Abstract
Recovery of skeletal muscle mass from immobilisation-induced atrophy is faster in young than older individuals, yet the cellular mechanisms remain unknown. We examined the cellular and molecular regulation of muscle recovery in young and older human subjects subsequent to 2 weeks of immobility-induced muscle atrophy. Retraining consisted of 4 weeks of supervised resistive exercise in 9 older (OM: mean age) 67.3, range 61–74 yrs) and 11 young (YM: mean age 24.4, range 21–30 yrs) males. Measures of myofibre area (MFA), Pax7-positive satellite cells (SCs) associated with type I and type II muscle fibres, as well as gene expression analysis of key growth and transcription factors associated with local skeletal muscle milieu, were performed after 2 weeks immobility (Imm) and following 3 days (+3d) and 4 weeks (+4wks) of retraining. OM demonstrated no detectable gains in MFA (vastus lateralis muscle) and no increases in number of Pax7-positive SCs following 4wks retraining, whereas YM increased their MFA (P < 0.05), number of Pax7-positive cells, and had more Pax7-positive cells per type II fibre than OM at +3d and +4wks (P < 0.05). No age-related differences were observed in mRNA expression of IGF-1Ea, MGF, MyoD1 and HGF with retraining, whereas myostatin expression levels were more down-regulated in YM compared to OM at +3d (P < 0.05). In conclusion, the diminished muscle re-growth after immobilisation in elderly humans was associated with a lesser response in satellite cell proliferation in combination with an age-specific regulation of myostatin. In contrast, expression of local growth factors did not seem to explain the age-related difference in muscle mass recovery.
Key points
Elderly individuals require a prolonged recovery phase in order to return to initial muscle mass levels following short-term immobilisation.
The cellular mechanisms responsible for the attenuated re-growth and associated molecular signalling processes in ageing human skeletal muscle are not fully understood.
The main study finding was the observation of a less marked muscle mass recovery after immobilisation in elderly compared to young individuals that was paralleled by an elevation in myogenic precursor cell content in young individuals only, whereas the elderly failed to demonstrate any change in myogenic precursor cells.
No age-related differences were observed in the expression of major myogenic regulating factors known to promote skeletal muscle hypertrophy or satellite cell proliferation (IGF-1Ea, MGF, MyoD1, myogenin, HGF gene products).
In contrast, the expression of myostatin demonstrated a more pronounced up-regulation following immobilisation along with an attenuated down-regulation in response to reloading in older compared to young individuals, which may have contributed to the present lack of satellite cell proliferation in ageing muscle.
Introduction
Human skeletal muscle is a highly plastic tissue, which is reflected by its ability to rapidly adapt to short-term changes in habitual loading intensity (Hespel et al. 2001; Jones et al. 2004; Hvid et al. 2011) and it has been demonstrated that elderly individuals require a prolonged recovery phase in order to return to initial muscle mass levels following short-term immobilisation (Suetta et al. 2009; Hvid et al. 2010). Yet, there is a paucity of studies examining the cellular mechanisms responsible for the attenuated re-growth and associated molecular signalling processes in ageing human skeletal muscle.
The regulation of muscle growth and maintenance of muscle mass are known to be influenced by a unique population of muscle resident stem cells referred to as satellite cells (SCs) or myogenic stem cells (Mauro, 1961; Moss & Leblond, 1970; Heslop et al. 2001). Notably, an impaired response to muscle damage has been documented as a consequence of ageing in mice (Conboy et al. 2003) and recently also demonstrated in human individuals when examining a subpopulation of individuals from the present intervention (Carlson et al. 2009). As suggested by the latter data, the age-related impairment in muscle re-growth following disuse could, at least in part, reside in an impaired capacity for myogenic stem cell proliferation and activation in aged myofibres (Carlson et al. 2009), but it is not known whether such changes are related to muscle fibre phenotype (type I vs. type II fibres). Further, systemic factors appear to play an important role in explaining the impaired proliferative capacity of SCs cultured from old vs. young human adults (Carlson et al. 2009) in close accordance with previous findings using the murine model (Conboy et al. 2005). There is, however, also evidence of local mechanisms influencing satellite cell activation (Sheehan et al. 2000; Horsley & Pavlath, 2003; Lorenzon et al. 2004; Mitchell & Pavlath, 2004) and recent data suggest a close relation between various systemic and local factors in the regulation of SC function in vivo (Chakkalakal et al. 2012). Furthermore, myogenic regulatory factors such as MyoD and myogenin, the growth and differentiation factor myostatin, as well as growth factors like hepatocyte growth factor (HGF), fibroblast growth factor (FGF) and insulin-like growth factor (IGF-I) have been shown to be involved in the regulation of muscle mass with changes in mechanical muscle loading while also affecting satellite cell activation, proliferation and differentiation (Mezzogiorno et al. 1993; Adams & Haddad, 1996; McPherron et al. 1997; McCroskery et al. 2003; Gopinath & Rando, 2008). However, it is not known to what extent the expression of these factors are associated with any age-related differences in recovery of muscle mass after a period of muscle immobilisation. Based on the previous findings, we hypothesised that satellite cell proliferation would be impaired especially in relation to type II myofibres along with a reduced expression of key anabolic genes in elderly compared to young individuals during rehabilitation after immobilisation of skeletal muscle.
Methods
Subjects
Twenty healthy males, 11 young males (YM; 24.4 years, range 21–30 years) and 9 older males (OM; 67.3 years, range 61–74 years) volunteered to participate in the study. Prior to inclusion all subjects were screened by a physician to exclude individuals with cardiovascular disease, diabetes, neural or musculoskeletal disease, inflammatory or pulmonary disorders or any known predisposition to deep venous thrombosis. The local ethics committee of Copenhagen and Frederiksberg approved the conditions of the study (KF01–322606) and all experimental procedures were performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants before inclusion in the study.
Intervention procedures
The intervention protocol along with data on changes in muscle contractile function and morphology have been described previously (Suetta et al. 2009; Hvid et al. 2010). In brief, immobility was accomplished by 2 weeks of randomised unilateral whole-leg casting using a lightweight whole-leg fibre cast extending from the malleoli to the proximal groin region. The retraining protocol comprised 4 weeks of surveyed and supervised unilateral strength training for the immobilised leg, with three sessions performed per week using a protocol consistently proven effective for inducing substantial gains in muscle size in elderly individuals with 12 weeks of training in our laboratory (Esmarck et al. 2001; Lange et al. 2002; Suetta et al. 2004a,b). After a 5–8 min warm-up on a stationary bike, subjects performed isolated knee extension and flexion, and leg press exercises. Each exercise was performed in 3–4 sets × 12 repetitions (reps) (at 15 rep maximum (RM)) in week 1, followed by 5 sets × 10 reps (at 12RM) in weeks 2 and 3, and 4 sets × 10 reps (at 12RM) in week 4. Training loads were determined at baseline and loads were progressively adjusted on a weekly basis by use of 5RM testing.
Muscle biopsy sampling
Muscle biopsies were obtained at ∼1 week prior to the immobilisation (Pre), immediately after 2 weeks of immobilisation (Imm), following 3 days of retraining (+3d) and finally after 28 days of retraining (+4wks). Subjects were prohibited from exercise for at least 2 days before the first biopsy and were carefully instructed only to eat a light meal in the morning of the day of biopsy sampling. Further, to minimise the influence of diurnal variation, all subjects were biopsied at the same time of the day (±1 h) at successive test sessions.
As described in detail elsewhere (Suetta et al. 2008; Hvid et al. 2010) biopsies were obtained from the middle portion of vastus lateralis muscle utilising the percutaneous needle biopsy technique of Bergström (Bergström, 1962), and careful efforts were made to extract tissue from the same depth and within ∼2–3 cm distance between each biopsy, to avoid the potential influence of muscle damage from repeated biopsies (Guerra et al. 2011). After dissecting the muscle samples of visible blood, adipose and connective tissue, samples were divided into two separate pieces, one oriented in embedding medium (Tissue-Tek, Sakura Finetek, USA) frozen in isopentane cooled with liquid nitrogen and stored at −80°C and the other directly frozen in liquid nitrogen and stored at −80°C until further analyses.
ATPase staining and muscle fibre area
Subsequently serial transverse sections (10 μm) were cut in a cryotome at −20°C and stained for myofibrillar ATPase at pH 9.4 after both alkaline (pH 10.3) and acid (pH 4.3 and 4.6) preincubations (Brooke & Kaiser, 1970). All samples of each individual person were stained in the same batch to avoid interassay variation. Based on the ATPase staining pattern muscle fibres were characterised as type I and type II and an average of 213 ± 39 fibres were analysed in each biopsy. For the determination of muscle fibre size only truly horizontal fibres were used, with a minimum of 50 fibres included for the analysis. A videoscope consisting of a microscope (Olympus BX 50) and colour video camera (Sanyo high resolution CCD) in combination with Tema Image Analysis System (Scanbeam, Denmark) were used to determine the mean fibre area of the muscle fibres.
