Abstract
Colitis, induced by trinitrobenzene sulfonic acid (TNBS) in guinea pig, leads to decreased purinergic neuromuscular transmission resulting in a reduction in inhibitory junction potentials (IJPs) in colonic circular muscle. We explored possible mechanisms responsible for this inflammation-induced neurotransmitter plasticity. Previous studies have suggested that the deficit in inflamed tissue involves decreased ATP release. We therefore hypothesized that decreased purinergic transmission results from inflammation-induced free radical damage to mitochondria, leading to decreased purine synthesis and release. Stimulus-induced release of purines was measured using high-performance liquid chromatography, and quantities of all purines measured were significantly reduced in the inflamed colons as compared to controls. To test whether decreased mitochondrial function affects the IJP, colonic muscularis preparations were treated with the mitochondrial ATP synthase inhibitors oligomycin or dicyclohexylcarbodiimide, which resulted in a significant reduction of IJP amplitude. Induction of oxidative stress in vitro, by addition of H2O2 to the preparation, also significantly reduced IJP amplitude. Purinergic neuromuscular transmission was significantly restored in TNBS-inflamed guinea pigs, and in dextran sodium sulfate-inflamed mice, treated with a free radical scavenger. Furthermore, propulsive motility in the distal colons of guinea pigs with TNBS colitis was improved by in vivo treatment with the free radical scavenger. We conclude that oxidative stress contributes to the reduction in purinergic neuromuscular transmission measured in animal models of colitis, and that these changes can be prevented by treatment with a free radical scavenger, resulting in improved motility.
Key points
Colitis is associated with an attenuation of purinergic inhibitory neuromuscular transmission.
In this study we tested the hypothesis that purine release is disrupted due to an effect of oxidative stress on mitochondrial purine synthesis.
Stimulus-induced release of purines was decreased in inflamed colons.
Disruption of mitochondrial purine synthesis, or induction of oxidative stress, mimicked the effects of inflammation on purinergic neuromuscular transmission.
Treatment of animals with a free radical scavenger resulted in a protection of the purinergic neuromuscular transmission.
Treatment with a free radical scavenger also resulted in an improvement of propulsive motility in inflamed colons.
Introduction
The enteric nervous system is a highly complex structure capable of a large amount of autonomous activity. One of the most important motor reflexes in the enteric nervous system is the peristaltic reflex, responsible for moving luminal contents in an oral to aboral direction. There is polarity to this circuitry: ascending interneurons synapse primarily on excitatory motor neurons, while descending interneurons synapse primarily on inhibitory motor neurons (Brookes, 2001; Furness, 2006). The resulting contraction above the stimulus and relaxation below the stimulus results in a pressure gradient and the effective movement of luminal contents in the aboral direction. Disruption of propulsive motility is a hallmark of colitis in both animal models and patients with inflammatory bowel disease (IBD) (Reddy et al. 1991), and better understanding of the mechanisms leading to this disruption will lead to better treatment options for these patients.
There are many key elements working in concert for propulsive motility to be effective, and disruption of any of these may lead to disruption in motility. For example, there is altered serotonin signalling in trinitrobenzene sulfonic acid (TNBS)-induced colitis, leading to increased serotonin (5-hydroxytryptamine, 5-HT) availability, which may disrupt motility through 5-HT receptor desensitization (Linden et al. 2003a, 2005). Another change seen in colitis is hyperexcitability of intrinsic primary afferent (AH) neurons. In the colon of TNBS colitis guinea pigs, AH neurons fire more action potentials and exhibit more spontaneous activity, and these changes are associated with a decrease in the amplitude of the afterhyperpolarization (AHP) (Linden et al. 2003b; Lomax et al. 2005). In the myenteric plexus of the TNBS guinea pig colon, this decrease in AHP is due, at least in part, to increased ionic flow through the Ih channel (Linden et al. 2003b), and blocking excess flow through this channel dramatically improves motility in these preparations (Hoffman et al. 2011). Another neuroplastic change seen in TNBS colitis is synaptic facilitation in both AH and S neurons (Linden et al. 2003b; Lomax et al. 2005; Krauter et al. 2007a).
While many of the changes seen in inflammation have been previously characterized, one area still under investigation is the inflammation-induced change that occurs at the neuromuscular junction. In the guinea pig TNBS model of colitis, pellet velocity through the colon is slowed or halted specifically at areas of ulceration. This led to studies examining neuromuscular transmission at areas of ulceration, and it was found that, while there is no change in the excitatory junction potential, there is a significant decrease in the amplitude of the inhibitory junction potential (IJP) (Strong et al. 2010). More specifically, there is a significant decrease in the amplitude of the fast, purinergic component of the IJP. This is not due to a decrease in the fibre density of inhibitory nerves or to a decrease in P2Y1 receptor responsiveness. Furthermore, antagonism of the P2Y1 receptor in a guinea pig propulsive motility assay results in significant slowing of pellet velocity, indicating that decreased purinergic transmission may be playing a significant role in the slowed motility seen in colitis.
The aim of the current study was to elucidate the mechanisms responsible for the decrease in purinergic neuromuscular transmission and to demonstrate that reversal of these changes improved propulsive motility. High-performance liquid chromatography (HPLC) was used to measure electrically stimulated release of purine neurotransmitter candidates β-NAD and ATP and their metabolites from normal and inflamed colonic preparations. To test the hypothesis that mitochondrial function is linked to the strength of purinergic signalling, IJPs were measured in normal colonic tissue after pharmacological disruption of mitochondrial function as well as after acute free radical damage. To examine whether the IJP could be protected in inflamed animals, the effect of in vivo administration of a free radical scavenger was tested in two animal models of intestinal inflammation, guinea pig TNBS colitis and murine dextran sodium sulfate (DSS) colitis. The results of this study indicate that oxidative stress, leading to disruption of mitochondrial function and decreased synthesis of purines, contributes to decreased purinergic neuromuscular transmission seen in guinea pig TNBS colitis as well as mouse DSS colitis, and that the prevention of these changes by treatment with a free radical scavenger represents a novel treatment strategy for patients suffering from IBD.
Methods
Animal preparations
All methods used in this study were approved by the University of Vermont Animal Care and Use Committee. Experiments were performed on albino guinea pigs of either sex weighing 250–350 g (Charles River, Montreal, Canada). The animals were housed in microfilter cages, had access to food and water ad libitum and were maintained on a 12/12 h light–dark cycle. To induce colitis, animals received a TNBS enema, as previously described (Linden et al. 2003a). Briefly, guinea pigs were anaesthetized (isoflurane, 4% induction, 2% maintenance), a polyethylene catheter was inserted 7 cm into the colon, and a 0.3 ml bolus of TNBS was delivered (Fluka, Milwaukee, WI, USA; 25 mg ml−1, dissolved in 30% ethanol to facilitate mucosal barrier disruption). Experiments were performed 6 days following TNBS administration.
