Abstract
We elucidated the autonomic mechanisms whereby heart rate (HR) is regulated by the muscle metaboreflex. Eight male participants (22 ± 3 years) performed three exercise protocols: (1) enhanced metaboreflex activation with partial flow restriction (bi-lateral thigh cuff inflation) during leg cycling exercise, (2) isolated muscle metaboreflex activation (post-exercise ischaemia; PEI) following leg cycling exercise, (3) isometric handgrip followed by PEI. Trials were undertaken under control (no drug), β1-adrenergic blockade (metoprolol) and parasympathetic blockade (glycopyrrolate) conditions. HR increased with partial flow restriction during leg cycling in the control condition (Δ11 ± 2 beats min−1; P < 0.05). The magnitude of this increase in HR was similar with parasympathetic blockade (Δ11 ± 2 beats min−1), but attenuated with β-adrenergic blockade (Δ4 ± 1 beats min−1; P < 0.05 vs. control and parasympathetic blockade). During PEI following leg cycling exercise, HR remained similarly elevated above rest under all conditions (Δ11 ± 2, Δ13 ± 3 and Δ9 ± 4 beats min−1, for control, β-adrenergic and parasympathetic blockade; P > 0.05 between conditions). During PEI following handgrip, HR was similarly elevated from rest under control and parasympathetic blockade (Δ4 ± 1 vs. Δ4 ± 2 beats min−1; P > 0.05 between conditions) conditions, but attenuated with β-adrenergic blockade (Δ0.2 ± 1 beats min−1; P > 0.05 vs. rest). Thus muscle metaboreflex activation-mediated increases in HR are principally attributable to increased cardiac sympathetic activity, and only following exercise with a large muscle mass (PEI following leg cycling) is there a contribution from the partial withdrawal of cardiac parasympathetic tone.
Key points
Heart rate increases during exercise due to withdrawal of cardiac parasympathetic tone and increased cardiac sympathetic nerve activity.
We investigated the autonomic mechanisms whereby heart rate is regulated by the activation of metabolically sensitive skeletal muscle afferents (muscle metaboreflex).
Heart rate responses elicited by partial flow restriction during leg cycling (enhanced metaboreflex activation) and post-exercise muscle ischemia following leg cycling and handgrip (isolated metaboreflex activation) were evaluated under control (no drug), β-adrenergic blockade and parasympathetic blockade conditions.
We show that the muscle metaboreflex principally elevates heart rate by increasing cardiac sympathetic activity, and only following dynamic exercise with a large muscle mass (post-exercise muscle ischemia following leg cycling) does the partial withdrawal of cardiac parasympathetic tone make a contribution to this heart rate response.
These findings may have implications for patient populations in which alterations in skeletal muscle afferent sensitivity have been identified.
Introduction
The classic work of Alam & Smirk (1937) indicated that the arterial blood pressure (BP) response to exercise is in part driven by a substance(s) present in the muscle and produced during exercise. Alam & Smirk found that making the skeletal muscle ischaemic augmented the BP response to exercise, and that BP remained elevated if the ischaemia was maintained during the post-exercise period despite the muscle remaining quiescent. More than 30 years later the reflex nature of this pressor response was shown, the activation of group III and IV muscle afferents implicated (Coote et al. 1971; McCloskey & Mitchell, 1972), and their discharge characteristics in response to mechanical (mechanoreflex) and metabolic (metaboreflex) perturbation elucidated (Kaufman & Rybicki, 1987). Mark et al. (1985) demonstrated that isolated activation of metabolically sensitive skeletal muscle afferents during post-exercise ischaemia (PEI) sustains the exercise-induced increase in sympathetic vasoconstrictor activity directed to the skeletal muscle vasculature. Animal investigations indicate that skeletal muscle afferent activation also increases sympathetic activity to the renal and adrenal vascular beds (Victor et al. 1989; Vissing et al. 1991; Hayes & Kaufman, 2002). However, how metaboreflex activation affects the autonomic control of the heart remains less well understood.
While BP remains elevated during PEI, the exercise-induced increase in heart rate (HR) is typically reported to return to baseline following isometric handgrip (Iellamo et al. 1994; Nishiyasu et al. 1994; Cui et al. 2001; Fisher et al. 2008, 2010) or isometric single leg knee extension (Iellamo et al. 1999). One potential explanation for these different effects is that the metaboreflex does not influence HR (Rowell & O’Leary, 1990), while an alternative explanation is that during PEI any sympathetic chronotropic affect is masked by reactivation of cardiac parasympathetic tone (O’Leary, 1993; Nishiyasu et al. 1994; Iellamo et al. 1999; Fisher et al. 2010). Reactivation of cardiac parasympathetic activity may result from baroreflex activation and/or loss of the inhibitory effects on cardiac parasympathetic tone from descending signals arising from higher brain centres in parallel with activation of motor pathways (‘central command’; Krogh & Lindhard, 1917; Goodwin et al. 1972) and the muscle mechanoreflex (Gladwell et al. 2005). Indirect support of this proposition in humans comes from the observation that HR variability-derived indices of cardiac parasympathetic tone are reported to be augmented during PEI (Nishiyasu et al. 1994; Iellamo et al. 1999). In addition, administration of the cholinergic muscarinic blocker glycopyrrolate unmasks a modest elevation in HR during PEI following low intensity handgrip, suggesting that the metaboreflex can augment cardiac sympathetic activity in humans (Fisher et al. 2010). However, in contrast to PEI following handgrip both HR and BP are markedly elevated during PEI following dynamic leg exercise (Alam & Smirk, 1938; Bonde-Petersen et al. 1978). The autonomic alterations underpinning these differential HR responses to isolated metaboreflex activation are presently unclear.
Elevations in HR during PEI following leg cycling are accompanied by reductions in spontaneous cardiovagal baroreflex sensitivity and HR variability (Hartwich et al. 2011, 2013). This apparent reduction in cardiac parasympathetic tone raises the possibility that isolated muscle metaboreflex activation following leg cycling reduces cardiac parasympathetic tone, although there is no direct evidence to support that contention. Notably, enhancement of muscle metaboreflex activation during dynamic exercise also causes an elevation in HR (Bonde-Petersen et al. 1978; Wyss et al. 1983; Sundberg & Kaijser, 1992; O’Leary, 1993; Sun et al. 1993). Pharmacological studies in dogs demonstrate that this increase in HR results from an increase in cardiac sympathetic activity and/or a reduction in cardiac parasympathetic activity (O’Leary, 1993) and studies in humans using indirect indices of cardiac autonomic activity (HR variability) support this (Sun et al. 1993; Hartwich et al. 2011, 2013). These findings raise the possibility that muscle metaboreflex activation may under certain circumstances reduce cardiac parasympathetic tone in humans. However, this contrasts with the more commonly accepted view that the elevation in HR due to muscle metaboreflex activation results from an increase in cardiac sympathetic activity and additional studies are required to resolve this issue.
