Abstract
The striated muscle activator of Rho signalling (STARS) pathway is suggested to provide a link between external stress responses and transcriptional regulation in muscle. However, the sensitivity of STARS signalling to different mechanical stresses has not been investigated. In a comparative study, we examined the regulation of the STARS signalling pathway in response to unilateral resistance exercise performed as either eccentric (ECC) or concentric (CONC) contractions as well as prolonged training; with and without whey protein supplementation. Skeletal muscle STARS, myocardian-related transcription factor-A (MRTF-A) and serum response factor (SRF) mRNA and protein, as well as muscle cross-sectional area and maximal voluntary contraction, were measured. A single-bout of exercise produced increases in STARS and SRF mRNA and decreases in MRTF-A mRNA with both ECC and CONC exercise, but with an enhanced response occurring following ECC exercise. A 31% increase in STARS protein was observed exclusively after CONC exercise (P < 0.001), while pSRF protein levels increased similarly by 48% with both CONC and ECC exercise (P < 0.001). Prolonged ECC and CONC training equally stimulated muscle hypertrophy and produced increases in MRTF-A protein of 125% and 99%, respectively (P < 0.001). No changes occurred for total SRF protein. There was no effect of whey protein supplementation. These results show that resistance exercise provides an acute stimulation of the STARS pathway that is contraction mode dependent. The responses to acute exercise were more pronounced than responses to accumulated training, suggesting that STARS signalling is primarily involved in the initial phase of exercise-induced muscle adaptations.
Key points
Myocellular protein signalling constitutes an important regulatory process influencing skeletal muscle cell size and remodelling as an adaptation to exercise and training.
Findings suggest that the striated muscle activator of Rho signalling (STARS) pathway is involved in exercise-induced muscle hypertrophy and/or remodelling, but its regulation by different exercise modes is not well understood.
In a comparative study including single-bout exercise and training, we investigated the mRNA and protein regulation of STARS and members of its signalling pathway in response to eccentric versus concentric resistance exercise and protein supplementation.
Our data show that components of the STARS signalling pathway exhibit transient regulation in response to resistance exercise, but not to resistance training, and show contraction mode-specific regulation at the level of gene and protein expression.
The results suggest that STARS signalling is important for the initiation of myocellular adaptations to resistance exercise that are dependent on contraction mode, but independent of protein supplement.
Introduction
Skeletal muscle is a highly adaptive tissue that is responsive to anabolic and catabolic stimuli. Skeletal muscle mass is maintained by a finely regulated balance between muscle protein synthesis and degradation. Numerous diseases, physical trauma and inactivity result in a loss of muscle mass that is associated with reduced protein synthesis, increased protein degradation or a combination of both (Mitch & Goldberg, 1996; Jagoe & Goldberg, 2001). Conversely, physical exercise is a potent inducer of muscle growth and remodelling (Bassel-Duby & Olson, 2006; Potthoff et al. 2007) and this exercise-induced response can be augmented with the supplementation of dietary protein (Andersen et al. 2005; Hartman et al. 2007). Maintaining skeletal muscle mass and function is vital for healthy living over the lifespan. Therefore, understanding the intracellular signalling mechanisms that may regulate muscle cell growth and remodelling in response to anabolic stimuli is of fundamental interest.
The striated muscle activator of Rho signalling (STARS) protein, also known as myocyte stress-1 (Ms1) protein and actin-binding Rho-activating protein (Abra), is an actin-binding protein highly enriched in cardiac, skeletal and smooth muscle (Arai et al. 2002; Mahadeva et al. 2002; Peng et al. 2008; Troidl et al. 2009; Ounzain et al. 2012). STARS promotes the binding of free G-actin to F-actin filaments, resulting in enhanced or stabilized actin polymerization (Arai et al. 2002). This results in the nuclear translocation of the serum response factor (SRF) transcriptional co-activator myocardin-related transcription factor-A (MRTF-A) that enhances SRF transcriptional activity (Kuwahara et al. 2005, 2007). SRF controls the transcription of numerous genes involved in muscle cell proliferation, differentiation and growth, as well as maintenance of cytoskeletal structure and integrity (Olson & Nordheim, 2010; Braun & Gautel, 2011). Accordingly, the STARS signalling pathway provides a link between actin dynamics and gene transcription.
Few studies have investigated the influence of exercise on STARS and members of its signalling pathway in human skeletal muscle, with most results limited to STARS mRNA levels. A single-bout of eccentric exercise provokes an early increase in STARS mRNA (MacNeil et al. 2010) and basal levels of STARS, MRTF-A and SRF mRNA are increased following 8 weeks of conventional hypertrophy-inducing resistance exercise training in parallel with increases in the basal levels of mRNAs coding for myofibrillar proteins such as MHCIIa and α-actin (Lamon et al. 2009). Furthermore, we recently showed that STARS, MRTF-A and SRF mRNA levels increased following 10 weeks of both endurance and resistance training (Lamon et al. 2013). Finally, STARS mRNA and protein levels are also increased in the early hours after single-leg endurance exercise (Wallace et al. 2011). However, following a period of exercise habituation STARS mRNA is only increased following a single bout of resistance but not endurance exercise (Lamon et al. 2013).
STARS signalling can be activated by different modes of exercise. The interplay between upstream mechano-sensitive membrane-bound molecules (Zhao et al. 2007; Huveneers & Danen, 2009; Zou et al. 2011) suggests that the modality of muscle contraction during exercise can influence STARS signalling. It is therefore possible that both the magnitude and the temporal regulation of STARS and members of its signalling pathway are dependent on the mode of exercise performed. As a consequence, this may dictate the type of muscle adaptation. However, at present no studies have investigated the effects of divergent modes of muscle contraction on the regulation of STARS and members of its signalling pathway.
In addition to mechanical stress, protein supplementation also increases actin polymerization (Badr et al. 2011). However, it is presently unknown if protein supplementation has an additive effect on the exercise-induced regulation of STARS and members of its signalling pathway.
Therefore the aim of the current study was to compare the effects of isolated single-bout eccentric versus concentric resistance exercise, as well as the cumulative effects of eccentric versus concentric resistance training, on the gene and protein regulation of STARS and members of its signalling pathway. To avoid the effect of unaccustomed exercise-induced stress, a 10-day period of eccentric and concentric exercise habituation was initially completed. Furthermore, we aimed to investigate if protein supplementation combined with exercise would have an additive effect on the regulation of the STARS signalling pathway.
We hypothesized that increases in the mRNA and protein levels of STARS and members of its signalling pathway would be augmented following both acute eccentric exercise and eccentric training, compared with concentric exercise and concentric training. Additionally, we hypothesized that the supplementation of whey protein would augment the STARS signalling response to both eccentric and concentric training, compared to an isocaloric placebo.
Methods
Ethical approval
All subjects were informed about the purpose and risks of the study and provided written informed consent in accordance with the Declaration of Helsinki. The study was approved by the local ethical committee of Region Midtjylland (j. no. M-20110003).
Subjects
Twenty-four healthy untrained young men (mean ± SEM; height: 181.5 ± 1.5 cm, weight: 78.1 ± 1.8 kg, age 23.9 ± 0.8 years, fat% 16.0 ± 0.9%) volunteered to participated in the study. Subject criteria for participation in the study comprised: (1) no participation in systematic resistance or high intensity training for lower extremity muscles 6 months prior to inclusion in the study; (2) no history of lower extremity musculoskeletal injuries; (3) no vegan diet or use of dietary supplements or prescription medicine of potential influence on muscle size.