Mouse experiments were performed on male Balb/C mice age 10–12 weeks (Charles River). Colitis was induced by dissolving DSS (molecular weight = 36–50 kDa; MP Biomedicals, LLC, Solon, OH, USA) to a concentration of 4% (w/v) in the drinking water. The mice drank the DSS solution until they were killed 6 or 7 days later.
All colitis animals were weighed daily and inspected twice each day for signs of pain (vocalization, lack of mobility) and distress (lack of grooming). If an animal lost >20% of its weight, it was killed.
All animals were killed by isoflurane overdose and exsanguination, and the distal colon was removed. A macroscopic damage score was used to assess severity of colitis, based on weight loss, intestinal wall thickness, presence and extent of ulceration, severity of adhesions, hyperaemia, blood in stool and diarrhoea. Macroscopic damage scores were consistent with previous reports (Linden et al. 2003a; Krauter et al. 2007a; Strong et al. 2010; Hoffman et al. 2011).
Motility analyses
The GastroIntestinal Motility Monitor (GIMM; Med Associates, St. Albans, VT, USA) was used to assess motility in guinea pig colons, as previously described (Hoffman et al. 2010). The distal colon was removed and loosely pinned in an organ chamber and continuously exposed to warmed and aerated Krebs solution (10 ml min−1; mmol l−1: NaCl, 121; KCl, 5.9; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 25; NaH2PO4, 1.2; and glucose 8; aerated with 95%O2/5%CO2; Sigma, St Louis, MO, USA) After a 30 min equilibration period, an epoxy-coated guinea pig faecal pellet was inserted into the oral end and tracked by video camera as it moved distally, and computer software was used to analyse the motility pattern over a 5 cm segment of colon. Three to five trials were attained for each experimental condition, with 5 min between trials. For some trials, the HCN channel blocker ZD 7288 (10 μm; Tocris Bioscience, Ellisville, MO, USA) was added to the circulating Krebs solution after baseline motility patterns were obtained.
Intracellular recording
The distal colon of mice and guinea pigs was removed and dissected in iced Krebs by cutting along the mesenteric border and removing mucosal and submucosal layers to expose the circular muscle layer. The tissue was then pinned flat in a Sylgard-lined recording chamber, and continuously exposed to warmed and aerated circulating Krebs solution. Nifedipine (5 μm; Sigma-Aldrich) and atropine (200 nm; Sigma-Aldrich) were added to reduce smooth muscle contractile activity. Glass microelectrodes used for recording were filled with 2 m KCl and had resistances ranging from 80 to 120 MΩ. An inverted microscope was used at 200× magnification to visualize the tissue. Voltage recordings were obtained with an Axoclamp-2A amplifier (Axon Instruments, Union City, CA, USA), and PowerLab Chart (version 5.01; AD Instruments, Castle Hill, NSW, Australia) was used to analyse the signals. Smooth muscle cells were impaled at random.
Transmural stimuli (0.3 ms pulse duration, 0.5 Hz, 50 V) were used to activate junction potentials. Due to the polarity of the descending inhibitory motor neurons, evoked IJPs were recorded aborally to transmural stimulating electrodes, and excitatory junction potentials (EJPs) were recorded oral to the field stimulation electrodes. In some studies, the nitrergic component of the IJP was isolated by blocking P2Y1 receptors with the antagonist MRS-2179 (3 μm, Sigma-Aldrich), whereas EJPs were isolated by blocking P2Y1 receptors with MRS-2179, and nitric oxide synthase with N-nitro-l-arginine.
Agonist-induced hyperpolarizations were induced by microinjection using a Picospritzer II (General Valve Corp., Fairfield, NJ, USA). Glass micropipettes were filled with 2-methyl thio-ATP (1 mm) and positioned close to the site of intracellular recording using a micromanipulator. Puffs of 2-methyl thio-ATP were delivered in 200 ms pulses at 10 p.s.i.
Dihydroethidium (DHE) staining for oxidative stress
Guinea pig and mouse colons were dissected as for intracellular recording and placed in a Krebs–Ringers–Hepes (KRH) solution of the following composition (mm): NaCl 130, KCl 1.3, CaCl2 2.2, MgSO4 1.2, KH2PO4 1.2, Hepes 1.0, glucose 0.09 (pH = 7.4; Sigma). Unfixed preparations were incubated in 2 μm DHE at 37°C for 60 min in light-protected tubes. The wholemounts were then washed three times with KRH solution and mounted on slides with Citiflour and coverslipped. Images were captured with an Optronics MagnaFire CCD camera (Goleta, CA, USA), attached to the Olympus AX70 microscope (Olympus America, Center Valley, PA, USA). Images were captured with identical exposure settings and no adjustments were made to brightness or contrast. Evaluations of relative intensities were made by visually comparing the images.
Purine release studies
Distal guinea pig colon was dissected as for electrophysiology recording. After a 30 min equilibration period in warmed, aerated Krebs solution, a 2 cm piece of tissue was moved into a 200 μl chamber with two transmural stimulating electrodes. The chamber was filled with Krebs solution, and samples were saved in a citrate phosphate buffer from each of the following conditions: 60 s with no stimulus (basal release); and 60 s with stimulation at 16 Hz, 0.5 ms duration pluses.
Endogenous purines that were released from colonic muscularis preparations were converted to 1,N6-etheno derivatives to increase detection sensitivity, as described previously (Durnin et al. 2012). Purines that were analysed include ATP, ADP, AMP, adenosine (ADO), and β-NAD plus ADPR plus cyclic ADPR that eluted as one chromatography signal as shown previously (Mutafova-Yambolieva et al. 2007; Hwang et al. 2011). In all systems tested so far, β-NAD is the predominant substance in the composite peak (Mutafova-Yambolieva et al. 2007; Hwang et al. 2011; Durnin et al. 2013) and therefore we refer to this peak as β-NAD throughout the study. Citrate phosphate buffer (150 μl, pH 4.0) was added to 250 μl of superfusate sample, and purines were etheno-derivatized by adding 10 μl of 2-chloroacetaldehyde for 40 min at 80°C. The resulting ethenopurines were assayed by HPLC with fluorescence detection (HPLC-FLD; Agilent Technologies, Inc., Wilmington, DE, USA), as described previously (Mutafova-Yambolieva et al. 2007; Durnin et al. 2012). Purine quantities present in each sample were calculated from purine standard calibration curves run simultaneously with every set of experimental samples. Results were normalized for sample volume and weight of tissue. The overflow of purines was expressed in fmol mg-1 tissue.
