Abstract
The natural ligands of two major human immunodeficiency virus type 1 (HIV-1) co-receptors, CXCR4 and CCR5, can profoundly inhibit the replication of HIV-1 that uses these co-receptors for entry into the target cells. It has been postulated that these natural chemokines inhibit HIV-1 infection by blocking common binding sites on CXCR4 or CCR5 that are required for HIV-1 envelope glycoprotein gp120 interaction with its co-receptor and/or by inducing receptor internalization. To investigate whether receptor internalization caused by stromal cell-derived factor (SDF)-1α, a natural ligand of CXCR4, plays a role in its anti-HIV activity, we applied the SMM (synthetically and modularly modified)-chemokine approach to generate a functional probe of SDF-1α that retains significant CXCR4 binding but does not induce CXCR4 internalization. The antiviral study of this functional probe analog versus wild-type SDF-1α showed that, despite the significant CXCR4 binding activity, this probe analog displayed a complete loss of effect in causing CXCR4 internalization and greatly diminished antiviral activity. Interestingly, this new analog also showed a decreased number of overlapping binding sites with HIV-1 on CXCR4 transmembrane and extracellular domains. The correlation of the decrease in the anti-HIV activity with the loss of CXCR4 internalization observed with this probe molecule suggests that receptor internalization may play an important role in the anti-HIV activity of SDF-1α and possibly other natural chemokines. This further implies that any modifications in SDF-1α that result in a reduction or loss of internalization activity may result in analogs that are not suitable as effective HIV-1 inhibitors that target CXCR4, unless such modifications also result in improved CXCR4 interaction with increased number of overlapping binding sites with HIV-1, thus leading to more effective steric hindrance against HIV-1.
Keywords: HIV-1, AIDS, CXCR4, chemokines, SDF-1α, vMIP-II
Introduction
The productive infection of human cells with human immunodeficiency virus type 1 (HIV-1) requires both CD4, the primary receptor on the target cell, and chemokine receptors that are members of the superfamily of G-protein coupled receptors. The chemokine receptors CXCR4 and CCR5 are the two principal HIV-1 co-receptors.1,2 The natural ligands of chemokine receptors are chemokines, a family of small proteins of about 70–80 residues. The chemokines can be categorized into four subfamilies based on the positions of two conserved cysteine residues in their amino (N)-termini: CC, CXC, CX3C and C chemokines.3,4 The CC chemokines, including `regulated on activation normal T-cell expressed and secreted' (RANTES) and macrophage inflammatory protein (MIP)-1α/β, can inhibit the entry of M-tropic (CCR5-preferring) HIV-1 strains, which are usually isolated from infected patients during the asymptomatic stage of HIV-1 infection.5,6 The CXC chemokines, such as stromal cell-derived factor (SDF)-1α, inhibit the cell fusion and infection by T-tropic (CXCR4-preferring) HIV-1 strains, which are isolated at late, symptomatic stages of acquired immunodeficiency syndrome (AIDS).7,8
In a plausible model, the initial binding of HIV-1 gp120 to CD4 results in conformational changes in gp120 that expose the co-receptor-binding determinants. The subsequent interaction between gp120 and the co-receptor induces a further conformational change in the HIV-1 envelope that results in insertion of the fusion peptide, gp41, into the target cell membrane.3,9 Two theories have been proposed for the mechanism(s) by which natural chemokines prevent this chemokine receptor-dependent HIV-1 entry. First, the natural chemokines of CXCR4 or CCR5 can inhibit HIV-1 infection10,11 by blocking the binding of HIV-1 gp120 to CXCR4 or CCR5. The mere occupancy of HIV-1 co-receptors by chemokines even in the absence of G-protein-mediated signaling is known to be sufficient for inhibition of HIV-1 infection.12,13 Alternatively or additionally, chemokines, such as SDF-1α, can inhibit HIV-1 entry by inducing receptor downregulation from the cell surface, thereby removing the essential co-receptors.14,15
