Abstract
Objective
Obesity and hypertension are comorbid in epidemic proportion, yet their biological connection is largely a mystery. The peptide chemerin is a candidate for connecting fat deposits around the blood vessel (perivascular adipose tissue, PVAT) to arterial contraction. We presently test the hypothesis that chemerin is expressed in PVAT and is vasoactive, supporting the existence of a chemerin axis in the vasculature.
Approach and Results
RT-PCR, immunohistochemistry and Western analyses supported the synthesis and expression of chemerin in PVAT, while the primary chemerin receptor ChemR23 was expressed both in the tunica media and endothelial layer. The ChemR23 agonist chemerin-9 caused receptor-, concentration-dependent contraction in the isolated rat thoracic aorta, superior mesenteric artery, and mesenteric resistance artery, and contraction was significantly amplified (over 100%) when nitric oxide synthase was inhibited, the endothelial cell mechanically removed or tone was placed on the arteries. The novel ChemR23 antagonist CCX832 inhibited phenylephrine- and PGF2α-induced contraction (+PVAT) suggesting that endogenous chemerin contributes to contraction. Arteries from animals with dysfunctional endothelium (obese or hypertensive) demonstrated a pronounced contraction to chemerin-9. Finally, mesenteric arteries from obese humans demonstrate amplified contraction to chemerin-9.
Conclusions
These data support a new role for chemerin as an endogenous vasoconstrictor that operates through a receptor typically attributed to function only in immune cells.
Keywords: chemerin, vasoconstriction, ChemR23, PVAT, adipose tissue
Three classic layers – intimal-, medial and adventitial – form the blood vessel as we know it. With the report by Soltis and Cassis in 1991 [1], the perivascular adipose tissue (PVAT) has earned its position as the fourth layer, being called the tunica adiposa [2]. The power of this tissue comes from its ability to synthesize and secrete a multitude of substances that include both vasoconstrictors and vasodilators that can act in a paracrine manner on the blood vessel [3, 4]. PVAT is in the unique position of enabling coordination of the inner compartment of the circulatory system to communicate with the external environment it supports. Most vessels, save the cerebral blood vessels and special vessels such as the tail artery, possess some amount and type of PVAT, varying from mostly brown fat (thoracic aorta) to mixed brown and white fat (mesenteric vessels). PVAT is found in healthy animals, suggesting it carries out a physiological function. Dozens of vasoactive substances are released from PVAT, with a majority of substances reducing arterial contraction [3,4].
Obesity is a situation in which fat is pathologically deposited in the body due to an imbalance of energy intake and expenditure. There is no question that increased visceral body fat (as opposed to subcutaneous body fat) is associated with higher risks of virtually every cardiovascular disease, including hypertension [5,6]. A low-grade inflammation is described as being part of obesity [7] and obesity is considered a significant risk factor for developing hypertension. We discovered that an adipokine previously believed to be found only in visceral fat stores and inflammatory cells is also produced by PVAT. Chemerin (tazarotene induced gene 2 or TIG2) is a peptide secreted as prochemerin from visceral fat and the liver [8-10]. Originally located in human inflammatory cells [11], its secretion is attributed to the fat cell itself [12]. Chemerin is described as a biomarker for adiposity as circulating chemerin levels associate strongly with BMI [13], is increased in nascent metabolic syndrome [14], and chemerin levels are reduced with weight and fat loss [15, 16]. Importantly, genetic knockout of the primary receptor for chemerin, ChemR23 (also known as chemokine like receptor 1, CMKLR1) is associated with reduced adiposity and body mass [16]. Best known for stimulating movement of dendritic cells and leukocytes, chemerin activates ChemR23 [17,18], a G protein-linked receptor, on monocytes and macrophages to elicit recruitment and stimulate an inflammation that could also play a role in obesity. Additionally, chemerin regulates adipocyte differentiation [18-20] and production of a number of proinflammatory cytokines. We hypothesized that a chemerin axis exists in blood vessels. We propose that chemerin and the primary receptor for chemerin, ChemR23, are present and mediate contraction in the vasculature.
Materials and Methods
Results
The arterial chemerin axis
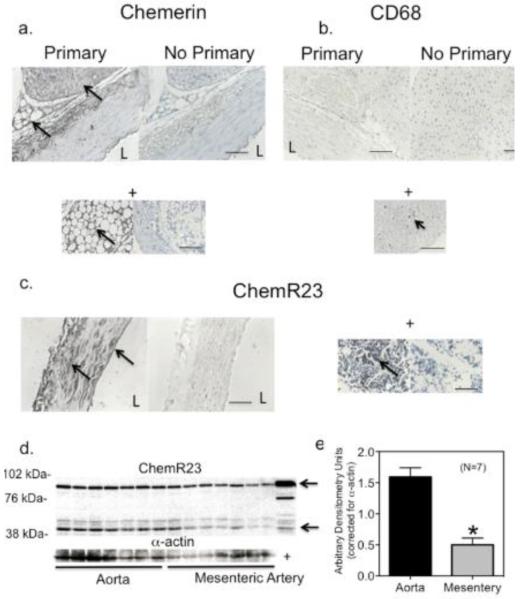
Isolated rat arteries express chemerin protein in the perivascular adipose tissue (PVAT; figure 1a). Real time RT-PCR supports the expression of chemerin (RARRES2) mRNA in the rat thoracic aortic PVAT (whole PVAT; CT = 22.78±0.35, β2microglobulin as control = 19.32±0.27, N=6). Chemerin signal does not wholly derive from resident mast cells, as there was negligible CD68 staining in PVAT (figure 1b, + control below) and staining for chemerin was, in many places, not punctate. Positive staining was observed within the cytoplasm of the fat cell, outside the rounded lipid droplet. The predominant receptor for chemerin, ChemR23, is expressed in the tunica media and endothelial cell layer (figure 1c) and is observed as three dominant bands in homogenates (−PVAT) of the thoracic aorta and superior mesenteric artery cleaned of PVAT (figure 1d, 1e). Two bands (at arrows) are consistent with that observed in a JAR (choriocarcinoma) positive control, and were 42 kDa in size (expected size for ChemR23) and ~84 kDa.
Figure 1. Chemerin Axis in Rat Arteries.
Immunohistochemical staining of isolated rat thoracic aorta for chemerin (a), CD68 (b) and ChemR23 (c). Representative of six different rats. + = bone marrow positive control (left image with antibody, right image without). d, Western analyses of homogenates of seven separate samples isolated from the thoracic aorta (left) and superior mesenteric artery (right) −PVAT from normal Sprague Dawley rats. + = human choriocarcinoma (JAR) whole cell lysate. Quantification of ChemR23 expression normalized to alpha actin is in e, presented as means±SEM for number of animals in parentheses. Bars = 100 microns. * p<0.05, unpaired Students t test aorta vs mesentery. L = arterial lumen.