Satellite cell analysis
SC analysis was carried out by microscopic evaluation of cryosections that had been immunohistochemically stained for Pax7, as previously described in detail (Mackey et al. 2010). A combination of immunoenzymatic and immunofluorescence methods was employed to allow the staining of Pax7, type I myosin and laminin on the same section. Sections were fixed for 5 min with a 5% formaldehyde solution (Histofix, Histolab, Gothenburg, Sweden), followed by incubation for 1 h with blocking buffer (0.05 m Tris-buffered saline (TBS) containing 0.01% Triton X-100, 1% bovine serum albumin, 1% skimmed milk powder and 0.1% sodium azide). Satellite cells were labelled with a mouse anti-Pax7 antibody (cat. no. MO15020; Neuromics, Edina, MN, USA), diluted 1:500 in the blocking buffer, and incubated overnight at 4°C. The next day, the slides were washed in two changes of TBS containing 0.01% Triton. A biotinylated goat anti-mouse secondary antibody (cat. no. E0433; Dako Denmark, Glostrup, Denmark) was then applied, followed by Vector Elite ABC kit (cat. no. PK6100; Vector Laboratories, Peterborough, UK). Horseradish peroxidase activity was visualised with the ImmPACT diaminobenzidine substrate (cat. no. SK-4105; Vector yes Laboratories). The sections were then incubated for 2 h at room temperature with a mixture of the two primary antibodies, mouse anti-A4.951 (cat. no. A4.951; Developmental Studies Hybridoma Bank, Iowa, IA, USA) and rabbit anti-laminin (cat. no. Z0098; Dako, Denmark), for visualisation of type I myosin and laminin, respectively. A mixture of Alexa Fluor 488 goat anti-rabbit (Molecular Probes cat. no. A11034; Invitrogen, Taastrup, Denmark) and Alexa Fluor 568 goat anti-mouse secondary antibodies (Molecular Probes, cat. no. A11031) was applied for 45 min. Washing in two changes of TBS was carried out between all steps, except for between the blocking and Pax7 incubation steps, where no washing was performed. 4′,6-Diamidino-2-phenylindole (DAPI) in the mounting medium (Molecular Probes ProLong Gold anti-fade reagent, cat. no. P36935) stained the nuclei, rendering nuclei blue, type I myosin red and laminin green. Sites of Pax7 antigenicity were stained brown, visible by light microscopy. The number of Pax7 cells associated with type I (A4.951-positive) or type II (A4.951-negative) fibres was counted separately and expressed relative to the total number of type I or type II fibres included in the assessment, as described in detail (Mackey et al. 2010; Fig. 2).
Figure 2. Pax7-positive cells associated with type I and II myofibres.
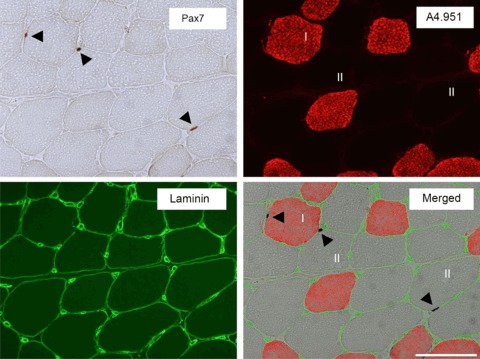
Immunohistochemically detected Pax7+ cells, type I myosin and laminin on a muscle biopsy cross-section. Pax7+ cells (black arrows) are visualised by light microscopy (brown; upper left, ‘Pax7’), and type I myosin staining (red) used to indicate Pax7+ cells associated with type I fibres (A4.951+; red) or type II fibres (unstained; upper right, ‘A4.951’). Laminin staining (green) was used to mark the myofibre basal membrane (lower left, ‘Laminin’). In the merged image of the three stainings for Pax7, type I myosin (A4.951) and laminin, a Pax7+ cell was located within a type I fibre while two Pax7+ cells were identified in type II fibres (lower right, ‘Merged’). Scale bar, 100 μm.
RNA purification
Total RNA was isolated from ∼20 mg of frozen muscle biopsy by phenol extraction (TriReagent; Molecular Research Center, OH, USA) as previously described (Kadi et al. 2004b). Intact RNA was confirmed by denaturing agarose gel electrophoresis.
Real-time PCR
The mRNA expression of IGF-1Ea, mechano growth factor (MGF, also known as IGF-1Ec) and RPLP0 was analysed by real-time PCR as described previously (Suetta et al. 2012). Total RNA (500 ng) was converted into cDNA in 20 μl using the OmniScript reverse transcriptase (Qiagen, CA, USA) according to the manufacturer's protocol. For each of the mRNA targets, 0.25 μl cDNA was amplified in a 25 μl SYBR Green PCR reaction containing 1 × Quantitect SYBR Green Master Mix (Qiagen) and 100 nm of each primer (Table 1A). The amplification was monitored real-time using the MX3000P real-time PCR machine (Stratagene, CA, USA). The threshold cycle (Ct) values were related to a standard curve made with the cloned PCR products and specificity ensured by melting curves analysis; the quantities were normalised to RPLP0. Quantitative real-time PCR of myostatin, Pax7, MyoD1, myogenin, FGF2, fibroblast growth factor receptor 1 (FGFR1), HGF, c-Met, CDKN1A (p21), CDKN1B (p27) and RPLP0 mRNA were performed in the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, UK) using ABI TaqMan Low Density Arrays (Applied Biosystems; Table 1B). Each sample was run in triplicate with four samples per card. cDNA, 250 ng, was mixed with 100 μl TaqMan Gene Expression Mastermix and loaded into two ports (2.5 ng cDNA per reaction). Raw data were extracted and analysed using the SDS 2.1 software (Applied Biosystems, UK) and qBasePlus (Biogazelle) was used to quality-check Ct values, assess triplicates, exclude runs when the difference among triplicates exceeded 0.5Ct and finally to normalise data to RPLP0 using the 2Ct method (Livak & Schmittgen, 2001).
Table 1.
Primers for qRT-PCR and TaqMan low density array (LDA) assay ID
| A. Primers for qRT-PCR using SYBR Green assay | ||
|---|---|---|
| Gene products | Sense primer | Antisense primer |
| MGF | GCCCCCATCTACCAACAAGAACAC | CGGTGGCATGTCACTCTTCACTC |
| IGF-1Ea | GACATGCCCAAGACCCAGAAGGA | CGGTGGCATGTCACTCTTCACTC |
| GAPDH | CCTCCTGCACCACCAACTGCTT | GAGGGGCCATCCACAGTCTTCT |
| RPLP0 | GGAAACTCTGCATTCTCGCTTCCT | CCAGGACTCGTTTGTACCCGTTG |
| B. Applied Biosystems TaqMan Low Density Array assay ID | ||
|---|---|---|
| Gene products | Assay ID | |
| MyoD | Hs02330075_g1 | |
| Myogenin | Hs01072232_m1 | |
| HGF | Hs00300159_m1 | |
| c-Met | Hs01565581_m1 | |
| FGF2 | Hs00266645_m1 | |
| FGFR1 | Hs01552926_m1 | |
| Pax7 | Hs00242962_m1 | |
| Myostatin | Hs00193363_m1 | |
| CDKN1A (p21) | Hs01121172_m1 | |
| CDKN1B (p27) | Hs00153277_m1 | |
| RPLP0 | Hs99999902_m1 | |
A. mRNA expression of MGF (IGF-1Ec), IGF-1Ea and RPLP0 was analysed by SYBR Green-based quantitative real-time RT-PCR. B. TaqMan-based quantitative real-time RT-PCRs of MyoD1, myogenin, HGF, c-Met, FGF2, FGFR1, Pax7, myostatin, CDKN1A, CDKN1B and RPLP0 mRNA were performed using ABI TaqMan Low Density Arrays.
Correlation analyses
Correlation analyses were performed at post immobilisation (Imm) to examine the relationship between myofibre area (fibre type I and type II) and number of Pax7-positive (+) cells (total, fibre type I and type II), for young and older individuals combined. Furthermore, correlation analyses were performed between changes during the retraining (4wks relative to Imm) in myofibre area (MFA, fibre type I and type II) and number of Pax7+ cells (total, fibre type I and type II) for young and older individuals combined.