The purpose of the present study was to elucidate the autonomic mechanisms whereby HR is increased by the muscle metaboreflex during various modalities of exercise (i.e. dynamic vs. static) and utilising variable muscle masses (i.e. one arm vs. two legs). HR and BP responses to separate trials of leg cycling with partial flow restriction (Protocol 1) and PEI (Protocol 2) were conducted under control (no drug) conditions, with β1-adrenergic blockade (metoprolol) and with cholinergic muscarinic blockade (glycopyrrolate). HR and BP were also monitored during separate bouts of isometric handgrip followed by PEI under control, β-adrenergic and parasympathetic blockade conditions (Protocol 3). We tested the hypothesis that HR returns to baseline levels during PEI following handgrip due to reactivation of cardiac parasympathetic nerve activity, but both withdrawal of cardiac parasympathetic tone and an increase in cardiac sympathetic activity contribute to the HR response to muscle metaboreflex activation during leg cycling with partial flow restriction and PEI.
Methods
The study protocol and procedures were approved by the ethical review committee in Copenhagen (Protocol no. H-3-2011-101) and performed in accordance with the Declaration of Helsinki. Eight male participants (age 22 ± 3 years; height 183 ± 6 cm; weight 74 ± 2 kg; mean ± SD) were recruited and after being informed of the study purpose and potential risks each participant provided written consent for participation. All participants were free from cardiovascular, pulmonary, renal, metabolic and neurological conditions, and none were taking any prescription or over-the-counter medications. Participants were requested to avoid strenuous exercise and alcohol consumption for 24 h, caffeine intake for 12 h, and food intake for 2 h prior to testing. Experiments were conducted in a room with a mean ambient temperature of ∼22°C.
Experimental measurements
HR was measured using a lead II electrocardiogram (ECG; BioAmp, ADInstruments, Bella Vista, NSW, Australia). A catheter (1.1 mm i.d., 20 gauge) was placed in the left brachial artery and connected to a pressure transducer (Baxter, Uden, the Netherlands) positioned at the height of the right atrium. ECG and BP signals were obtained through a Dialogue 2000 monitor (IBC-Danica, Copenhagen, Denmark) interfaced with an analog-to-digital convertor (Powerlab, ADInstruments) and personal computer equipped with data acquisition software (LabChart, ADInstruments). Cardiovascular variables were sampled at 1 kHz and beat-to-beat values of HR, systolic BP, diastolic BP and mean arterial pressure (MAP) calculated. When requested participants provided a rating of perceived exertion (RPE) using a 6–20 scale (Borg, 1982) as this has been associated with central command (Mitchell, 1990). Study drugs were administered through a catheter inserted into a left forearm vein.
Experimental protocol
The experimental protocol was conducted over two laboratory visits separated by ∼7–10 days. On the first experimental visit the control trial was conducted, followed by either the β-adrenergic or parasympathetic blockade trial. On the second experimental visit the remaining trial was undertaken in a counterbalanced order. The control trials were conducted first to minimise repeated catheterisations and to allow the accurate determination of the leg cycling workload, which was based on target HR. Time was given to permit the participant to recover between each trial (∼15–20 min).
Metoprolol was administered in stepwise infusions of 1 mg, with full β-adrenergic blockade considered achieved when HR was unchanged to consecutive doses (mean group dose 0.15 ± 0.002 mg kg−1). Glycopyrrolate was also administered in stepwise infusions of 0.2 mg, and complete cardiac parasympathetic blockade identified when consecutive doses caused no further increases in HR (mean group dose 17.9 ± 1.2 μg kg−1). If HR was changed from post-drug resting baseline values between trials, supplementary doses of metoprolol (0.011 ± 0.001 mg kg−1) or glycopyrrolate (4.4 ± 0.6 μg kg−1) were administered until no further change in HR occurred.
Protocol 1: leg cycling with partial flow restriction
Participants performed leg cycling exercise in a semi-recumbent position at 60 r.p.m. using a cycle ergometer (Krogh, 1913). After a 3 min baseline period, subjects performed ∼14 min of leg cycling at a low intensity workload (target HR of 100 beats min−1; 44 ± 8 W). The first ∼3.5 min was used to adjust the workload to reach the target HR. Following ∼3.5 min of steady-state cycling (Ex1), bilateral thigh cuffs were inflated to 100 mmHg (Tournipress Automatic, Speidel and Keller, Germany) in order to partially restrict blood flow to the exercising muscles and engage the metaboreflex. After ∼3.5 min the thigh cuffs were deflated and a further 3.5 min of steady-state leg cycling under free-flow conditions was performed (Ex2). RPE was obtained at the end of both periods of free-flow leg cycling and leg cycling with partial flow restriction.
Protocol 2: leg cycling and PEI
As described for Protocol 1, after a baseline period (3 min) participants performed semi-recumbent cycling for ∼14 min. The first ∼3.5 min was used to adjust the workload and reach a target HR of 100 beats min−1 (44 ± 8 W), and once established ∼10 min of steady-state cycling was performed. Fifteen seconds before the end of the exercise bout, bilateral thigh cuffs were inflated to 240 mmHg and they remained inflated for ∼3.5 min, thus isolating the activation of the muscle metaboreflex.
Protocol 3: isometric handgrip and PEI
While resting in a semi-recumbent position, participants held a dynamometer in their right hand. The maximum voluntary contraction (MVC) was determined as the greatest of 3–5 efforts, each separated by 1 min. After a 3 min resting baseline period participants performed 3 min of isometric handgrip at 25% MVC. During handgrip the force elicited was displayed on a computer screen to provide feedback to the subject. In the PEI trials, a cuff (Hokanson Inc., Bellevue, WA, USA) placed around the upper arm was inflated to 240 mmHg, 15 s before the end of exercise in order to occlude the circulation to the exercising muscles. The cuff remained inflated for ∼3.5 min to isolate muscle metaboreflex activation. One subject did not tolerate the PEI following handgrip and was omitted from the analyses of data from this protocol.
Data analysis
Comparisons of variables were made using repeated-measures ANOVA. Significant main effects and interactions were explored using post hoc Student–Newman–Keuls tests. Statistical significance was set at P < 0.05. Analyses were conducted using SigmaStat for Windows (Jandel Scientific Software, SPSS, Chicago, IL, USA).
Results
Protocol 1: leg cycling with partial flow restriction
As intended, resting HR increased with parasympathetic blockade and tended to be reduced with β-adrenergic blockade (P= 0.064; Table 1 and Fig. 1). HR increased during leg cycling under all conditions. In the control condition, partial flow restriction to enhance muscle metaboreflex activation during leg cycling caused a further increase in HR (Δ11 ± 2 beats min−1; P < 0.05 vs. Ex1). HR also increased during partial flow restriction with parasympathetic blockade, and the magnitude of the increase was similar to that under control conditions (Δ11 ± 2 beats min−1). In contrast, with β-adrenergic blockade the HR response to partial flow restriction was significantly attenuated in comparison to control and parasympathetic blockade conditions (Δ4 ± 1 beats min−1) and there was only a tendency for HR to increase from the prevailing level established during leg cycling (P= 0.09 vs. Ex1). Upon cessation of partial flow restriction HR fell under control and parasympathetic blockade conditions and returned to steady-state exercise levels (P > 0.05 Ex 1 vs. Ex2).