Data analysis
Statistical analyses were performed using GraphPad Prism software (version 6 for Macintosh, GraphPad Software, San Diego, CA, USA). For comparisons of two sets of data, an unpaired Student's t test was used. When three or more sets of data were compared, we used a two-way analysis of variance (ANOVA) with Bonferroni post-test. We used the ANOVA to evaluate percentage reduction of the junction potentials in response to oligomycin, and for the guinea pig and mouse experiments involving the free radical scavenger Tempol. A P value of <0.05 was considered to be statistically significant. For motility experiments, n values represent the number of animals tested; for electrophysiological experiments, n values represent the number of cells tested; for purine release studies, n values represent the number of preparations tested. Data presented are means ± SEM for n animals.
Results
Stimulus-induced release of purines is decreased in TNBS colitis
In the guinea pig distal colon, TNBS colitis is associated with a decrease in the amplitude of the purinergic component of the IJP (Strong et al. 2010). It was demonstrated that this is not due to a decrease in responsiveness of smooth muscle to purines, as the relaxation response of muscle strips to ATP is unchanged in TNBS ulcerated regions. Neither does a decrease in motor neuron fibre density appear to be a factor, based on immunohistochemistry studies. Therefore, to test whether altered release of purines from the muscularis is responsible for the decrease in purinergic neuromuscular transmission, HPLC was used to analyse the quantities of purines released from muscularis preparations into a fixed volume of Krebs in response to electrical stimulation. The parameters for electrical stimulation (0.5 ms pulse duration; 16 Hz for 60 s) were chosen based on train parameters that we have used previously to evaluate neurogenic purine release in mouse, monkey and human colons (Mutafova-Yambolieva et al. 2007; Hwang et al. 2011), and intracellular recording parameters (Strong et al. 2010; current study).
In the current study, the effects of TNBS colitis on release of ATP, ADP, AMP, ADO, β-NAD, separately, and all purines collectively was evaluated. Samples were collected under both basal and stimulated release conditions, and the results are presented as the difference between stimulated and basal release to more accurately reflect the quantities of purines released by the electrical field stimulation. Release of all purines tested was decreased in muscularis samples from TNBS-inflamed colons as compared to controls (Fig. 1; ATP, P= 0.01; ADP, P= 0.005; AMP, P= 0.015; ADO, P= 0.004; β-NAD, P= 0.04; total purines, P= 0.006; n= 5 per group; unpaired t test). These results support the concept that a decrease in purine release from neuromuscular nerve terminals contributes to the deficit in purinergic neuromuscular transmission in the inflamed colon.
Figure 1. Stimulus-induced release of purines is reduced in TNBS colitis.
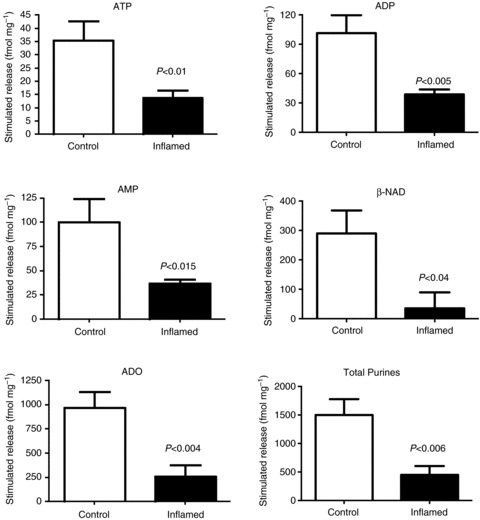
Graphs illustrating levels of purines, measured in the Krebs bathing solution, from muscularis preparations in normal and TNBS-inflamed guinea pig colons. These graphs represent stimulus-induced release as levels are presented as stimulated minus basal release; n= 5 per group; unpaired t test.
Exposure of normal tissue to ATP synthase inhibitors leads to a decrease in the amplitude of the IJP
One possible mechanism for the decrease in purine release and associated attenuation of the IJP is a disruption of purine synthesis within the nerve terminals. Therefore, we tested the hypothesis that inflammation-induced decreases in purinergic neuromuscular transmission can be mimicked by disrupting mitochondrial ATP synthesis in normal preparations. Evoked IJPs were measured from circular smooth muscle cells from distal guinea pig colon before and after exposure to the ATP synthase inhibitor oligomycin. After 30 min exposure to 2.0 μm oligomycin, the amplitude of the IJP was reduced by 55% (Fig. 2A and B; P < 0.0001; unpaired t test; control, n= 91 cells; oligomycin, n= 73 cells from six guinea pigs). In three preparations, the time course of the decrease in IJP amplitude was investigated. In each case, the onset of the response occurred at about 10 min following administration of oligomycin, and the amplitude of the IJP decreased progressively for the next 20 min.
Figure 2. Inhibition of ATP synthase leads to a decrease in the amplitude of the IJP.
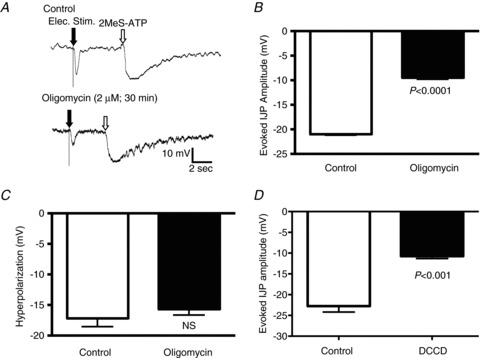
IJPs were evoked by transmural electrical field stimulation oral to the impaled circular muscle cell. A, representative traces for IJPs before (top trace) and after (bottom trace) exposure to the ATP synthase inhibitor oligomycin (2 μm). The IJPs in both traces are followed by representative hyperpolarizations in response to pressure microinjection of a P2Y1 agonist. B, quantification of the IJP amplitude in preparations exposed to oligomycin for 30 min versus control (control, n= 91 cells; oligomycin, n= 73 cells). C, graph illustrating the hyperpolarization amplitude in response to the P2Y1 agonist, 2-MeSATP in the presence of oligomycin versus control (control, n= 11 cells; oligomycin, n= 14 cells). This indicates that the cells were viable and responsive to P2Y1 receptor stimulation. D, graph showing IJP amplitude in preparations exposed to the ATP synthase inhibitor DCCD (0.1 mm; > 30 min exposure) versus control (n= 14 cells per group). Data were compared with an unpaired t test.