Statistical analyses
Statistical analyses were performed with SigmaPlot v11.0. Interaction between Age and Time was tested with a two-way repeated measures ANOVA. Differences over time were tested with one-way repeated measures ANOVA for Young and Old separately. If an overall time difference was found, the different time points were compared using Student–Newman–Keuls post hoc test. Pair-wise comparisons between Young and Old at each time point were obtained from the two-way repeated measures ANOVA (to include global variance) and Bonferroni corrected.
Non-parametric statistical analysis was used to evaluate changes in muscle fibre cross-sectional area, since not all data were normally distributed. To evaluate the effect of intervention over time, the Friedmann two-way analysis of variance by ranks of related samples was used with subsequent analysis using the Wilcoxon signed rank test for paired samples. Intergroup differences were evaluated using the Kruskal–Wallis signed rank test. Correlation analysis was performed using the Spearman's rho (rs) method. Data are presented as mean values ± SEM or for mRNA data geometric means ± back-transformed SEM. A P value of less than 0.05 was considered significant.
Results
Muscle fibre cross-sectional area
Young individuals showed a 21.3% increase in type I fibre area (4440.4 ± 499.9 vs. 5386.3 ± 508.9 μm2, P < 0.05) along with a 35.5% increase in type II myofibre area (4347.5 ± 419.5 vs. 5904.4 ± 483.3 μm2, P < 0.05) after retraining (+4wks; Fig. 1). In contrast, no increases in type I fibre area (4830.3 ± 517.5 vs. 4848.2 ± 336.9 μm2) or type II fibre area (4004.3 ± 467.4 vs. 4225.1 ± 276.2 μm2) were observed in the older individuals. Furthermore type II fibre area was restored with retraining in young individuals to reach higher levels than observed in older individuals (YM: 5386.3 ± 508.9 μm2, OM: 4225.1 ± 276.2 μm2, P < 0.05). No difference was observed in fibre-type distribution between YM (type I: 55.5%; type II: 44.5%) and OM (type I: 56.1%; type II: 43.9%) at Pre or following the interventions (Hvid et al. 2010).
Figure 1. Changes in myofibre cross-sectional area following 2 weeks of immobilisation and 4 weeks of retraining in young and older human individuals.
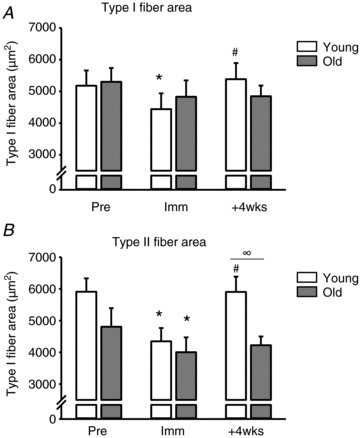
A, type I myofibre cross-sectional area. B, type II myofibre cross-sectional area. Open bars represent young (n= 9) and grey bars represent older individuals (n= 7). Pre, ∼1 week prior to the immobilisation; Imm, after 2 weeks of immobilisation; +4wks, after 28 days of retraining. *Time effect, P < 0.05, compared to Pre. #Time effect, P < 0.05 compared to Imm. ∞Age effect, P < 0.05 young compared to old within time point. Group mean data ± SEM.
Satellite cells: association with fibre type
To analyse for satellite cells, Pax7+ cells were assessed for type I and II fibres separately, as illustrated in Fig. 2. An overall effect of age was found for Pax7+ cells in relation to type II (P < 0.01), but not type I fibres (Fig. 3). The number of Pax7+ cells in the young subjects increased compared to Pre in both type I and II fibres at all time points. No changes in the elderly individuals were seen over time and in the type II fibres at +3d and +4wks; this was significantly different to the young individuals.
Figure 3. Pax7-positive cells pre- and post immobilisation and following 4 weeks of retraining: association with fibre type.
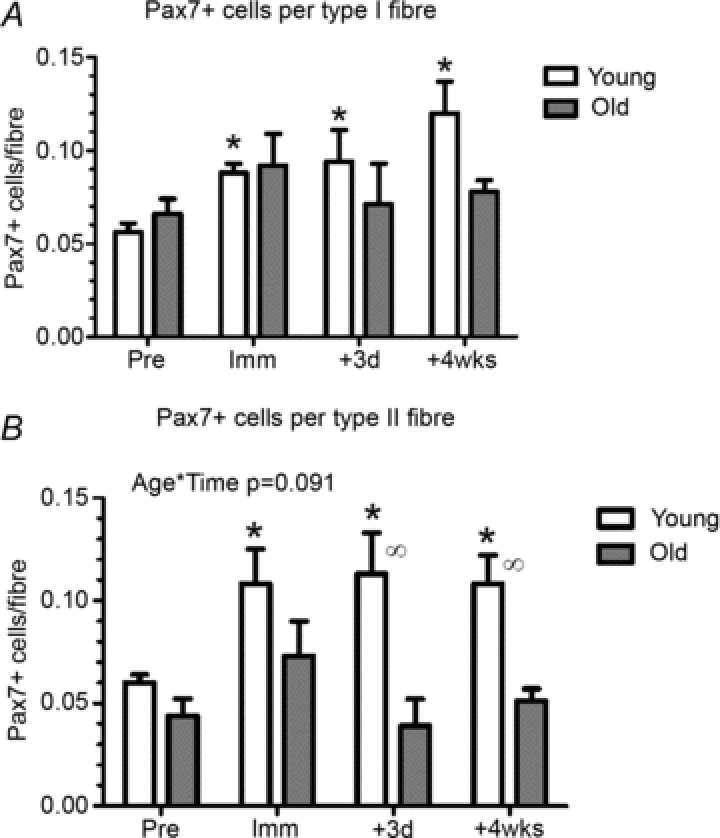
A, number of Pax7+ cells associated with fibre type I. B, number of Pax7-positive satellite cells associated with fibre type II. +3d, after 3 days of retraining. *Time effect, P < 0.05 compared to Pre. ∞Age effect, P < 0.05 difference between young and old within time point. Data are means ± SEM.
mRNA expression levels at baseline
At baseline (Pre) there was no age-related differences in the expression levels of IGF-1Ea, MGF, MyoD1, myogenin, HGF, c-Met, FGF2, FGFR1, Pax7, CDKN1A (p21) or CDKN1B (p27) whereas the expression levels of myostatin mRNA and GAPDH were lower in old compared to young (Fig. 4, P < 0.05).
Figure 4. mRNA values relative to the young group at baseline (Pre).
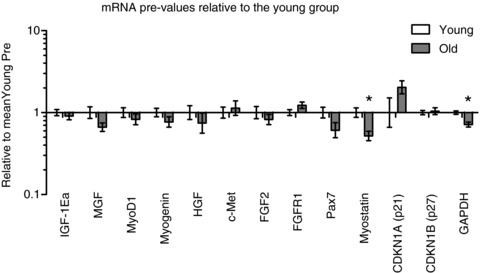
*Age effect, P < 0.05 young compared to old. Data are geometric means ± back-transformed SEM.
IGF-1Ea and MGF
Expression levels of IGF-1Ea and MGF (IGF-1Ec) mRNA increased in young individuals with immobilisation (Fig. 5A and B). Expression levels of IGF-1Ea demonstrated a subsequent marked decrease after 3 days of retraining in young individuals and after 4 weeks of retraining mRNA expression levels were increased compared to baseline levels as well as post immobilisation (Imm) in both young and old (Fig. 5A, P < 0.05). Expression levels of MGF mRNA remained elevated after 3 days of retraining in the young (P < 0.05), and after 4 weeks of retraining mRNA expression levels were further increased compared to baseline and post immobilisation (Imm) levels, respectively, in the young (Fig. 5B, P < 0.05).
Figure 5. mRNA expression levels relative to baseline following 2 weeks of immobilisation and 4 weeks of retraining.