Table 1.
Heart rate (HR) and blood pressure during leg cycling before (Ex1) and following (Ex2) partial flow restriction (PFR), for the control, β-adrenergic blockade and parasympathetic blockade conditions
| Experimental phase | P value | ||||||
|---|---|---|---|---|---|---|---|
| Rest | Exl | PFR | Ex2 | Drug | Phase | Interaction | |
| HR (beats min-1) | |||||||
| Control | 64 ± 4 | 97 ± 2* | 110 ± 3*†‡ | 100 ± 2* | <0.001 | <0.001 | <0.001 |
| β-Block | 57 ± 3 | 83 ± 3*§ | 89 ± 4*§ | 85 ± 3*§ | |||
| Parasympathetic block | 114 ± 3§¶ | 132 ± 4*§¶ | 145 ± 5*†‡§¶ | 135 ± 4*§¶ | |||
| SBP (mmHg) | |||||||
| Control | 121 ± 3 | 136 ± 6* | 157 ± 7*†‡ | 137 ± 6* | 0.426 | <0.001 | 0.048 |
| β-Block | 116 ± 2 | 127 ± 4* | 150 ± 5*†‡ | 129 ± 5* | |||
| Parasympathetic block | 120 ± 6 | 131 ± 5* | 148 ± 7*†‡ | 131 ± 5* | |||
| MAP (mmHg) | |||||||
| Control | 82 ± 2 | 86 ± 3* | 105 ± 3*†‡ | 87 ± 3* | 0.290 | <0.001 | <0.001 |
| β-Block | 81 ± 2 | 84 ± 3 | 105 ± 3*†‡ | 85 ± 3 | |||
| Parasympathetic block | 90 ± 3* | 91 ± 4 | 107 ± 5*†‡ | 91 ± 4 | |||
| DBP (mmHg) | |||||||
| Control | 63 ± 2 | 65 ± 2 | 83 ± 3*†‡ | 65 ± 3 | 0.133 | <0.001 | <0.001 |
| β-Block | 64 ± 2 | 62 ± 3 | 83 ± 3*†‡ | 63 ± 2 | |||
| Parasympathetic block | 75 ± 3§¶ | 69 ± 4* | 87 ± 4*†‡ | 70 ± 4* | |||
Values represent means (±SEM) at rest (3 min) and last minute of each experimental phase. SBP, systolic blood pressure; MAP, mean arterial pressure; DBP, diastolic blood pressure. P values are derived from ANOVA examining main effects of drug, phase and interaction (drug × phase). *P < 0.05 vs. rest, †P < 0.05 vs. Ex1, ‡P < 0.05 vs. Ex2, §P < 0.05 vs. control, ¶P < 0.05 vs. β-blockade.
Figure 1. Heart rate (HR) and mean arterial pressure (MAP) during leg cycling before (Ex1) and following (Ex2) partial flow restriction (PFR), for control, β-adrenergic blockade and parasympathetic blockade conditions.
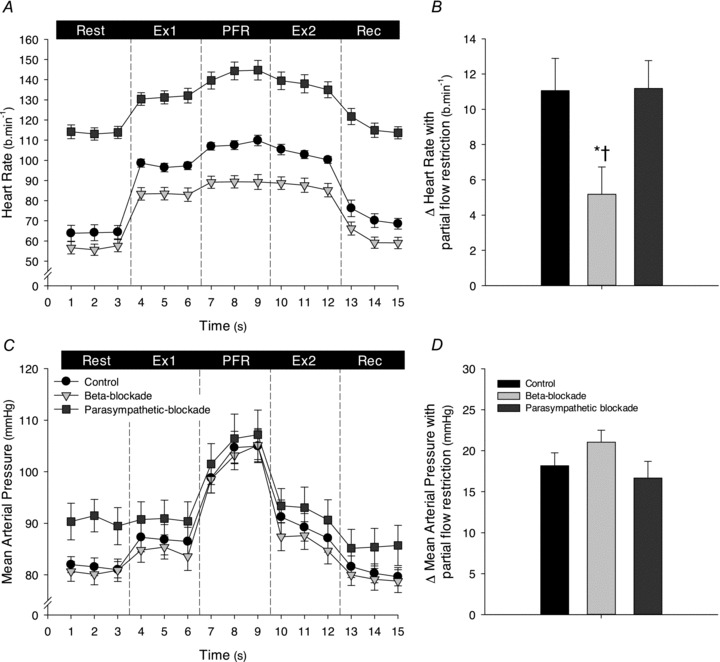
A, 1 min averages of HR during all experimental phases; B, change in HR elicited by partial flow restriction (PFR) from the HR during leg cycling (average of last minute of Ex1 and Ex2). C, 1 min averages of MAP during all experimental phases; D, change in MAP elicited by partial flow restriction (PFR) from the MAP during leg cycling (average of last minute of Ex1 and Ex2). Control, black symbols and bars; β-adrenergic blockade, light grey symbols and bars; parasympathetic blockade, dark grey symbols and bars. Ex1, leg cycling exercise prior to PFR; Ex2, leg cycling following PFR; Rec, recovery. *P < 0.05 vs. control, †P < 0.05 vs. β-blockade.
Resting MAP was unaltered with β-adrenergic blockade (P > 0.05 vs. control; Table 1 and Fig. 1), but increased with parasympathetic blockade (P < 0.05 vs. control). MAP increased slightly during exercise in all conditions, but this increase only reached statistical significance in the control condition. Partial flow restriction increased MAP (P < 0.05) to a similar extent in all conditions (Δ18 ± 2, Δ21 ± 1 and Δ17 ± 2 mmHg in the control, β-adrenergic and parasympathetic blockade conditions, respectively; P > 0.05 between conditions) and fell following cessation of partial flow restriction to steady-state exercise levels (P > 0.05 Ex1 vs. Ex2). RPE was significantly increased during partial flow restriction to a similar extent in all conditions (Ex1, 9 ± 0.5, 9 ± 1 and 9 ± 0.4; partial flow restriction, 13 ± 0.5, 14 ± 1 and 14 ± 1; Ex2, 9 ± 0.5, 9 ± 1 and 9 ± 0.5 arbitrary units (a.u.), for the control, β-adrenergic and parasympathetic blockade conditions, respectively; P > 0.05 between conditions).