Experimental design
The complete study comprised a single-bout exercise study to investigate acute exercise responses which was extended to form a training study to investigate accumulated exercise responses. Among the 24 subjects completing the habituation and acute trials, five subjects dropped out before the training trial was initiated. Another five subjects were recruited and completed the training trial with the remaining 19 subjects.
To investigate the acute and accumulated effects of eccentric contraction mode versus concentric contraction mode, the two legs of each subject were randomly assigned to eccentric or concentric contraction mode. This within-subject design was used to minimize the potential differences in the training response that are inherent with group designs (e.g. initial training status, habitual nutritional intake and/or hormonal status). Furthermore, to investigate the acute and accumulated exercise-induced effects of a whey protein hydrolysate (WPH) supplementation versus isocaloric placebo (PLA) supplementation, subjects were randomly divided into two groups of 12 subjects and dietary supplements were provided in a double blinded fashion.
An overview of pre-single-bout trial procedures and the single-bout trial is shown in Fig. 1A. Three days prior to the start of the study, the subjects were asked to abstain from extra physical activity other than normal daily activities. A basal muscle biopsy sample was then taken from one randomly chosen leg. Three days following the muscle biopsy, the subjects commenced a period of exercise habituation. On three occasions during 7 days, the subjects completed eccentric and concentric exercise on alternate legs using a protocol similar to the single-bout exercise trial. Three days following the end of the exercise-habituation period, muscle biopsies were sampled from both legs after an overnight fast and after at least 30 min of supine rest. These biopsies provided contraction mode-specific habituated basal levels for eccentric (Basal ECC) and concentric (Basal CONC) exercise. Three days after this, the subjects completed the single-bout exercise trial (see Fig. 1B). Approximately 7 days after the biopsies, the subjects commenced a 12 week training period. A final (post training) biopsy was sampled from ECC and CONC trained legs the day after the last exercise session of the training period.
Figure 1. Exercise-habituation and single-bout exercise protocols.
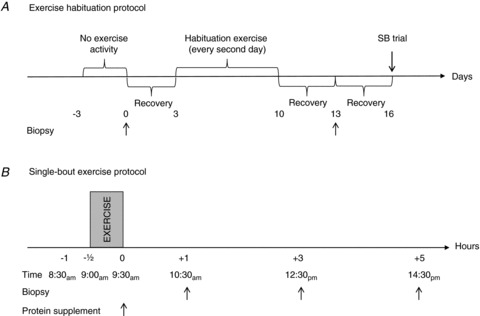
A, exercise-habituation protocol. Three days prior to a harvesting of a pre-exercise-habituation biopsy, subjects refrained from exercise (−3 days). A pre-exercise-habituation biopsy (arrow) was then sampled from a randomly selected leg (0 days). Subjects were then allowed to recover for 3 days prior to initiation of the exercise-habituation period (3 days). The exercise-habituation period included 3 exercise sessions during a 7 day period, with 48 h interspacing each exercise session. Exercise sessions were conducted as 6 sets of 10 maximal eccentric or concentric repetitions at 30 deg s−1 angular velocity, with 1 min of recovery between sets (10–13 days). Three days of recovery was then interspaced prior to sampling of post-exercise-habituation biopsies (Becc and Bconc;13 days). Another 3 days of recovery were then interspaced before completion of single-bout exercise trial (16 days). All pre- and post-exercise biopsies were sampled after an overnight fast. B, single-bout exercise protocol. After an overnight fast, the subjects reported to the laboratory at 08.30 h (−1 h). After 30 min of supine rest (−1/2 to 0 h), subjects completed a single-bout exercise session, conducted exactly as during exercise habituation. Immediately after exercise, a protein or a placebo supplement was ingested (0 h) and during the post-exercise recovery, biopsies were sampled at 1, 3 and 5 h from both eccentrically and concentrically working legs.
Exercise-habituation and single-bout exercise protocols
All exercise-habituation sessions and the single-bout exercise session conducted on the single-bout trial day were conducted in an isokinetic dynamometer (Humac Norm, CSMi Medical Solutions, Stoughton, MA, USA), as six sets of 10 maximal eccentric or concentric repetitions at 30°deg s−1 angular velocity, with 1 min of recovery between sets. All sets were completed using the same leg before switching to the other leg, with the eccentric and concentric exercises conducted in randomized order.
The overall protocol of the single-bout trial is represented in Fig. 1B. Subjects had been instructed to abstain from physical activity in the days prior to the single-bout trial and arrived after an overnight fast at 08.00 h on the day of the single-bout trial. Prior to the exercise session, the subjects rested in the supine position for approximately 30 min. At 08.30 h, subjects commenced the exercise session; the same protocol was used for the exercise-habituation sessions. Immediately after completion of the exercise session, the subjects ingested a whey protein supplement (see below). The subjects then continued fasting for another 5 h post exercise (except for water provided ad libitum), and biopsies were taken from each leg at time points corresponding to 1, 3 and 5 h after exercise. To control for potential effects of circadian rhythm, the absolute daily time points and time resolution of the protocol were strictly adhered to, as far as possible, for all subjects.
Supplementation
On the single-bout trail day, immediately after completion of the exercise session, subjects of the WPH group were given a single bolus solution consisting of 0.30 g whey protein + 0.30 g CHO per kilogram lean body mass, while subjects of the PLA-group were given an isocaloric solution consisting of 0.60 g CHO per kilogram lean body mass. The supplements were diluted in artificially flavoured water.
The BCAA content of the whey protein supplement (produced by Arla Foods Ingredients, Viby J., Denmark) was 27.7% (leucine 14.2%, isoleucine 6.6%, valine 6.9%), which is considered high compared to standard milk-based whey protein sources (Hulmi et al. 2010).
On all training days, the subjects in both groups received a fixed amount of the respective supplements. Accordingly, each intake consisted of an 8% solution (equal to 663 kJ), with the WPH drink consisting of 19.5 g whey protein +19.5 g of carbohydrate and the placebo drink consisting of 39 g of carbohydrate. Half of the solution was ingested just before each training session and the other half was ingested immediately after each training session. The subjects were instructed not to eat or drink anything calorie-containing for 1½ h prior to and during the immediate 1 h after the exercise session.
Training protocol
The subjects completed 33 exercise sessions over a 12 week training period. Training was aimed to promote muscle hypertrophy and was conducted as a progressive overload resistance training programme. Exercise training frequency was three times per week. All exercise sessions commenced with a standardized warm-up consisting of 5 min light bicycling exercise. An exercise session consisted of six sets gradually increasing to 12 sets × 15 repetitions, then gradually decreasing to six repetitions, with repetition loading equal to repetition maximum and with 2 min recovery interspaced between sets. Isotonic exercise mode sessions were performed as isolated unilateral knee extensions (Technogym-Selection line, Technogym, Italy), with one leg working eccentrically and one leg working concentrically. Eccentric load was aimed at 120% relative to concentric loading, with a training supervisor assisting to allow isolated exercise modality of the two legs. Subjects were instructed to perform each repetition in a controlled manner during both the concentric and the eccentric part of the exercise (∼2 s pace). All training was closely supervised and monitored by training instructors to ensure proper execution and loading.
Preparation of muscle biopsies
Muscle biopsies were harvested from the middle section of the m. vastus lateralis muscle using the Bergström needle technique, with at least 3 cm between incision sites of each biopsy and care taken to reach the identical sampling depth between biopsies.