Due to the importance of chemokines and their receptors in numerous physiological and pathological processes, most notably in AIDS, we have been working towards the development of a systematic strategy based on the full-length chemokine structures, aiming to synthesize a new family of unnatural chemokines called SMM (synthetically and modularly modified)-chemokines.16,17 In this approach, synthetic chemistry is applied to introduce unnatural amino acids or novel chemical modifications into the important functional sequence modules of the native chemokines to yield new mechanistic probes of receptor functions and inhibitors of pathological processes. We previously demonstrated that this SMM-chemokine approach can be applied to convert the non-selective viral macrophage protein (vMIP)-II into highly selective ligands for CXCR4 or CCR5 in terms of their binding, signaling and antiviral activities.16,17 Using this approach, we have also obtained new insights into the distinct signaling pathways of neuronal apoptosis associated with HIV-associated dementia, which is activated by different chemokine receptor ligands serving either as agonists or as antagonists.18 Similarly, Hartley's group has recently described the role of CCR5 internalization on the inhibitory activities of RANTES analogs by modifying the amino (N)-terminus of PSC-RANTES.19 Despite the subtle differences in sequence, volume and mass among these modified RANTES compounds, they showed very distinct characteristics with respect to G protein-linked signaling and receptor sequestration. In the present study, we applied the SMM-chemokine approach to determine if CXCR4 internalization caused by SDF-1α plays any role in its anti-HIV activity. By synthetically and selectively removing the ability of SDF-1α to cause CXCR4 internalization, while retaining significant CXCR4 binding, we developed a novel chemical probe analog of SDF-1α, which now allows us to investigate the mechanistic question of whether CXCR4 receptor internalization plays an important role in the anti-HIV activity of SDF-1α, and possibly other natural chemokines.
Materials and mthods
Total chemical synthesis of SDF-1α analogs
The automated stepwise incorporation of protected amino acids was performed using an Applied Biosystems 433A peptide synthesizer (Foster City, CA, USA) with a CLEAR amide resin (Peptides International, Louisville, KY, USA) as the solid support. Fmoc-chemistry was employed for the synthesis. 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) and N-hydroxybenzotriazole (HOBt) were used as coupling reagents in the presence of diisopropylethylamine. In certain coupling steps with potentially slow reaction rates, double coupling followed by capping of the unreacted amino functional groups was performed. After incorporation of the 50th residue, 2% vol./vol. of dimethyl sulfoxide was introduced to the solution to enhance the coupling reaction. After removing N-terminal Fmoc protection, the protein was cleaved from the resin support by adding a cleavage cocktail comprised of phenol (4% wt./vol.), thioanisole (5% vol./vol.), water (5% vol./ vol.), ethanedithiol (2.5% vol./vol.), triisopropylsilane (1.5% vol./vol.) and trifluoroaceticacid (TFA; 82% vol./vol.). The protein was precipitated by adding ice-cold tert-butyl methyl ether and washed repeatedly in cold ether. The crude protein was dissolved in 25% CH3CN in water containing 0.1% TFA before being lyophilized, and it was dissolved in water and purified using semipreparative reverse phase-high performance liquid chromatography (RP-HPLC). Folding of the purified protein was performed in 1 mol/L guanidinium hydrochloride and 0.1 mol/L Trizma base at pH 8.5 (1 mg protein/mL folding buffer), and was monitored by analytical RP-HPLC using a Vydac C-18 column (Grace, Deerfield, IL, USA; 0.46 × 15 cm, 5 μm) with a flow rate of 1 mL/min, solvent: A, water with 0.1% TFA; solvent: B, 20% water in CH3CN with 0.1% TFA, and a linear gradient 30–70% B over 30 min. Protein desaltation and purification were then performed. The purified protein was characterized by matrix-assisted laser desorption/ionization-time-of-flight-mass spectrometry.
Transfection of adherent 293 cells
Wild-type CXCR4 or CCR5 was transfected into 293 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The selective medium containing G418 (800 μg/mL) was used to isolate stably transfected cells that were subsequently cloned from a single colony.
