 2008). Although the AC6 deletion did not affect the protein content of Epac1 or Rap1, the protein content of the PKA regulatory subunit was increased (Fig. 5C). Tang et al. (2008) noted that the protein content of the PKA catalytic subunit is not affected in AC6−/− mice.
AC6 is required for cAMP/PKA pathway activation in pancreatic acini
To determine the importance of AC6 in pancreatic acini, we first measured cAMP formation. VIP, secretin and forskolin increased cAMP formation in acini from WT mice up to 8-, 3- and 6-fold, respectively. Deletion of AC6 did not affect basal cAMP levels in pancreatic acini, although the absence of AC6 was associated with a reduction of cAMP formation stimulated by VIP (10 nm), secretin (100 nm) or forskolin (20 μm) by 42, 46 and 53%, respectively (Fig. 6A). We also studied the effect of AC6 deletion on PKA activity. VIP, secretin and forskolin increased PKA activity in pancreatic acini from WT mice up to 4-, 1.75- and 2-fold, respectively. Deletion of AC6 reduced the activation of PKA induced by VIP, secretin and forskolin by 78, 35 and 65%, respectively (Fig. 6B). These findings indicate that AC6 is the dominant cAMP formation-stimulating isoform for VIP-, secretin- and forskolin-mediated PKA activation.
Figure 6. Deletion of AC6 reduces the stimulated cAMP formation and PKA activation in pancreatic acini.
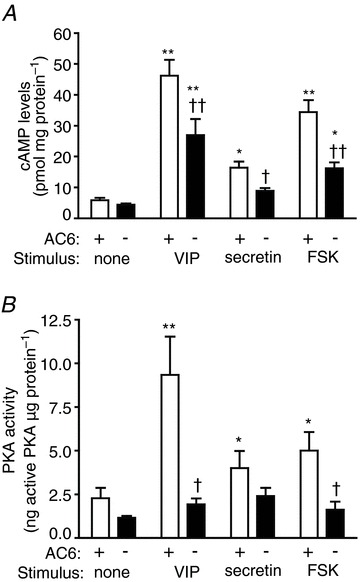
The absence of AC6 reduces cAMP formation (A) and PKA activity (B) in pancreatic acini. FSK, forskolin. n = 6 mice. *P < 0.05 and **P < 0.01 vs. control; †P < 0.05 and ††P < 0.01 vs. WT mice.
As a consequence of AC6 deletion, the enhancement of VIP-stimulated cAMP levels observed in the presence of the calcium chelator BAPTA-AM was reduced in AC6−/− mice by 40% (Fig. 7).
Figure 7. AC6 is negatively regulated by calcium in pancreatic acini.
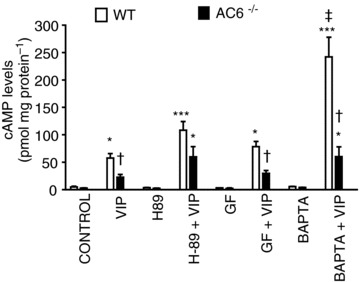
The lack of AC6 largely impairs the enhancement of VIP-stimulated cAMP levels observed in the presence of the calcium chelator BAPTA. GF, GF109203X. Values are means ± SEM; n = 5 mice. *P < 0.05 and ***P < 0.001 vs. control; †P < 0.05 vs. WT mice; ‡P < 0.001 vs. VIP alone.
AC6 is required for cAMP/PKA pathway activation in pancreatic duct
Basal cAMP formation from mouse duct fragments was not affected by the deletion of AC6 (Fig. 8A). VIP, secretin and forskolin evoked an increase in cAMP formation from WT mice up to 26-, 16- and 44-fold, respectively. The increase in cAMP formation induced by VIP, secretin and forskolin was reduced from AC6−/− mice by 70, 60 and 75%, respectively (Fig. 8A). VIP, secretin and forskolin increased PKA activity from WT mice up to 4-, 2.5- and 4-fold, respectively. The lack of AC6 essentially abolished the activation of PKA induced by VIP, secretin and forskolin (Fig. 8B).
Figure 8. The lack of AC6 reduces the stimulated cAMP formation and PKA activity in isolated pancreatic ducts.