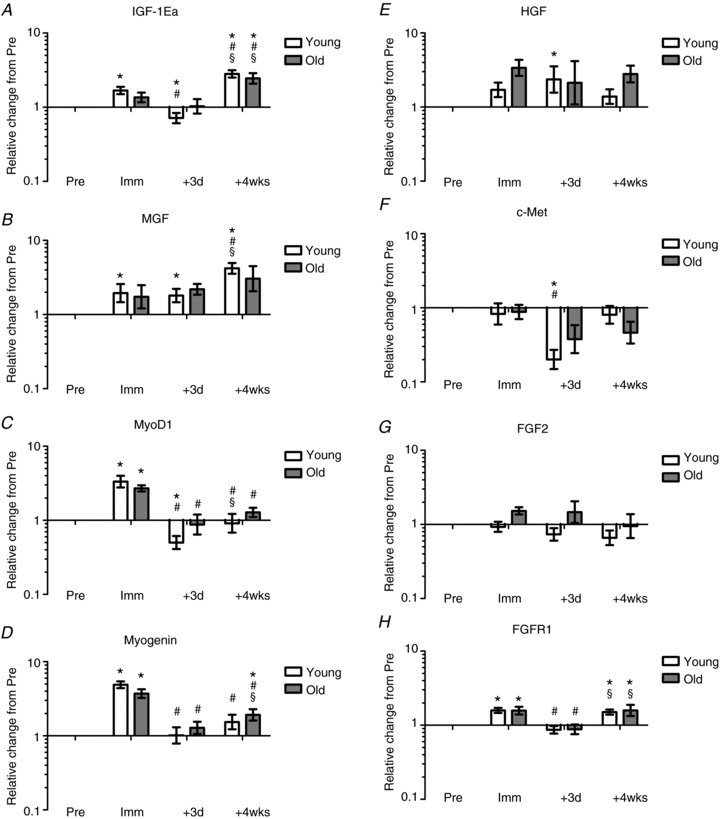
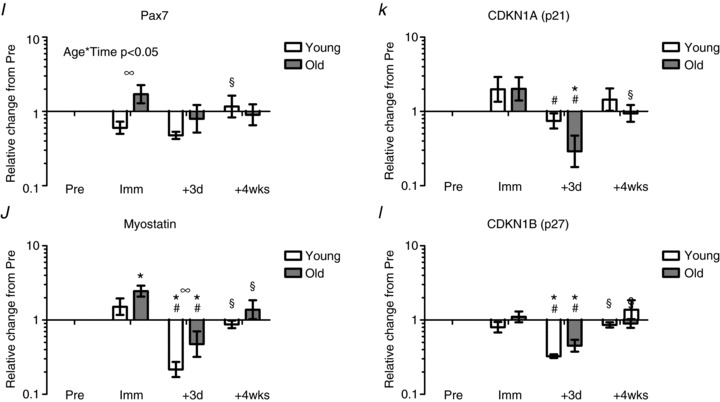
A, IGF-1Ea; B, MGF; C, MyoD1; D, myogenin; E, HGF; F, c-Met; G, FGF2; H, FGFR1; I, Pax7; J, myostatin; K, CDKN1A (p21); L, CDKN1B (p27). *Time effect, P < 0.05 compared to Pre. #Time effect, P < 0.05 compared to Imm. §Time effect, P < 0.05 compared to +3d. ∞Age effect, P < 0.05 young compared to old. Data are geometric means ± back-transformed SEM.
MyoD1 and myogenin
MyoD1 expression increased in both young and older males with immobilisation, and in addition showed a marked decrease following 3 days of retraining in both young and old (P < 0.05, Fig. 5C). After 4 weeks of retraining MyoD1 mRNA levels returned to baseline levels in both young and old (Fig. 5C). Myogenin mRNA expression increased markedly in both young and old, while decreasing after 3 days of retraining in both young and old (Fig. 5D, P < 0.05). After 4 weeks of retraining myogenin expression levels remained reduced compared to post immobilisation levels (Fig. 5D).
HGF and c-Met
Expression levels of HGF and c-Met mRNA increased in young individuals in the early phase of retraining (+3d, P < 0.05; Fig. 5E and F) but remained unaltered after immobilisation and more prolonged retraining. In old individuals no change in expression levels of HGF and c-Met was observed at any time point.
FGF2 and FGFR1
FGF2 mRNA levels remained unaltered at all time points in young as well as older individuals (P < 0.05, Fig. 5G). Conversely, FGFR1 mRNA expression increased in both young and old following immobilisation, followed by marked decreases after 3 days of retraining in both young and old (+3d; Fig. 5H). After 4 weeks of retraining, FGFR1 mRNA expression increased compared to baseline and the initial training phase (+3d) approaching values similar to the expression levels observed following immobilisation (Fig. 5H).
Pax7
The expression of Pax7 mRNA following immobilisation was higher in the older males compared to the young males (Fig. 5I). Although there was an interaction (Age × Time, P < 0.05), it was not possible to pinpoint whether this difference was due to an increase in the elderly or a decrease in the young, or both. No differences with time or age were detected at the retraining time points, except for a lower level at +3d compared to +4wks in the young.
Myostatin
Myostatin mRNA expression increased in the elderly with immobilisation, and showed a marked decrease after 3 days of retraining (+3d) in both young and old (P < 0.05, Fig. 5J). After 4 weeks of retraining, myostatin expression levels returned to baseline levels in both young and old. The temporal changes in myostatin mRNA expression differed between young and old individuals, with a less pronounced down-regulation of myostatin expression levels in the initial phase of retraining (+3d) in older individuals (P < 0.05, Fig. 5J).
CDKN1A (p21) and CDKN1B (p27)
Expression levels of CDKN1A (p21) remained unchanged with immobilisation (Imm) while decreasing in both young and older males after 3 days of retraining (P < 0.05; Fig. 5K). After 4 weeks of retraining CDKN1A mRNA expression returned to baseline levels in both young and old (P < 0.05, Fig. 5K). No change in the expression level of CDKN1B (p27) was observed with immobilisation; however, a marked decrease was observed in both young and old after 3 days of retraining (P < 0.05, Fig. 5L). After 4 weeks of retraining CDKN1B mRNA expression levels returned to baseline levels in both young and older individuals (Fig. 5L).
Correlation analysis
After muscle disuse, the number of Pax7+ cells was positively related to myofibre area (rs= 0.712, P < 0.01; n= 16; Fig. 6A). Comparison within fibre type showed that this relationship was true for both type I (rs= 0.779; P < 0.001; n= 16) and type II fibres (rs= 0.694; P < 0.01; n= 16). Notably, analysing the retraining phase, relative changes in MFA following 4 weeks retraining (relative to post immobilisation) were positively associated with the change in the total number of Pax7+ SCs (rs= 0.693, P < 0.01, n= 15; Fig. 6B). Comparison within each fibre type revealed a strong correlation for type I fibres (rs= 0.864, P < 0.001, n= 15), whereas there was no significant correlation for type II fibres (Fig. 6B).
Figure 6. Association between changes in myofibre area and number of Pax7+ cells.
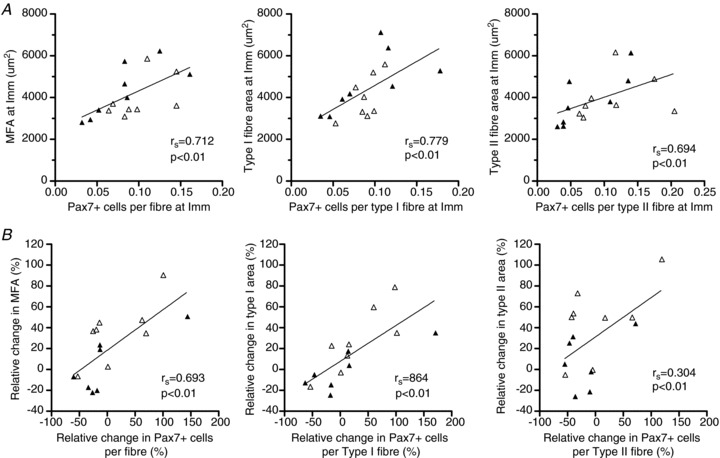
A, number of Pax7+ cells versus fibre area for all fibres collapsed or separated into type I or II fibres post immobilisation (Imm). B, relative changes in myofibre area (MFA, type I or type II) following 4 weeks of retraining (relative to post immobilisation) versus the change in number of Pax7+ cells (total, type I associated and type II associated). Open triangles, young individuals; filled triangles, older individuals.
Discussion
In the present study, we report measures of myofibre area and myogenic stem cell number associated with type I and type II muscle fibres in young and older humans, in combination with transcriptional data from regulatory signalling pathways related to skeletal muscle re-growth, many of which have not been previously studied in the immobilised and retrained ageing human muscle. The main study finding was the observation of a less marked muscle mass recovery after immobilisation in elderly compared to young individuals that was paralleled by an elevation in Pax7+ satellite cell content in young individuals only, whereas the elderly failed to demonstrate any change in Pax7+ cells. This potential coupling of satellite cell proliferation and recovery in myofibre area in young individuals occurred despite no age-related differences in the expression of major myogenic regulatory factors known to promote skeletal muscle hypertrophy or satellite cell proliferation gene products (IGF-1Ea, MGF, MyoD1, myogenin, HGF). However, the expression of myostatin demonstrated a more pronounced up-regulation following immobilisation along with an attenuated down-regulation in response to reloading in older compared to young individuals, which may have contributed to the present lack of satellite cell proliferation in ageing muscle.