To ensure that the decrease in IJP amplitude was not due to a decrease in the viability of the tissue, we compared the hyperpolarization induced by pressure microinjection of the P2Y1 agonist 2-methyl thio-ATP (2-MeSATP) before and after the addition of oligomycin. In these experiments, there was no significant difference between the hyperpolarization induced by 2-MeSATP (Fig. 2A and C; P= 0.34; unpaired t test; control, n= 11 cells; oligomycin, n= 14 cells). The resting membrane potential was not altered by oligomycin (control, −50.1 ± 0.7 mV; oligomycin, −50.9 ± 1.0 mV; P= 0.55; unpaired t test).
Oligomycin inhibits ATP synthase by blocking the F0 subunit of the proton channel, but it is known to be somewhat non-selective. Therefore, we tested the effects of another inhibitor of mitochondrial ATP synthesis, dicyclohexylcarbodiimide (DCCD), which acts by blocking the F0 and F1 subunits of the mitochondrial proton channel. After a 30 min exposure to 0.1 mm DCCD the amplitude of the IJP was significantly reduced (Fig. 2D; P < 0.001; unpaired t test; n= 14 cells per group from four guinea pigs). This treatment did not affect the resting membrane potential of the smooth muscle cells (control, −49.4 ± 0.7 mV; DCCD, −49.9 ± 0.8 mV; unpaired t test). These results demonstrate that disruption of mitochondrial purine production leads to a decrease in purinergic neuromuscular transmission.
In our initial studies of the effects of inflammation on inhibitory neuromuscular transmission, we found that purinergic neurotransmission was affected to a greater extent than the nitrergic component of the IJP or the cholinergic EJP. There was a 16.2% reduction in stimulus-induced nitrergic transmission, and a 2% increase in the EJP, neither of which reached significance (nitrergic IJP, P= 0.065; EJP, P= 0.88; unpaired t test) (Strong et al. 2010). In the present study, we tested the effects of oligomycin on the nitrergic component of the IJP by blocking the purinergic component with the P2Y1 receptor antagonist MRS-2179 (3 μm). After 30 min exposure to oligomycin, the amplitude of the nitrergic component of the IJP was reduced by 19% (Fig. 3A; P < 0.02; unpaired t test; control, n= 15; oligomycin, n= 12 from three guinea pigs). We also tested the effect of oligomycin on the EJP (IJPs were inhibited with 3 μm MRS-2179 plus 100 μm–N-nitro-l-arginine and we recorded oral to the stimulating electrode), and detected a 24% reduction (Fig. 3A; P < 0.0001; unpaired t test; control, n= 11; oligomycin, n= 10 from four guinea pigs). Therefore, the nitrergic component of the IJP, and the cholinergic EJP are sensitive to mitochondrial ATP synthesis inhibition, but as with the response to inflammation, they are not affected as much as the purinergic IJP (ANOVA; Fig. 3C).
Figure 3. Oligomycin causes less extensive decreases in the nitrergic component of the IJP, and in the cholinergic EJP.
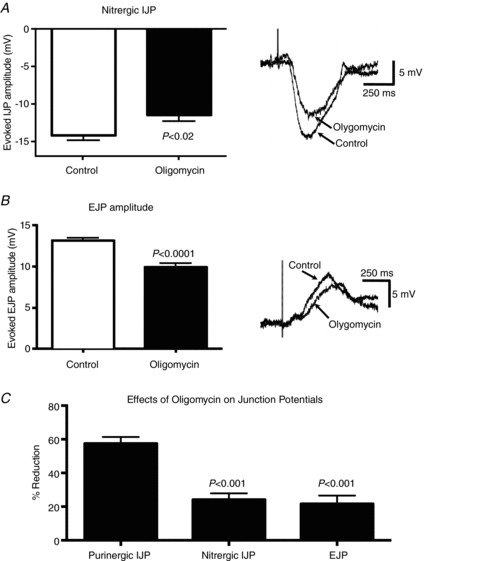
A, graph and intracellular recordings showing the nitrergic IJP amplitude in preparations exposed to 2 μm oligomycin versus control (controls, n= 15 cells; oligomycin, n= 12 cells). The nitrergic component of the IJP was isolated by inhibiting the purinergic component with the P2Y1 receptor antagonist MRS-2179. B, graph and intracellular recordings showing the effects of 2 μm oligomycin on the EJP (controls, n= 11 cells; oligomycin, n= 10 cells). While oligomycin reduced the nitric IJP and cholinergic EJP by 19 and 24% respectively, the purinergic IJP was more sensitive to ATP synthase inhibition as it was decreased by 55% (see Fig. 2). Data in A and B were compared with an unpaired t test. C, graph demonstrating that the oligomycin-induced reduction of the purinergic IJP is significantly more extensive than the reduction of the nitrergic IJP or the EJP. Data in C were compared by ANOVA.
Induction of oxidative stress in vitro reduces the IJP
Oxidative stress is a common feature of inflammatory responses, and previous studies have demonstrated that free radical levels are elevated in the muscularis of animals with TNBS or DSS colitis (Shi et al. 2011). It is also known that free radicals damage mitochondria and their ability to generate ATP (Navarro & Boveris, 2007). As we learned from the experiments described above that the IJP is sensitive to decreased mitochondrial ATP formation, we next tested the hypothesis that, in normal tissue, acute free radical damage would decrease the amplitude of the IJP. Evoked IJPs were measured from circular smooth muscle cells from distal guinea pig colon before and after exposure to the free radical donor hydrogen peroxide (H2O2). After 30 min exposure to 0.1 mm H2O2, the amplitude of the IJP was reduced by 50% (Fig. 4A; P < 0.0001; unpaired t test; control, n= 20 cells; H2O2, n= 16 cells from 11 guinea pigs). As with the oligomycin studies, to ensure that the decrease in IJP amplitude was not due to a decrease in the viability of the tissue, we compared hyperpolarizations induced by pressure microejection of 2-MeSATP before and after the addition of H2O2 and found that there was no significant difference (Fig. 4B; control, −15.4 ± 1.4 mV, n= 11; H2O2, −15.6 ± 0.8 mV, n= 8; P= 0.9; unpaired t test). Furthermore, the resting membrane potential was not altered by H2O2 (control, –50.9 ± 0.8 mV; H2O2, −49.1 ± 0.7 mV; P= 0.19; unpaired t test). These results demonstrate that the decreased IJP measured in colitis can be mimicked by incurring oxidative stress in normal tissue, thereby linking oxidative stress to mitochondrial function and purinergic neuromuscular transmission.
Figure 4. Exposure of normal tissue to the free radical donor H2O2 reduced the amplitude of the IJP.