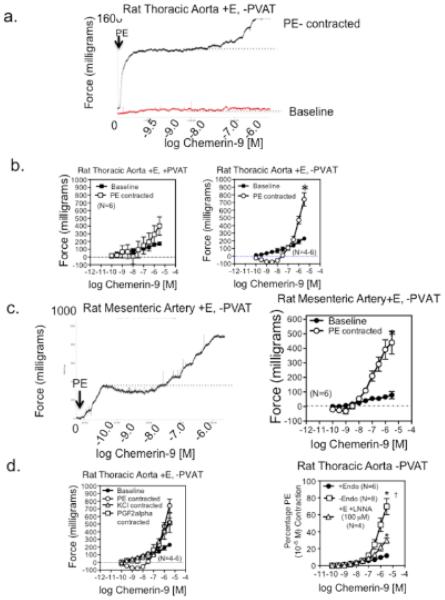
In endothelium-intact (+E) rat aorta (figure 2a) and rat superior mesenteric artery without PVAT (figure 2c, left panel), the ChemR23 agonist chemerin-9 [21] caused a small concentration-dependent contraction from baseline. When arteries were contracted with phenylephrine prior to chemerin-9, chemerin-9 first caused a modest relaxation that converted to an amplified contraction. This was readily visualized in tissues with no PVAT (figures 2b and 2c, right panel). Amplification occurred regardless of whether an agonist of G-protein coupled receptors [prostaglandin F2alpha (PGF2alpha) or the adrenergic agonist phenylephrine (PE)] or a depolarizing stimulus (KCl) was used to contract the tissues (figure 2d, left). The L type calcium channel antagonist nifedipine (100 nM) reduced not only agonist tone (KCl in this example; contraction to KCl abolished by nifedipine) but the amplified contraction to chemerin-9 (figure SI). Removal of nitric oxide synthase activity by using N-ω-nitro-L-arginine (LNNA) and, to a greater extent, removal of the endothelial cell amplified chemerin-9-induced arterial contraction (figure 2d, right). Similar PE-induced amplification of chemerin-9 contractions were observed in arteries from diet-induced obese and stroke prone spontaneously hypertensive rats (SHRSP) when compared to controls (figure SII). Chemerin-15, a putative anti-inflammatory chemerin [22], did not stimulate baseline or agonist-enhanced contraction (figure SIII). When chemerin-9 was acutely infused into the LNNA treated rat (0.1 mg/μL at 200 μL/min for 5 minutes), mean arterial blood pressure was not elevated (control = 102±3 mm Hg; during infusion = 104±4 mm Hg; N=3). This concentration/dose of chemerin-9 was sufficient to stimulate vasoconstriction in the isolated artery.
Figure 2. Chemerin-9-induced Arterial Contraction.
a, Concentration-dependent response to chemerin from baseline and above PE-induced contraction in the endothelium intact (+E) isolated thoracic aorta. Dotted line is point from which contraction was measured. b, Quantification of data in tissues in which PVAT was left intact (left) or removed (right). * significant difference of maximum response in baseline vs PE contracted tissue (p <0.05). c, Concentration-dependent response to chemerin-9 in a PE-induced contraction of the endothelium-intact isolated superior mesenteric artery, quantified on the far right. * significant difference in maximum response in baseline vs PE contracted tissue (p<0.05). d, Chemerin-9-induced contraction in thoracic aorta (no PVAT, endothelium intact) contracted with different agonists (left) and effect of endothelium removal and LNNA on baseline induced contraction (right). * significantly different from baseline values, † different from LNNA values. Points represent means±SEM for number of animals in parentheses.
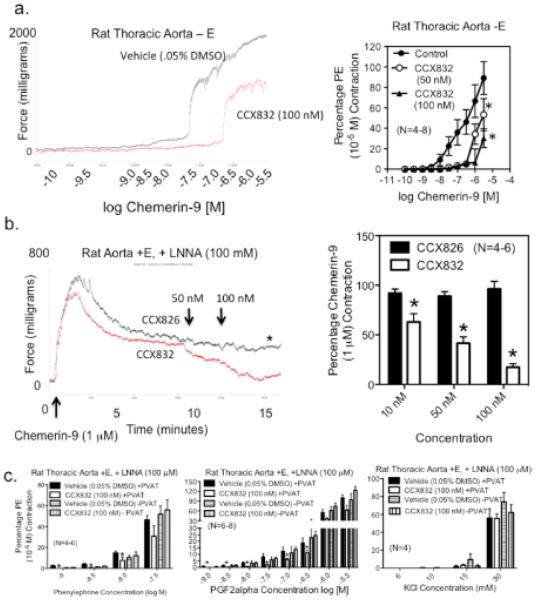
CCX832 was developed as a ChemR23 antagonist. Table 1 reports the low nanomolar affinity of CCX832 for ChemR23 receptors of the human, mouse and rat. CCX832 does not possess appreciable affinity for two other chemerin receptors -- GPR1 and CCLR2 – as well as a host of related chemokine receptors (supplemental Table 1). CCX823 inhibited chemerin-9-induced calcium mobilization and chemotaxis in multiple cell types. The significant affinity of CCX832 at rat ChemR23 supported our use of CCX832 as a receptor antagonist in isolated tissue bath studies. CCX832 rightward shifted chemerin-9 induced contraction (figure 3a; −log EC50 [M] vehicle = 6.53±0.05; 50 nM = 5.60±0.41; 100 nM = 5.20±0.06; p<0.05) and reversed chemerin-9 induced contraction while the inactive analog of CCX832, CCX826, did not (figure 3b, tracing left, quantitation right). Endogenous chemerin from PVAT supports agonist-induced contraction as CCX832 reduced contraction to low concentrations of α1-adrenergic agonist phenylephrine (figure 3c, left panel); CCX832 was without effect on phenylephrine-induced contraction in tissues without PVAT. CCX832 also reduced PGF2alpha-induced contraction in the rat thoracic aorta +PVAT (figure 3c, middle panel). By contrast, CCX832 was ineffective in reducing KCl-induced contraction in tissues with PVAT (figure 3c, right). Thoracic aorta from the global ChemR23 knockout mouse and wild type mouse were unresponsive to human chemerin-9 (figure SIVa). These same aortae contracted with half-maximal PE also did not contract or relax to chemerin-9 (data not shown, N=4 for WT and KO). ChemR23 protein was observed immunohistochemically in mouse thoracic aorta (figure SIVb).
Table 1.