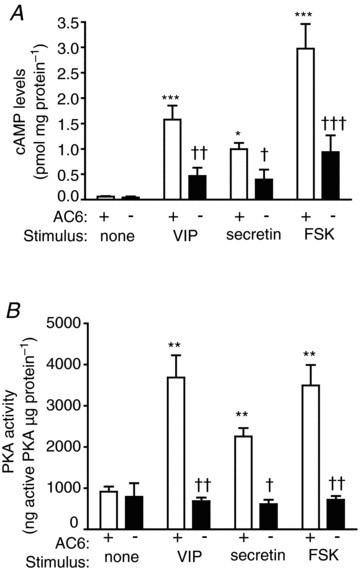
Deletion of AC6 reduces cAMP formation (A) and PKA activity (B) in pancreatic ducts. FSK, forskolin. Values are means ± SEM; n = 6 mice. *P < 0.05, **P < 0.01 vs. control; †P < 0.05 and ††P < 0.01 vs. WT mice.
AC6 is required for amylase secretion
To study the functional relevance of AC6 in pancreatic acini, amylase secretion was studied. Amylase release was increased by VIP, secretin and forskolin up to 2-, 1.7- and 2-fold, respectively. Although AC6 deletion did not affect basal amylase secretion, a reduction in VIP-stimulated amylase secretion by 31%, as well as that evoked by secretin (28%) and forskolin (36%) was observed in AC6−/− mice (Fig. 9A and B).
Figure 9. Stimulated amylase secretion is reduced from AC6−/− mice.
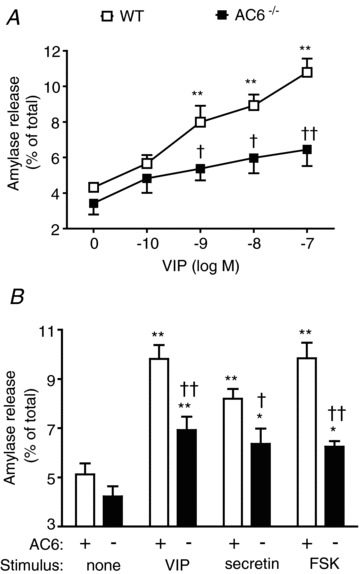
A, concentration–response curve to VIP on amylase secretion. B, effect of a single concentration of VIP (10 nm), secretin (100 nm) and forskolin (FSK) (20 μm) on amylase secretion. n = 6 mice. *P < 0.05 and **P < 0.01 vs. control. †P < 0.05 and ††P < 0.01 vs. AC6 mice.
AC6 is necessary for evoked pancreatic fluid
The physiological consequence of AC6 deletion on pancreatic fluid secretion was further studied both in vitro and in vivo. Isolated sealed pancreatic ducts from WT and AC6−/− mice were incubated for 16 min at 37°C in a bicarbonate-rich medium. The average size of pancreatic duct fragments without stimulation was 5604 ± 284 μm2 in WT mice and 5980 ± 278 μm2 in AC6−/− mice. No changes in the lumen area between WT and AC6−/− mice were observed during this period. Upon VIP stimulation, a progressive expansion in the lumen was observed in duct fragments from WT mice up to 2-fold, but there was no change in lumen area of ducts from AC6−/− mice (Fig. 10A). A representative image of a duct fragment before and after VIP stimulation (18 min) from WT and AC6−/− mice is shown in Fig. 11. Further evaluation of the pancreatic fluid secretion in vivo revealed no change in the basal secretion from AC6−/− mice. The administration of VIP (0.5 μg kg−1, i.v.) and secretin (2 U kg−1, i.v.) increased pancreatic fluid from WT mice up to 2-fold, but there was no significant increase in AC6−/− mice (Fig. 10B).
Figure 10. The absence of AC6 impairs the response to secretagogues in pancreatic fluid secretion in isolated duct fragments and in anaesthetized mice.
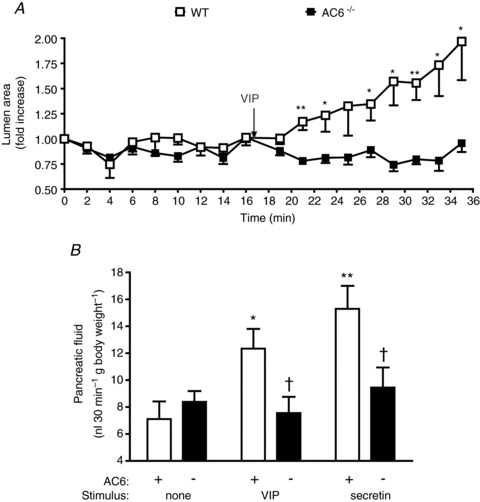
A, an expansion in the lumen of sealed isolated duct fragments was observed in WT mice upon VIP (10 nm) stimulation while no change in lumen area was observed in duct from AC6−/− mice. n = 8–9 duct fragments in each group. B, stimulation by VIP (0.5 μg kg−1, i.v.) or secretin (2 U kg−1, i.v.) increased pancreatic fluid secretion in anaesthetized WT mice but not in AC6−/− mice. Values are means ± SEM; n = 3–4 mice. *P < 0.05, **P < 0.01 vs. control. †P < 0.05 vs. WT mice.