Competition receptor binding assays using labeled chemokines
Ligand-binding experiments were performed using a single concentration (0.2 nmol/L) of 125I-SDF-1α or 125I-MIP-1β in a final volume of 100 μL binding buffer (50 mmol/L HEPES, pH 7.4, 1 mmol/L CaCl2, 5 mmol/L MgCl2, 0.1% bovine serum albumin [BSA]) containing 5 105 cells in 96-well plates in the presence of various concentrations of unlabeled chemokines. Non-specific binding was determined by adding 150 nmol/L unlabeled SDF-1α or 100 nmol/L unlabeled vMIP-II. Samples were incubated for 60 min at room temperature. The cells were washed with 200 μL binding buffer. Bound ligands were determined by counting gamma emissions. The binding data were analyzed using the PRISM program (GraphPad Inc, San Diego, CA, USA).
Intracellular calcium measurements
Sup T1 cells (107 cells/mL) were loaded with 2 μmol/L fura-2/AM (Molecular Probes, Eugene, OR, USA) and 0.01% Pluronic F-127 (Sigma, St Louis, MO, USA) in Hank's balanced salt saline (140 mmol/L NaCl, 5 mmol/L KCl, 10 mmol/L HEPES, pH 7.4, 1 mmol/L CaCl2, 1 mmol/L MgCl2, 1 mg/mL glucose and 0.025% BSA) for 20 min at room temperature. The cells were washed and re-suspended in the same buffer to 106 cells/mL. Fura-2 fluorescence was measured on the fluorescence spectrophotometer (ISA SPEX FluoroMax-2; Horiba, Ann Arbor, MI, USA) using excitation wavelengths of 340 and 380 nm, and an emission wavelength of 510 nm.
Internalization assays
293 cells stably expressing HA-CXCR4 (3 × 105 cells/well) were plated onto 24-well tissue culture plates pretreated with 0.01% poly-l-lysine (Sigma) for 30 min. The cells were incubated with an SMM-chemokine analog at increasing concentrations (for 90 min) or incubation time (at 100 nmol/L). Non-transfected 293 cells were used as the background. Fixing the cells for five minutes at room temperature with 4% paraformaldehyde/PBS (phosphate-buffered saline) stopped the reactions. After blocking the non-specific binding with 1% BSA/PBS and incubating the cells for 45 min, a monoclonal antibody HA.11 (Covance Inc, Princeton, NJ, USA) was added. The cells were incubated with HA.11 for one hour at room temperature. The cells were washed with PBS and re-blocked with 1% BSA/PBS for 15 min at room temperature. The cells were incubated with goat anti-mouse conjugated alkaline phosphate (Bio-Rad, Richmond, CA, USA) for one hour at room temperature. The cells were washed with PBS before colorimetric alkaline phosphate substrate BCIP-NBT (Bio-Rad) was added. The plate was continuously shaken until an adequate color change occurred (~1 h). The absorbance readings were taken using the Wallac Victor2 1420 Multilabel counter (PerkinElmer, Waltham, MA, USA).
Single-round virus inhibition assays
293T human embryonic kidney and Cf2Th canine thymocytes (ATCC, St Louis, MO, USA) were grown at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM; Cambrex, Walkersville, MD, USA) containing 10% fetal bovine serum (FBS; Cambrex) and 100 μg/mL penicillin-streptomycin (P/S; Cambrex). Cf2Th cells stably expressing human CD4 and CXCR420 were grown in the medium supplemented with 0.4 mg/mL G418 (Cambrex) and 0.15 mg/mL hygromycin B (Roche Diagnostics, Basel, Switzerland). 293 T-cells were co-transfected with vectors expressing the pCMVΔP1Δenv HIV Gag-Pol packaging construct,21 the envelope glycoproteins of HIV-1 isolates (HXBc2 or JR-FL) and a firefly luciferase reporter gene, at a DNA ratio of 1:1:3 μg using Effectene transfection reagent (Qiagen, Valencia, CA, USA). Co-transfection produced single-round, replication-defective viruses. Virus-containing supernatants were harvested 24–30 h after transfection, filtered (0.45 μm), ali-quoted and frozen at −80°C until further use. The reverse transcriptase activities of all the viruses were measured as described previously.22 To determine the infection by single-round luciferase viruses, Cf2Th-CD4-CXCR4 target cells were seeded at a density of 6 × 103 cells/well in 96-well luminometer-compatible tissue culture plates (Dynex, Chantilly, VA, USA) 24 h before infection. On the day of infection, synthetic chemokines were added to the target cells and incubated for one hour at 37°C. Following the incubation, recombinant viruses (10,000 RT units), to a final volume of 50 μL, were added to the chemokine-cell mixtures and incubated for 48 h at 37°C. The medium was removed from each well, and the cells were lysed with 30 μL passive lysis buffer (Promega, Madison, WI, USA), and by three freeze–thaw cycles. An EG&G Berthold Microplate Luminometer LB 96V (Berthold, Oak Ridge, TN, USA) was used to measure the luciferase activity of each well after the addition of 100 μL luciferin buffer (15 mmol/L MgSO4, 15 mmol/L KPO4, pH 7.8, 1 mmol/L ATP and 1 mmol/L dithiothreitol) and 50 μL of 1 mmol/L d-luciferin potassium salt (BD Pharmingen, San Diego, CA, USA).