CCX832 is a potent inhibitor of ChemR23 across multiple species
| Assay Format | h = human, m = mouse, r = rat |
|---|---|
| Human ChemR23 | Potency (nM); |
| hChemerin Radioligand Binding (hChemR23/Baf-3, buffer IC50) | 2.4 |
| hC9 Ca2+ Mobilization (hChemR23/Baf-3, buffer A2) | 1.7 |
| hC9 Chemotaxis (hChemR23/Baf-3, buffer IC50) | 3.0 |
| Mouse ChemR23 | |
| hChemerin Radioligand Binding (mChemR23/Baf-3, buffer IC50) | 5.0 |
| hChemerin Radioligand Binding (mChemR23/Baf-3, plasma IC50) | 39 |
| mC9 Ca2+ Mobilization (mChemR23/Baf-3, buffer A2) | 3.0 |
| mC9 Chemotaxis (mChemR23/Baf-3, buffer A2) | 2.0 |
| Rat ChemR23 | |
| hChemerin Radioligand Binding (rChemR23/Baf-3, buffer IC50) | 2 |
| hChemerin Radioligand Binding (rChemR23/Baf-3, plasma IC50) | 22 |
| mC9 Ca2+ Mobilization (rChemR23/Baf-3, buffer A2) | 0.6 |
| mC9 Chemotaxis (rChemR23/Baf-3, buffer A2) | 1.4 |
IC50= concentration of drug necessary to reduce measure by 50% =, A2 values were calculated from the following equation: pA2 = p[drug(M)] – p[(A’/A)-1] where A reflects the potency of the agonist in the absence of antagonist and A’ reflects the potency of the agonist in the presence of antagonist at a given concentration of drug ([drug(M)]).
Figure 3. Amplification and ChemR23-dependence of Chemerin-9 Contraction.
a, left: Sample tracing of the ability of chemerin-9 to cause concentration-, ChemR23-dependent contraction in the aorta with endothelium removed (−E). Right, Quantification of the ability of CCX832 to inhibit chemerin-9 induced contraction. b, left: representative tracing of CCX832 reversal of chemerin-9-induced contraction. Right demonstrates quantification of the effects of equivalent and increasing concentrations of CCX826 (inactive analog, DMSO) and CCX832 (ChemR23 antagonist, DMSO) in reversing chemerin-9-induced contraction. Bars/points represent means±SEM for number of animals in parentheses. * significantly different from CCX826 (p<0.05). c, Ability of ChemR23 receptor antagonist CCX832 to inhibit PE (left), PGF2α (middle) but not KCl (right) -induced contraction in tissues + PVAT, * = significantly different from appropriate (+ or −PVAT) vehicle-incubated responses.
Upregulated chemerin axis in hypertension
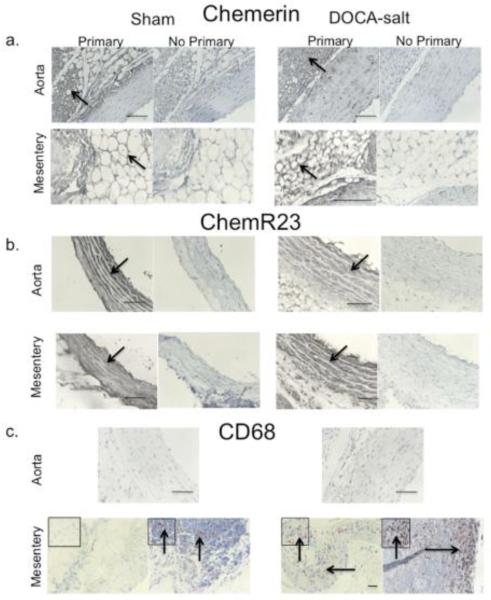
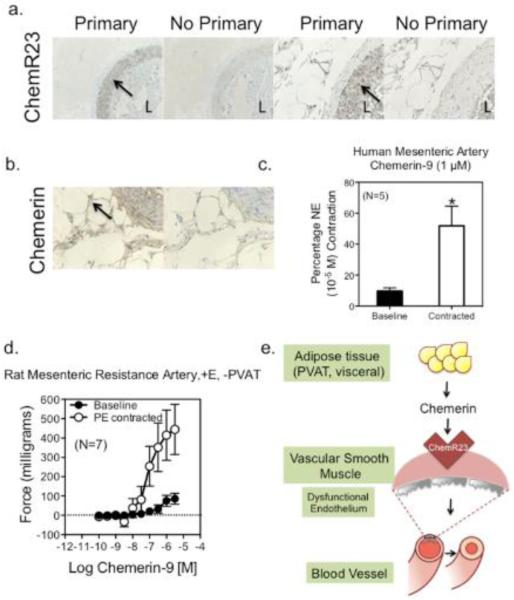
Chemerin protein was detected in PVAT around the aorta and superior mesenteric artery of both the sham normotensive (left) and DOCA-salt hypertensive rat. Chemerin was detected in the cytoplasm of the fat cell (figure 4a). ChemR23 expression was evident in the media of the aorta and superior mesenteric artery of the sham and DOCA-salt rat (figure 4b). Western analysis supported no significant hypertension-related difference in ChemR23 expression in aorta (sham = 48.5±5.7, DOCA-salt 41.9±10.0 densitometry units/α actin expression, p>0.05) or superior mesenteric artery (sham = 21.0±6.0, DOCA-salt = 32.2±5.2, p>0.05; figure SV). In the mesentery, CD68 positive cells were visible in the media, adventitia and strongly observed in mesenteric lymph nodes, especially of the DOCA-salt tissue (figure 4c, bottom row). By contrast, CD68 staining was not observed in the thoracic aorta, and lymph nodes were not observed in the fat around the thoracic aorta. Baseline chemerin-9-induced contraction was significantly increased in the aorta and superior mesenteric artery of the DOCA-salt hypertensive rat (figure 5a, b). The relationship between chemerin-9-induced maximum contraction in rat aorta and superior mesenteric artery from baseline and endothelial function [acetylcholine-induced relaxation (1 μM) in tissues half-maximally contracted to PE] was inverse. Results from thirty-two different samples from normal Sprague Dawley, Sprague Dawley + LNNA or endothelium removed, diet-induced obese, sham normotensive, DOCA-salt hypertensive, and SHRSP rats are included in figure 5c. Tissues that relaxed to ACh greater than 20% had a modest contraction to chemerin-9 from baseline. In tissues with less than a 20% relaxation (dashed line), chemerin-9-induced contraction from baseline was significantly increased. The relevance of the chemerin axis to human arterial function is supported by the expression of ChemR23 in the media (figure 6a, higher magnification right), chemerin protein expression in PVAT and adipose cell cytoplasm (figure 6b) and the ability of chemerin-9 to stimulate both baseline and agonist amplified contraction in the isolated human small mesenteric artery (figure 6c). Importantly, resistance arteries from the same area of the rat (mesentery) demonstrate baseline constriction and agonist potentiated contraction to chemerin-9 (figure 6d).
Figure 4. The Chemerin Axis in DOCA-salt Hypertension.
Immunohistochemical staining of chemerin (a), ChemR23 (b) and CD68 (c) in thoracic aorta and superior mesenteric artery from the sham normotensive (left) and DOCA-salt hypertensive rat (right). Representative of 4-5 different animals for each group. Arrows are regions of interest with boxed insets magnified. Bars = 100 microns.
Figure 5. Amplification of Chemerin-9 induced Contraction in DOCA-salt hypertension.