Figure 11. The absence of AC6 impairs the secretory response to VIP in isolated duct fragments.
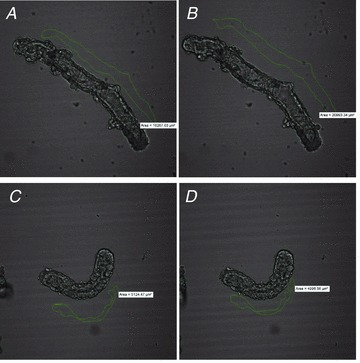
A and B, a representative duct fragment before (A) and after VIP stimulation (10 nm) (B) from WT mice. C and D, a representative duct fragment before (C) and after VIP stimulation (D) from AC6−/− mice. The lumen area is drawn in green. An expansion in lumen area is observed in sealed duct fragments from WT mice (area: 16267.03 μm2 vs 20993.34 μm), but not from AC6−/− mice (area: 5124.47 μm2 vs 4996.56 μm2).
Response to exogenous cAMP is affected by AC6 deletion
To address whether the decrease in amylase and fluid secretion in AC6−/− mice is a consequence solely of impaired cAMP formation and PKA activation, two cAMP agonists were used: 8-Br-cAMP and CPT-cAMP. The cAMP analogues increased PKA activation in isolated pancreatic acini from WT mice, but their effects were also reduced in AC6−/− mice (Fig. 12A, left). 8-Br-cAMP increased PKA activation in duct fragments from WT mice, but not in AC6−/− mice (Fig. 12A, right). In isolated pancreatic acini, deletion of AC6 also impaired the response to CPT-cAMP on amylase secretion (Fig. 12B). However, deletion of AC6 did not affect the response to either the Epac selective analogue 8-pCPT-2′-O-Me-cAMP, or the calcium-dependent secretagogues CCK or carbachol (Fig. 12B, right: only concentration–response to CCK is shown). When we studied the effect of exogenous cAMP derivatives in isolated pancreatic duct fragments, we found that 8-Br-cAMP evoked a ductal lumen expansion in WT mice, but not in AC6−/− mice (Fig. 12C). These findings indicate that the deletion of AC6 affects not only the response to cAMP-dependent secretagogues, but also the response to exogenous cAMP, while not affecting the response to secretagogues signalling through other pathways.
Figure 12. The response to exogenous cAMP is impaired in AC6−/− mice.
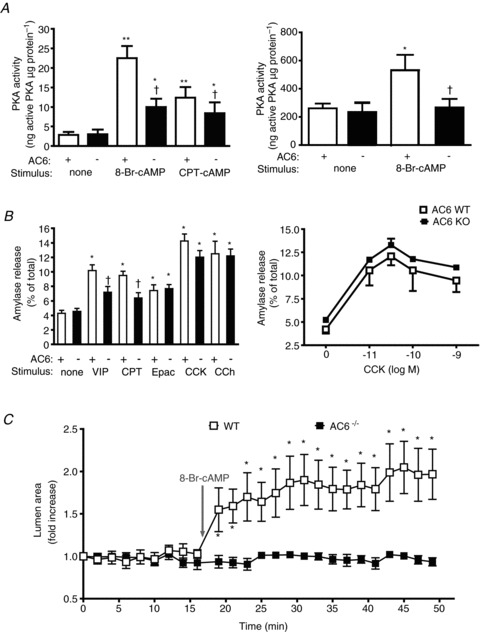
In the presence of cAMP analogues PKA activation (A), amylase secretion (B) and lumen expansion (C) were studied in WT and AC6−/− mice. A, the absence of AC6 reduces the effect of cAMP analogues 8-Br-cAMP (500 μm) and CPT-cAMP (500 μm) to activate PKA in isolated pancreatic acini (left panel) while it abolishes the effect of 8-Br-cAMP in isolated duct fragments (right panel); n = 5–12. B (left), deletion of AC6 reduces the effect of VIP (10 nm) and CPT-cAMP (CPT) (100 μm), but not 8-pCPT-2′-O-Me-cAMP (Epac) (100 μm), CCK (30 pm) or CCh (10 μm) on amylase secretion; n = 5–9. B (right), concentration–response curve to CCK; n = 5 in each group. C, an expansion in the lumen of sealed isolated pancreatic duct fragments is observed in WT mice upon 8-Br-cAMP (500 μm) stimulation while no change in lumen area is observed in duct from AC6−/− mice; n = 5–7 duct fragments from six mice in each group. *P < 0.05, **P < 0.01 vs. control. †P < 0.05 vs. WT mice.