Changes in myofibre size with reloading
Reloading of disuse-induced skeletal muscle atrophy, by means of resistive types of exercise, is known to restore muscle mass in young individuals by increased myofibrillar protein synthesis and restoration of fibre-type area (Hespel et al. 2001; Jones et al. 2004; Machida & Booth, 2004). In line with our previous data on whole quadriceps muscle volume measured by magnetic resonance imaging (Suetta et al. 2009), young men in the present study responded positively to the reloading protocol, showing robust increases in both type I and type II myofibre area (Fig. 1). In contrast, no changes in type I or type II myofibre area emerged with 4 weeks of retraining in aged individuals (Fig. 1). This demonstrates an attenuated response to short-term reloading in old compared to young humans. However, due to the relatively short observation period, we cannot conclude to what extent the recovery of skeletal muscle mass in elderly undergoing short-term immobilisation is impaired or just occurs more slowly than in young counterparts. In support of the second view, observations over more prolonged periods of reloading (e.g. 12 weeks) have demonstrated that the elderly can fully recover whole muscle cross-sectional area and myofibre area after long-term (months to years) muscle disuse due to hip osteoarthritis and subsequent elective hip-replacement surgery, but only if the reloading phase comprises a systematic use of resistance-based exercise (Suetta et al. 2004b, 2008).
Changes in myogenic progenitor cells with reloading
In skeletal muscle the myogenic stem cells, also referred to as satellite cells (SCs), are known to play a key role in the maintenance, growth and repair of myofibres (Mauro, 1961; Moss & Leblond, 1970; Heslop et al. 2001; Pallafacchina et al. 2012). During the process of load-induced muscle hypertrophy, satellite cells are thought to proliferate, differentiate and eventually fuse with existing myofibres (McCormick & Schultz, 1994). The resulting donation of new myonuclei by the fusion of SCs with existing myofibres is thought to ensure that myonuclear domain size stays within certain functional limits in situations of marked myofibre hypertrophy (Kadi et al. 2004b; Petrella et al. 2008). In humans, skeletal muscle SCs seems to be maintained into the seventh decade of life (Roth et al. 2000; Petrella et al. 2006; Hikida, 2011), with a decline in content and activation capabilities with progressive ageing (Renault et al. 2002; Kadi et al. 2004a; Verdijk et al. 2007) accompanied by a reduced migration capacity of existing SCs in turn resulting in a reduced regeneration potential after muscle injury and disuse (Carlson & Faulkner, 1989; Mitchell & Pavlath, 2004; Conboy et al. 2005; Carlson & Conboy, 2007). Despite a mean age of only ∼70 years in our aged individuals signs of impaired SC proliferation with immobilisation and subsequent retraining were observed compared to young subjects (∼25 years old), indicating an attenuated myogenic response to changes in exercise pattern (Fig. 3A and B). Interestingly, compared to the changes induced by 2 weeks of disuse, more accentuated age-related differences in SC activation were observed in the acute (+3d) as well as the prolonged (+4wk) phase of reloading (Fig. 3A and B). The latter trend is in line with Dreyer et al. (2006) reporting a greater increase in SC content in young compared to aged skeletal muscle within 24 h following 92 maximal eccentric muscle contractions (Dreyer et al. 2006). The importance of SC number in relation to muscle size across the age-span was underlined by Kim and co-workers who demonstrated a positive linear association between muscle size and SC number in baseline muscle biopsies obtained from young and older human individuals (Kim et al. 2005b). Extending those data, here we report for the first time in human individuals that a positive relationship exists between SC number and myofibre area following disuse atrophy (Fig. 5A) as well as in response to subsequent reloading (Fig. 5B).
Since human ageing is associated with a preferential reduction in muscle fibre type II size (Andersen, 2003; Aagaard et al. 2010) it has been speculated that SC content might decrease more in type II fibres compared to type I fibres with ageing (Kim et al. 2005b). In young human individuals, SC appears to be similar between type I and type II muscle fibres (Kadi et al. 2006; Verdijk et al. 2007). In contrast, age-related type II muscle fibre atrophy is accompanied by a type II muscle fibre-specific reduction in SC content (Verdijk et al. 2007; Verney et al. 2008). In support of these observations, young and old adults demonstrating marked exercise-induced gains in myofibre area (i.e. ‘hypertrophy responders’) are characterised by a greater concurrent up-regulation in myogenic SCs compared to individuals with a less robust hypertrophy response (Petrella et al. 2008). However, although the regenerative capacity of human skeletal muscle seems to decline at a more advanced age (reflected by a decline in SC number and/or proliferative capacity), the impairment in SC function does not seem to prevent a significant capacity for muscle hypertrophy provided that the intervention period is sufficiently long (months), as reported even at very old age (Thornell et al. 2003; Dedkov et al. 2003; Shefer et al. 2006).
Changes in IGF-1 expression with reloading
Numerous growth factors are known to regulate satellite cell activity, among which insulin-like growth factor 1 (IGF–1) is known to play an essential role in the process of muscle hypertrophy (Rosenblatt et al. 1994; Adams & Haddad, 1996; Suetta et al. 2010). The discovery of distinct IGF–1 isoforms (mechano growth factor (MGF) and IGF-1Ea) has suggested different roles for IGF–1, namely that MGF mainly triggers satellite cell activation and proliferation, while IGF-1Ea is thought to mainly promote differentiation of proliferating SCs (Yang & Goldspink, 2002). In line with those findings the present study demonstrated a differentiated regulation in IGF-1Ea and MGF expression, with both an acute and a sustained up-regulation of MGF mRNA expression in response to retraining (+3d and +4wks), whereas IGF-1Ea expression was up-regulated in the later phase of reloading (+4wks) only, suggesting a supportive role of paracrine/autocrine IGF signalling in the process of human muscle hypertrophy, at least when recovering from short-term muscle disuse. In contrast to earlier findings in rodent and human skeletal muscle (Owino et al. 2001; Hameed et al. 2003) the present up-regulation in MGF mRNA expression with reloading (post 4 weeks resistance training) was not attenuated in older vs. young individuals in the present study.
Changes in MyoD1 and myogenin expression with reloading
In the present study MyoD1 and myogenin expression were markedly up-regulated with immobilisation in both age groups, whereas subsequent reloading led to both an acute (+3d) and a sustained (+4wks) decrease in MyoD1 and myogenin expression in young as well as aged skeletal muscle. The rise in MyoD1 and myogenin mRNA expression following immobilisation independently of age may represent a compensatory signalling pathway for partial muscle retention during periods of acute muscle loss, while also observed in other atrophy situations (Alway et al. 2001). Following resistance-type exercise increased expression of MyoD1 and myogenin mRNA has been observed in both young and older adults (Psilander et al. 2003; Kim et al. 2005a; Kosek et al. 2006; Raue et al. 2006; Costa et al. 2007; McKay et al. 2008). In the present study, however, only a modest up-regulation in myogenin expression compared to the basal non-immobilised state was observed following the reloading phase consisting of 4 weeks of resistance training, suggesting that retraining after short-term disuse atrophy may evoke different molecular signalling stimuli compared to regular resistance exercise intervention.
Changes in HGF and c-Met expression with reloading
Hepatocyte growth factor (HGF) is generally considered to be one of the most important growth factors involved in organ regeneration (Zarnegar, 1995) as well as a key regulator of satellite cell activity during muscle regeneration (Jennische et al. 1993). The presence of HGF transcripts in newly regenerated myotubes and in satellite cells suggests that HGF activity is mediated primarily through paracrine/autocrine mechanisms (Anastasi et al. 1997; Sheehan & Allen, 1999). Furthermore, HGF is a potent growth factor that has the ability to stimulate quiescent satellite cells to enter the cell cycle early in vitro as well as in vivo (Allen et al. 1995; Tatsumi et al. 1998) and is therefore considered most important during the early phase of re-growth (Tatsumi et al. 1998). In support of this, we observed a significant increase in HGF mRNA expression in young individuals in response to early retraining (+3d) but not after more sustained retraining (+4wks) (Fig. 5E). In contrast to HGF, a marked decrease in the HGF receptor c-Met expression was observed in response to early retraining (+3d) in young individuals, which may support the hypothesis previously proposed that increasing concentrations of HGF reduces c-Met which forms a negative feedback mechanism inducing SC quiescence in regenerating muscle (Tatsumi et al. 2009; Yamada et al. 2010).