Results and discussion
The design of SDF-1α probe analogs and their CXCR4 binding activities
Given that natural chemokines, such as SDF-1α, are known to inhibit HIV infection and to cause receptor internalization, it is important to determine if this receptor internalization caused by natural chemokines is involved in their anti-HIV activities.14,15 To achieve this goal, we designed and synthesized new synthetic analogs as functional probes of SDF-1α by employing the SMM-chemokine approach16 to modify the N-terminal (1–8) sequence module of SDF-1α (termed RCP211 here; Table 1). Our analog design was based on the notion and hypothesis that the N-terminus of SDF-1α is the key determinant of its functions, such as its promotion of CXCR4 internalization,23–25 and that synthetic modifications in this region of SDF-1α may generate new analogs with changed activities with respect to CXCR4 internalization. An N-terminal (1–8) sequence truncated SDF-1α analog (termed RCP212; Table 1) showed a significant loss in its binding activity to CXCR4 (Figure 1; Table 1), and this analog was used as the negative control for this study. We generated a functional probe of SDF-1α that retains significant CXCR4 binding, but shows no CXCR4 internalization activity by synthesizing a novel d-amino acid-containing SDF-1α analog, termed d(1–8)-SDF-1α (or RCP214; Table 1). In this analog, the N-terminal (1–8) residues were all replaced with d-amino acids. The binding affinity of RCP214 for CXCR4 was tested using 125I-SDF-1α competition binding assays. As previously demonstrated by our laboratory,16 despite the introduction of d-amino acids into the N-terminal sequence, this novel probe molecule RCP214 retained significant CXCR4 binding with the IC50 of 25 nmol/L (Figure 1; Table 1). In control experiments designed to verify that this analog is specific for CXCR4, its binding activity was tested in CCR5 competition binding assays using 125I-MIP-1β. As expected, RCP214 did not show any CCR5 binding activity (Table 1).
Table 1.
List of stromal cell-derived factor (SDF)-1α analogs, their sequences, modifications, and binding affinity and selectivity for CXCR4 and CCR5*
| Analog | Modification diagrams | Amino acid sequences† | CXCR4 binding (nmol/L) | CCR5 binding (nmol/L) |
|---|---|---|---|---|
| RCP211 |
|
KPVSLSYRCPCRFFESHVARANVKHLKILNTPNCALQIVARLKNNNRQVCIDPKLKWIQEYLEKALNK | 4 | >2700 |
| RCP212 |
|
CPCRFFESHVARANVKHLKILNTPNCALQIVARLKNNNRQVCIDPKLKWIQEYLEKALNK | >2700 | >2700 |
| RCP214 |
|
KPVSLSYRCPCRFFESHVARANVKHLKILNTPNCALQIVARLKNNNRQVCIDPKLKWIQEYLEKALNK | 25 | >2700 |
The binding activity of each SDF-1α analog is shown by its IC50 value determined by competition receptor binding assays using labeled chemokines
d-Amino acids are shown in italic and underlined
Figure 1.