Protocol 2: leg cycling and PEI
Resting HR was increased with parasympathetic blockade (P < 0.05) and decreased with β-adrenergic blockade (P < 0.05; Table 2 and Fig. 2). HR increased during leg cycling under all conditions, but the magnitude of the increase in HR with parasympathetic blockade (Δ18 ± 3 beats min−1) was less than that under control (Δ33 ± 3 beats min−1) and β-adrenergic blockade (Δ29 ± 3 beats min−1) conditions (P < 0.05). During PEI, HR remained elevated in all conditions (P < 0.05). The magnitude of the elevation in HR during PEI was not significantly different under control (Δ11 ± 2 beats min−1), β-adrenergic blockade (Δ13 ± 3 beats min−1), and parasympathetic blockade (Δ9 ± 4 beats min−1) conditions.
Table 2.
Heart rate (HR) and blood pressure during leg cycling (Ex) and post-exercise ischaemia (PEI) for the control, β-adrenergic blockade and parasympathetic blockade conditions
| Experimental phase | P value | |||||
|---|---|---|---|---|---|---|
| Rest | Ex | PEI | Drug | Phase | Interaction | |
| HR (beats min-1) | ||||||
| Control | 66 ± 3 | 99 ± 2* | 77 ± 2*† | <0.001 | <0.001 | <0.001 |
| β-Block | 56 ± 3§ | 85 ± 3*§ | 69 ± 2*† | |||
| Parasympathetic block | 114 ± 3§¶ | 132 ± 4*§¶ | 123 ± 5*†§¶ | |||
| SBP (mmHg) | ||||||
| Control | 116 ± 2 | 132 ± 4* | 132 ± 3* | 0.656 | <0.001 | <0.001 |
| β-Block | 112 ± 2 | 123 ± 3* | 133 ± 4*† | |||
| Parasympathetic block | 117 ± 4 | 127 ± 4* | 126 ± 5* | |||
| MAP (mmHg) | ||||||
| Control | 78 ± 2 | 84 ± 2* | 94 ± 2*† | 0.177 | <0.001 | <0.001 |
| β-Block | 78 ± 2 | 81 ± 2 | 100 ± 3*† | |||
| Parasympathetic block | 89 ± 3§¶ | 88 ± 3 | 99 ± 4*† | |||
| DBP (mmHg) | ||||||
| Control | 61 ± 2 | 62 ± 2 | 75 ± 2*† | 0.041 | <0.001 | <0.001 |
| β-Block | 61 ± 2 | 61 ± 3 | 80 ± 3*† | |||
| Parasympathetic block | 74 ± 3§¶ | 68 ± 3* | 83 ± 4*† | |||
Values represent means (±SEM) at rest (3 min) and last minute of each experimental phase. SBP, systolic blood pressure; MAP, mean arterial pressure; DBP, diastolic blood pressure. P values are derived from ANOVA evaluation of main effects of drug, phase and interaction (drug × phase). *P < 0.05 vs. rest, †P < 0.05 vs. Ex, §P < 0.05 vs. control, ¶P < 0.05 vs. β-blockade.
Figure 2. Heart rate (HR) and mean arterial pressure (MAP) during leg cycling and post-exercise ischaemia (PEI) for the control, β-adrenergic blockade and parasympathetic blockade conditions.
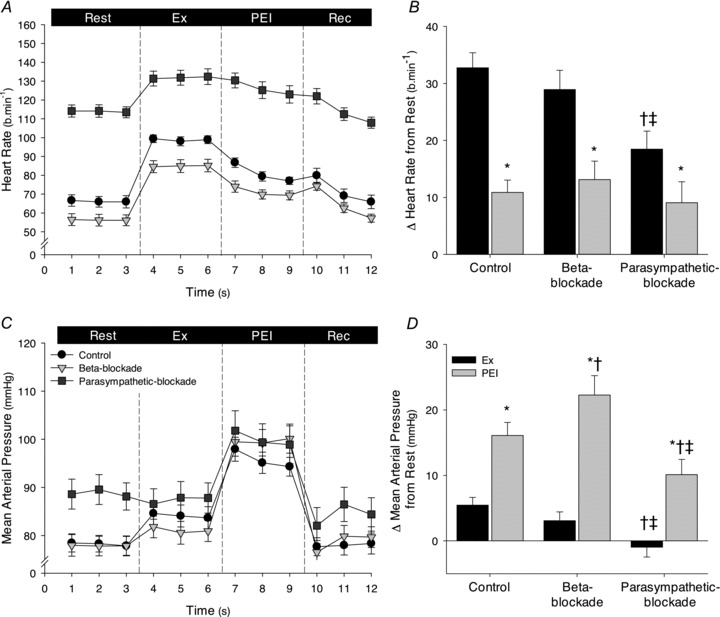
A and C, 1 min averages of absolute HR and MAP, respectively, during all experimental phases of the control (black symbols), β-adrenergic blockade (light grey symbols) and parasympathetic blockade (dark grey symbols) conditions. B and D, change in HR and MAP, respectively, elicited at the last minute of leg cycling (Ex, black bars) and PEI (dark grey bars) in each condition. Rec, recovery. *P < 0.05 vs. Ex, †P < 0.05 vs. control; ‡P < 0.05 vs. β-blockade.
Resting MAP was increased with parasympathetic blockade (P < 0.05 vs. control and β-adrenergic blockade), but unaltered with β-adrenergic blockade (P > 0.05 vs. control). MAP was slightly increased from rest during leg cycling under control conditions, but not with parasympathetic or β-adrenergic blockade (Table 2). During PEI, MAP was elevated above rest and exercise levels (P < 0.05) in all conditions. The elevation was greater with β-adrenergic blockade (Δ22 ± 3 mmHg) than control conditions (Δ16 ± 2 mmHg; P < 0.05), and lower with parasympathetic blockade (Δ10 ± 2 mmHg; P < 0.05 vs. control and β-adrenergic blockade; Fig. 2). RPE was similar during leg cycling under all conditions (9 ± 0.5, 9 ± 1 and 9 ± 1 a.u., for the control, β-adrenergic and parasympathetic blockade conditions, respectively; P > 0.05).
Protocol 3: isometric handgrip and PEI
Resting HR was increased with parasympathetic blockade (P < 0.05) and tended to be decreased with β-adrenergic blockade prior to isometric handgrip (P= 0.079; Table 3 and Fig. 3). During handgrip, HR increased similarly under all conditions (Δ26 ± 3, Δ18 ± 2 and Δ18 ± 3 beats min−1 for control, β-adrenergic and parasympathetic blockade conditions). During PEI, HR was slightly elevated from rest under control conditions (Δ4 ± 1 beats min−1, P < 0.05 vs. rest) and tended to be elevated with parasympathetic blockade (Δ4 ± 2 beats min−1, P= 0.06 vs. rest). In contrast, with β-adrenergic blockade HR was not significantly elevated from rest (Δ0.2 ± 1 beats min−1).
Table 3.