The muscle samples were quickly dissected free of visible fat and connective tissue, weighed and divided into smaller parts for protein and RNA extraction and stored at −80°C until further investigation.
Immunoblotting
Frozen muscle biopsy sample material (from 40–50 mg) was homogenized in ice-cold solubilization buffer containing: 20 mm Tris, 50 mm NaCl, 5 mm Na4P2O7, 50 mm NaF, 250 mm sucrose, 2 mm DTT, 1% Triton-X 100, 2 μg ml−1 aprotinin, 5 μg ml−1 leupeptin, 0.5 μg ml−1 pepstatin, 10 μg ml−1 antipain, 1.5 mg ml−1 benzamidine, and 100 μmol l−1 4-(-2-aminoethyl)-benzenesulfonyl fluoride, hydrochloride (pH 7.4). Insoluble materials were removed by centrifugation at 16,000 g for 20 min at 4°C. Total protein content was determined using the Bradford protein assay kit (BioRad, Hercules, CA, USA). A representative blot is shown in Fig. 3. Electrophoresis was performed using a 4–12% NuPAGE® Novex Bis-Tris Gel (Invitrogen, Carlsbad, CA, USA) in NuPAGE® SDS Mops Running Buffer (Invitrogen). Protein transfer was performed in a Bjerrum buffer containing 50 mm Tris, 17 mm glycine and 10% methanol using PVDF membranes. The membranes were blocked with 5% BSA in PBS, after which they were incubated at 4°C with the following primary antibodies diluted 1:1000 in 5% BSA in PBS: STARS (Institute of Medical and Veterinary Science, Adelaide, SA, Australia); SRF (Santa Cruz, sc-335; CA, USA); MRTF-A (Santa Cruz, sc-32909); phospho-SRF (Abcam, ab53130; Cambridge, MA, USA). Following overnight incubation, the membranes were washed and incubated for 1 h with a goat anti-rabbit IgG antibody labelled with an infrared-fluorescent 800 nm dye (Alexa Fluor® 800, Invitrogen) diluted 1:5000 in PBS containing 50% Odyssey® blocking buffer (LI-COR Biosciences, Lincoln, NE, USA) and 0.01% SDS. After washing, the proteins were exposed on an Odyssey® Infrared Imaging System (LI-COR Biosciences) and individual protein band optical densities were determined using the Odyssey® Infrared Imaging System software. All blots were normalized against the GAPDH protein (G8795; Sigma-Aldrich, Sydney, Australia).
Figure 3. Examples of Western blots.
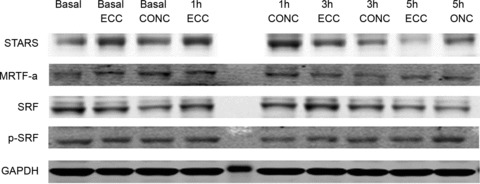
Examples of representative Western blots are shown for STARS, MRTF-A, SRF, pSRF and GAPDH for contraction mode-specific effects of exercise habituation and single-bout exercise.
Real-time PCR
Total RNA (from approximately 20 mg of muscle) was extracted using the guanidinium thiocyanate–phenol–chloroform extraction method previously described by (Chomczynski & Sacchi, 1987) and the concentration was determined spectrophotometrically using a Nanodrop 1000 (Thermo Fischer Scientific, Wilmington, DE, USA). Real-time PCR was carried out using the Stratagene MX3000 PCR system (Agilent Technologies, Santa Clara, CA, USA) to measure mRNA levels for STARS, MRTF-A, SRF, α-actin, JUNB, insulin-like growth factor 1 (IGF-1) and carnitine palmitoyltransferase-1β (CPT-1β). Primer sequences and PCR conditions have been published previously by our group (Lamon et al. 2009; Wallace et al. 2011). To compensate for variations in input RNA amounts and efficiency of the reverse transcription, data were normalized to ribosomal protein 36B4 (also known as RPLPO) mRNA.
Muscle cross-sectional area
Magnetic resonance imaging (MRI) of thigh muscle was performed with a 1.5-T scanner (Philips Achieva, Best, the Netherlands), as previously described (Farup et al. 2012). In brief, MRI scans were performed on both legs and knee extensor muscle cross-sectional area (CSA; mm. vastus lateralis, vastus medialis, vastus intermedius and rectus femoris) was determined as mid-level CSA. All analyses were conducted by an investigator blinded to the intervention. The CSA calculations were performed three times (mean ICC = 0.99) and the mean value was used for further data analysis.
Isometric strength performance
Subsequent to a standardized warm-up consisting of 5 min low intensity exercise on a stationary ergometer cycle (Monark, Varberg, Sweden), the subjects were seated in an isokinetic dynamometer (Humac Norm, CSMI, Stoughton, MA, USA) with 90 deg hip flexion and restraining straps crossing the torso and tested leg. Both legs were tested since they were trained differently and the test order was randomized between the eccentric and concentric leg. The transverse axis of the subject's knee was aligned with the axis of the dynamometer. The test leg was attached to the dynamometer arm while the other leg was placed behind a stabilization bar. The dynamometer was adjusted individually so the contact point between the subject's leg and the dynamometer arm was 3 cm proximal to the malleolus medialis.
Maximal voluntary contraction (MVC) was measured at 70 deg knee flexion (0 deg equals full extension). Before starting the test the subject's lower leg was weighed to enable gravity correction of the measured torque. The subject was allowed four trials (however, if a subject continued to improve additional trials were provided) and all contractions were interspaced with 1 min recovery time. Maximal voluntary contraction was determined as the highest peak torque from the best of the four trials.
Statistical analyses
The effect of time (pre versus post), group (WHD versus PLA) and contraction mode (eccentric versus concentric) and their interactions on quadriceps CSA and MVC were assessed using a mixed-effect three-way ANOVA with repeated measures for time and contraction mode.
Post-habituation basal arbitrary protein and mRNA levels were normalized to pre-habituation basal arbitrary levels (no supplement intervention during exercise habituation) and were analysed only for effect of contraction mode using a one-way repeated measures ANOVA.
Post-single-bout arbitrary protein and mRNA levels were normalized to post-habituation basal arbitrary levels and analysed for contraction mode (eccentric versus concentric) × supplement type (whey versus placebo) × time using the mixed-effect three-way ANOVA with repeated measures for time. As no effects of dietary supplementation type were observed during single-bout exercise, data were merged by supplementation and the effects of contraction mode × time were instead assessed using two-way repeated measures ANOVA.
Post-training basal arbitrary protein levels were normalized to pre-habituation basal arbitrary levels and were also shown to be independent of dietary supplementation type. Accordingly, both exercise and, training changes were analysed only for effect of contraction mode using a one-way repeated measures ANOVA.
When a significant effect of contraction mode and/or time was found, significant pairwise differences were assessed using the Student–Newman–Keuls post hoc test. Data are presented as mean percentage changes ± SEM. The level of significance was set at P < 0.05.
Results
Effect of exercise habituation on the STARS pathway
Exercise habituation did not result in any changes in STARS, MRTF-A or SRF mRNA levels (P > 0.05) (Fig. 2A, C and E). For the STARS protein, a treatment effect was observed (P < 0.001), with both ECC and CONC habituation exercise increasing STARS by 40 ± 11% (P < 0.001) and 27 ± 10% (P < 0.05), respectively, compared to basal levels. The effects of ECC and CONC habituation exercise were not significantly different from each other (P > 0.05; Fig. 2B). For the MRTF-A protein, a between-treatment effect was observed (P < 0.05), with ECC habituation exercise increasing MRTF-A by 95 ± 33% (P < 0.05), compared to basal levels (Fig. 2D). Exercise habituation did not result in any changes in total or phosphorylated SRF protein levels (Fig. 2F and G). Representative examples of Western blots for all protein targets are shown in Fig. 3 (compare Basal, Basal ECC and Basal CONC).