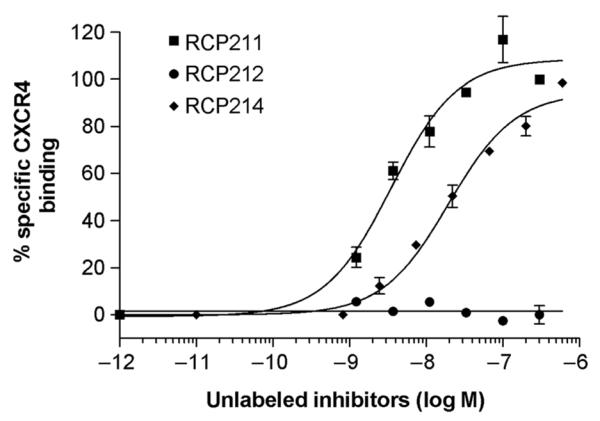
CXCR4-binding activities of stromal cell-derived factor (SDF)-1α analogs. 125I-SDF-1α competition binding assays were used to determine their IC50 values. The binding data were analyzed using the PRISM program (GraphPad Inc, San Diego, CA, USA). All data are shown as mean±SD from at least three independent experiments
Signaling activity of RCP214
In addition to receptor binding, another important biological property of these de novo designed ligands is signaling activity. Thus, calcium (Ca2+) mobilization assays were performed. In contrast to the rapid Ca2+ mobilization induced by SDF-1α, neither RCP212 nor RCP214 was able to induce any mobilization of Ca2+ in Sup T1 cells expressing CXCR4 (Figure 2). This demonstrates that CXCR4 signaling activity is encoded in the N-terminal (1–8) residue sequence of SDF-1α, which is consistent with the previous findings that Lys1 and Pro2 of SDF-1α are involved in signal transduction.24,26 Whether SDF-1α analogs may interfere with the normal Ca2+ signaling activated by SDF-1α was also examined. Consistent with the binding data, RCP214 (Figure 2a) interfered with the Ca2+ mobilization induced by SDF-1α in a dose-dependent manner, whereas RCP212 did not even when its concentration was increased up to 1 μmol/L (Figure 2b).
Figure 2.
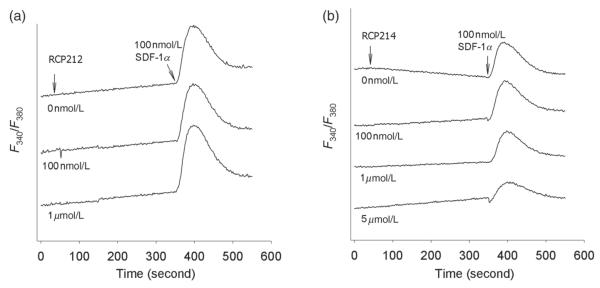
Signaling activities of stromal cell-derived factor (SDF)-1α analogs. Intracellular Ca2+ influx in Sup T1 cells expressing CXCR4 was measured in response to RCP212 (a) or RCP214 (b). For inhibition assays, Sup T1 cells were preincubated with each of the SDF-1α analogs for five minutes, and then stimulated with 100 nmol/L SDF-1α. At least three independent experiments were performed
Internalization activity of RCP214
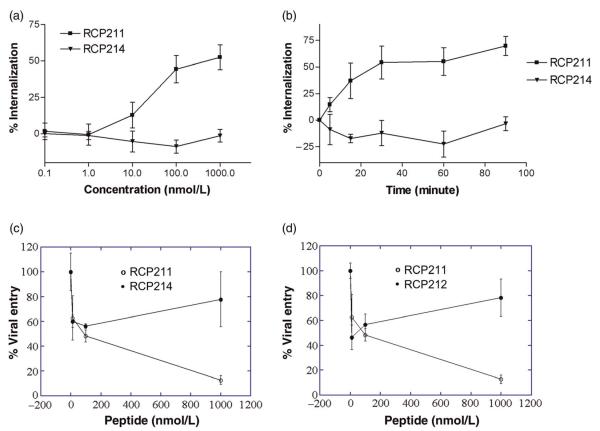
We used CXCR4 internalization studies to examine whether RCP214, which retains significant CXCR4 binding as described above, shows any alternations in its ability to promote CXCR4 internalization. As shown in Figure 3a, an increase in the concentration of RCP214 from 0.1 nmol/L to 1 μmol/L with the total incubation time of 90 min for each concentration failed to cause CXCR4 downregulation. Similarly, a variation in the incubation time had no impact on the receptor internalization. Even after 90 min of incubation, 100 nmol/L RCP214 did not elicit any significant internalization of CXCR4 (Figure 3b). However, its parent ligand SDF-1α, which is known to cause CXCR4 internalization,14,15 induced a 50% receptor loss when its concentration was increased up to 1 μmol/L or after 30-min incubation (Figure 3). These results demonstrate that RCP214 is an SDF-1α analog with a changed functional profile. Even though it has significant CXCR4 binding affinity, it does not cause CXCR4 internalization.