Discussion
This study demonstrates that pancreatic exocrine cells express multiple AC isoforms: AC3, AC4, AC6 and AC9 are expressed in both pancreatic acini and ducts whereas AC7 is expressed only in pancreatic ducts.
Depending on the relative levels of the isoforms expressed in a cell type, extracellular signals received by the G-protein coupled receptor can be differently integrated. AC activity can be regulated by α subunits of Gs, Gi, Gz and Go, βγ subunits of G protein, PKA, PKC isoforms, changes in membrane potential and calcium in a direct or indirect manner (Sadana & Dessauer, 2009). Specifically in the exocrine pancreas, an increase in diacylglycerol and calcium through 1,4,5-IP3 receptors induces an activation of PKC (Williams & Yule, 2012), which in turn could activate AC3, and AC7. Calcium can activate calcineurin (Groblewski et al. 1998; Gurda et al. 2008), and thereby could inhibit AC9, and via activation of calmodulin kinase II (Duan et al. 1994) inhibit AC3. In this study we found that AC6 is the primary isoform regulating the response to cAMP-mobilized secretagogues because: (1) the response to VIP was enhanced by PKA and PKC inhibitors, as well as by a depletion of calcium. No change in the response to VIP was observed in the presence of either the calmodulin inhibitor or the calcineurin inhibitor, which indicates that both the calcium/calmodulin-stimulated AC3 and the calcium/calcineurin-inhibited AC9 are unlikely to participate in VIP-stimulated cAMP formation; and (2) isolated pancreatic acini from mice with targeted AC6 deletion showed a reduction in cAMP formation and PKA activity in response to VIP, secretin and forskolin, and as a consequence, a reduction in amylase secretion from AC6−/− mice was shown. We also studied the importance of AC6 in the function of pancreatic ducts. We observed that in AC6−/− mice both cAMP formation and PKA activation were abolished in response to VIP, secretin and forskolin. We also found that AC6 plays a physiologically relevant role in pancreatic ducts because the VIP-stimulated expansion of the lumen observed in pancreatic ducts from WT mice upon VIP stimulation was absent in duct fragments from AC6−/− mice. Collection of pancreatic fluid in vivo also showed a decrease in fluid secretion from AC6−/− mice.
The deletion of AC6 not only affected the response to cAMP-dependent secretagogues, but also the response to cAMP replacement using 8-Br-cAMP or CPT-cAMP, although Western blotting showed an increase in protein content of the PKA regulatory subunit. Unlike cAMP/PKA signalling, the deletion of AC6 did not affect either calcium or Epac1 signalling because the effect of calcium-mobilized secretagogues or the Epac1 selective analogue on amylase secretion was not modified in AC6−/− mice. No changes in the morphology of the pancreas or in the protein content of other molecular elements of the exocrine pancreas, such as amylase, keratin 19, Epac1 and Rap1, have been observed. These findings indicate that AC6 plays an important selective role in cAMP/PKA signalling and that the increase in protein content of the PKA regulatory subunit is not enough to compensate for the lack of AC6. We suggest that AC6 deletion impairs the PKA pathway and, as a consequence, these cells no longer respond to cAMP. If so, it emphasizes the importance of AC6 in these cells.