Figure 2. Effect of exercise habituation on STARS pathway mRNA and signalling proteins.
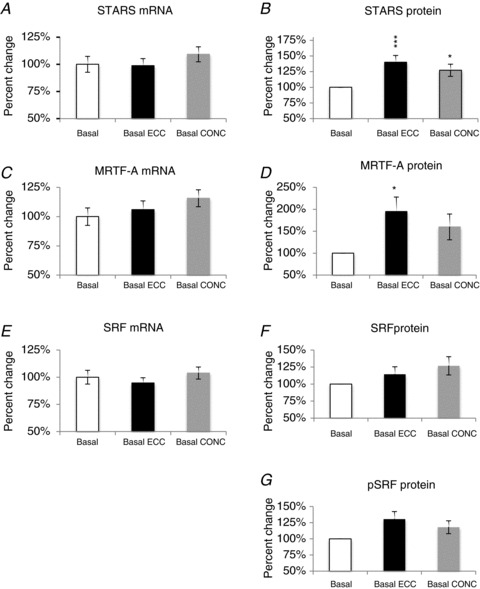
Contraction mode-specific effects (ECC versus CONC) of exercise habituation (pre-habituation basal versus post-habituation basal) are shown as % change (mean ± SEM)from basal set to a fixed value of 100% for: STARS (A and B); MRTF-A (C and D); SRF and pSRF (E–G). Difference from basal level: *P < 0.05, ***P < 0.001.
Effect of single-bout exercise following exercise habituation on the STARS pathway
There was no effect of whey protein supplementation on any of the mRNA or protein targets measured (data not shown). Therefore the data of both groups were pooled. Following the period of ECC and CONC exercise habituation, the effect of an acute bout of ECC and CONC exercise on the STARS signalling pathway was investigated (Fig. 4).
Figure 4. Effect of single-bout exercise on STARS pathway mRNA and signalling proteins.
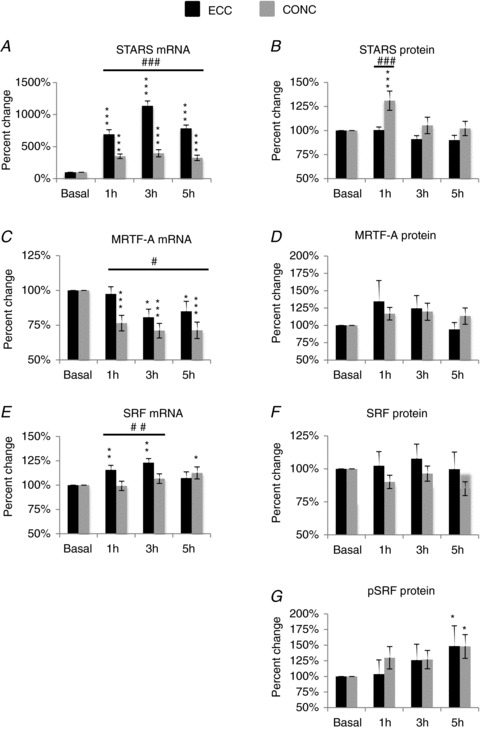
Contraction mode-specific effects (ECC versus CONC) of single-bout exercise (post-habituation basal versus post-exercise recovery time points) are shown as % change (mean ± SEM) from basal set to a fixed value of 100% for: STARS (A and B); MRTF-A (C and D); SRF and pSRF (E–G). Difference from ECC or CONC basal level: *P < 0.05, **P < 0.01, ***P < 0.001. Difference between contraction modes, #P < 0.05, ##P < 0.01, ###P < 0.001.
In regards to changes in gene expression, a contraction mode × time interaction was observed (P < 0.001) for STARS mRNA (Fig. 4A). Accordingly, ECC exercise increased STARS mRNA levels by 591 ± 74%, 1035 ± 77% and 685 ± 52% at 1 h, 3 h and 5 h post exercise, respectively, compared to basal levels (P < 0.001). CONC exercise also increased STARS mRNA levels by 249 ± 35%, 297 ± 56% and 224 ± 42% at 1 h, 3 h and 5 h post exercise, respectively, compared to basal levels (P < 0.001). At all time points post exercise the increase in STARS mRNA elicited by ECC exercise was greater than the increases elicited by CONC exercise (P < 0.001).
For MRTF-A mRNA levels (Fig. 4C), a time effect was observed (P < 0.001). ECC exercise decreased MRTF-A mRNA levels by 19 ± 6% and 15 ± 7% at 3 h and 5 h post exercise, respectively, compared to basal levels (P < 0.05). CONC exercise decreased MRTF-A mRNA levels by 24 ± 6%, 29 ± 5% and 29 ± 6% at 1 h, 3 h and 5 h post exercise, respectively, compared to basal levels (P < 0.001). An effect of contraction mode was observed, with a larger decrease in MRTF-A mRNA following CONC exercise compared to ECC exercise (P < 0.05).
For SRF mRNA (Fig. 4E), a contraction mode × time interaction was observed (P < 0.001). ECC exercise increased SRF mRNA by 16 ± 5% and 23 ± 4% at 1 h and 3 h post exercise, respectively, compared to basal levels. CONC exercise did not influence SRF mRNA levels when measured 1 h and 3 h post exercise, but there was an increase in SRF mRNA levels at 5 h post CONC exercise. SRF mRNA levels were greater following ECC than CONC exercise at 1 h and 3 h post exercise (P < 0.01); no difference in SRF mRNA was observed for contraction modes at 5 h post exercise (P > 0.05).
For the STARS protein a contraction mode × time interaction was observed (P < 0.001). Accordingly, CONC, but not ECC exercise increased STARS protein levels by 31 ± 10% at 1 h post exercise compared to basal levels (P < 0.001; Fig. 4B).
There was a tendency for MRTF-A protein levels to increase with time but this did not reach statistical significance (P= 0.07); Fig. 4D).
A time effect was observed for pSRF protein (P < 0.05). Accordingly, ECC and CONC exercise increased pSRF levels by 48 ± 33% and 48 ± 19%, respectively, at 5 h post exercise compared to basal levels (P < 0.05) (Fig. 4G). No changes were observed for total SRF (Fig. 4F).
Representative examples of Western blots for all protein targets are shown in Fig. 3.
Effect of single-bout exercise following exercise habituation on SRF gene targets
As pSRF levels were increased by both contraction modes, suggesting increased SRF activity, it was of interest to measure the expression levels of several known SRF gene targets, including α-actin, JUNB, IGF-1 and CPT-1β.
For α-actin (Fig. 5A), a time effect was observed (P < 0.01). CONC exercise increased α-actin mRNA levels by 21 ± 9% at 5 h post exercise compared to basal level (P < 0.05).
Figure 5. Effect of single-bout exercise on SRF gene targets.