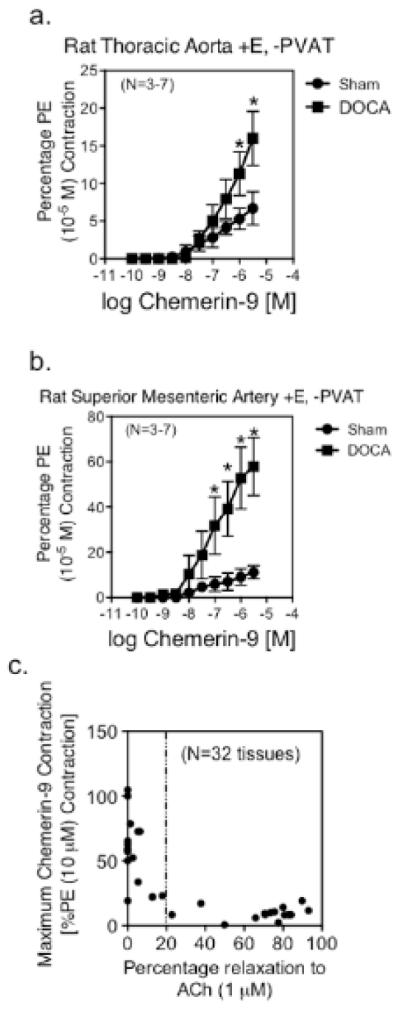
Baseline contraction of the thoracic aorta (a) and superior mesenteric artery (b) from sham normotensive and DOCA-salt hypertensive (+E, −PVAT). Points represent means±SEM for number of animals in parentheses, * Significant differences from sham response (p<0.05). c, relationship of state of endothelial cell integrity (relaxation of acetylcholine, x-axis) to magnitude of contraction to chemerin-9 (1 μM). Thirty-two different samples (aorta, superior mesenteric artery) from rodents (sham, DOCA-salt, SHRSP, WKY, diet-induced obese and control).
Figure 6. The Chemerin Axis in Human Arteries.
Immunohistochemical localization of ChemR23 (a, higher magnification right) and chemerin (b) in small mesenteric arteries isolated from intestinal bariatric specimens (obese humans). Representative of seven (7) different humans. c, Chemerin-9 induced contraction from baseline and in norepinephrine contracted arteries. Bars are means±SEM for number of patients in parentheses. * indicates statistically significant increase from baseline (p<0.05, unpaired Students t test). d, Chemerin-9 induced contraction from baseline and in PE contracted resistance arteries from the mesenteric arcade of the normal male Sprague-Dawley rat. e, Depiction of hypothesis. Chemerin, released from fat (PVAT, visceral fat) or tissue (liver), stimulates the ChemR23 receptor on vascular smooth muscle to elicit contraction, a process that is revealed in the presence of damaged endothelial function (gray endothelial cell).
Discussion
In the last two years, reports from human studies support a positive association of circulating chemerin concentration with both systolic and diastolic blood pressure [13, 15, 23-30]. Patient populations in these studies include those with obstructive sleep apnea, preeclampsia, non-alcoholic fatty liver disease, obesity, metabolic syndrome, type 2 diabetes, and type 2 diabetes with hypertension. In healthy humans, plasma chemerin is estimated to be from 4-40 nM [13, 15]. Plasma is, however, just one of the sources of chemerin to be considered in vascular function. Because chemerin is produced within PVAT, it is likely the concentration of chemerin experienced by the vasculature is significantly higher than that which is circulating. Importantly, the concentrations we use experimentally are in this physiological range. The effects of chhemerin are most probably balanced or synergized by a host of other PVAT substances, such as adiponectin, that promote arterial relaxation [31]. A recent report suggests that the chemerin/adiponectin ratio can be used as a predictor of metabolic syndrome [32]. We are beginning to understand the potential impact of chemerin on human health with reports such as that by Min et al [33]. This study is a multi-institution, world-wide study of gene loci associated with metabolic syndrome, a syndrome defined by a group of risk factors that include a large waistline, high plasma triglycerides, low HDL cholesterol, hypertension and high fasting blood glucose (http://www.nhlbi.nih.gov/health/health-topics/topics/ms/). The gene for chemerin, RARRES2, was one of two that was elevated to a positive association with metabolic syndrome. This syndrome presents as a constellation of events that are seemingly unrelated in terms of cause. However, our finding that chemerin, a product of adipose tissue (both visceral and PVAT), stimulates receptor-dependent contraction links at least two of the risk factors in metabolic syndrome (large waistline and hypertension).
Chemerin
Endogenous sources of chemerin include white fat, the liver, the platelet and PVAT. Chemerin is initially produced as a 163 amino acid precursor (pro-chemerin, TIG2) and processing of prochemerin by proteases is considered the key regulatory mechanism affecting chemerin concentration. Proteases that activate prochemerin include cathepsin G, elastase, tryptase, plasmin and carboxypeptidase N and B [10, 34-40]. Similarly, inactivation of active chemerin peptides is carried out by proteases that include neutrophil proteinase, mast cell chymase and angiotensin converting enzyme [41]. The present work describes PVAT as a source of chemerin. The presence, function and balance of these proteases in PVAT are key for determining the production of chemerin peptides that influence vascular function. This includes both the final concentration of peptides and the specific chemerin protein produced. The first N-terminal 20 amino acids are cleaved by an unknown protease to form prochemerin, chemerin 20-163. Proteolytic processing of the C terminus results in the multitude of chemerin peptides that have varied activity in promoting chemotaxis (the only endpoint consistently measured for chemerin; last number is the amino acid residue): chemerin-158 (low activity), chemerin-157 (high activity), chemerin-156 (high activity), chemerin-155 (inactive), chemerin-154 (inactive), chemerin-152 (unknown) [8, 34]. The antibody used in the immunohistochemical experiments shared in this paper recognizes all these chemerin peptides, as the antibody is directed towards amino acids 29-67. Thus, we do not yet know the chemerin isoforms produced in different vascular beds, nor how this might change in disease. However, the inflammogen TNF-α stimulates chemerin production from adipocytes, thereby linking chemerin to inflammation [42]. In cultured endothelial cells, chemerin increases generation of mitochondrial reactive oxygen species, thereby perpetuating a cycle of events that potentially underly disease [43]. These findings place the protein chemerin as a link between obesity and inflammation.
New Role for ChemR23
ChemR23, as supported by data in this manuscript, has to be ascribed the new function of modifying vascular tone. Pieces of the potential chemerin axis in the vasculature have been discovered. ChemR23 expression was observed in cultured endothelial and cultured human venous smooth muscle cells [44, 45], and an atypical chemerin receptor CCRL2 was found in cultured human and mouse vascular endothelial cells [46]. Chemerin has been detected in epicardial and aortic adipose tissue [47]. Chemerin incubation of isolated vessels for 24 hours increased arterial sensitivity to endothelin -1 [48]. However, never before have all the elements of the chemerin axis been identified in non-cultured vascular smooth muscle as they are in the present report. Importantly, the finding that ChemR23 serves the function of contractility attributes a new function to this receptor. Loss of endothelial function, as occurs in multiple cardiovascular diseases, potentiates the ability of chemerin to increase arterial tone. This suggests that chemerin interacts at both the level of the endothelial cell and smooth muscle cell, supported by ChemR23 expression in both locations. We have not pursued the mechanism of chemerin-induced relaxation, but this will be important given the equivocal effects of chemerin-9 infusion. One reason for the lack of blood pressure elevation is that chemerin-9, at the dose given, may still exert an effect on the endothelium that would support vasorelaxation. Involvement of chemerin in the cardiovascular system becomes increasingly important with discoveries that chemerin stimulates angiogenesis [49], might promote atherosclerosis [50, 51] and diabetic nephropathy [52].