Changes in FGF2 and FGFR1 expression with reloading
In recent years, fibroblast growth factors (FGFs) and their receptors (FGFRs) have gained increased focus as major players in both embryonic development and skeletal muscle tissue repair (Coutu & Galipeau, 2012). Moreover, somatic stem cells have been suggested as major targets of FGF signalling in both tissue homeostasis and repair where FGFs appear to promote self-renewing proliferation and inhibit cellular senescence in nearly all tissues tested to date (Coutu & Galipeau, 2012). Fibroblast growth factor 2 (FGF2) is a polypeptide growth factor that stimulates SC proliferation in already activated SCs (Allen & Boxhorn, 1989; Mezzogiorno et al. 1993). However, despite our findings of marked SC proliferation with reloading, expression levels of FGF2 remained unchanged at all time points examined, suggesting that FGF may be less important for SC proliferation in human skeletal muscle, at least in relation to reloading subsequent to immobilisation.
Changes in Pax7 expression with reloading
The observed differences in immunohistochemical Pax7+ cell content of the vastus lateralis muscle in young and older individuals during immobilisation and subsequent retraining (Fig. 3) were not associated with corresponding changes in mRNA for Pax7. In fact, the expression level of Pax7 was down-regulated in young and up-regulated in old individuals following 2 weeks of immobilisation (Fig. 5I), indicating that factors other than Pax7 mRNA levels may influence the content of Pax7+ SCs during immobilisation and retraining conditions in humans.
Changes in myostatin expression with reloading
Myostatin is a member of the transforming growth factor-β superfamily and a strong negative regulator of skeletal muscle mass, known to inhibit myogenic SC activation (McPherron et al. 1997; Trendelenburg et al. 2009). Although the mechanisms are not fully understood, myostatin is thought to modulate key regulators of the cell cycle such as cyclin-dependent kinase inhibitors p21cip and p27kip (Kim et al. 2005a), thereby inhibiting SC cycle progression from G0 to S phase (McCroskery et al. 2003). Down-regulated myostatin mRNA expression has been observed in response to exercise training in both young and elderly individuals (Roth et al. 2003; Kim et al. 2005a; Raue et al. 2006; Costa et al. 2007), with some studies reporting an attenuated response with ageing (Kim et al. 2005b; Haddad & Adams, 2006). In line with the latter findings, McKay and colleagues recently demonstrated a markedly blunted myogenic response in older compared to younger individuals (McKay et al. 2012). Although stem cell-specific myostatin levels did not appear to differ at baseline, acute resistance exercise (75% 1RM) was found to evoke ∼70% greater content of myostatin-positive type II-associated SCs in old versus young adults at 24 h post exercise, suggesting that the greater co-localisation of myostatin with SCs may provide a mechanism for the impaired myogenic capacity of aged muscle (McKay et al. 2012). Somewhat unexpectedly, the expression level of myostatin mRNA was lower in old compared to young at baseline in the present study, which might reflect the rather high activity level (equal to that of young) of the present group of elderly individuals. Despite this, an age-specific difference in the regulation of myostatin was also observed in the present study, manifested by a reduced suppression with reloading in aged vs. young individuals (Fig. 5J). This observation may explain, at least in part, the impaired capacity for SC proliferation and re-growth in aged skeletal muscle observed in the present study, although the interpretation of these data is limited if only assessing transcript levels.
Changes in cyclin-dependent kinase inhibitors with reloading
In line with the regulation in myostatin mRNA, expression levels of cyclin-dependent kinase inhibitors CDKN1A (p21) and CDKN1B (p27) decreased in response to acute loading in the present study. Both CDKN1A and CDKN1B are known to block cell cycle progression and induce SC cell cycle withdrawal (Coqueret, 2003). Furthermore, ectopic expression of CDKN1B has been shown to block the IGF–I-induced increase in satellite cell proliferation (Chakravarthy et al. 2000) and thus CDKN1B is considered a key regulatory factor in the regulation of satellite cell cycle progression (Machida et al. 2003).
Conclusions
Collectively, the present data suggest that significant age-specific differences may exist for the ability of human skeletal muscle to regenerate after immobility-induced muscle atrophy. More specifically, our results indicate an attenuated – or at least delayed – response in aged individuals to active reloading subsequent to short-term disuse, as reflected by attenuated gains in myofibre area and SC number despite no age-related differences being observed in local growth factors responsible for promoting skeletal muscle hypertrophy (IGF-1Ea, MGF, MyoD1, myogenin, HGF). These disparate trends may partly reside on a reduced cellular sensitivity to paracrine/autocrine growth factors as basal MRF mRNA expression appears to be chronically up-regulated in senescent muscle (Edström & Ulfhake, 2005; Kim et al. 2005a; Kosek et al. 2006; Raue et al. 2006). Our findings of an age-specific regulation in myostatin expression levels may also have contributed to the apparent lack of increase in SC number and myofibre area with ageing in response to reloading. Gaining an improved understanding of the ability of human skeletal muscle to recover from atrophy has important implications for the development of effective molecular and rehabilitative countermeasures against physical frailty in the continuously growing population of elderly adults.
Translational perspective
We report measures of myofiber area and myogenic stem cell number (SC) associated with type I and type II muscle fibres in young and older humans, in combination with transcriptional data from regulatory pathways related to skeletal muscle re-growth in immobilised and re-trained aging human muscle. The main study finding was a less marked muscle mass recovery after immobilisation in elderly compared to young individuals that was paralleled by an elevation in SC content in young only, whereas elderly failed to demonstrate any change in SC's. This potential coupling of SC proliferation and recovery in myofiber area in young individuals occurred despite no age related differences in the expression of myogenic regulating genes normally known to promote skeletal muscle hypertrophy or SC proliferation. However, expression of myostatin was more pronounced after immobilisation along with an attenuated down-regulation in response to re-loading in older compared to young individuals, which may have contributed to the lack of SC proliferation in aging muscle. The age-specific regulation in myostatin expression may also have contributed to the apparent lack of increase in SC number and myofiber area with aging in response to re-loading. Collectively, the present findings underlines that elderly have an impaired ability to recover from disuse muscle atrophy and thus, elderly may need longer time to recover from periods of disuse or disease compared to younger ones.
Gaining insight in the ability to recover muscle from atrophy has implications for effective molecular and rehabilitative countermeasures against frailty in the growing population of elderly.
Acknowledgments
We wish to express our gratitude to the subjects who participated in this study for their abundant efforts and contribution to this work.
Glossary
- CDKN1A (p21)
cyclin-dependant kinase inhibitor 1A
- CDKN1B (p27)
cyclin-dependant kinase inhibitor 1B
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- HGF
hepatocyte growth factor
- IGF-1Ea
insulin-like growth factor-1Ea
- MFA
mean fibre area
- MGF
mechano growth factor
- MFA
myofibre area
- OM
older males
- RM
repetition maximum
- SC
satellite cell
- YM
young males
Additional information
Competing interests
None.
Author contributions
The study was performed at the Institute of Sports Medicine Copenhagen, Bispebjerg Hospital, Denmark. Conception and design of the study: C.S., U.F., P.S., P.A. and M.K. Collection, analysis and interpretation of data: C.S., U.F., A.L.M., L.J., L.G.H., M.L.B., S.J.P., H.D.S., J.L.A. and P.S. Writing or revising the manuscript critically: C.S., U.F., A.L.M., P.S., P.A., L.J., L.G.H. and M.K. All authors have approved the final version of the manuscript.
Funding
This study was supported by grants from the Danish Medical Research Council, the Danish Rheumatology Association, Faculty of Health Sciences, University of Copenhagen, The Danish Ministry of Culture, Lundbeck Foundation, EU 7th framework programme ‘Myoage’, and Nordea Foundation (Healthy Ageing Grant).