Figure 3.
Internalization and antiviral activities of stromal cell-derived factor (SDF)-1α analogs. The data represent the mean values of three independent assays with the error bars indicating the standard deviations. (a) An increase in the concentration of RCP214 from 0.1 nmol/L to 1 μmol/L failed to cause CXCR4 down-regulation, while 1 μmol/L RCP211 induced 50% receptor loss. The cells were incubated for 90 min at each concentration. (b) The longer incubation of cells with 100 nmol/L RCP214 did not elicit any significant internalization of CXCR4. In contrast, 100 nmol/L RCP211 induced 50% receptor loss after 30-min incubation. (c) The antiviral activity of RCP214 was compared with that of the natural chemokine, RCP211. (d) Similarly, the antiviral activities of RCP212 versus RCP211 were compared
Antiviral activity of RCP214
The development of this novel SDF-1α analog, RCP214, with its loss of CXCR4 internalization activity as described above, provided us with a tool to investigate the impact of the loss of CXCR4 internalization on the anti-HIV activity of SDF-1α. As such, single-round virus inhibition assays were carried out for RCP214 and compared with the similar tests using its parent ligand SDF-1α (RCP211). RCP214 showed a greatly impaired ability to inhibit T-tropic HIV-1 (HXBc2) entry compared with RCP211, especially at concentrations greater than 200 nmol/L (Figure 3c). RCP212 and 214 only showed a maximum inhibition of 40–50% versus 90% for RCP211, and their inhibitory effects were consistently lower than that of RCP211 at concentrations greater than 200 nmol/L. It is striking to note that RCP214, which has significant CXCR4 binding, is almost equally impotent in inhibiting HIV-1 entry as the negative control molecule RCP212, which has no CXCR4 binding (Figure 3d). This suggests that CXCR4 internalization may play an important role in the anti-HIV activity of SDF-1α. Otherwise, RCP214, which retains significant CXCR4 binding, would be expected to show higher anti-HIV activity than the non-binding RCP212. This conclusion is consistent with the previous findings that the HIV-1 suppressive effects of natural chemokines, including SDF-1α, can be markedly reduced when receptor endocytosis does not occur.14,15 Interestingly, based on our previous mutational mapping analysis of the binding sites of RCP214,27 RCP214 showed a decreased number of overlapping binding sites with HIV-1 on CXCR4 transmembrane and extracellular domains, compared with RCP168 (17), a highly potent antiviral vMIP-II analog with d-amino acids substituted at the N-terminal (1–10) sequence module of vMIP-II or RCP222,17 an SDF-1α analog with the N-terminal (1–8) residues replaced with all d-forms of (1–10) sequence module of vMIP-II. The distinct sites required for the binding activities of RCP168 and RCP222 include Tyr45, Phe87, Asp97, Tyr121, Asp171, Asp187, Tyr219, Trp252, Tyr255, Asp262, Glu288 and Phe292, many of which play important roles in HIV-1 co-receptor activity.27 In contrast, RCP214 requires only Tyr45, Phe87, Asp171, Asp187 and Glu288 for its interaction with CXCR4. Taken together, these finding suggest that in addition to the important role of CXCR4 internalization in the anti-HIV activity of SDF-1α, less efficient steric interference with the binding of HIV-1 gp120 to CXCR4 mediated by RCP214 may also lead to the overall impaired antiviral activity of RCP214.