There are two different components of chemerin contractile function in the vasculature. First, chemerin directly causes contraction in arteries without PVAT. It does this from baseline, but contraction is magnified when tone is applied to the tissue before hand. Amplification of chemerin-induced contraction occurred whether an activator of a G protein coupled receptor (adrenergic, prostaglandin) or non-G protein coupled receptor was used to establish tone. This suggests that a mechanism that underlies elevation of vascular tone, such as influx of extracellular calcium, is key to potentiating chemerin-induced contraction. The ability of nifedipine to reduce agonist (KCl)-induced contraction and potentiation of chemerin-9-induced contraction supports the necessity of having tone to amplify chemerin-induced contraction. This is a post-receptor mechanism of interaction of chemerin and the other agonists. Because all three agonists (PE, PGF and KCl) depend on activation of the L-type calcium channel, we suggest that their similar ability to potentiate chemerin-induced contraction is likely due to elevation of intracellular calcium.
Second, chemerin may participate in arterial contraction (+PVAT) initiated by other agonists. This is distinct from agonist-induced potentiation of chemerin-induced contraction and is a pre-receptor mechanism of interaction. In this instance, we are not adding chemerin to this experiment exogenously, but rather are causing its release as interpreted by the ability of the ChemR23 antagonist CCX832 to reduce agonist-induced contraction. PE and PGF2alpha-induced contraction was reduced by CCX832, while that of KCl was not. Our data suggest that PE and PGF2alpha initiate a series of events in PVAT that promotes chemerin release, and this does not occur with KCl. In the future we hope to understand the physiological stimuli that can release chemerin from PVAT. These initial experiments were important in that they suggest stimuli normally experienced by the vasculature can release and use chemerin in their vasoconstriction.
Two other receptors for chemerin exist. Chemokine receptor like 2 (CCLR2) is not thought to mediate biological signals of chemerin but to chaperone the chemerin molecule to ChemR23 for biological effect [8,9]. G protein-receptor 1 (GPR1) binds chemerin but its role in mediating biological signals of chemerin is unknown. CCX832 has negligible affinity for both of these receptors, underscoring the importance of ChemR23 as a receptor for chemerin. CCX832 was effective in reducing chemerin-9 induced contraction in all tissues examined. More recently, other agonists of ChemR23 have been identified. The Resolvin E1 (RvE1) family members have been suggested to bind to ChemR23 in transfected CHO cells [53, 54]. The presence of a chemerin axis in the artery opens the door for chemerin and potentially resolvin to have functions within the blood vessel itself. The clinical relevance of this chemerin axis is supported by the qualitatively similar findings in the rat and human arteries. These findings contrast with those observed in the mouse thoracic aorta. The thoracic aorta of the wild type mouse did not contract to chemerin-9, but ChemR23 was present. One explanation for this outcome is that human chemerin-9 cannot activate the mouse receptor. Human and mouse chemerin-9 differ in the first two N terminal amino acids [55], and this difference could play a role in reactivity. Another explanation revolves around a dichotomy between mouse and rat contractility that has been observed before in this very tissue. Unlike the rat thoracic aorta, the mouse thoracic aorta does not contract to angiotensin II and endothelin-1 [56]; other groups have observed this in response to endothelin-1 and urotensin [57-59]. The inability of the mouse thoracic aorta ChemR23, similar to the endothelin receptors, to couple to contraction is one interpretation of these findings. Finally, it will be interesting to determine if other arteries of the mouse are similarly unresponsive.
Looking forward
It will be critical to understand the role, in vivo, of chemerin and ChemR23 in the regulation of blood pressure. Initial experiments infusing chemerin-9 suggested that in vivo functions of chemerin will not be straightforward. Chemerin-9 infusion did not elevate blood pressure. However, chemerin peptides have not been designed for use in vivo, and appear to be largely ineffective given their rapid metabolism [59]. We are unable to prevent this metabolism because we do not know those proteases responsible for chemerin-9 degradation. This will necessitate design of available peptides that are resistant to metabolism [55], and investigation of the in vivo properties of CCX832. This finding may also illustrate the dual role played by chemerin. Though not investigated presently, low concentrations of chemerin-9 caused a modest relaxation in the isolated rat thoracic aorta, rat superior mesenteric artery and rat mesenteric resistance artery. Thus, two events may be occurring simultaneously such that blood pressure is ultimately not modified. We also cannot discount the possibility that chemerin-9 exerts central nervous system effects to modify blood pressure as ChemR23 has been identified in the brain [12]. Finally, it is unclear how infusion of chemerin-9 adds to already circulating levels of chemerin, and whether this dose was sufficient to initiate a physiological change. Circulating levels of chemerin in the rat models used are not known. All of these important issues deserve formal attention.
Conclusions
The present work is timely in that investigating PVAT function is a current focus in understanding arterial function. The intimate apposition of PVAT to the arterial wall makes PVAT perfectly poised to affect the function of the artery and link adiposity to hypertension and other diseases (figure 6e). PVAT is not the only source of chemerin, but PVAT should be recognized as immediately important to arterial function. Chemerin peptides become new members of the vasoactive PVAT factors, adipokines that may serve as a connector between obesity and a change in arterial tone.
Methods and Materials
Materials
Unless stated otherwise, chemicals and salts are from Sigma Chemical Co. (St. Louis, MO, USA). Human chemerin-9 was purchased from GenScript (cat # RP20248; Piscataway, NJ, USA), and chemerin-15 synthesized to specification (AGEDPHGYFLPGQFA) by Biosynthesis (Lewisville, TX USA). CCX832 and CCX826 were discovered and synthesized by ChemoCentryx (Mountain View, CA, USA).
Animal use
Procedures were performed in accordance with the institutional guidelines and animal use committee of Michigan State University. All guidelines are in accordance with the “The Guide for the Care and Use of Laboratory Animals”(8th Edition, Revised 2011).
Normal male Sprague-Dawley rats (8-10 weeks of age, 250–300 g; Charles River Laboratories, Inc., Portage, MI, USA) were used unless otherwise stated.
Genetic hypertension
The stroke prone spontaneously hypertensive rats (SHRSP, bred at Michigan State University, ~12 weeks of age) were used and normotensive Wistar Kyoto rats (WKY) purchased from Charles River Laboratories (Portage, MI, USA). Systolic blood pressures of SHRSP were 201±3 mm Hg, WKY were 110±5 mm Hg (tail cuff, conscious measurement).