References
- Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. 2010;20:49–64. doi: 10.1111/j.1600-0838.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- Adams GR, Haddad F. The relationships among IGF–1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol. 1996;81:2509–2516. doi: 10.1152/jappl.1996.81.6.2509. [DOI] [PubMed] [Google Scholar]
- Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol. 1989;138:311–315. doi: 10.1002/jcp.1041380213. [DOI] [PubMed] [Google Scholar]
- Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol. 1995;165:307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- Alway SE, Dawn AL, Kuangjen DC. The effects of age and hindlimb supension on the levels of expression of the myogenic regulatory factors MyoD and myogenin in rat fast and slow skeletal muscles. Exp Physiol. 2001;86:509–517. doi: 10.1113/eph8602235. [DOI] [PubMed] [Google Scholar]
- Anastasi S, Giordano S, Sthandier O, Gambarotta G, Maione R, Comoglio P, Amati P. A natural hepatocyte growth factor/scatter factor autocrine loop in myoblast cells and the effect of the constitutive Met kinase activation on myogenic differentiation. J Cell Biol. 1997;137:1057–1068. doi: 10.1083/jcb.137.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports. 2003;13:40–47. doi: 10.1034/j.1600-0838.2003.00299.x. [DOI] [PubMed] [Google Scholar]
- Bergström J. Muscle electrolytes in man. Scand J Clin Lab Invest. 1962;14(Suppl. 68):1–110. [Google Scholar]
- Brooke MH, Kaiser KK. Muscle fibre types: How many and what kind. Arch Neurol. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol Cell Physiol. 1989;256:C1262–C1266. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- Carlson ME, Conboy IM. Loss of stem cell regenerative capacity within aged niches. Aging Cell. 2007;6:371–382. doi: 10.1111/j.1474-9726.2007.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ME, Suetta C, Conboy MJ, Aagaard P, Mackey A, Kjaer M, Conboy I. Molecular aging and rejuvenation of human muscle stem cells. EMBO Mol Med. 2009;1:381–391. doi: 10.1002/emmm.200900045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy MV, Abraha TW, Schwartz RJ, Fiorotto ML, Booth FW. Insulin-like growth factor-I extends in vitro replicative life span of skeletal muscle satellite cells by enhancing G1/S cell cycle progression via the activation of phosphatidylinositol 3′-kinase/Akt signaling pathway. J Biol Chem. 2000;275:35942–35952. doi: 10.1074/jbc.M005832200. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Coqueret O. New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment. Trends Cell Biol. 2003;13:65–70. doi: 10.1016/s0962-8924(02)00043-0. [DOI] [PubMed] [Google Scholar]
- Costa A, Dalloul H, Hegyesi H, Apor P, Csende Z, Racz L, Vaczi M, Tihanyi J. Impact of repeated bouts of eccentric exercise on myogenic gene expression. Eur J Appl Physiol. 2007;101:427–436. doi: 10.1007/s00421-007-0510-z. [DOI] [PubMed] [Google Scholar]
- Coutu DL, Galipeau J. Roles of FGF signalling in stem cell self-renewal, senescence and aging. Aging (Albany NY) 2012;3:920–933. doi: 10.18632/aging.100369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedkov EI, Kostrominova TY, Borisov AB, Carlson BM. MyoD and myogenin protein expression in skeletal muscles of senile rats. Cell Tissue Res. 2003;311:401–416. doi: 10.1007/s00441-002-0686-9. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve. 2006;33:242–253. doi: 10.1002/mus.20461. [DOI] [PubMed] [Google Scholar]
- Edström E, Ulfhake B. Sarcopenia is not due to lack of regenerative drive in senescent skeletal muscle. Aging Cell. 2005;4:65–77. doi: 10.1111/j.1474-9728.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M, Kjaer M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol. 2001;535:301–311. doi: 10.1111/j.1469-7793.2001.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath SD, Rando TA. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7:590–598. doi: 10.1111/j.1474-9726.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- Guerra B, Gómez-Cabrera MC, Ponce-González JG, Martinez-Bello VE, Guadalupe-Grau A, Santana A, Sebastia V, Viña J, Calbet JA. Repeated muscle biopsies through a single skin incision do not elicit muscle signalling, but IL–6 mRNA and STAT3 phosphorylation increase in injured muscle. J Appl Physiol. 2011;110:1708–1715. doi: 10.1152/japplphysiol.00091.2011. [DOI] [PubMed] [Google Scholar]
- Haddad F, Adams GR. Aging-sensitive cellular and molecular mechanisms associated with skeletal muscle hypertrophy. J Appl Physiol. 2006;100:1188–1203. doi: 10.1152/japplphysiol.01227.2005. [DOI] [PubMed] [Google Scholar]
- Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD. Expression of IGF–I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol. 2003;547:247–254. doi: 10.1113/jphysiol.2002.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop L, Beauchamp JR, Tajbakhsh S, Buckingham ME, Partridge TA, Zammit PS. Transplanted primary neonatal myoblasts can give rise to functional satellite cells as identified using the Myf5nlacZl+ mouse. Gene Ther. 2001;8:778–783. doi: 10.1038/sj.gt.3301463. [DOI] [PubMed] [Google Scholar]
- Hespel P, Op't Eijnde B, Van Leemputte M, Urso B, Greenhaff PL, Labarque V, Dymarkowski S, Van Hecke P, Richter EA. Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humans. J Physiol. 2001;536:625–633. doi: 10.1111/j.1469-7793.2001.0625c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida RS. Aging changes in satellite cells and their functions. Curr Aging Sci. 2011;4:279–297. doi: 10.2174/1874609811104030279. [DOI] [PubMed] [Google Scholar]
- Horsley V, Pavlath GK. Prostaglandin F2α stimulates growth of skeletal muscle cells via an NFATC2-dependent pathway. J Cell Biol. 2003;161:111–118. doi: 10.1083/jcb.200208085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvid L, Aagaard P, Justesen L, Bayer ML, Andersen JL, Ortenblad N, Kjaer M, Suetta C. Effects of aging on muscle mechanical function and muscle fibre morphology during short-term immobilization and subsequent retraining. J Appl Physiol. 2010;109:1628–1634. doi: 10.1152/japplphysiol.00637.2010. [DOI] [PubMed] [Google Scholar]
- Hvid LG, Ortenblad N, Aagaard P, Kjaer M, Suetta C. Effects of ageing on single muscle fibre contractile function following short-term immobilisation. J Physiol. 2011;589:4745–4757. doi: 10.1113/jphysiol.2011.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennische E, Ekberg S, Matejka GL. Expression of hepatocyte growth factor in growing and regenerating rat skeletal muscle. Am J Physiol Cell Physiol. 1993;265:C122–C128. doi: 10.1152/ajpcell.1993.265.1.C122. [DOI] [PubMed] [Google Scholar]
- Jones SW, Hill RJ, Krasney PA, O’Conner B, Peirce N, Greenhaff PL. Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J. 2004;18:1025–1027. doi: 10.1096/fj.03-1228fje. [DOI] [PubMed] [Google Scholar]
- Kadi F, Charifi N, Denis C, Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve. 2004a;29:120–127. doi: 10.1002/mus.10510. [DOI] [PubMed] [Google Scholar]
- Kadi F, Charifi N, Henriksson J. The number of satellite cells in slow and fast fibres from human vastus lateralis muscle. Histochem Cell Biol. 2006;126:83–87. doi: 10.1007/s00418-005-0102-0. [DOI] [PubMed] [Google Scholar]
- Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, Andersen JL. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol. 2004b;558:1005–1012. doi: 10.1113/jphysiol.2004.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Cross JM, Bamman MM. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab. 2005a;288:E1110–E1119. doi: 10.1152/ajpendo.00464.2004. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kosek DJ, Petrella JK, Cross JM, Bamman MM. Resting and load-induced levels of myogenic gene transcripts differ between older adults with demonstrable sarcopenia and young men and women. J Appl Physiol. 2005b;99:2149–2158. doi: 10.1152/japplphysiol.00513.2005. [DOI] [PubMed] [Google Scholar]
- Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol. 2006;101:531–544. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- Lange KH, Andersen JL, Beyer N, Isaksson F, Larsson B, Rasmussen MH, Juul A, Bulow J, Kjaer M. GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. J Clin Endocrinol Metab. 2002;87:513–523. doi: 10.1210/jcem.87.2.8206. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lorenzon P, Bandi E, de Guarrini F, Pietrangelo T, Schäfer R, Zweyer M, Wernig A, Ruzzier F. Ageing affects the differentiation potential of human myoblasts. Exp Gerontol. 2004;39:1545–1554. doi: 10.1016/j.exger.2004.07.008. [DOI] [PubMed] [Google Scholar]
- McCormick KM, Schultz E. Role of satellite cells in altering myosin expression during avian skeletal muscle hypertrophy. Dev Dyn. 1994;199:52–63. doi: 10.1002/aja.1001990106. [DOI] [PubMed] [Google Scholar]
- McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162:1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida S, Booth FW. Regrowth of skeletal muscle atrophied from inactivity. Med Sci Sports Exerc. 2004;36:52–59. doi: 10.1249/01.MSS.0000106175.24978.84. [DOI] [PubMed] [Google Scholar]
- Machida S, Spangenburg EE, Booth FW. Forkhead transcription factor FoxO1 transduces insulin-like growth factor's signal to p27Kip1 in primary skeletal muscle satellite cells. J Cell Physiol. 2003;196:523–531. doi: 10.1002/jcp.10339. [DOI] [PubMed] [Google Scholar]
- McKay BR, O’Reilly CE, Phillips SM, Tarnopolsky MA, Parise G. Co-expression of IGF–1 family members with myogenic regulatory factors following acute damaging muscle-lengthening contractions in humans. J Physiol. 2008;586:5549–5560. doi: 10.1113/jphysiol.2008.160176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky M, Parise G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J. 2012;26:2509–2521. doi: 10.1096/fj.11-198663. [DOI] [PubMed] [Google Scholar]
- Mackey AL, Andersen LL, Frandsen U, Suetta C, Sjogaard G. Distribution of myogenic progenitor cells and myonuclei is altered in women with vs. those without chronically painful trapezius muscle. J Appl Physiol. 2010;109:1920–1929. doi: 10.1152/japplphysiol.00789.2010. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzogiorno A, Coletta M, Zani BM, Cossu G, Molinaro M. Paracrine stimulation of senescent satellite cell proliferation by factors released by muscle or myotubes from young mice. Mech Ageing Dev. 1993;70:35–44. doi: 10.1016/0047-6374(93)90057-x. [DOI] [PubMed] [Google Scholar]
- Mitchell PO, Pavlath GK. Skeletal muscle atrophy leads to loss and dysfunction of muscle precursor cells. Am J Physiol Cell Physiol. 2004;287:C1753–C1762. doi: 10.1152/ajpcell.00292.2004. [DOI] [PubMed] [Google Scholar]
- Moss FP, Leblond CP. Nature of dividing nuclei in skeletal muscle of growing rats. J Cell Biol. 1970;44:459–462. doi: 10.1083/jcb.44.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owino V, Yang SY, Goldspink G. Age-related loss of skeletal muscle function and the inability to express the autocrine form of insulin-like growth factor-1 (MGF) in response to mechanical overload. FEBS Lett. 2001;505:259–263. doi: 10.1016/s0014-5793(01)02825-3. [DOI] [PubMed] [Google Scholar]
- Pallafacchina G, Blaauw B, Schiaffino S. Role of satellite cells in muscle growth and maintenance of muscle mass. Nutr Metab Cardiovasc Dis. 2012 doi: 10.1016/j.numecd.2012.02.002. (in press; DOI: 10.1016/j.numecd.2012.02.002) [DOI] [PubMed] [Google Scholar]
- Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab. 2006;291:E937–E946. doi: 10.1152/ajpendo.00190.2006. [DOI] [PubMed] [Google Scholar]
- Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell mediated myonuclear addition: a cluster analysis. J Appl Physiol. 2008;104:1736–1742. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- Psilander N, Damsgaard R, Pilegaard H. Resistance exercise alters MRF and IGF-I mRNA content in human skeletal muscle. J Appl Physiol. 2003;95:1038–1044. doi: 10.1152/japplphysiol.00903.2002. [DOI] [PubMed] [Google Scholar]
- Raue U, Slivka D, Jemiolo B, Hollon C, Trappe SW. Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J Appl Physiol. 2006;101:53–59. doi: 10.1152/japplphysiol.01616.2005. [DOI] [PubMed] [Google Scholar]
- Renault V, Rolland E, Thornell LE, Mouly V, Butler-Browne G. Distribution of satellite cells in the human vastus lateralis muscle during aging. Exp Gerontol. 2002;37:1513–1514. doi: 10.1016/s0531-5565(02)00095-5. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Yong D, Parry DJ. Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve. 1994;17:608–613. doi: 10.1002/mus.880170607. [DOI] [PubMed] [Google Scholar]
- Roth SM, Martel GF, Ferrell RE, Metter EJ, Hurley BF, Rogers MA. Myostatin gene expression is reduced in humans with heavy-resistance strength training: a brief communication. Exp Biol Med (Maywood) 2003;228:706–709. doi: 10.1177/153537020322800609. [DOI] [PubMed] [Google Scholar]
- Roth SM, Martel GF, Ivey FM, Lemmer JT, Metter EJ, Hurley BF, Rogers MA. Skeletal muscle satellite cell populations in healthy young and older men and women. Anat Rec. 2000;260:351–358. doi: 10.1002/1097-0185(200012)260:4<350::AID-AR30>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Sheehan SM, Allen RE. Skeletal muscle satellite cell proliferation in response to members of the fibroblast growth factor family and hepatocyte growth factor. J Cell Physiol. 1999;181:499–506. doi: 10.1002/(SICI)1097-4652(199912)181:3<499::AID-JCP14>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Sheehan SM, Tatsumi R, Temm-Grove CJ, Allen RE. HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve. 2000;23:239–245. doi: 10.1002/(sici)1097-4598(200002)23:2<239::aid-mus15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetta C, Aagaard P, Rosted A, Jakobsen AK, Duus B, Kjaer M, Magnusson SP. Training-induced changes in muscle CSA, muscle strength, EMG, and rate of force development in elderly subjects after long-term unilateral disuse. J Appl Physiol. 2004a;97:1954–1961. doi: 10.1152/japplphysiol.01307.2003. [DOI] [PubMed] [Google Scholar]
- Suetta C, Andersen JL, Dalgas U, Berget J, Koskinen S, Aagaard P, Magnusson SP, Kjaer M. Resistance training induces qualitative changes in muscle morphology, muscle architecture, and muscle function in elderly postoperative patients. J Appl Physiol. 2008;105:180–186. doi: 10.1152/japplphysiol.01354.2007. [DOI] [PubMed] [Google Scholar]
- Suetta C, Clemmensen C, Andersen JL, Magnusson SP, Schjerling P, Kjaer M. Coordinated increase in skeletal muscle fibre area and expression of IGF–I with resistance exercise in elderly post-operative patients. Growth Horm IGF Res. 2010;20:134–140. doi: 10.1016/j.ghir.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Suetta C, Frandsen U, Jensen L, Jensen MM, Jespersen JG, Hvid LG, Beyer M, Petersson SJ, Schrøder HD, Andersen JL, Heinemeier K, Aagaard P, Schjerling P, Kjaer M. Aging affects the transcriptional regulation of human skeletal muscle disuse atrophy. PLoS One. 2012;12:1–13. doi: 10.1371/journal.pone.0051238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol. 2009;107:1172–1180. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- Suetta C, Magnusson SP, Rosted A, Aagaard P, Jakobsen AK, Larsen LH, Duus B, Kjaer M. Resistance training in the early postoperative phase reduces hospitalization and leads to muscle hypertrophy in elderly hip surgery patients–a controlled, randomized study. J Am Geriatr Soc. 2004b;52:2016–2022. doi: 10.1111/j.1532-5415.2004.52557.x. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Sankoda Y, Anderson JE, Sato Y, Mizunoya W, Shimizu N, Suzuki T, Yamada M, Rhoads RP, Jr, Ikeuchi Y, Allen RE. Possible implication of satellite cells in regenerative motoneuritogenesis: HGF upregulates neural chemorepellent Sema3A during myogenic differentiation. Am J Physiol Cell Physiol. 2009;297:C238–C252. doi: 10.1152/ajpcell.00161.2009. [DOI] [PubMed] [Google Scholar]
- Thornell LE, Lindstrom M, Renault V, Mouly V, Butler-Browne GS. Satellite cells and training in the elderly. Scand J Med Sci Sports. 2003;13:48–55. doi: 10.1034/j.1600-0838.2003.20285.x. [DOI] [PubMed] [Google Scholar]
- Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signalling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296:C1258–C1270. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab. 2007;292:E151–E157. doi: 10.1152/ajpendo.00278.2006. [DOI] [PubMed] [Google Scholar]
- Verney J, Kadi F, Charifi N, Feasson L, Saafi MA, Castells J, Piehl-Aulin K, Denis C. Effects of combined lower body endurance and upper body resistance training on the satellite cell pool in elderly subjects. Muscle Nerve. 2008;38:1147–1154. doi: 10.1002/mus.21054. [DOI] [PubMed] [Google Scholar]
- Yamada M, Tatsumi R, Yamanouchi K, Hosoyama T, Shiratsuchi S, Sato A, Mizunoya W, Ikeuchi Y, Furuse M, Allen RE. High concentrations of HGF inhibit skeletal muscle satellite cell proliferation in vitro by inducing expression of myostatin: a possible mechanism for reestablishing satellite cell quiescence in vivo. Am J Physiol Cell Physiol. 2010;298:C465–C476. doi: 10.1152/ajpcell.00449.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SY, Goldspink G. Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett. 2002;522:156–160. doi: 10.1016/s0014-5793(02)02918-6. [DOI] [PubMed] [Google Scholar]
- Zarnegar R. Regulation of HGF and HGFR gene expression. EXS. 1995;74:33–49. doi: 10.1007/978-3-0348-9070-0_3. [DOI] [PubMed] [Google Scholar]