The deletion of AC6 slightly reduced VIP-, secretin- or forskolin-stimulated amylase secretion. It is likely that the residual responses were mediated by other AC isoforms expressed in pancreatic acini and/or by the cAMP-dependent PKA-independent pathway, Epac1, which has participated in cAMP-stimulated amylase secretion (Chaudhuri et al. 2007; Sabbatini et al. 2008). The result showing the deletion of AC6 does not affect the response to the Epac1 analogue 8-pCPT-2′-O-Me-cAMP on amylase secretion supports this hypothesis. Unlike amylase secretion, cAMP-dependent secretagogue-stimulated fluid secretion was highly dependent on AC6 activity because the deletion of AC6 completely blocked the expansion of the duct fragment in vitro, as well as pancreatic fluid secretion in vivo. A relatively higher transcript level of AC6 in both pancreatic acini and ducts can explain its physiological relevance. It is also possible that the intracellular conditions favour the activation of AC6. For example, AC6 may have a greater sensitivity to Gs-activated secretin and VPAC receptors than other AC isoforms.
AC activation increases cAMP accumulation in isolated ducts, which in turn activates PKA, which phosphorylates CFTR in the apical membrane of the duct cells (Argent et al. 2012). Elevation of intracellular cAMP by stimulation with forskolin significantly inhibits the Na/H exchanger and this, like the stimulation of the apical anion exchanger, may occur through a direct physical interaction with CFTR (Argent et al. 2012). The basolateral Cl−/HCO3− exchanger (AE) does not seem to be directly activated by forskolin (Lee et al. 1999). Based on this knowledge, it is likely that AC6 is responsible for the activation of CFTR, and indirectly, for the inhibition of the Na/H exchanger and the apical anion exchanger. Because the direct evidence of AC6 on the activity of transporters is beyond the scope of the paper, we did not test this hypothesis further.
Given that AC6 is the only AC isoform regulated by Gαi, the presence of AC6 in the exocrine pancreas has previously been suggested because in rabbit pancreatic acini the inhibition of Gαi activity by pertussis toxin stimulates cAMP formation without affecting calcium mobilization or amylase secretion (Willems et al. 1987). In addition to this observation, because somatostatin inhibits AC activity through somatostatin type 2 receptor (ss2R) receptors coupled to the Gαi subunit (Matsushita et al. 1993; Ohnishi et al. 1994), it is likely that AC6 mediates the response to somatostatin in pancreatic acini.
AC7 participates in the synergistic effect of Gα12/13 on Gs-stimulated cAMP production in HEK cells (Jiang et al. 2008). The lack of AC7 expression in pancreatic acini is consistent with our previous results which demonstrated that the expression of p115-RGS, which is an regulator of G protein signaling domain of p115-RhoGEF and an inhibitor of Gα12/13 (Chen et al. 2003), does not modify VIP-induced cAMP production in mouse pancreatic acini (Sabbatini et al. 2010). The significance of the higher expression of this isoform in pancreatic ducts remains to be determined.
Changes in cytosolic calcium levels are known to either enhance or impair cAMP generation through calcium-sensitive AC isoforms. AC1 and AC8 are the major calcium-activated isoforms whereas AC5 and AC6 are calcium-inhibited AC isoforms. The presence of only AC6 indicates the existence of a mechanism of regulation which is activated by an increase in cytosolic calcium. AC8 has been shown to be responsible for calcium stimulation of cAMP production in mouse parotid acini (Watson et al. 2000). The authors showed the lack of a stimulatory effect of calcium on cAMP generation in isolated parotid acini from AC8−/− mice. In parotid acini, unlike in pancreatic acini, calcium is able to increase cAMP formation, which is the primary signal for parotid amylase secretion (Seino & Shibasaki, 2005). The lack of AC8 expression in mouse pancreatic acini is consistent with the lack of a stimulatory response to calcium in cAMP generation.
AC9 is the most divergent in sequence of the membrane-bound isoforms and the only isoform which is not stimulated by forskolin (Premont et al. 1996; Sadana & Dessauer, 2009). AC9 is inhibited by calcineurin (Cooper, 2003), which is present in pancreatic acini (Groblewski et al. 1998; Gurda et al. 2008), and involved in pancreatic adaptive growth (Tashiro et al. 2004), as well as in caerulein-induced intracellular pancreatic zymogen activation (Husain et al. 2007). AC9 is stimulated by calcium/CaMKII phosphorylation (Cumbay & Watts, 2005), which is also present in pancreatic acini and activated by CCK (Duan et al. 1994). The currently available validated antibodies allowed us to document the presence of AC9 at the protein level in the pancreas. Unfortunately, antibodies to other AC isoforms we tested did not work on mouse pancreas. Future studies may be better carried out on human tissues because most large-scale antibody projects use human sequences as antigens.
cAMP can be produced either by membrane-associated AC or by the soluble AC (AC10) (Buck et al. 1999). Recently, AC10 has been found in pancreatic acini and shown to be possibly involved in the development of pancreatitis, but not in physiological events (Kolodecik et al. 2012).