Heart rate (HR) and blood pressure during isometric handgrip and post-exercise ischaemia (PEI) for the control, β-adrenergic blockade and parasympathetic blockade conditions
| Experimental phase | P value | |||||
|---|---|---|---|---|---|---|
| Rest | Handgrip | PEI | Drug | Phase | Interaction | |
| HR (beats min-1) | ||||||
| Control | 68 ± 5 | 94 ± 4* | 72 ± 4*† | <0.001 | <0.001 | <0.001 |
| β-Block | 58 ± 4 | 76 ± 4*§ | 58 ± 3†§ | |||
| Parasympathetic block | 116 ± 3§¶ | 133 ± 4*§¶ | 120 ± 4†§¶ | |||
| SBP (mmHg) | ||||||
| Control | 122 ± 4 | 152 ± 6 | 147 ± 6 | 0.091 | 0.001 | 0.635 |
| β-Block | 110 ± 2 | 140 ± 3 | 137 ± 5 | |||
| Parasympathetic block | 120 ± 4 | 150 ± 6 | 143 ± 5 | |||
| MAP (mmHg) | ||||||
| Control | 82 ± 2 | 122 ± 4 | 108 ± 4 | 0.039 | 0.001 | 0.077 |
| β-Block | 76 ± 2 | 109 ± 3 | 103 ± 4 | |||
| Parasympathetic block | 90 ± 3 | 118 ± 4 | 111 ± 4 | |||
| DBP (mmHg) | ||||||
| Control | 63 ± 2 | 87 ± 3 | 82 ± 3 | <0.001 | <0.001 | 0.080 |
| β-Block | 60 ± 2 | 90 ± 3 | 82 ± 4 | |||
| Parasympathetic block | 73 ± 3 | 99 ± 3 | 92 ± 4 | |||
Values represent means (±SEM) at rest (3 min) and last minute of each experimental phase. SBP, systolic blood pressure; MAP, mean arterial pressure; DBP, diastolic blood pressure. P values are derived from ANOVA examining main effects of drug, phase and interaction (drug × phase). *P < 0.05 vs. rest, †P < 0.05 vs. Ex, §P < 0.05 vs. control, ¶P < 0.05 vs. β-blockade.
Figure 3. Heart rate (HR) and mean arterial pressure (MAP) during isometric handgrip and post-exercise ischaemia (PEI), for the control, β-adrenergic blockade and parasympathetic blockade conditions.
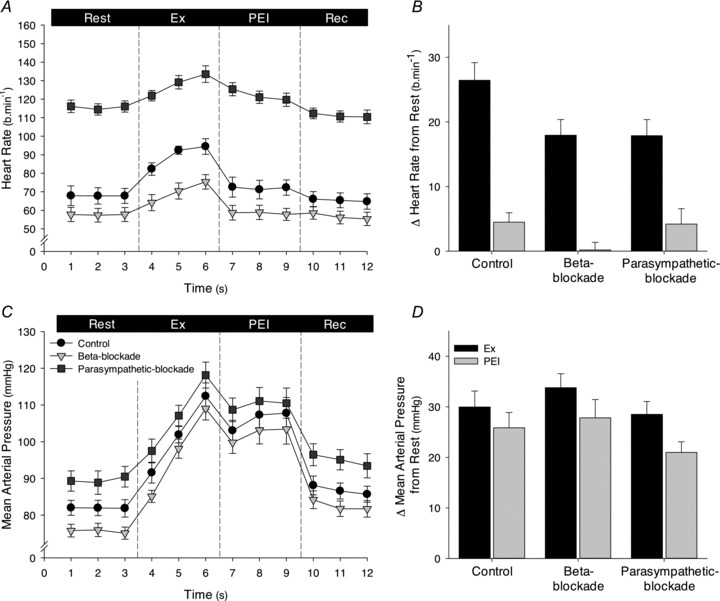
A and C, 1 min averages of HR and MAP, respectively, during all experimental phases of the control (black symbols), β-adrenergic blockade (light grey symbols) and parasympathetic blockade (dark grey symbols) conditions. B and D, change in HR and MAP, respectively, elicited during the last minute of handgrip (Ex, black bars) and PEI (dark grey bars) in each condition. Rec, recovery.
MAP was increased with parasympathetic blockade at all experimental phases (P < 0.05 vs. control and β-adrenergic blockade), but unaltered with β-adrenergic blockade (P > 0.05 vs. control). During handgrip, MAP was increased from rest similarly in all conditions, and also remained elevated during PEI to a similar extent under all conditions (P < 0.05 vs. rest; P > 0.05 between conditions; Table 3 and Fig. 3). RPE was similar during handgrip under all conditions (17 ± 1, 17 ± 1 and 16 ± 1 beats min−1, for the control, β-adrenergic and parasympathetic blockade conditions, respectively; P > 0.05).
Discussion
We aimed to determine the sympathetic and parasympathetic contribution to the HR response elicited by partial flow restriction during leg cycling (enhanced metaboreflex activation) and post-exercise muscle ischaemia following leg cycling and handgrip (isolated metaboreflex activation). The main novel findings are: (1) increases in HR elicited by partial flow restriction during leg cycling were attenuated by β-adrenergic blockade, but unaffected by parasympathetic blockade; (2) the elevation in HR during PEI following leg exercise was the same under control (no drug) conditions, β-adrenergic blockade, and parasympathetic blockade. In contrast, the modest elevation in HR during PEI following handgrip was abolished with β-adrenergic blockade, as has been shown previously (Fisher et al. 2010). Taken together these findings indicate that the muscle metaboreflex principally increases HR by an increase in cardiac sympathetic activity, and only following dynamic exercise with a large muscle mass (i.e. PEI following leg cycling) does the partial withdrawal of cardiac parasympathetic tone make a contribution to this HR response.
Following seminal work in dogs (O’Leary, 1993), evidence from studies in humans has indicated that during PEI following handgrip exercise there is an augmentation of both cardiac sympathetic and parasympathetic activity. Nishiyasu et al. (1994) noted that indices of cardiac parasympathetic tone, derived from HR variability analyses, were augmented during PEI following isometric handgrip, although HR had returned to baseline levels. During modest muscle metaboreflex activation with PEI following low intensity isometric handgrip, pharmacological blockade of cardiac parasympathetic tone reveals a sympathetically mediated increase in HR (Fisher et al. 2010). This suggests that the effect of the metaboreflex on cardiac sympathetic nerve activity can be antagonised by enhanced parasympathetic tone during PEI (O’Leary, 1993; Nishiyasu et al. 1994; Iellamo et al. 1999; Fisher et al. 2010). This may in part be due to the cessation of central command and muscle mechanoreceptor activation at the termination of exercise, since they have been reported to inhibit cardiac parasympathetic control during muscular contraction (Mitchell et al. 1989; McWilliam & Yang, 1991; McWilliam et al. 1991; Gladwell et al. 2005). In addition, the elevation in BP during exercise may stimulate the arterial baroreceptors and elicit a reflex increase in cardiac parasympathetic tone (O’Leary, 1993; Nishiyasu et al. 1994; Iellamo et al. 1999; Fisher et al. 2010). In the present study, and as shown during PEI following high intensity handgrip (Fisher et al. 2010), when the metaboreflex is robustly activated HR is modestly elevated during PEI and although this is unaltered by parasympathetic blockade it is attenuated with β-adrenergic blockade. Thus, with robust metaboreflex activation increases in cardiac sympathetic nerve activity may prevail over enhanced cardiac parasympathetic tone.