Conclusion
In this study, we have demonstrated a chemical biology approach that uses the SMM-chemokine concept for the design of novel functional probes of SDF-1α to understand the mechanism of the anti-HIV activity of SDF-1α. Even though the effective steric hindrance of CXCR4 mediated by SDF-1α is required to achieve the maximum antiviral activity of SDF-1α to prevent the docking of HIV-1 gp120 onto the same receptor, our data also suggest an important role of receptor internalization in the overall HIV-1 suppressive activity of SDF-1α and possibly of other natural chemokines. In particular, any modifications in SDF-1α that result in a reduction or loss of internalization activity may result in analogs that are not suitable as effective CXCR4 inhibitors, unless such modifications also result in improved CXCR4 interaction with increased number of overlapping binding sites with HIV-1, as demonstrated by our previous binding site mapping experiments.27 Similar chemical biology strategies may be employed to study other physiological or pharmaceutical activities of many different chemokines.
ACKNOWLEDGEMENT
This work was supported by grants from the National Institutes of Health.
Footnotes
Author contributions: C-ZD, ST, NM, SK, W-TC, DL, JGS, ZH and JA designed the experiments and wrote the manuscript. C-ZD and SK synthesized SDF-1α probe analogs. C-ZD, ST and NM determined binding, internaliz ation and antiviral activities of SDF-1α analogs.
REFERENCES
- 1.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 2.Trkola A, Dragic T, Arthos J, Binley JM, Olson WC, Allaway GP, Cheng-Mayer C, Robinson J, Maddon PJ, Moore JP. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–7. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 3.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 4.Proudfoot A. Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol. 2002;2:106–15. doi: 10.1038/nri722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–8. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 6.Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–73. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 7.Cheng-Mayer C, Seto D, Tateno M, Levy JA. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988;240:80–2. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- 8.Tersmette M, Lange JM, de Goede RE, de Wolf F, Eeftink-Schattenkerk JK, Schellekens PT, Coutinho RA, Huisman JG, Goudsmit J, Miedema F. Association between biological properties of human immunodeficiency virus variants and risk for AIDS and AIDS mortality. Lancet. 1989;1:983–5. doi: 10.1016/s0140-6736(89)92628-7. [DOI] [PubMed] [Google Scholar]
- 9.Wild C, Dubay JW, Greenwell T, Baird TJ, Oas TG, McDanal C, Hunter E, Matthews T. Propensity for a leucine zipper-like domain of human immunodeficiency virus type 1 gp41 to form oligomers correlates with a role in virus-induced fusion rather than assembly of the glycoprotein complex. Proc Natl Acad Sci USA. 1994;91:12676–80. doi: 10.1073/pnas.91.26.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–33. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 11.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–5. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 12.Oravecz T, Pall M, Norcross MA. Beta-chemokine inhibition of monocytotropic HIV-1 infection interference with a postbinding fusion step. J Immunol. 1996;157:1329–32. [PubMed] [Google Scholar]
- 13.Arenzana-Seisdedos F, Virelizier JL, Rousset D, Clark-Lewis I, Loetscher P, Moser B, Baggiolini M. HIV blocked by chemokine antagonist. Nature. 1996;383:400. doi: 10.1038/383400a0. [DOI] [PubMed] [Google Scholar]