In conclusion, in the exocrine pancreas activation of AC occurs after the stimulation by secretagogues VIP and secretin, which acting through activation of VPACs and secretin receptors, respectively, activates Gαs. Multiple AC isoforms are differentially expressed in mouse pancreatic acini and ducts: AC3, AC4, AC6 and AC9 are expressed in pancreatic acini and ducts whereas AC7 is expressed only in pancreatic ducts. We found that in pancreatic exocrine cells AC6 plays a critical role in cAMP/PKA-mediated signalling. AC6 deletion decreased cAMP generation, as well as in the activity of PKA in both pancreatic acini and ducts. As a consequence, a decrease in amylase secretion and pancreatic fluid occurred. The role and importance of other AC isoforms will require future studies in which these isoforms are deleted or inhibited.
Translational perspective
Adenylyl cyclase (AC) isoforms are differentially expressed in mouse pancreatic acini, ducts and islets. To study the importance of AC6 in pancreatic physiology, AC6-deficient mice were used. Deletion of AC6 has a negative effect on the function of pancreatic exocrine cells: the lack of AC6 not only reduces amylase secretion but also impairs fluid secretion from pancreatic ducts. The participation of AC6 in pancreatic pathophysiology is of clinical interest.
Acknowledgments
We thank Brad Nelson for his excellent assistant with immunohistochemistry.
Glossary
- AC
adenylyl cyclase
- 8-Br-cAMP
8-bromoadenosine 3′,5′cyclic monophosphate sodium salt
- cAMP
cyclic AMP
- CCh
carbachol
- CCK-8
sulfated cholecystokinin octapeptide
- CFTR
cystic fibrosis transmembrane conductance regulator
- CPT-cAMP
8-(4-chlorophenylthio)adenosine 3′,5′-cyclic monophosphate sodium salt
- FSK
forskolin
- IBMX
3-isobutyl-1-methylxantine
- 8-pCPT-2′-O-Me-cAMP
8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate
- PMA
phorbol 12-myristate 13-acetate
- PK
protein kinase
- SBTI
soybean trypsin inhibitor
- VIP
vasoactive intestinal polypeptide
- VPAC
vasoactive intestinal polypeptide receptor
Additional information
Competing interests
None
Authors’ contributions
M.E.S. conceived and designed the experiments, collected, analysed and interpreted the data, and wrote the paper; L.D. conducted some experiments, contributed with some tools and revised the manuscript; S.I.L. conducted some experiments, contributed with some tools, and revised the manuscript; T.T. provided AC6−/− mice and revised the manuscript; J.A.W. conceived and designed the experiments, analysed and interpreted the data, and revised the manuscript. All the authors read and approved the final version of the manuscript.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-41122 (to J.A.W.), American Heart Association 11GRNT7610059 (to T.T.), Morphology and Image Analysis Core of the Michigan Diabetes Research and Training Centre (P60DK020572) and the Protein Identification and Localization Core of the Michigan Gastrointestinal Peptide Centre (Grant P30 DK-34933).