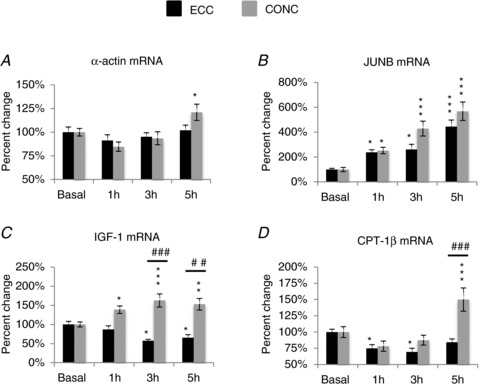
Contraction mode-specific effects (ECC versus CONC) of single-bout exercise (post-habituation basal versus post-exercise recovery time points) are shown as % change (mean ± SEM) from basal set to a fixed value of 100% for: α actin (A); JUNB (B); IGF-1 (C) and CPT-1β (D). Difference from ECC or CONC basal level: *P < 0.05, **P < 0.01, ***P < 0.001. Difference between contraction modes: ##P < 0.01, ###P < 0.001.
A time effect was also observed for JUNB (Fig. 5B), (P < 0.001). With ECC exercise, JUNB mRNA levels increased by 139 ± 39% (P < 0.05), 161± 42% (P < 0.05) and 345 ± 52% (P < 0.001) at 1 h, 3 h and 5 h post exercise, respectively, compared to basal level. With CONC exercise, JUNB mRNA levels increased by 152 ± 26% (P < 0.05), 329 ± 60% (P < 0.001) and 468 ± 74% (P < 0.001) at 1 h, 3 h and 5 h post exercise, respectively, compared to basal level. No specific effect of contraction mode was observed at any of the time points.
For IGF-1 (Fig. 5C), a contraction mode × time interaction was observed (P < 0.001). With ECC exercise, IGF-1 mRNA levels decreased by 43 ± 4% (P < 0.05) and 35 ± 8% (P < 0.05) at 3 h and 5 h post exercise, respectively, compared to basal level. With CONC exercise, IGF-1 mRNA levels increased by 39 ± 10% (P < 0.05), 63 ± 17% (P < 0.001) and 53 ± 15% (P < 0.01) at 1 h, 3 h and 5 h post exercise, respectively, compared to basal level. The increase in IGF-1 mRNA elicited by CONC exercise was greater than the increase elicited by ECC exercise at 3 h (P < 0.001) and 5 h (P < 0.01) post exercise.
For CPT-1β (Fig. 5D), a contraction mode × time interaction was observed (P < 0.001). With ECC exercise, CPT-1β mRNA levels decreased by 25 ± 6% (P < 0.05) and 31 ± 6% (P < 0.05) at 1 h and 3 h post exercise, respectively, compared to basal level. With CONC but not ECC exercise (P < 0.001), CPT-1β mRNA levels increased by 50 ± 18% (P < 0.001) at 5 h post exercise compared to basal level.
Effect of training without and with whey protein supplementation on the STARS pathway
To ascertain the efficacy of the training programme and the effect of whey supplementation, measurements of quadriceps CSA and MVC were made. A group × time interaction was observed (P < 0.001) for quadriceps CSA, with whey supplementation combined with either ECC or CONC training having a greater effect on CSA than placebo supplementation (P < 0.01). In the whey protein group, ECC and CONC training increased quadriceps CSA by 8.3 ± 1.3% and by 5.8 ± 1.0%, respectively (P < 0.001); no difference was observed between contraction modes. In the PLA group, ECC and CONC training increased quadriceps CSA by 2.7 ± 1.1% and by 2.2 ± 0.7%, respectively (P < 0.01), with no difference between contraction modes. MVC increased by 15.6 ± 3.5% (P < 0.001). This response was independent of contraction mode, with no effect of protein supplementation observed.
No measurements of mRNA levels were made after training. The effect of ECC and CONC exercise training are shown in Fig. 6. There was no effect of whey protein supplementation on any of the proteins measured (data not shown) therefore the data of both groups were pooled. There was no effect of training mode on STARS and total SRF protein levels (Fig. 6A and C). For the MRTF-A protein, a treatment effect was observed (P < 0.01), with ECC and CONC training both increasing MRTF-A protein levels by 43 ± 24% (P < 0.01) and 31 ± 17% (P < 0.05), respectively, compared to basal levels (Fig. 6B).
Figure 6. Training-induced changes in STARS pathway protein expression.
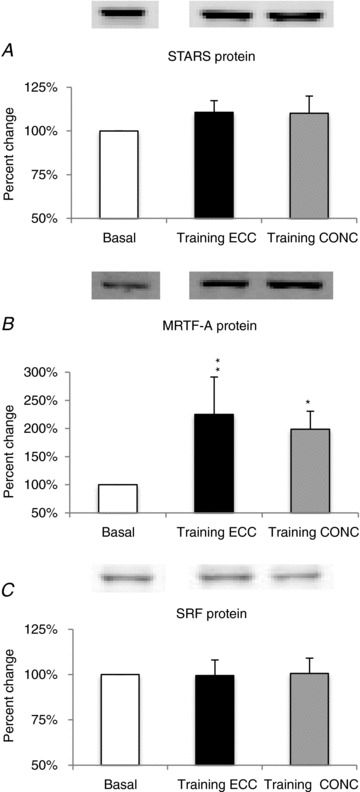
Contraction mode-specific effects (ECC versus CONC) of prolonged training (pre-habituation basal versus post-training basal) are shown as % change (mean ± SEM) from basal set to a fixed value of 100% for: STARS (A); MRTF-A (B); SRF (C). Different from basal level: *P < 0.05, **P < 0.01.
Discussion
STARS and members of its signalling pathway are upregulated following exercise (Lamon et al. 2009, 2013; Pollanen et al. 2010; Wallace et al. 2011). However the magnitude and temporal regulation of STARS and members of its signalling pathway with divergent modes of muscle contraction (eccentric versus concentric) has not been investigated. In the present study, we demonstrate that the regulation of STARS and members of its signalling pathway are sensitive to the effects of short-term exercise habituation, acute resistance exercise, prolonged resistance training, and different modes of muscle contraction. Furthermore, we demonstrate that several downstream SRF gene targets are differentially expressed by different modes of muscle contraction. Finally, we provide evidence that the effect of training on the STARS signalling pathway is not influenced by protein supplementation.
Effect of exercise habituation on the STARS signalling pathway
Prior to the acute exercise bout, the subjects completed three exercise sessions over a 7 day period to reduce the potential acute non-exercise effects of unaccustomed muscle stress (especially following eccentric exercise) on the STARS signalling pathway. It was observed that the exercise habituation phase increased STARS and MRTF-A protein levels; a response that was independent of the mode of muscle contraction. No changes in gene expression were observed following the habituation phase. These results again raise important questions, previously put forward by us, about how control and/or familiarisation procedures can best be implemented in human exercise study designs in order to optimise the ability to distinguish between true exercise responses and responses due to other stressors inherent in the exercise study protocol itself (Vissing et al. 2005, 2011, 2012).
Acute effects of single-bout resistance exercise on the STARS signalling pathway
To investigate the acute effects of resistance exercise mode on members of the STARS signalling pathway, the participants of our study completed a single bout of unilateral ECC versus CONC resistance exercise with and without whey protein supplementation. Since no effects were observed with protein supplementation, data were merged for contraction mode. A 10-fold increase in STARS mRNA was observed following acute ECC resistance exercise, supporting a previous observation made following muscle-damaging eccentric exercise (MacNeil et al. 2010). A 3-fold increase in STARS mRNA was observed following CONC resistance exercise, supporting results observed following a concentric single-bout of one-legged cycling (Wallace et al. 2011). Similarly, but to a smaller extent, we also observed an increase in SRF mRNA following acute resistance exercise. Again, this increase was greater following ECC than CONC resistance exercise. Interestingly, SRF gene targets α-actin, JUNB, IGF-1 and CPT-1β all displayed an early upregulation following CONC resistance exercise. These results are partially in line with those of Wallace and co-workers (2011) following a single bout of CONC endurance exercise. However, despite a greater increase in SRF mRNA, ECC resistance exercise had no effect on α-actin mRNA and induced a decrease in IGF-1 and CPT-1β mRNA levels. JUNB mRNA levels were still increased following ECC resistance exercise, but to a smaller extent when compared to CONC resistance exercise. These results potentially indicate a role for alternative, SRF-independent signalling pathways in the early regulation of these genes following ECC resistance exercise.