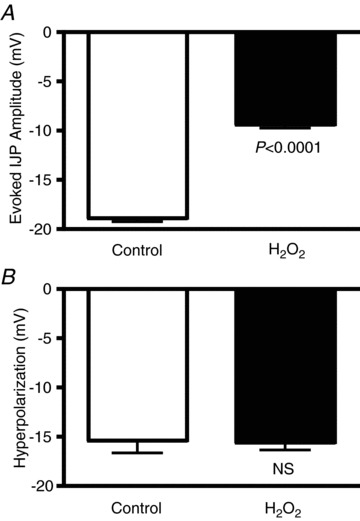
A, quantification of the IJP amplitude in preparations exposed to 0.1 mm H2O2 (≥30 min) versus control (control, n= 20 cells; H2O2, n= 16 cells). B, quantification of agonist-induced hyperpolarizations of circular muscle cells before and after exposure to H2O2 (control, n= 11 cells; H2O2, n= 8 cells; P= 0.9). Data were compared with an unpaired t test.
The free radical scavenger Tempol decreases oxidative stress and protects the IJP from the effects of inflammation
If oxidative stress is responsible for the decrease in purinergic neuromuscular transmission that occurs in colitis, then it is possible that treatment with a free radical scavenger could restore the IJP amplitude. Therefore, guinea pigs were treated with the antioxidant Tempol (1.0 mm in drinking water) beginning the same day that they were treated with TNBS. Tempol was chosen because it has been shown to reduce oxidative stress in a guinea pig model of gallstone disease (Gomez-Pinilla et al. 2010).
The effectiveness of Tempol as an antioxidant was verified by evaluating the intensity of fluorescence in muscularis samples treated with DHE, which stains intracellular reactive oxygen species (Demel et al. 2010). As can be seen in Fig. 5A, the intensity of DHE fluorescence is dramatically increased in TNBS colitis as compared to the control preparations, and the fluorescence intensity was reduced to the control level in preparations from TNBS inflamed animals that were treated with Tempol (n= 5 animals per group). This indicates that Tempol is capable of reducing oxidative stress in this model at the concentration that was used. Note that the macroscopic damage scores were comparable for animals treated with TNBS alone or TNBS plus Tempol (TNBS, 5.9 ± 0.9; TNBS + Tempol, 5.9 ± 0.5; P= 0.95; unpaired t test; TNBS n= 6; TNBS + Tempol n= 5).
Figure 5. The free radical scavenger Tempol decreases oxidative stress and protects the IJP from the effects of inflammation.
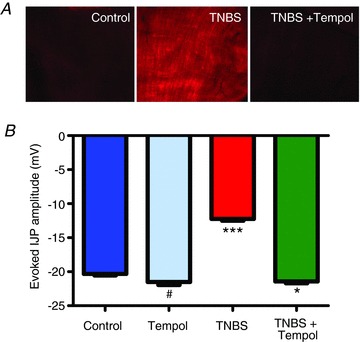
A, representative images, obtained with identical camera settings, showing DHE staining for oxidative stress in muscularis samples from control, TNBS- and TNBS + Tempol-treated animals. The fluorescence intensity was dramatically increased in the TNBS-inflamed preparation, and this was inhibited by Tempol. B, graph showing the IJP amplitude in control, and Tempol-, TNBS- and TNBS + Tempol-treated preparations (#P= not significant versus control and versus TNBS + Tempol; ***P < 0.001 versus all three other groups; *P < 0.05 versus control, ANOVA; n values: control, 15; Tempol, 19; TNBS, 14; TNBS + Tempol, 20).
When the effect of the antioxidant on the IJP was evaluated, we found that it did not alter the amplitude of the IJP in non-inflamed animals, but in animals with TNBS colitis, the IJP was comparable to the control IJP in preparations from animals treated with Tempol (Fig. 5B; control vs. TNBS, P < 0.0001; ANOVA; n= 14–20 per group). The resting membrane potentials of the smooth muscle cells were not affected by these conditions (ANOVA; control, −49.2 ± 0.6 mV; Tempol, −49.7 ± 0.7 mV; TNBS, −49.4 ± 0.9 mV; TNBS + Tempol, −49.4 ± 0.8 mV).
The free radical scavenger Tempol improves motility in TNBS colitis
Descending inhibition of colonic smooth muscle, mediated in part by purinergic neuromuscular transmission, is an important component of the peristaltic reflex because it provides a space for the forward movement of luminal contents. Previous studies have shown that blocking P2Y1 receptors significantly slows motility (Strong et al. 2010), although there are many other upstream components that are also altered in inflammation that contribute to dysmotility. Therefore, it is possible that the restoration of purinergic neuromuscular transmission could at least partially improve the disrupted motility seen in the TNBS guinea pig model of colitis. In Tempol-treated TNBS guinea pigs, propulsive motility was improved compared to TNBS guinea pigs, but was not restored to a normal linear pattern in all cases (see Fig. 6A). We have previously demonstrated that hyperexcitability of AH neurons also contributes to dysmotility in colitis, and that normalization of AH neuron excitability in inflamed preparations with the HCN channel blocker ZD7288 dramatically improves motility (Hoffman et al. 2011). Therefore, we tested whether administration of ZD7288 (10 μm) to inflamed colon preparations from animals treated with Tempol would result in a further improvement of motility. As can be seen in Fig. 6B and C, ZD7288 enhanced propulsive motility in these preparations. This suggests both that disruptions in purinergic neuromuscular transmission and that AH neuron hyperexcitability contribute to dysmotility in the inflamed colon.
Figure 6. The free radical scavenger Tempol improves propulsive motility in TNBS colitis.
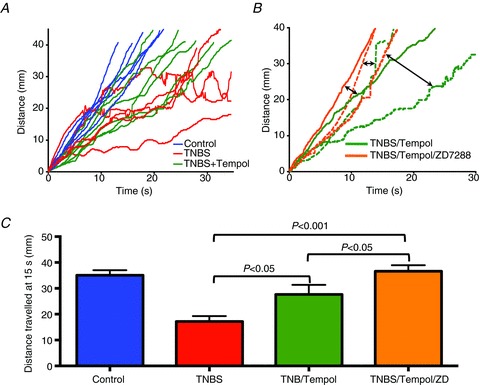
A, time–distance plots showing typical propulsive motility patterns from control (blue), TNBS- (red) and TNBS plus Tempol-treated (green) animals. Note that in control colons, propulsive motility is relatively rapid and linear, whereas in the TNBS-inflamed colons, the progress of the faecal pellet was slowed and ultimately halted. In colons from TNBS-inflamed animals treated with Tempol, the propulsive motility was not as rapid or as linear as the controls, but in each case the pellet proceeded through the entire colon. Each plot represents a colon from a different animal. B, time–distance plots of three TNBS-inflamed colons from animals treated with Tempol showing further improvements in propulsive motility following addition of 10 μm ZD7288 to the bathing solution. Data from the three separate colons are indicated as solid, dashed and stippled lines. Two headed arrows highlight pairs of data from the same colons. C, graph demonstrating the progress of a faecal pellet along the colon at a 15 s time point under the different conditions. As previously demonstrated (Linden et al. 2003a; Strong et al. 2010; Hoffman et al. 2011), propulsive motility was slowed in colons with TNBS colitis. Motility was improved by treatment of animals with Tempol, and further improved when the colons of Tempol-treated, TNBS-inflamed colons were exposed to the HCN channel blocker ZD7288, which dampens the hyperexcitability of AH neurons that occurs in TNBS colitis. Data were compared by ANOVA.