Supplementary material
Supplemental Table S1-S2
References
- Akiyama T, Hirohata Y, Okabayashi Y, Imoto I, Otsuki M. Supramaximal CCK and CCh concentrations abolish VIP potentiation by inhibiting adenylyl cyclase activity. Am J Physiol. 1998;275:G1202–1208. doi: 10.1152/ajpgi.1998.275.5.G1202. [DOI] [PubMed] [Google Scholar]
- Argent BE, Gray MA, Steward MC, Case RM. Cell physiology of pancreatic ducts. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 5th edn. Amsterdam: Elsevier; 2012. pp. 1399–1423. [Google Scholar]
- Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signalling molecule in mammals. Proc Natl Acad Sci U S A. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham DB, McChesney DJ, Thurston KC, Williams JA. Interaction of cholecystokinin and vasoacive intestinal polypeptide on function of mouse pancreatic acini in vitro. J Physiol. 1984;349:475–482. doi: 10.1113/jphysiol.1984.sp015168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham DB, Williams JA. Activation of protein kinase activity in pancreatic acini by calcium and cAMP. Am J Physiol. 1984;246:G500–G508. doi: 10.1152/ajpgi.1984.246.5.G500. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Husain SZ, Kolodecik TR, Grant WM, Gorelick FS. Cyclic AMP-dependent protein kinase and Epac mediate cyclic AMP responses in pancreatic acini. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1403–1410. doi: 10.1152/ajpgi.00478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Singer WD, Wells CD, Sprang SR, Sternweis PC. Mapping the Gα13 binding interface of the rgRGS domain of p115RhoGEF. J Biol Chem. 2003;278:9912–9919. doi: 10.1074/jbc.M212695200. [DOI] [PubMed] [Google Scholar]
- Collen MJ, Sutliff VE, Pan GZ, Gardner JD. Postreceptor modulation of action of VIP and secretin on pancreatic enzyme secretion by secretagogues that mobilize cellular calcium. Am J Physiol. 1982;242:G423–G428. doi: 10.1152/ajpgi.1982.242.4.G423. [DOI] [PubMed] [Google Scholar]
- Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem J. 2003;375:517–529. doi: 10.1042/BJ20031061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumbay MG, Watts VJ. Novel regulatory properties of human type 9 adenylate cyclase. J Pharmacol Exp Ther. 2004;310:108–115. doi: 10.1124/jpet.104.065748. [DOI] [PubMed] [Google Scholar]
- Cumbay MG, Watts VJ. Gαq potentiation of adenylate cyclase type 9 activity through a Ca2+/calmodulin-dependent pathway. Biochem Pharmacol. 2005;69:1247–1256. doi: 10.1016/j.bcp.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Duan RD, Guo YJ, Williams JA. Conversion to Ca2+-independent form of Ca2+/calmodulin protein kinase II in rat pancreatic acini. Biochem Biophys Res Commun. 1994;199:368–373. doi: 10.1006/bbrc.1994.1238. [DOI] [PubMed] [Google Scholar]
- Gardner JD, Sutliff VE, Walker MD, Jensen RT. Effects of inhibitors of cyclic nucleotide phosphodiesterase on actions of cholecystokinin, bombesin, and carbachol on pancreatic acini. Am J Physiol. 1983;245:G676–G680. doi: 10.1152/ajpgi.1983.245.5.G676. [DOI] [PubMed] [Google Scholar]
- Groblewski GE, Yoshida M, Bragado MJ, Ernst SA, Leykam J, Williams JA. Purification and characterization of a novel physiological substrate for calcineurin in mammalian cells. J Biol Chem. 1998;273:22738–22744. doi: 10.1074/jbc.273.35.22738. [DOI] [PubMed] [Google Scholar]
- Gurda GT, Guo L, Lee SH, Molkentin JD, Williams JA. Cholecystokinin activates pancreatic calcineurin-NFAT signalling in vitro and in vivo. Mol Biol Cell. 2008;19:198–206. doi: 10.1091/mbc.E07-05-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain SZ, Grant WM, Gorelick FS, Nathanson MH, Shah AU. Caerulein-induced intracellular pancreatic zymogen activation is dependent on calcineurin. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1594–1599. doi: 10.1152/ajpgi.00500.2006. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Lindsay AR, Case RM. Accumulation of intracellular HCO3− by Na+-HCO3− cotransport in interlobular ducts from guinea-pig pancreas. J Physiol. 