In contrast to STARS and SRF mRNA, MRTF-A mRNA levels exhibited a modest, yet significant decrease following both modes of exercise; however, this change was slightly more pronounced with acute CONC rather than with ECC resistance exercise. A similar decrease in MRTF-A mRNA levels following a single-bout of resistance exercise performed in a training-habituated state was recently reported by our group (Lamon et al. 2013), but the influence of the ECC and CONC phase of contraction was not investigated.
The upregulation of STARS and SRF mRNA with acute resistance exercise may indicate that a potential ‘priming’ of the STARS signalling pathway is occurring to assist with the transcription of specific myofibrillar genes as part of the adaptation to resistance exercise. Furthermore, the greater ability of ECC resistance exercise than CONC resistance exercise to promote the upregulation of STARS and SRF mRNA, suggests the potential activation of a transcriptional programme important for protecting against contractile-induced muscle damage and/or muscle regeneration. For example SRF, as well as regulating its own gene expression and that of STARS (Chong et al. 2012), transcribes a number of genes that participate in maintaining cytoskeletal and sarcomere integrity (Olson & Nordheim, 2010).
STARS protein exhibited an early increase in response to acute CONC but not ECC resistance exercise, whereas p-SRF exhibited an increase later, post exercise, that was contraction mode independent. No changes were observed for SRF and MRTF-A proteins. These findings are partly in line with the study by Wallace et al. (2011), where STARS and SRF protein levels were increased following concentric single-leg cycling exercise. The lack of observed change in MRTF-A protein may be due to its measurement in whole-muscle homogenate. As MRTF-A is known to translocate to the nucleus (Vartiainen et al. 2007; Wallace et al. 2011), its measurement in nuclear extracts may be required to detect potential changes. Indeed, the study by Wallace and co-workers (2011) reported a small upregulation of MRTF-A protein in the nuclear fraction, following acute concentric single-leg cycling.
On several occasions changes in mRNA and protein levels were not consistent. It is well known that not all increases in mRNA result in an increase in their encoded proteins. Protein activity does not necessarily depend upon changes in total protein levels. Post-translational modifications such a phosphorylation, methylation or nitrosylation can also regulate protein activity and physiological function. It is presently unknown if these post-translational modifications occur with the STARS protein to regulate its activity and consequent downstream signals. STARS signalling may also be more important for the initiation of adaptation processes, rather than for the maintenance of such processes over time. Pressure overload-induced upregulation of STARS mRNA in rat cardiac muscle precedes the physiological response of cardiac hypertrophy (Kuwahara et al. 2007). In fact, STARS mRNA levels return to baseline prior to the observed increase in cardiac hypertrophy. With this in mind, it is possible that the STARS protein may have been increased at a time point earlier in the training programme.
Our results support the notion that STARS signalling is responsive to muscle contraction. However, our results at the protein level do not completely support our hypothesis that acute ECC resistance exercise would elicit a greater regulation of the STARS pathway when compared to CONC resistance exercise. Our observations suggest that in response to acute resistance exercise, SRF activation is not exclusively dependent upon the STARS–MRTF-A axis but is also influenced by other upstream transcriptional co-activators (Chen & Schwartz, 1996; Belaguli et al. 2000; Sepulveda et al. 2002; Davis et al. 2003). For example, SRF can also be activated by p38 MAPK (Thuerauf et al. 1998).
Effect of resistance training and whey protein supplementation
Following the single-bout trials, the participants completed 12 weeks of unilateral ECC versus CONC resistance training. Additionally, subjects also received a whey protein supplement or an isocaloric placebo. In line with previous findings, our resistance training programme resulted in an increase in muscle hypertrophy that was associated with an increase in muscle strength (Andersen et al. 2005; Hartman et al. 2007). In regard to the ability of divergent contraction modes to produce hypertrophy, the higher contraction forces and lower energy cost during ECC resistance exercise training suggest that ECC resistance exercise training has superior potential to promote muscle protein synthesis and hypertrophy than CONC resistance exercise training (Beltman et al. 2004; Moore et al. 2005). However, this is still debated (Roig et al. 2009). In the current study, there were no differences in the magnitude of muscle hypertrophy produced by ECC versus CONC resistance training. We cannot exclude the possibility that this lack of difference may be due to the relatively high volume of total training work performed with each contraction mode. Accordingly, meta-analysis indicates that the rate of increase in muscle hypertrophy may decline at higher training volumes (Wernbom et al. 2007). Surprisingly, post-training levels of STARS, which exhibited acute upregulation following a single bout of exercise, and SRF were not upregulated following training. However, both ECC and CONC training did elicit an increase in MRTF-A protein levels, a response not observed following a single-bout of resistance exercise. In two previous studies by us, standard resistance training increased STARS, MRTF-A and SRF gene expression after 8 and 10 weeks of resistance training, respectively (Lamon et al. 2009, 2013). Nuclear SRF protein levels were increased following 8 weeks of resistance training, but we failed to detect any change in the STARS signalling proteins following 10 weeks of resistance training. It has been shown in rat cardiac muscle that pressure overload by aortic banding causes an upregulation in STARS mRNA that precedes cardiac hypertrophy and that the increase in STARS returned towards baseline following the induction of cardiac hypertrophy (Mahadeva et al. 2002). This seems to support the notion that STARS signalling is more important for the initiation of adaptation processes rather than for the maintenance of such processes over time. As such, the STARS protein may have been increased at a time point earlier in the training programme.
Eccentric and concentric resistance training induced a similar level of muscle hypertrophy. Therefore, the mode-specific difference in STARS regulation is not due to differing effects on muscle hypertrophy dependent on mode of resistance training. The greater response in gene expression induced by eccentric exercise could be due to the greater stress induced by the lengthening contractions; stress that is inherent in this type of contraction. It is known that strenuous eccentric exercise/stretch invokes micro-damage to myofibrillar components. This stress may activate membrane-bound mechano-transducing molecules (Olson & Nordheim, 2010) that activate pathways promoting the transcriptional regulation of STARS signalling pathway genes, as a part of an adaptive remodelling process required for muscle repair and regeneration. With this in mind, very few sporting/exercise activities contain the large degree of controlled eccentric contraction performed during this study and therefore under physiological conditions eccentric stress may not have the same impact as observed here.
In regard to protein supplementation we found that resistance training-induced muscle hypertrophy was augmented by protein supplementation (Farup et al. 2013); an observation previously noted in some studies (Andersen et al. 2005; Hartman et al. 2007), but not others (Hulmi et al. 2009). This fits with our expectations, as our protein supplement was high in BCAA, especially leucine, content, even compared to standard milk-based whey protein sources (Hulmi et al. 2010), and as BCAAs, and especially leucine, are considered as a very potent trigger of muscle protein synthesis and hypertrophy signalling (Atherton & Smith, 2012; Breen & Phillips, 2012). This is the first study to investigate the effect of protein supplementation on STARS signalling. As protein supplementation can increase actin polymerization (Badr et al. 2011), we hypothesized that protein supplementation could enhance the exercise-induced increase in STARS signalling. However, this was not the case in our study. This observation suggests that the regulation of STARS is protein independent or that the effect of resistance exercise saturates the mechanism regulating STARS. Future studies should look at the effect of protein supplementation, without resistance exercise, on the regulation of STARS and members of its signalling pathway.