Inhibitory neuromuscular transmission is suppressed in murine DSS colitis, and it is normalized by treatment with a free radical scavenger
Based on our previous studies and the findings described above, TNBS colitis is associated with a decrease in purinergic neurotransmission that probably involves oxidative stress-induced disruption of mitochondrial function. To test whether this is a species and/or inflammation model-specific phenomenon, we induced colitis in mice using DSS. Whereas TNBS colitis involves primarily an adaptive immune response that is largely Th1 mediated (Krauter et al. 2007b; Shi et al. 2011), DSS colitis involves an innate immune response as DSS colitis occurs in immunodeficient mice (Reddy et al. 1991; Terry et al. 2009).
IJPs were evaluated in the mouse distal colon, where the IJP is predominantly purinergic (Gallego et al. 2012; Hwang et al. 2012). The amplitude of the IJP in DSS mice was significantly smaller than that recorded in normal mice (Fig. 7A; P < 0.001; ANOVA; control, n= 79 from six mice; DSS, n= 81 from five mice). If the decrease in the IJP measured in DSS mice is due to a similar mechanism as the one causing the decrease in IJP amplitude in TNBS guinea pig, then in vivo treatment with Tempol should protect purinergic neuromuscular transmission in DSS mice as it did in TNBS guinea pigs. In mice with both DSS and Tempol added to their drinking water, the amplitude of the IJP was comparable to that measured in non-inflamed mice, and significantly greater than the IJP in mice treated with DSS alone (Fig. 7A; P= 0.96 vs. control; P= 0.001 vs. DSS alone, ANOVA, n= 23 cells from five mice). The resting membrane potential of the smooth muscle cells was not affected by these conditions (ANOVA; control, −49.3 ± 0.8 mV; DSS, −49.2 ± 0.7 mV; DSS + Tempol, −49.3 ± 1.3 mV). DHE fluorescence evaluation confirmed that oxidative stress is increased in the DSS-inflamed colon and reduced to an intensity near that of control in the Tempol-treated DSS colons (Fig. 7B). These findings demonstrate that: (1) colitis leads to a decrease in IJP amplitude in the mouse as well as the guinea pig; (2) that DSS colitis as well as TNBS colitis reduces the IJP; and (3) that the IJP is normalized by in vivo treatment with a free radical scavenger.
Figure 7. Inhibitory neuromuscular transmission is suppressed in murine DSS colitis, and it is normalized by treatment with the free radical scavenger Tempol.
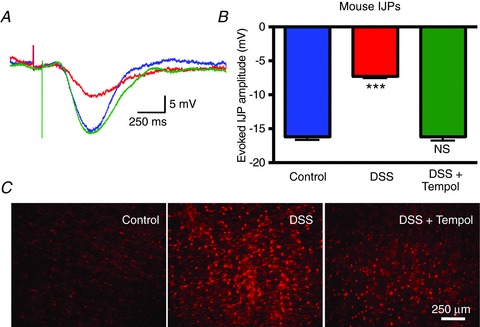
A, overlaid representative traces of IJPs in control mice (blue), DSS-treated mice (red) and DSS- plus Tempol-treated mice (green). B, graph indicating that the IJP amplitude is comparable to control in mice treated with DSS plus Tempol (***P < 0.001 versus the other groups; NS, P= NS versus control, ANOVA; n values: control, 79; DSS, 81; DSS plus Tempol, 23). C, representative images of DHE staining, obtained with identical camera settings, demonstrating that the intensity of oxidative stress in muscularis samples from Tempol- treated mice is reduced as compared to mice treated with DSS alone.
Discussion
The goal of this investigation was to determine the mechanisms responsible for the selective decrease in purinergic neuromuscular transmission in colitis. Colitis disrupts the peristaltic reflex circuitry at several different sites, including at the neuromuscular junction, where we have previously demonstrated a decrease in the amplitude of the purinergic IJP (Strong et al. 2010). The findings of the current investigation indicate that oxidative stress from the inflammatory process disrupts mitochondrial function, which in turn leads to decreased release of purines from the nerve terminals. The decrease in purinergic IJP measured in colitis can be mimicked in normal tissue by disrupting purine synthesis with ATP synthase inhibitors as well as by acutely causing free radical damage to normal tissue with hydrogen peroxide. The attenuation in purinergic neuromuscular transmission in inflamed tissue was prevented by in vivo treatment with a free radical scavenger. Notably, motility is also somewhat improved in free radical scavenger-treated animals, indicating the importance of effective purinergic transmission in motility. These results have been replicated in a mouse DSS colitis model, demonstrating that this mechanism is neither species nor inflammation-model specific.
The results of this study are consistent with the emerging concept that multiple purines are formed and released in nerve terminals at the neuroeffector junction. Previous studies in mouse and primate preparations have shown that tissue superfusates contain a variety of purines, including β-NAD and ATP and their metabolites, although the exact contributions of the various purines to the inhibitory purinergic post-junctional response have not been conclusively determined (Mutafova-Yambolieva et al. 2007; Hwang et al. 2011; Durnin et al. 2013). Hyperpolarization in response to purines is mediated by post-junctional P2Y1 receptors (Gallego et al. 2006, 2012; Wang et al. 2007; King & Townsend-Nicholson, 2008; Hwang et al. 2012), which are activated by both β-NAD and ATP.
In the current experiments, stimulus-induced release of β-NAD and ATP was decreased in inflamed preparations as compared to controls. Therefore, regardless of which purine(s) mediate inhibitory neuromuscular neurotransmission in the colon, the data reported here indicate that release of these compounds is decreased in the inflamed colon. These findings suggest that lower levels of purines are available for synaptic transmission.