- 14.Amara A, Gall SL, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J-L, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1α-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–46. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Förster R, Kremmer E, Schubel A, Breitfeld D, Kleinschmidt A, Nerl C, Bernhardt G, Lipp M. Intracellular and surface expression of the HIV-1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J Immunol. 1998;160:1522–31. [PubMed] [Google Scholar]
- 16.Kumar S, Choi WT, Dong CZ, Madani N, Tian S, Liu D, Wang Y, Pesavento J, Wang J, Fan X, Yuan J, Fritzsche WR, An J, Sodroski JG, Richman DD, Huang Z. SMM-chemokines: a class of unnatural synthetic molecules as chemical probes of chemical receptor biology and leads for therapeutic development. Chem Biol. 2006;13:69–79. doi: 10.1016/j.chembiol.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Dong CZ, Kumar S, Choi WT, Madani N, Tian S, An J, Sodroski JG, Huang Z. Different stereochemical requirements for CXCR4 binding and signaling functions as revealed by an anti-HIV, D-amino acid-containing SMM-chemokine ligand. J Med Chem. 2005;48:7923–4. doi: 10.1021/jm050829u. [DOI] [PubMed] [Google Scholar]
- 18.Choi WT, Kaul M, Kumar S, Wang J, Kumar IM, Dong CZ, An J, Lipton SA, Huang Z. Neuronal apoptotic signaling pathways probed and intervened by synthetically and modularly modified (SMM) chemokines. J Biol Chem. 2007;282:7154–63. doi: 10.1074/jbc.M611599200. [DOI] [PubMed] [Google Scholar]
- 19.Gaertner H, Cerini F, Escola JM, Kuenzi G, Melotti A, Offord R, Rossitto-Borlat I, Nedellec R, Salkowitz J, Gorochov G, Mosier D, Hartley O. Highly potent, fully recombinant anti-HIV chemokines: reengineering a low-cost microbicide. Proc Natl Acad Sci USA. 2008;105:17706–11. doi: 10.1073/pnas.0805098105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard NP, Gerard C, Sodroski J, Choe H. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell. 1999;96:667–76. doi: 10.1016/s0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- 21.Parolin C, Taddeo B, Palu G, Sodroski J. Use of cis- and trans-acting viral regulatory sequences to improve expression of human immunodeficiency virus vectors in human lymphocytes. Virology. 1996;222:415–22. doi: 10.1006/viro.1996.0438. [DOI] [PubMed] [Google Scholar]
- 22.Rho HM, Poiesz B, Ruscetti FW, Gallo RC. Characterization of the reverse transcriptase from a new retrovirus (HTLV) produced by a human cutaneous T-cell lymphoma cell line. Virology. 1981;112:355–60. doi: 10.1016/0042-6822(81)90642-5. [DOI] [PubMed] [Google Scholar]
- 23.Crump MP, Gong JH, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, Virelizier JL, Baggiolini M, Sykes BD, Clark-Lewis I. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997;16:6996–7007. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heveker N, Montes M, Germeroth L, Amara A, Trautmann A, Alizon M, Schneider-Mergener J. Dissociation of the signaling and antiviral properties of SDF-1-derived small peptides. Curr Biol. 1998;8:369–76. doi: 10.1016/s0960-9822(98)70155-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhou N, Luo Z, Luo J, Fan X, Cayabyab M, Hiraoka M, Liu D, Han X, Pesavento J, Dong CZ, Wang Y, An J, Kaji H, Sodroski JG, Huang Z. Exploring the stereochemistry of CXCR4-peptide recognition and inhibiting HIV-1 entry with D-peptides derived from chemokines. J Biol Chem. 2002;277:17476–85. doi: 10.1074/jbc.M202063200. [DOI] [PubMed] [Google Scholar]
- 26.Loetscher P, Gong JH, Dewald B, Baggiolini M, Clark-Lewis I. N-terminal peptides of stromal cell-derived factor-1 with CXC chemokine receptor 4 agonist and antagonist activities. J Biol Chem. 1998;273:22279–83. doi: 10.1074/jbc.273.35.22279. [DOI] [PubMed] [Google Scholar]
- 27.Choi WT, Tian S, Dong CZ, Kumar S, Liu D, Madani N, An J, Sodroski JG, Huang Z. Unique ligand binding sites on CXCR4 probed by a chemical biology approach: implications for the design of selective human immunodeficiency virus type 1 inhibitors. J Virol. 2005;79:15398–404. doi: 10.1128/JVI.79.24.15398-15404.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]