Deoxycorticosterone Acetate (DOCA) Salt hypertension
Male Sprague Dawley rats underwent left uninephrectomy (isoflurane anesthesia) and a DOCA pellet was implanted subcutaneously (200 mg/kg). Shams underwent uninephrectomy only. Rats were given standard rat chow ad libitum. Animals receiving DOCA also received water supplemented with 1% NaCl and 0.2% KCl. After four weeks, the systolic blood pressure of sham rats was 128±1 mm Hg and that of the DOCA-salt rat 211±2 mm Hg (tail cuff, conscious measurement).
Diet-Induced Obesity
Male Sprague-Dawley rats were fed a high fat-diet (36% fat; 15.2% saturated, 20.8% unsaturated, 0.4% sodium, and 0.6% potassium; cat # F3283, Bioserve, Frenchtown, NJ) from 3 - 20 weeks of age. Control rats received normal chow (4.4% fat; 2.5% saturated, 1.9% unsaturated, 0.39% sodium, and 1.0% potassium; Harlan). At 20 weeks, high fat fed rats were heavier (511±21 grams) than controls (447±9; p<0.05) and possessed a greater amount of visceral fat (total control = 9.17±0.85 grams; high-fat = 24.2±2.84 grams) that elevated the percentage of body fat from 2.04 to 4.68 percent.
ChemR23 WT and KO mice
Thoracic aortae were dissected from WT and KO male mice (2-3 months old) and shipped overnight from Canada in cold physiological salt solution for isometric contractile experiments and ChemR23 immunohistochemistry.
Prior to all dissection, rats were anesthetized with Fatal Plus® (60 mg/kg, i.p.) and tissues dissected.
RT-PCR
RNA was extracted from PVAT (rat thoracic aorta) using a RNeasy Lipid Tissue kit (Qiagen Valencia, CA). One μg of RNA was reverse transcribed using SuperScriptVILO (cat #11754; Invitrogen, Grand Island, NY). Real-time PCR was performed using TaqMan primers (Applied Biosystems, Foster City CA) specific for Chemerin (Rarres2; Rn01451853_m1) and β2microglobin (Rn00560865_m1). Results were expressed as the mean ± SEM of the CT value obtained.
Histochemistry
Tissues were formalin-fixed or fresh frozen. Sections (8 micron) were taken through immunohistochemistry using a species-specific Vector kit (Burlingame CA, USA; rabbit: cat # PK-4001; rat = cat # PK-4004). Sections were incubated 24 hours with chemerin (rabbit TIG-2; 1:200, cat #H-002-52, Phoenix Pharmaceuticals Inc, Belmont CA USA), ChemR23 (rabbit 1:50-1:100, cat # APO6779PU-N, Acris Antibodies, Inc, San Diego CA USA), CD68 (rat, 1:50, cat #MAB1435, Millipore, Temecula, CA USA) or no primary antibody at 4 °C. Sections were developed using a DAB (3, 3-diaminobenzidine) developing solution (cat # SK-4100; Vector Laboratories, Burlingame CA, USA). Slides were counterstained with Vector Hematoxylin (cat # H-3401, 30 seconds). Sections were photographed on a Nikon TE2000 inverted microscope using MMI® Cellcut Software.
Western analysis
Standard protein isolation, western blotting and transfer procedures using Immobilon-FL (IPFL10100, Millipore, Temecula, CA USA) were performed on cleaned arteries, loading equivalent amounts of total protein per lane [1]. Primary antibody (ChemR23, 1:1000, cat #APO6779PU-N, Acris Antibodies, San Diego CA USA) was incubated with blots overnight at 4 °C. Blots were rinsed thrice in TBS + Tween (0.1%) with a final rinse in TBS and incubated with IRDye ® 800 goat anti-rabbit IgG (1:1000, cat #926-32211, LI-COR Biosciences, Lincoln, NE, USA) for 1 hour at 4 °C with rocking. Blots were visualized using an Odyssey® Infrared Imaging System LICOR CE (LI-COR Biosciences, Lincoln, NE, USA) and reprobed for α-actin (cat #A14, EMD Chemicals, Gibbstown NJ, USA).
CCX832 Pharmacology
CCX832 was developed as a ChemR23 receptor antagonist. Methods for determining the affinity of CCX832 for human, mouse and rat ChemR23 and ability to inhibit activation of the same receptors are reported in supplemental methods. Supplemental Table 1 contains information regarding the ability of CCX832 to bind to and/or inhibit related chemokine receptors.
Isometric Contraction and Pharmacology: large and small arteries
Arteries (endothelium intact or removed; thoracic aorta of mouse and rat; superior mesenteric or mesenteric resistance of rat) cleaned of fat (−PVAT) or with fat intact (+PVAT) were mounted in tissue baths or a wire myograph for isometric tension recordings using Grass FT03 transducers and PowerLab data Acquisitions (ADInstruments, Colorado Springs, CO, USA). Baths contained physiological salt solution (PSS) [NaCl 130; KCl 4.7; KH2PO4 1.8; MgSO4 * 7H2O 1.7; NaHCO3 14.8; dextrose 5.5; CaNa2EDTA 0.03, CaCl2 1.6 (pH 7.2), 95% O2/CO2]. Rings were placed under optimum resting tension [4 grams for rat thoracic aorta, 500 mg for mouse thoracic aorta, 1.2 grams for rat superior mesenteric artery, 400 mg for rat mesenteric resistance artery] and equilibrated for one hour. An initial concentration of 10 μM phenylephrine (PE) tested arterial viability and the status of the endothelial cell layer tested by acetylcholine (1 μM)-induced relaxation of a half-maximal PE-induced contraction. Tissues were washed and one of the following experiments performed.
From baseline
chemerin peptides were added cumulatively in a concentration-dependent fashion.
From contraction
tissues were half-maximally contracted with agonist (PE, KCl, PGF2alpha). When agonist-induced contraction plateaued, either vehicle (water) or chemerin peptides were added in a cumulative fashion.
Antagonist
Tissues were incubated for one hour with vehicle (0.05% DMSO or water) or drug (LNNA, CCX832, CCX826) prior to cumulative addition of chemerin peptide or other agonist. Alternatively, tissues were contracted with chemerin-9 (1 μM) and either CCX826 or CCX832 added in parallel tissue baths. CCX826, solubilized in DMSO, provides both a negative control (inactive congener) and vehicle control for CCX832.
Human samples
Small arteries (~500 micron diameter) from the intestinal/fat border were removed from small intestinal samples from de-identified obese humans during bariatric surgery (MSU IRB BIRB approved, with formal consent granted), and placed in a wire myograph (Tungsten .0008 wire, California Fine Wire, Grover Beach, CA USA). Arteries equilibrated for 30 minutes, passive tension placed (~800 milligrams), and equilibrated another 30 minutes. Tissues were challenged with a maximal concentration of NE, washed, and challenged again with vehicle or a half-maximal concentration of NE (~3 μM) prior to addition of chemerin-9 (1 μM).
In vivo Chemerin-9 administration
Isoflurane-anesthetized normal male Sprague Dawley rats, treated with LNNA (0.5 gram) for one week prior to experimentation, were implanted with a femoral arterial and venous line for measurement of blood pressure and chemerin-9 infusion, respectively. Chemerin-9 was infused through a syringe pump at a rate of 200 μL/minutes for 5 minutes and recordings of mean arterial blood pressure monitored before and after infusion.