The findings of the current study indicate that levels of ATP and β-NAD metabolites do not appear to be increased during electrical stimulation of the inflamed tissue. This supports the concept that the decrease in purinergic transmission seen in colitis is not likely to be due to an increase in breakdown of purines, which would presumably lead to an increase in the metabolites in inflamed preparations. This is a relevant issue because Neshat et al. (2009) have demonstrated that purinergic neurovascular transmission is diminished in the inflamed colon due to an upregulation of nucleoside triphosphate diphosphohydrolase-1 (NTPDase1, also known as CD39), the purine catabolizing ectonucleotidase.
The decrease in stimulus-induced purine secretion from inflamed preparations that was detected in the release studies suggests that purine synthesis might be decreased in the inhibitory nerve terminals of the inflamed colon. As purine synthesis occurs in the mitochondria, and mitochondria are particularly sensitive to oxidative stress (Navarro & Boveris, 2007; Keating, 2008), we tested the effects of mitochondrial ATP synthase inhibitors and free radicals on purinergic IJPs. One of the novel findings of this study is the tight temporal link between mitochondrial function and purinergic neuromuscular signalling. Within 30 min of exposure to ATP synthase inhibitors, the amplitude of the IJP was significantly reduced, indicating that continuous purine synthesis is necessary for the maintenance of normal inhibitory neuromuscular transmission. A similar decrease in the IJP was detected when the preparations were exposed to the free radical donor H2O2.
If the decrease in purinergic neurotransmission does involve oxidative stress, it stands to reason that the IJP could be protected by treatment of the animals with free radical scavengers. Indeed, administration of the membrane-permeable superoxide dismutase mimetic Tempol to guinea pigs and mice in which colitis was induced with TNBS or DSS, respectively, resulted in a protection of the IJP. Furthermore, propulsive motility was improved in inflamed guinea pigs treated with Tempol as compared to the disrupted motility seen in distal colons from animals with TNBS colitis. Note that in these preparations, an additional improvement in propulsive motility was detected when AH neuron hyperexcitability was dampened by the HCN channel blocker ZD7288, which had previously been shown to improve motility in this model (Hoffman et al. 2011). Taken together, these findings suggest that both disruptions in purinergic neuromuscular transmission, as well as AH neuron hyperexcitability, contribute to dysmotility in the inflamed colon.
Some forms of neuroplasticity that we have detected in guinea pig TNBS colitis, including AH neuron hyperexcitability and interneuronal synaptic facilitation, persist for at least 4 weeks following recovery from inflammation (Krauter et al. 2007b). In contrast, purinergic neuromuscular transmission is completely recovered at this 4 week time point (Strong et al. 2010). This is consistent with the concept that oxidative stress is probably a feature of the active inflammatory process and probably reverses as the inflammation resolves. This means that unlike the more persistent inflammation-induced changes in the neuronal circuitry, it is doubtful that decreased neuromuscular transmission contributes to post-infectious irritable bowel syndrome or irritable bowel syndrome-like symptoms during remission from IBD.
While the effects of in vivo antioxidant administration have not been examined extensively in IBD, they have been studied in cardiovascular disease. Recently, using a rat hypertension model, Demel et al. (2010) showed that there is a selective decrease in release of ATP, but not noradrenaline, from sympathetic nerves innervating mesenteric arteries. Furthermore, purinergic transmission was improved with chronic in vivo antioxidant treatment, although the mechanism remains to be determined. Taken together, results point to the potential of free radical scavengers for other conditions associated with increased oxidative stress, such as ageing, obesity and diabetes.
The current study has concentrated on the neuromuscular junction, but the findings reported here could have implications at other purinergic synapses in the enteric neural circuitry where oxidative stress is increased. It is worth noting, however, that decreased purinergic synaptic transmission is not a global feature of colitis. In fact, enhanced interneuronal synaptic activity is a feature of guinea pig TNBS colitis in both submucosal (Lomax et al. 2005) and myenteric (Linden et al. 2003b; Krauter et al. 2007a) ganglia. In distal colonic myenteric ganglia, roughly half of fast excitatory postsynaptic potentials have a purinergic component that is mediated by P2X receptors, and this purinergic neurotransmission is not altered in TNBS colitis (Krauter et al. 2007a). In the submucosal plexus of the guinea pig distal colon, fast synaptic transmission is predominantly nicotinic in normal tissue, but in inflammation, 78% of fast synaptic potentials have a non-cholinergic component that includes purines acting on P2X receptors. Therefore, while purinergic neuromuscular transmission is attenuated in the circular muscle, interneuronal purinergic synaptic transmission is unaltered in myenteric ganglia, and augmented in the submucosal plexus. These results illustrate the specific regional and sub-regional responses to inflammation, and highlight the importance of studies that examine specific changes caused by inflammation in a systematic fashion.
A limitation of the current study was an inability to resolve elevation in oxidative stress in the nerve terminals that provide purinergic input to the circular muscle, let alone in neuronal cell bodies. The DHE staining technique that we used to assess the inflammation-induced elevation in oxidative stress, and following free radical scavenger treatment, resulted in a diffuse staining pattern in guinea pig whole mounts and a more punctate nuclear staining pattern in the mouse. The latter pattern was similar to that observed in wholemounts of rat mesenteric arteries (Demel et al. 2010). In future studies to evaluate implications of the current findings in other conditions, it would be a significant advantage to be able to evaluate the cells and nerve terminals that are undergoing oxidative stress at a higher resolution.
In conclusion, these findings provide evidence that oxidative stress plays an important role in the decreased purinergic neuromuscular transmission seen in colitis. Dysmotility is a hallmark of colitis, and a very important clinical problem. We recently demonstrated that inflammation-induced hyperexcitability of intrinsic sensory neurons is one factor that contributes to dysmotility in colitis. It is likely that decreased purinergic neuromuscular signalling is another contributing factor. Propulsive motility is decreased in response to P2Y1 antagonist (Strong et al. 2010) and in P2Y1 knockout mice (Hwang et al. 2012), and restoration of purinergic transmission improves motility in colitis. Moreover, these findings underscore the importance of enteric neural integrity for the normal function of the bowel, and demonstrate that restoring normal neural activity can improve function in the inflamed colon.
Acknowledgments
We thank Dr James J. Galligan for valuable discussion.
Glossary
- 2-MeSATP
2-methylthioadenosine 5′-triphosphate
- AHP
afterhyperpolarization
- DCCD
dicyclohexylcarbodiimide
- DHE
dihydroethidium
- DSS
dextran sodium sulfate
- EJP
excitatory junction potential
- HPLC
high-performance liquid chromatography
- IBD
inflammatory bowel disease
- IJP
inhibitory junction potentials
- TNBS
trinitrobenzene sulfonic acid
Additional information
Competing interests
None.
Author contributions
All authors contributed to the conception and design of experiments, analysis and interpretation of data, and drafting the article or revising it critically for important intellectual content. J.A.R. performed the electrophysiological studies and L.D. performed the HPLC studies of purine concentrations.