Whereas little to no elevation in HR is noted during PEI following handgrip (Nishiyasu et al. 1994; Iellamo et al. 1999; Fisher et al. 2010), HR may be elevated substantially during PEI following leg exercise (Alam & Smirk, 1938; Bonde-Petersen et al. 1978). This too may be explained by a robust metaboreflex activation evoking a profound increase in cardiac sympathetic activity that overpowers any increase in parasympathetic activity. However, indirect indices of cardiac parasympathetic activity derived from HR variability analyses are reduced to below baseline levels during PEI following leg cycling exercise (Hartwich et al. 2011, 2013). Therefore, withdrawal of cardiac parasympathetic activity may also contribute to the increase in HR under these conditions. We found an elevation in HR during PEI following leg exercise under control conditions that was no different to that with β-adrenergic or parasympathetic blockade. Thus, both the withdrawal of cardiac parasympathetic activity and/or the activation of cardiac sympathetic activity appear able to elicit the increase in HR during PEI following leg exercise. This apparent redundancy in the autonomic neural control of HR by the metaboreflex highlights the complexity of cardiac sympathetic–parasympathetic interactions during exercise (Levy, 1971). It is difficult to provide an explanation for the differential effects of isolated muscle metaboreflex activation in the forearm and legs on the autonomic regulation of HR, but differences in muscle mass, exercise modality (isometric vs. dynamic exercise) and skeletal muscle fibre type remain as possibilities.
An alternative approach to PEI for studying the autonomic effects of the muscle metaboreflex is via a reduction in perfusion to the exercising skeletal muscles, thereby inducing an oxygen demand/delivery mismatch and consequent local metabolite accumulation. Partially occluding the terminal aorta and attenuating blood flow to the hindlimb of treadmill running dogs is well established in this regard (Wyss et al. 1983; O’Leary, 1993). As a non-invasive analogue, humans studies have utilised chambers to apply positive pressure over the exercising muscles (Sundberg & Kaijser, 1992; Sun et al. 1993) or inflated cuffs proximal to the exercising limbs (Bonde-Petersen et al. 1978; Hartwich et al. 2011). In both human and animal studies a pronounced HR and pressor response is evoked by these manoeuvres. Notably, O’Leary (1993) reported that progressively decreasing hindlimb perfusion in canines running on a treadmill at a constant workload induced an increase in HR, despite β-adrenergic blockade, although the increase in HR for a given reduction in hindlimb flow was somewhat attenuated. These findings suggest that metaboreflex activation in exercising canines is at least in part attributable to a reduction in cardiac parasympathetic activity. We observed that the chronotropic response to partial flow restriction during leg cycling was attenuated with β-adrenergic blockade, suggesting that it was in part sympathetically mediated in humans. We also observed reductions in cardiovagal baroreflex sensitivity and indices of cardiac parasympathetic activity derived from HR variability analysis during leg cycling with partial flow restriction (Hartwich et al. 2011, 2013). We speculate that enhanced metaboreflex activation during exercise in humans inhibits cardiac parasympathetic tone. However, administration of glycopyrrolate did not influence the chronotropic response to partial flow restriction suggesting that cardiac parasympathetic withdrawal does not contribute to the HR response to enhanced metaboreflex activation during exercise in humans. Although somewhat as a paradox with β-adrenergic blockade a small increase in HR persisted with partial flow restriction, meaning that some inhibition of parasympathetic withdrawal may have occurred.
During isolated muscle metaboreflex (PEI) following either handgrip or leg cycling, HR was lower than end-exercise levels in all conditions. This is in contrast to what has been observed in dogs, where during PEI following treadmill running HR remained elevated at exercise values with parasympathetic blockade (O’Leary, 1993). The reason for these contrasting findings may be attributable to species differences (Rowell & O’Leary, 1990) or differences in relative exercise intensities. Since HR falls from end-exercise levels during PEI with cardiac parasympathetic blockade it can be inferred that central command and/or the muscle mechanoreflex can increase HR by augmenting cardiac sympathetic nerve activity during moderate intensity leg cycling and handgrip exercise.
An assumption inherent in the use of PEI is that metabolically sensitive skeletal muscle afferents are activated in the absence of central command or muscle mechanoreceptors, and account for the resultant autonomic and haemodynamic changes observed. However, this may not faithfully represent the conditions under which the metaboreflex is usually activated (Kaufman, 2010; Hartwich et al. 2011). While a similar population of muscle afferents is likely to be activated by hypoperfusion of the exercising muscle a change in intramuscular pressure may also stimulate those muscle afferents that are mechanically sensitive (Kaufman & Rybicki, 1987; McClain et al. 1993; Haouzi et al. 1999). Furthermore, the sensitivity of some mechanically sensitive muscle afferents may be enhanced by an accumulation of metabolites within the exercising skeletal muscles (Kaufman & Rybicki, 1987; Fisher & White, 2004). The contribution of these populations of skeletal muscle afferents to the autonomic responses we observed cannot be excluded; however, we feel that it is unlikely because they are inconsistent with their described effects in humans. Isolated muscle mechanoreflex activation with passive calf muscle stretch evokes a modest increase in HR (∼5 beats min−1) that is abolished with pharmacological blockade of cardiac parasympathetic nerve activity or parasympathetic withdrawal evoked with low intensity rhythmic handgrip (10% MVC; Gladwell et al. 2005). However, the HR responses to partial flow restriction we observed were more marked (∼11 beats min−1) and preserved with cardiac parasympathetic blockade. Furthermore, the HR responses to passive calf stretch also appear to be independent of the level of metabolites within the muscles being lengthened (Fisher et al. 2005). In contrast to passive stretch, muscle mechanoreflex activation induced by external compression of the calf with a wide cuff inflated to 300 mmHg does not increase HR even when applied during PEI following high intensity isometric calf exercise (50–70% MVC; Bell & White, 2005). Passive muscle stretch (Cui et al. 2006) and venous distension (Cui et al. 2011) increase sympathetic outflow to the skeletal muscle vasculature, but the effects on cardiac sympathetic activity in humans remain unclear. In addition, thigh cuff inflation increased RPE suggesting that central command was increased, possibly as a result of a skeletal muscle afferent activation on central motor drive, or a change in skeletal muscle fibre recruitment and leg cycling mechanical efficiency (Gandevia, 2001; Amann et al. 2009).
Pharmacological blockade of cardiac parasympathetic nerve activity was undertaken using glycopyrrolate because unlike atropine it does not cross the blood–brain barrier (Proakis & Harris, 1978). However, we used a β-adrenergic blocker (metoprolol) which does cross the blood–brain barrier, thus a direct effect within the central nervous system cannot be ruled out (Neil-Dwyer et al. 1981). It is also a limitation of the present investigation that cardiac output was not assessed. Some controversy surrounds the effect of the muscle metaboreflex on cardiac output and the potential influence of the muscle group examined (Pawelczyk et al. 1997; Bastos et al. 2000; Crisafulli et al. 2003), thus additional studies are warranted.