Data Analysis
Data are reported as means±SEM for number of animals indicated in parentheses (N). Immunohistochemical results are shown in sections incubated with and without primary antibody, and are representative of a minimum of four (4) separate animals. Adjustments in brightness and contrast were made to the whole panel of a photograph, not a portion. Westerns were quantified using Image J (NIH) and normalized for actin expression. Contraction is reported as means±SEM as force (milligrams) or as a percentage of the initial contraction to phenylephrine (PE) or norepinephrine (NE). Relaxation is reported as a percentage of the initial contraction to chemerin-9 (1 μM) or half-maximal contraction to agonist. The contraction caused by the contractant (PE, KCl, PGF2alpha) was subtracted out to result in the data presented. Potency values (−log EC50, M) were calculated as concentrations necessary to cause a half-maximal effect. Where a maximum was not achieved, the values are estimated and true potencies equal or greater than that reported. Either an unpaired Student’s t test or repeated measures ANOVA performed after confirming the normality of data distribution. Normality of data variances was tested using the F test (StatPlus/Mac 2009). Where variances were not equivalent, a Mann-Whitney U test was conducted as a nonparametric measure of two independent means where appropriate. p< 0.05 was considered statistically significant.
Supplementary Material
Figure SI. Extracellular Calcium and Agonist-driven tone is Necessary for Potentiation of Chemerin-9-induced contraction. Nifedipine reduces KCl-induced half-maximal contraction and reduces the potentiation of chemerin-9-induced contraction in isolated rat thoracic aorta +E without PVAT. Points represent means±SEM for number of animals in parentheses. * significant differences compared to responses of vehicle-incubated tissues.
Figure SII. Chemerin-9-induced Arterial Contraction in Disease Models. a, Concentration-dependent response to chemerin from baseline and above PE-induced contraction in the endothelium intact (+E) isolated thoracic aorta from control and obese rats. b, Concentration-dependent response to chemerin from baseline and above PE-induced contraction in the endothelium intact (+E) isolated thoracic aorta from normotensive WKY and hypertensive SHRSP rats. Points represent means±SEM for number of animals in parentheses.
Figure SIII. Chemerin-9 vs Chemerin-15 Contraction. Comparison of chemerin-9 vs chemerin-15 induced contraction in normal male Sprague-Dawely rats from baseline (a) and above a PE-induced contraction (b). Points represent means±SEM for number of animals in parentheses.
Figure SIV. Lack of Chemerin-9 Induced Contraction in Mouse Thoracic Aorta. a, Lack of ability of chemerin-9 to cause baseline contraction in the endothelium intact, cleaned thoracic aorta +/− LNNA (100 μM) from the wild type or chemerin R23 receptor mouse knockout. Points represent means±SEM for number of animals in parentheses. Parenthetic values in key report the magnitude of contraction to PE (10 μM), validating these tissues were living. b, Immunohistochemical localization of ChemR23 in the wild type receptor knockout thoracic aorta. Arrows point to medial staining. Representative of three individual mice.
Figure SV. ChemR23 expression in Sham and DOCA-salt arteries. Full sized blot for expression of ChemR23 in SMA (superior mesenteric artery) and RA (rat aorta) from sham and DOCA-salt rats. Each lane represents an individual animal (N=4 total). Arrows point to those bands recognized in the positive control of the JAR cell lysate (figure 1) and which were used in quantitative analysis/densitometry. Stripped blot was reprobed for smooth muscle α-actin expression and densitometry values were normalized to this measure.
Supplemental Table I. Activity of CCX832 against a panel of chemokine receptors.
Acknowledgements
a) Acknowledgements :none
b) Source of Funding: The work in SWW and GDF laboratories was supported by NIH P01HL70687.
Footnotes
Watts: Chemerin as a vasoconstrictor
Disclosure: The authors have no conflicts of interest to disclose.
Author Contributions S.W.W. conceived the study, performed all contractile experiments, ChemR23 immunohistochemical experiments and provided financial support. A.M.D. provided the diet-induced obese and stroke prone spontaneously hypertensive rodents and performed real time RT-PCR for chemerin. M.E.P. provided CCX832 and CCX826 and consultation. B. S. performed the chemerin-9 infusions. J.R. and C. S. provided the ChemR23 WT and KO mice. T.J.S. and T.T.C. discovered and developed CCX832 and CCX826. J.M.T performed the Western analyses. R.B. performed chemerin immunohistochemistry experiments. G.D.F. and S. W. W. provided financial support and consultation. S.W.W. wrote the paper with comments from all authors and all authors read the final version of this manuscript.
Significance We discovered that the protein chemerin, typically found in visceral fat, is produced in the fat that is outside of arteries. ChemR23, a receptor for chemerin, is expressed in the artery such that chemerin can change the function of the artery. In conditions where the endothelium is not functional, chemerin causes a contraction. This work highlights that the chemerin axis, largely known for its function in immune cells, is important to the arterial circulation.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens. 1991;13:277–296. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 2.Chaldakov GN, Beltowsky J, Ghenev PI, Fiore M, Panayotov P, Rancic G, Aloe L. Adipoparacrinology: vascular periadventitial adipose tissue (tunica adipose) as an example. Cell Biology Int. 2011;36:327–330. doi: 10.1042/CBI20110422. [DOI] [PubMed] [Google Scholar]
- 3.Brandes RP. The fatter the better: Perivascular adipose tissue attenuates vascular contraction through different mechanisms. Br J Pharmacol. 2007;151:303–304. doi: 10.1038/sj.bjp.0707229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gollasch M, Dubrovska G. Paracrine role for periadventitial adipose tissue in the regulation of arterial tone. Trends Pharmacol Sci. 2004;25:647–653. doi: 10.1016/j.tips.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Achike FL, To NH, Wang H, Kwan CY. Obesity, metabolic syndrome, adipocytes and vascular function: a holistic viewpoint. Clin Exp Pharmacol Physiol. 2011;38:1–10. doi: 10.1111/j.1440-1681.2010.05460.x. [DOI] [PubMed] [Google Scholar]