Conclusion
Our results show that the magnitude of regulation of STARS and members of its signalling pathway following resistance exercise is dependent on the mode of contraction, but independent of protein supplementation. The regulation of both the mRNA and protein of members of the STARS pathway may be controlled by specific transcriptional and translational mediators that are sensitive to the different intracellular stress signals elicited by ECC and CONC muscle contractions. While the precise role of the STARS signalling pathway in skeletal muscle is not yet known, our results suggest that STARS signalling is important for the initiation of adaptation processes related to growth and/or remodelling and that it is tightly regulated in a temporal manner.
Acknowledgments
We thank the subjects for their participation in the project. Steffen Ringgaard (MR-Research Centre, Institute of Clinical Medicine, Aarhus University Hospital, Aarhus, Denmark), Cuno Rasmussen and Gitte Hartvigsen (both Section of Sport Science, Department of Public Health, Aarhus University, Aarhus, Denmark) are thanked for technical assistance.
Glossary
- Abra
actin-binding Rho activating (protein)
- BCAA
branched chain amino acid
- CONC
concentric contraction
- CPT-1β
carnitine palmitoyltransferase-1β
- CSA
cross-sectional area
- ECC
eccentric contraction
- IGF-1
insulin-like growth factor 1
- MAPK
mitogen activated protein kinase
- MRI
magnetic resonance imaging
- MRTF-A
myocardin-related transcription factor-A
- Ms1
myocyte stress-1
- mTOR
mammalian target of rapamycin
- MVC
maximal voluntary contraction
- STARS
striated muscle activator of Rho signalling
Additional information
Competing interests
The authors declare that there is no conflict of interest.
Author contributions
Conception and design of the experiments: K.V., S.K.R. and J.F. Collection, analysis and interpretation of data and drafting the article or revising it critically for important intellectual content and drafting the article or revising it critically for important intellectual content: K.V., S.K.R., J.F., S.L., R.J.S., M.A.W., A.R. and M.H.V.
Funding
We thank Arla Foods Ingredients Group P/S DK for funding the project.
References
- Andersen LL, Tufekovic G, Zebis MK, Crameri RM, Verlaan G, Kjaer M, Suetta C, Magnusson P, Aagaard P. The effect of resistance training combined with timed ingestion of protein on muscle fibre size and muscle strength. Metabolism. 2005;54:151–156. doi: 10.1016/j.metabol.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Arai A, Spencer JA, Olson EN. STARS, a striated muscle activator of Rho signalling and serum response factor-dependent transcription. J Biol Chem. 2002;277:24453–24459. doi: 10.1074/jbc.M202216200. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Smith K. Muscle protein synthesis in response to nutrition and exercise. J Physiol. 2012;590:1049–1057. doi: 10.1113/jphysiol.2011.225003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr G, Mohany M, Metwalli A. Effects of undenatured whey protein supplementation on CXCL12- and CCL21-mediated B and T cell chemotaxis in diabetic mice. Lipids Health Dis. 2011;10 doi: 10.1186/1476-511X-10-203. 203–511X-10–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- Belaguli NS, Sepulveda JL, Nigam V, Charron F, Nemer M, Schwartz RJ. Cardiac tissue enriched factors serum response factor and GATA-4 are mutual coregulators. Mol Cell Biol. 2000;20:7550–7558. doi: 10.1128/mcb.20.20.7550-7558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltman JG, van der Vliet MR, Sargeant AJ, de Haan A. Metabolic cost of lengthening, isometric and shortening contractions in maximally stimulated rat skeletal muscle. Acta Physiol Scand. 2004;182:179–187. doi: 10.1111/j.1365-201X.2004.01338.x. [DOI] [PubMed] [Google Scholar]
- Braun T, Gautel M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat Rev Mol Cell Biol. 2011;12:349–361. doi: 10.1038/nrm3118. [DOI] [PubMed] [Google Scholar]
- Breen L, Phillips SM. Nutrient interaction for optimal protein anabolism in resistance exercise. Curr Opin Clin Nutr Metab Care. 2012;15:226–232. doi: 10.1097/MCO.0b013e3283516850. [DOI] [PubMed] [Google Scholar]
- Chen CY, Schwartz RJ. Recruitment of the tinman homolog Nkx-2.5 by serum response factor activates cardiac alpha-actin gene transcription. Mol Cell Biol. 1996;16:6372–6384. doi: 10.1128/mcb.16.11.6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chong NW, Koekemoer AL, Ounzain S, Samani NJ, Shin JT, Shaw SY. STARS is essential to maintain cardiac development and function in vivo via a SRF pathway. PLoS One. 2012;7:e40966. doi: 10.1371/journal.pone.0040966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FJ, Gupta M, Camoretti-Mercado B, Schwartz RJ, Gupta MP. Calcium/calmodulin-dependent protein kinase activates serum response factor transcription activity by its dissociation from histone deacetylase, HDAC4. Implications in cardiac muscle gene regulation during hypertrophy. J Biol Chem. 2003;278:20047–20058. doi: 10.1074/jbc.M209998200. [DOI] [PubMed] [Google Scholar]
- Farup J, Kjolhede T, Sorensen H, Dalgas U, Moller AB, Vestergaard PF, Ringgaard S, Bojsen-Moller J, Vissing K. Muscle morphological and strength adaptations to endurance vs. resistance training. J Strength Cond Res. 2012;26:398–407. doi: 10.1519/JSC.0b013e318225a26f. [DOI] [PubMed] [Google Scholar]
- Farup J, Rahbek SK, Vendelbo MH, Matzon A, Hindhede J, Bejder A, Ringgard S, Vissing K. Whey protein hydrolysate augments tendon and muscle hypertrophy independent of resistance exercise contraction mode. Scand J Med Sci Sports. 2013 doi: 10.1111/sms.12083. doi: 10.1111/sms.12083. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr. 2007;86:373–381. doi: 10.1093/ajcn/86.2.373. [DOI] [PubMed] [Google Scholar]
- Hulmi JJ, Kovanen V, Selanne H, Kraemer WJ, Hakkinen K, Mero AA. Acute and long-term effects of resistance exercise with or without protein ingestion on muscle hypertrophy and gene expression. Amino Acids. 2009;37:297–308. doi: 10.1007/s00726-008-0150-6. [DOI] [PubMed] [Google Scholar]