Alterations in skeletal muscle afferent sensitivity have been identified in a number of cardiovascular diseases, such as hypertension (Smith et al. 2006; Delaney et al. 2010) and heart failure (Piepoli et al. 1996; Middlekauff et al. 2004). An inappropriate cardiovascular response to exercise could impair exercise tolerance and increase the risk of an acute cardiovascular event (e.g. stroke, myocardial infarction). However, much of the human work examining the influence of chronic cardiovascular conditions on muscle afferent sensitivity has focused on autonomic control of peripheral vasculature (e.g. muscle sympathetic nerve activity) rather than that of the heart. Given the cardioprotective actions of cardiac parasympathetic tone and the link between heightened cardiac sympathetic drive and arrhythmia in exercise (Billman et al. 2006), it is important to elucidate the influence of skeletal muscle afferents on the autonomic regulation of the heart in both health and disease.
In summary, autonomic regulation of HR by the muscle metaboreflex was investigated and the extent to which it is influenced by the mode of activation evaluated. Our findings indicate that partial flow restriction during leg cycling to enhance metaboreflex activation principally evokes a sympathetically mediated increase in HR. Similarly, the modest elevation in HR during isolated metaboreflex activation with PEI following handgrip appears to be sympathetically mediated, while in contrast PEI following leg cycling leads to a partial maintenance of the exercise-induced increase in HR due to increased cardiac sympathetic activity and/or withdrawal of cardiac parasympathetic tone. These observations indicate that the mode of muscle metaboreflex activation and the muscle group examined are important determinants of the autonomic control of HR.
Acknowledgments
The time and effort expended by all volunteer subjects is greatly appreciated. We thank Peter Nissen for his excellent technical assistance.
Glossary
- BP
blood pressure
- DBP
diastolic blood pressure
- HR
heart rate
- MAP
mean arterial pressure
- MVC
maximum voluntary contraction
- PEI
post-exercise ischaemia
- PFR
partial flow restriction
- RPE
rating of perceived exertion
- SBP
systolic blood pressure
Additional information
Competing interests
None.
Author contributions
J.P.F. contributed to study design, data acquisition, data analysis, data interpretation, writing the first draft and critical review of the manuscript. A.M.A. contributed to data acquisition, data analysis and critical review of the manuscript. A.S, J.F.S and H.S. contributed to data acquisition and critical review of the manuscript. N.H.S. contributed to study design, data acquisition, data interpretation and critical review of the manuscript. Experiments were undertaken at the Department of Anaesthesia, Rigshospitalet, University of Copenhagen, Denmark. All authors approved the final version of the manuscript.
Funding
This work was in part funded by the Royal Society (JPF).
References
- Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M, Smirk FH. Observations in man on a pulse-accelerating reflex from the voluntary muscles of the legs. J Physiol. 1938;92:167–177. doi: 10.1113/jphysiol.1938.sp003592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587:271–283. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos BG, Williamson JW, Harrelson T, Nobrega AC. Left ventricular volumes and hemodynamic responses to postexercise ischemia in healthy humans. Med Sci Sports Exerc. 2000;32:1114–1118. doi: 10.1097/00005768-200006000-00012. [DOI] [PubMed] [Google Scholar]
- Bell MP, White MJ. Cardiovascular responses to external compression of human calf muscle vary during graded metaboreflex stimulation. Exp Physiol. 2005;90:383–391. doi: 10.1113/expphysiol.2004.029140. [DOI] [PubMed] [Google Scholar]
- Billman GE, Kukielka M, Kelley R, Moustafa-Bayoumi M, Altschuld RA. Endurance exercise training attenuates cardiac β2-adrenoceptor responsiveness and prevents ventricular fibrillation in animals susceptible to sudden death. Am J Physiol Heart Circ Physiol. 2006;290:H2590–H2599. doi: 10.1152/ajpheart.01220.2005. [DOI] [PubMed] [Google Scholar]
- Bonde-Petersen F, Rowell LB, Murray RG, Blomqvist GG, White R, Karlsson E, Campbell W, Mitchell JH. Role of cardiac output in the pressor responses to graded muscle ischemia in man. J Appl Physiol. 1978;45:574–580. doi: 10.1152/jappl.1978.45.4.574. [DOI] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol. 1971;215:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisafulli A, Scott AC, Wensel R, Davos CH, Francis DP, Pagliaro P, Coats AJ, Concu A, Piepoli MF. Muscle metaboreflex-induced increases in stroke volume. Med Sci Sports Exerc. 2003;35:221–228. doi: 10.1249/01.MSS.0000048639.02548.24. discussion, p. 229. [DOI] [PubMed] [Google Scholar]
- Cui J, Blaha C, Moradkhan R, Gray KS, Sinoway LI. Muscle sympathetic nerve activity responses to dynamic passive muscle stretch in humans. J Physiol. 2006;576:625–634. doi: 10.1113/jphysiol.2006.116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Leuenberger UA, Gao Z, Sinoway LI. Sympathetic and cardiovascular responses to venous distension in an occluded limb. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1831–R1837. doi: 10.1152/ajpregu.00170.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Shibasaki M, Hodges NA, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during posthandgrip muscle ischemia in humans. J Appl Physiol. 2001;91:1679–1686. doi: 10.1152/jappl.2001.91.4.1679. [DOI] [PubMed] [Google Scholar]
- Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol. 2010;299:H1318–H1327. doi: 10.1152/ajpheart.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Bell MP, White MJ. Cardiovascular responses to human calf muscle stretch during varying levels of muscle metaboreflex activation. Exp Physiol. 2005;90:773–781. doi: 10.1113/expphysiol.2005.030577. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Seifert T, Hartwich D, Young CN, Secher NH, Fadel PJ. Autonomic control of heart rate by metabolically sensitive skeletal muscle afferents in humans. J Physiol. 2010;588:1117–1127. doi: 10.1113/jphysiol.2009.185470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, White MJ. Muscle afferent contributions to the cardiovascular response to isometric exercise. Exp Physiol. 2004;89:639–646. doi: 10.1113/expphysiol.2004.028639. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Young CN, Fadel PJ. Effect of muscle metaboreflex activation on carotid-cardiac baroreflex function in humans. Am J Physiol Heart Circ Physiol. 2008;294:H2296–H2304. doi: 10.1152/ajpheart.91497.2007. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gladwell VF, Fletcher J, Patel N, Elvidge LJ, Lloyd D, Chowdhary S, Coote JH. The influence of small fibre muscle mechanoreceptors on the cardiac vagus in humans. J Physiol. 2005;567:713–721. doi: 10.1113/jphysiol.2005.089243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haouzi P, Hill JM, Lewis BK, Kaufman MP. Responses of group III and IV muscle afferents to distension of the peripheral vascular bed. J Appl Physiol. 1999;87:545–553. doi: 10.1152/jappl.1999.87.2.545. [DOI] [PubMed] [Google Scholar]