Funding
This work was supported by NIH grants DK62267 (to G.M.M.) and PO1 DK41315 (to V.M.-Y.). K.A.S. is an Alberta Innovates-Health Solutions Medical Scientist and holds the Crohn's and Colitis Foundation of Canada Chair in IBD Research at the University of Calgary.
References
- Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Demel SL, Dong H, Swain GM, Wang X, Kreulen DL, Galligan JJ. Antioxidant treatment restores prejunctional regulation of purinergic transmission in mesenteric arteries of deoxycorticosterone acetate-salt hypertensive rats. Neuroscience. 2010;168:335–345. doi: 10.1016/j.neuroscience.2010.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnin L, Hwang SJ, Ward SM, Sanders KM, Mutafova- Yambolieva VN. Adenosine 5-diphosphate-ribose is a neural regulator in primate and murine large intestine along with β-NAD(+) J Physiol. 2012;590:1921–1941. doi: 10.1113/jphysiol.2011.222414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnin L, Sanders KM, Mutafova-Yambolieva VN. Differential release of β-NAD(+) and ATP upon activation of enteric motor neurons in primate and murine colons. Neurogastroenterol Motil. 2013;25:e194–204. doi: 10.1111/nmo.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB. The Enteric Nervous System. Malden, MA: Blackwell Publishing, Inc; 2006. [Google Scholar]
- Gallego D, Gil V, Martinez-Cutillas M, Mane N, Martin MT, Jimenez M. Purinergic neuromuscular transmission is absent in the colon of P2Y1 knocked out mice. J Physiol. 2012;590:1943–1956. doi: 10.1113/jphysiol.2011.224345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego D, Hernandez P, Clave P, Jimenez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G584–594. doi: 10.1152/ajpgi.00474.2005. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla PJ, Camello PJ, Tresguerres JA, Pozo MJ. Tempol protects the gallbladder against ischemia/reperfusion. J Physiol Biochem. 2010;66:161–172. doi: 10.1007/s13105-010-0021-y. [DOI] [PubMed] [Google Scholar]
- Hoffman JM, Brooks EM, Mawe GM. Gastrointestinal Motility Monitor (GIMM) J Vis Exp. 2010 doi: 10.3791/2435. http://www.jove.com/index/Details.stp?ID=2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JM, McKnight ND, Sharkey KA, Mawe GM. The relationship between inflammation-induced neuronal excitability and disrupted motor activity in the guinea pig distal colon. Neurogastroenterol Motil. 2011;23:673–e279. doi: 10.1111/j.1365-2982.2011.01702.x. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Blair PJ, Durnin L, Mutafova-Yambolieva V, Sanders KM, Ward SM. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol. 2012;590:1957–1972. doi: 10.1113/jphysiol.2011.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Durnin L, Dwyer L, Rhee PL, Ward SM, Koh SD, Sanders KM, Mutafova-Yambolieva VN. β-Nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology. 2011;140:608–617. doi: 10.1053/j.gastro.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating DJ. Mitochondrial dysfunction, oxidative stress, regulation of exocytosis and their relevance to neurodegenerative diseases. J Neurochem. 2008;104:298–305. doi: 10.1111/j.1471-4159.2007.04997.x. [DOI] [PubMed] [Google Scholar]
- King BF, Townsend-Nicholson A. Involvement of P2Y1 and P2Y11 purinoceptors in parasympathetic inhibition of colonic smooth muscle. J Pharmacol Exp Ther. 2008;324:1055–1063. doi: 10.1124/jpet.107.131169. [DOI] [PubMed] [Google Scholar]
- Krauter EM, Linden DR, Sharkey KA, Mawe GM. Synaptic plasticity in myenteric neurons of the guinea-pig distal colon: presynaptic mechanisms of inflammation- induced synaptic facilitation. J Physiol. 2007a;581:787–800. doi: 10.1113/jphysiol.2007.128082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauter EM, Strong DS, Brooks EM, Linden DR, Sharkey KA, Mawe GM. Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol Motil. 2007b;19:990–1000. doi: 10.1111/j.1365-2982.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003a;285:G207–216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- Linden DR, Foley KF, McQuoid C, Simpson J, Sharkey KA, Mawe GM. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol Motil. 2005;17:565–574. doi: 10.1111/j.1365-2982.2005.00673.x. [DOI] [PubMed] [Google Scholar]
- Linden DR, Sharkey KA, Mawe GM. Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. J Physiol. 2003b;547:589–601. doi: 10.1113/jphysiol.2002.035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax AE, Mawe GM, Sharkey KA. Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig. J Physiol. 2005;564:863–875. doi: 10.1113/jphysiol.2005.084285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. β-Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci U S A. 2007;104:16359–16364. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292:C670–686. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- Neshat S, deVries M, Barajas-Espinosa AR, Skeith L, Chisholm SP, Lomax AE. Loss of purinergic vascular regulation in the colon during colitis is associated with upregulation of CD39. Am J Physiol Gastrointest Liver Physiol. 2009;296:G399–405. doi: 10.1152/ajpgi.90450.2008. [DOI] [PubMed] [Google Scholar]
- Reddy SN, Bazzocchi G, Chan S, Akashi K, Villanueva-Meyer J, Yanni G, Mena I, Snape WJ., Jr Colonic motility and transit in health and ulcerative colitis. Gastroenterology. 1991;101:1289–1297. doi: 10.1016/0016-5085(91)90079-z. [DOI] [PubMed] [Google Scholar]
- Shi XZ, Winston JH, Sarna SK. Differential immune and genetic responses in rat models of Crohn's colitis and ulcerative colitis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G41–51. doi: 10.1152/ajpgi.00358.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong DS, Cornbrooks CF, Roberts JA, Hoffman JM, Sharkey KA, Mawe GM. Purinergic neuromuscular transmission is selectively attenuated in ulcerated regions of inflamed guinea pig distal colon. J Physiol. 2010;588:847–859. doi: 10.1113/jphysiol.2009.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry PD, Villinger F, Bubenik GA, Sitaraman SV. Melatonin and ulcerative colitis: evidence, biological mechanisms, and future research. Inflamm Bowel Dis. 2009;15:134–140. doi: 10.1002/ibd.20527. [DOI] [PubMed] [Google Scholar]
- Wang GD, Wang XY, Hu HZ, Liu S, Gao N, Fang X, Xia Y, Wood JD. Inhibitory neuromuscular transmission mediated by the P2Y1 purinergic receptor in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1483–1489. doi: 10.1152/ajpgi.00450.2006. [DOI] [PubMed] [Google Scholar]