- Hulmi JJ, Lockwood CM, Stout JR. Effect of protein/essential amino acids and resistance training on skeletal muscle hypertrophy: A case for whey protein. Nutr Metab (Lond) 2010;7:51. doi: 10.1186/1743-7075-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huveneers S, Danen EH. Adhesion signalling – crosstalk between integrins, Src and Rho. J Cell Sci. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- Jagoe RT, Goldberg AL. What do we really know about the ubiquitin-proteasome pathway in muscle atrophy. Curr Opin Clin Nutr Metab Care. 2001;4:183–190. doi: 10.1097/00075197-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Kuwahara K, Barrientos T, Pipes GC, Li S, Olson EN. Muscle-specific signalling mechanism that links actin dynamics to serum response factor. Mol Cell Biol. 2005;25:3173–3181. doi: 10.1128/MCB.25.8.3173-3181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara K, Teg Pipes GC, McAnally J, Richardson JA, Hill JA, Bassel-Duby R, Olson EN. Modulation of adverse cardiac remodeling by STARS, a mediator of MEF2 signalling and SRF activity. J Clin Invest. 2007;117:1324–1334. doi: 10.1172/JCI31240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamon S, Wallace MA, Leger B, Russell AP. Regulation of STARS and its downstream targets suggest a novel pathway involved in human skeletal muscle hypertrophy and atrophy. J Physiol. 2009;587:1795–1803. doi: 10.1113/jphysiol.2009.168674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamon S, Wallace MA, Stefanetti RJ, Rahbek SK, Vendelbo MH, Russell AP, Vissing K. Regulation of the STARS signalling pathway in response to endurance and resistance exercise and training. Pflugers Arch. 2013 doi: 10.1007/s00424-013-1265-5. (in press), DOI: 10.1007/s00424-013-1265-5. [DOI] [PubMed] [Google Scholar]
- MacNeil LG, Melov S, Hubbard AE, Baker SK, Tarnopolsky MA. Eccentric exercise activates novel transcriptional regulation of hypertrophic signalling pathways not affected by hormone changes. PLoS One. 2010;5:e10695. doi: 10.1371/journal.pone.0010695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadeva H, Brooks G, Lodwick D, Chong NW, Samani NJ. Ms1, a novel stress-responsive, muscle-specific gene that is up-regulated in the early stages of pressure overload-induced left ventricular hypertrophy. FEBS Lett. 2002;521:100–104. doi: 10.1016/s0014-5793(02)02833-8. [DOI] [PubMed] [Google Scholar]
- Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335:1897–1905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab. 2005;288:E1153–E1159. doi: 10.1152/ajpendo.00387.2004. [DOI] [PubMed] [Google Scholar]
- Olson EN, Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol. 2010;11:353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ounzain S, Kobayashi S, Peterson RE, He A, Motterle A, Samani NJ, Menick DR, Pu WT, Liang Q, Chong NW. Cardiac expression of ms1/STARS, a novel gene involved in cardiac development and disease, is regulated by GATA4. Mol Cell Biol. 2012;32:1830–1843. doi: 10.1128/MCB.06374-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YB, Guan HP, Fan B, Zhao SH, Xu XW, Li K, Zhu MJ, Yerle M, Liu B. Molecular characterization and expression pattern of the porcine STARS, a striated muscle-specific expressed gene. Biochem Genet. 2008;46:644–651. doi: 10.1007/s10528-008-9178-2. [DOI] [PubMed] [Google Scholar]
- Pollanen E, Fey V, Tormakangas T, Ronkainen PH, Taaffe DR, Takala T, Koskinen S, Cheng S, Puolakka J, Kujala UM, Suominen H, Sipila S, Kovanen V. Power training and postmenopausal hormone therapy affect transcriptional control of specific co-regulated gene clusters in skeletal muscle. Age (Dordr) 2010;32:347–363. doi: 10.1007/s11357-010-9140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Olson EN, Bassel-Duby R. Skeletal muscle remodeling. Curr Opin Rheumatol. 2007;19:542–549. doi: 10.1097/BOR.0b013e3282efb761. [DOI] [PubMed] [Google Scholar]
- Roig M, O’Brien K, Kirk G, Murray R, McKinnon P, Shadgan B, Reid WD. The effects of eccentric versus concentric resistance training on muscle strength and mass in healthy adults: a systematic review with meta-analysis. Br J Sports Med. 2009;43:556–568. doi: 10.1136/bjsm.2008.051417. [DOI] [PubMed] [Google Scholar]
- Sepulveda JL, Vlahopoulos S, Iyer D, Belaguli N, Schwartz RJ. Combinatorial expression of GATA4, Nkx2–5, and serum response factor directs early cardiac gene activity. J Biol Chem. 2002;277:25775–25782. doi: 10.1074/jbc.M203122200. [DOI] [PubMed] [Google Scholar]
- Thuerauf DJ, Arnold ND, Zechner D, Hanford DS, DeMartin KM, McDonough PM, Prywes R, Glembotski CC. p38 Mitogen-activated protein kinase mediates the transcriptional induction of the atrial natriuretic factor gene through a serum response element. A potential role for the transcription factor ATF6. J Biol Chem. 1998;273:20636–20643. doi: 10.1074/jbc.273.32.20636. [DOI] [PubMed] [Google Scholar]
- Troidl K, Ruding I, Cai WJ, Mucke Y, Grossekettler L, Piotrowska I, Apfelbeck H, Schierling W, Volger OL, Horrevoets AJ, Grote K, Schmitz-Rixen T, Schaper W, Troidl C. Actin-binding rho activating protein (Abra) is essential for fluid shear stress-induced arteriogenesis. Arterioscler Thromb Vasc Biol. 2009;29:2093–2101. doi: 10.1161/ATVBAHA.109.195305. [DOI] [PubMed] [Google Scholar]
- Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749–1752. doi: 10.1126/science.1141084. [DOI] [PubMed] [Google Scholar]
- Vissing K, Andersen JL, Schjerling P. Are exercise-induced genes induced by exercise. FASEB J. 2005;19:94–96. doi: 10.1096/fj.04-2084fje. [DOI] [PubMed] [Google Scholar]
- Vissing K, McGee SL, Farup J, Kjolhede T, Vendelbo MH, Jessen N. AMPK vs mTORC1 signalling: genuine exercise effects of differentiated exercise in humans. Response to letter to editor by Dr A. K. Yamada. Scand J Med Sci Sports. 2012;22:580–581. doi: 10.1111/j.1600-0838.2012.01450.x. [DOI] [PubMed] [Google Scholar]
- Vissing K, McGee SL, Farup J, Kjolhede T, Vendelbo MH, Jessen N. Differentiated mTOR but not AMPK signalling after strength vs endurance exercise in training-accustomed individuals. Scand J Med Sci Sports. 2011 doi: 10.1111/j.1600-0838.2011.01395.x. DOI: 10.1111/j.1600-0838.2011.01395.x. [DOI] [PubMed] [Google Scholar]
- Wallace MA, Hock MB, Hazen BC, Kralli A, Snow RJ, Russell AP. Striated muscle activator of Rho signalling (STARS) is a PGC-1α/oestrogen-related receptor-alpha target gene and is upregulated in human skeletal muscle after endurance exercise. J Physiol. 2011;589:2027–2039. doi: 10.1113/jphysiol.2011.205468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernbom M, Augustsson J, Thomee R. The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans. Sports Med. 2007;37:225–264. doi: 10.2165/00007256-200737030-00004. [DOI] [PubMed] [Google Scholar]
- Zhao XH, Laschinger C, Arora P, Szaszi K, Kapus A, McCulloch CA. Force activates smooth muscle α-actin promoter activity through the Rho signalling pathway. J Cell Sci. 2007;120 doi: 10.1242/jcs.001586. [DOI] [PubMed] [Google Scholar]
- Zou K, Meador BM, Johnson B, Huntsman HD, Mahmassani Z, Valero MC, Huey KA, Boppart MD. The α7β1-integrin increases muscle hypertrophy following multiple bouts of eccentric exercise. J Appl Physiol. 2011;111:1134–1141. doi: 10.1152/japplphysiol.00081.2011. [DOI] [PubMed] [Google Scholar]