1996;495:169–178. doi: 10.1113/jphysiol.1996.sp021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LI, Collins J, Davis R, Fraser ID, Sternweis PC. Regulation of cAMP responses by the G12/13 pathway converges on adenylyl cyclase VII. J Biol Chem. 2008;283:23429–23439. doi: 10.1074/jbc.M803281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodecik TR, Shugrue CA, Thrower EC, Levin LR, Buck J, Gorelick FS. Activation of soluble adenylyl cyclase protects against secretagogue stimulated zymogen activation in rat pancreaic acinar cells. PLoS One. 2012;7:e41320. doi: 10.1371/journal.pone.0041320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Wigley WC, Zeng W, Noel LE, Marino CR, Thomas PJ, Muallem S. Regulation of Cl−/HCO3− exchange by cystic fibrosis transmembrane conductance regulator expressed in NIH 373 and HEK 293 cells. J Biol Chem. 1999;274:3414–3421. doi: 10.1074/jbc.274.6.3414. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Okabayashi Y, Hasegawa H, Koide M, Kido Y, Okutani T, Sugimoto Y, Kasuga M. In vitro inhibitory effect of somatostatin on secretin action in exocrine pancreas of rats. Gastroenterology. 1993;104:1146–1152. doi: 10.1016/0016-5085(93)90286-l. [DOI] [PubMed] [Google Scholar]
- O'Sullivan AJ, Jamieson JD. Protein kinase A modulates Ca2+- and protein kinase C-dependent amylase release in permeabilized rat pancreatic acini. Biochem J. 1992;287:403–406. doi: 10.1042/bj2870403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi H, Mine T, Kojima I. Inhibition by somatostatin of amylase secretion induced by calcium and cyclic AMP in rat pancreatic acini. Biochem J. 1994;304:531–536. doi: 10.1042/bj3040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont RT, Matsuoka I, Mattei MG, Pouille Y, Defer N, Hanoune J. Identification and characterization of a widely expressed form of adenylyl cyclase. J Biol Chem. 1996;271:13900–13907. doi: 10.1074/jbc.271.23.13900. [DOI] [PubMed] [Google Scholar]
- Sabbatini ME, Bi Y, Ji B, Ernst SA, Williams JA. CCK activates RhoA and Rac1 differentially through Gα13 and Gαq in mouse pancreatic acini. Am J Physiol Cell Physiol. 2010;298:C592–601. doi: 10.1152/ajpcell.00448.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbatini ME, Chen X, Ernst SA, Williams JA. Rap1 activation plays a regulatory role in pancreatic amylase secretion. J Biol Chem. 2008;283:23884–23894. doi: 10.1074/jbc.M800754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbatini ME, Villagra A, Davio CA, Vatta MS, Fernandez BE, Bianciotti LG. Atrial natriuretic factor stimulates exocrine pancreatic secretion in the rat through NPR-C receptors. Am J Physiol Gastrointest Liver Physiol. 2003;285:G929–937. doi: 10.1152/ajpgi.00010.2003. [DOI] [PubMed] [Google Scholar]
- Sadana R, Dessauer CW. Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals. 2009;17:5–22. doi: 10.1159/000166277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans MD, Sabbatini ME, Ernst SA, D’Alecy LG, Nishijima I, Williams JA. Secretin is not necessary for exocrine pancreatic development and growth in mice. Am J Physiol Gastrointest Liver Physiol. 2011;301:G791–798. doi: 10.1152/ajpgi.00245.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- Stryjek-Kaminska D, Piiper A, Zeuzem S. EGF inhibits secretagogue-induced cAMP production and amylase secretion by Gi proteins in pancreatic acini. Am J Physiol. 1995;269:G676–682. doi: 10.1152/ajpgi.1995.269.5.G676. [DOI] [PubMed] [Google Scholar]
- Tang T, Gao MH, Lai NC, Firth AL, Takahashi T, Guo T, Yuan JX, Roth DM, Hammond HK. Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation. 2008;117:61–69. doi: 10.1161/CIRCULATIONAHA.107.730069. [DOI] [PubMed] [Google Scholar]
- Tashiro M, Samuelson LC, Liddle RA, Williams JA. Calcineurin mediates pancreatic growth in protease inhibitor-treated mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G784–790. doi: 10.1152/ajpgi.00446.2003. [DOI] [PubMed] [Google Scholar]
- Watson EL, Jacobson KL, Singh JC, Idzerda R, Ott SM, DiJulio DH, Wong ST, Storm DR. The type 8 adenylyl cyclase is critical for Ca2+ stimulation of cAMP accumulation in mouse parotid acini. J Biol Chem. 2000;275:14691–14699. doi: 10.1074/jbc.275.19.14691. [DOI] [PubMed] [Google Scholar]
- Willems PH, Tilly RH, de Pont JJ. Pertussis toxin stimulates cholecystokinin-induced cyclic AMP formation but is without effect on secretagogue-induced calcium mobilization in exocrine pancreas. Biochim Biophys Acta. 1987;928:179–185. doi: 10.1016/0167-4889(87)90119-4. [DOI] [PubMed] [Google Scholar]
- Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- Williams JA, Yule DI. Stimulus-secretion coupling in pancreatic acinar cells. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 5th edn. Amsterdam: Elsevier; 2012. pp. 1361–1398. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