- Hartwich D, Aldred S, Fisher JP. Influence of menstrual cycle phase on muscle metaboreflex control of cardiac baroreflex sensitivity, heart rate and blood pressure in humans. Exp Physiol. 2013;98:220–232. doi: 10.1113/expphysiol.2012.066498. [DOI] [PubMed] [Google Scholar]
- Hartwich D, Dear WE, Waterfall JL, Fisher JP. Effect of muscle metaboreflex activation on spontaneous cardiac baroreflex sensitivity during exercise in humans. J Physiol. 2011;589:6157–6171. doi: 10.1113/jphysiol.2011.219964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SG, Kaufman MP. MLR stimulation and exercise pressor reflex activate different renal sympathetic fibers in decerebrate cats. J Appl Physiol. 2002;92:1628–1634. doi: 10.1152/japplphysiol.00905.2001. [DOI] [PubMed] [Google Scholar]
- Iellamo F, Hughson RL, Castrucci F, Legramante JM, Raimondi G, Peruzzi G, Tallarida G. Evaluation of spontaneous baroreflex modulation of sinus node during isometric exercise in healthy humans. Am J Physiol Heart Circ Physiol. 1994;267:H994–H1001. doi: 10.1152/ajpheart.1994.267.3.H994. [DOI] [PubMed] [Google Scholar]
- Iellamo F, Pizzinelli P, Massaro M, Raimondi G, Peruzzi G, Legramante JM. Muscle metaboreflex contribution to sinus node regulation during static exercise: insights from spectral analysis of heart rate variability. Circulation. 1999;100:27–32. doi: 10.1161/01.cir.100.1.27. [DOI] [PubMed] [Google Scholar]
- Kaufman MP. Metaboreflex control of the heart. J Physiol. 2010;588:1037–1038. doi: 10.1113/jphysiol.2010.189241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res. 1987;61:I60–65. [PubMed] [Google Scholar]
- Krogh A. A bicycle ergometer and respiration apparatus for the experimental study of muscular work. Skand Arch Physiol. 1913;30:375–394. [Google Scholar]
- Krogh A, Lindhard J. A comparison between voluntary and electrically induced muscular work in man. J Physiol. 1917;51:182–201. doi: 10.1113/jphysiol.1917.sp001795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MN. Sympathetic-parasympathetic interactions in the heart. Circ Res. 1971;29:437–445. doi: 10.1161/01.res.29.5.437. [DOI] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- McClain J, Hardy C, Enders B, Smith M, Sinoway L. Limb congestion and sympathoexcitation during exercise. Implications for congestive heart failure. J Clin Invest. 1993;92:2353–2359. doi: 10.1172/JCI116840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliam PN, Yang T. Inhibition of cardiac vagal component of baroreflex by group III and IV afferents. Am J Physiol Heart Circ Physiol. 1991;260:H730–H734. doi: 10.1152/ajpheart.1991.260.3.H730. [DOI] [PubMed] [Google Scholar]
- McWilliam PN, Yang T, Chen LX. Changes in the baroreceptor reflex at the start of muscle contraction in the decerebrate cat. J Physiol. 1991;436:549–558. doi: 10.1113/jphysiol.1991.sp018566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi J, Patel J. Muscle mechanoreceptor sensitivity in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1937–H1943. doi: 10.1152/ajpheart.00330.2004. [DOI] [PubMed] [Google Scholar]
- Mitchell JH. J.B. Wolffe memorial lecture. Neural control of the circulation during exercise. Med Sci Sports Exerc. 1990;22:141–154. [PubMed] [Google Scholar]
- Mitchell JH, Reeves DR, Jr, Rogers HB, Secher NH, Victor RG. Autonomic blockade and cardiovascular responses to static exercise in partially curarized man. J Physiol. 1989;413:433–445. doi: 10.1113/jphysiol.1989.sp017662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil-Dwyer G, Bartlett J, McAinsh J, Cruickshank JM. Beta-adrenoceptor blockers and the blood-brain barrier. Br J Clin Pharmacol. 1981;11:549–553. doi: 10.1111/j.1365-2125.1981.tb01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyasu T, Tan N, Morimoto K, Nishiyasu M, Yamaguchi Y, Murakami N. Enhancement of parasympathetic cardiac activity during activation of muscle metaboreflex in humans. J Appl Physiol. 1994;77:2778–2783. doi: 10.1152/jappl.1994.77.6.2778. [DOI] [PubMed] [Google Scholar]
- O’Leary DS. Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol. 1993;74:1748–1754. doi: 10.1152/jappl.1993.74.4.1748. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA, Pawelczyk RA, Warberg J, Mitchell JH, Secher NH. Cardiovascular and catecholamine responses to static exercise in partially curarized humans. Acta Physiol Scand. 1997;160:23–28. doi: 10.1046/j.1365-201X.1997.00115.x. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation. 1996;93:940–952. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- Proakis AG, Harris GB. Comparative penetration of glycopyrrolate and atropine across the blood–brain and placental barriers in anaesthetized dogs. Anesthesiology. 1978;48:339–344. doi: 10.1097/00000542-197805000-00007. [DOI] [PubMed] [Google Scholar]
- Rowell LB, O’Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol. 1990;69:407–418. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol. 2006;577:1009–1020. doi: 10.1113/jphysiol.2006.121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Eiken O, Mekjavic IB. Autonomic nervous control of heart rate during blood-flow restricted exercise in man. Eur J Appl Physiol Occup Physiol. 1993;66:202–206. doi: 10.1007/BF00235094. [DOI] [PubMed] [Google Scholar]
- Sundberg CJ, Kaijser L. Effects of graded restriction of perfusion on circulation and metabolism in the working leg; quantification of a human ischaemia-model. Acta Physiol Scand. 1992;146:1–9. doi: 10.1111/j.1748-1716.1992.tb09386.x. [DOI] [PubMed] [Google Scholar]
- Victor RG, Rotto DM, Pryor SL, Kaufman MP. Stimulation of renal sympathetic activity by static contraction: evidence for mechanoreceptor-induced reflexes from skeletal muscle. Circ Res. 1989;64:592–599. doi: 10.1161/01.res.64.3.592. [DOI] [PubMed] [Google Scholar]
- Vissing J, Wilson LB, Mitchell JH, Victor RG. Static muscle contraction reflexly increases adrenal sympathetic nerve activity in rats. Am J Physiol Regul Integr Comp Physiol. 1991;261:R1307–R1312. doi: 10.1152/ajpregu.1991.261.5.R1307. [DOI] [PubMed] [Google Scholar]
- Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol. 1983;245:H481–H486. doi: 10.1152/ajpheart.1983.245.3.H481. [DOI] [PubMed] [Google Scholar]