- 6.Vykoukal D, Davis ME. Vascular biology of metabolic syndrome. J Vasc Surg. 2011;54:819–831. doi: 10.1016/j.jvs.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Med Inflam. 2010 doi: 10.1155/2010/535918. article ID 535918 doi:10.1155/2010/535918 (online only) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrin Metab. 2010;21:660–667. doi: 10.1016/j.tem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Iannone F, Lapadula G. Chemerin/ChemR23 pathway: a system beyond chemokines. Arthritis Res Ther. 2011;13:104. doi: 10.1186/ar3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshimura T, Oppenheim JJ. Chemerin reveals its chimeric nature. J Exp Med. 2008;205:2187–2190. doi: 10.1084/jem.20081736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wittamer V, Franssen J-D, Vulcano M, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song S-H, Fukui K, Nakajima K, Kozakai T, Sasaki S, Roh S-G, Katoh K. Cloning, expression analysis, and regulatory mechanisms of bovine chemerin and chemerin receptor. Dom Animal Endocrinol. 2010;39:97–105. doi: 10.1016/j.domaniend.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinol. 2007;148:4687–4694. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 14.Jialal I, Devaraj S, Kaur H, Adams-Huet B, Bremer AA. Increased chemerin and decreased omentin-1 in both adipose tissue and plasma in nascent metabolic syndrome. J. Clin. Endocrinol. Metab. 2013;98:E514–E517. doi: 10.1210/jc.2012-3673. [DOI] [PubMed] [Google Scholar]
- 15.Sell H, Divoux A, Poitou C, Basdevant A, Bouillot J-L, Bedossa P, Tordjman J, Eckel J, Clement K. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J Clin Endocrinol Metab. 2010;95:2892–2896. doi: 10.1210/jc.2009-2374. [DOI] [PubMed] [Google Scholar]
- 16.Ernst MC, Haidl IA, Zuniga LA, Dranse HJ, Rourke JL, Zabel BA, Butcher EG, Sinal CJ. Disruption of the chemokine-like receptor-1 (CMKLR1) gene is associated with reduced adiposity and glucose intolerance. Endocrinol. 2012;153:672–682. doi: 10.1210/en.2011-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart R, Greaves DR. Chemerin contributes to inflammation by promoting macrophage adhesion to VCAM-1 and fibronectin through clustering of VLA-4 and VLA-5. J Immunol. 2010;185:3728–3739. doi: 10.4049/jimmunol.0902154. [DOI] [PubMed] [Google Scholar]
- 18.Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 19.Muruganandan S. Chemerin, a novel peroxisome proliferator-activated receptor γ (PPARγ) target gene that promotes mesenchymal stem cell adipogenesis. J Biol Chem. 2011;286:23982–23995. doi: 10.1074/jbc.M111.220491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roh SG, Song SH, Choi KC, Katoh K, Wittamer V, Parmentier M, Sasaki SI. Chemerin-a new adipokine that modulates adipogenesis via its own receptor. Biochem Biophys Res Comm. 2007;362:1013–1018. doi: 10.1016/j.bbrc.2007.08.104. [DOI] [PubMed] [Google Scholar]
- 21.Wittamer V, Gregoire F, Robberecht P, Vassart G, Communi D, Parmentier M. The C-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J Biol Chem. 2004;279:9956–9962. doi: 10.1074/jbc.M313016200. [DOI] [PubMed] [Google Scholar]
- 22.Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, Hendrick AG, Carlton MB, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med. 2008;205:767–775. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong B, Ji W, Zhang Y. Elevated serum chemerin levels are associated with the presence of coronary artery disease in patients with metabolic syndrome. Intern Med. 2011;50:1093–1097. doi: 10.2169/internalmedicine.50.5025. [DOI] [PubMed] [Google Scholar]
- 24.Feng X, Li P, Zhour C, Jia X, Kang J. Elevated levels of serum chemerin in patients with obstructive sleep apnea syndrome. Biomarkers. 2012;17:248–253. doi: 10.3109/1354750X.2012.658864. [DOI] [PubMed] [Google Scholar]
- 25.Hu W, Feng P. Elevated serum chemerin concentrations are associated with renal dysfunction in type 2 diabetic patients. Diabetes Res Clin Pract. 2011;91:159–163. doi: 10.1016/j.diabres.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Kukla M, Zwirska-Korczala K, Hartleb M, Waluga M, Chwist A, Kajor M, Ciupinska-Kajor M, Berdowska A, Wozniak-Grygiel E, Buldak R. Serum chemerin and vaspin in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2010;45:235–242. doi: 10.3109/00365520903443852. [DOI] [PubMed] [Google Scholar]
- 27.Shin HY, Lee DC, Chu SH, Jeon JY, Lee MK, Im JA, Lee JW. Chemerin levels are positively correlated with abdominal visceral fat accumulation. Clin Endocrinol. 2012;77:47–50. doi: 10.1111/j.1365-2265.2011.04217.x. [DOI] [PubMed] [Google Scholar]
- 28.Stejskal D, Karpisek M, Hanulova Z, Svestak M. Chemerin is an independent marker of the metabolic syndrome in a Caucasian population – a pilot study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152:217–221. doi: 10.5507/bp.2008.033. [DOI] [PubMed] [Google Scholar]
- 29.Stepan H, Philipp A, Roth I, Kralisch S, Jank A, Schaarschmidt W, Lossner U, Kratzsch J, Bluher M, Stumvoll M, Fasshauer M. Serum levels of the adipokine chemerin are increased in preeclampsia during the 6 months after pregnancy. Regul Pept. 2011;168:69–72. doi: 10.1016/j.regpep.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Yang M, Yang G, Dong J, Liu Y, Zong H, Liu H, Boden G, Li L. Elevated plasma levels of chemerin in newly diagnosed type 2 diabetes mellitus with hypertension. J Investig Med. 2010;58:883–886. doi: 10.231/JIM.0b013e3181ec5db2. [DOI] [PubMed] [Google Scholar]
- 31.Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, Gollasch M. Adiponectin is a novel humoral vasodilator. Cardiovasc Res. 2007;75:719–727. doi: 10.1016/j.cardiores.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Chu SH, Lee WK, Ahn KY, Im J-A, Park MS, Lee D-C, Jeon JY, Lee JW. Chemerin and adiponectin contribute reciprocally to metabolic syndrome. PLoS ONE. 2012;7(4):e34710. doi: 10.1371/journal.pone.0034710. Doi:10.1371/journal.pone.0034710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Min JL, Nicholson G, Halgrimsdottir I, et al. Coexpression network analysis in abdominal and gluteal adipose tissue reveals regulatory genetic loci for metabolic syndrome and related phenotypes. PLoS Genet. 2012;(2):e1002505. doi: 10.1371/journal.pgen.1002505. Doi:10.1371/journalpgen.1002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du X-Y, Leung LLK. Proteolytic regulatory mechanism of chemerin bioactivity. Acta Biochim Biophys Sin. 2009;41:973–979. doi: 10.1093/abbs/gmp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schultz S, Beck-Sickinger AG. Chemerin and Vaspin: possible targets to treat obesity? ChemMedChem. 2012 doi: 10.1002/cmdc.201200448. doi: 10.1002/cmdc.201200448. [DOI] [PubMed] [Google Scholar]
- 36.Guillabert A, Wittamer V, Bondue B, Godot V, Imbault V, Parmentier M, Communi D. Role of neutrophil proteinase 3 and mast cell chymase in chemerin proteolytic regulation. J Leukoc Biol. 2008;84:1530–1539. doi: 10.1189/jlb.0508322. [DOI] [PubMed] [Google Scholar]
- 37.Zabel BA, Allen SJ, Kulig P, Allen JA, Cichy J, Handel TM, Butcher EC. Chemerin activation by serine proteases of the coagulation, fibrinolytic and inflammatory cascades. J Biol Chem. 2005;280:34661–34666. doi: 10.1074/jbc.M504868200. [DOI] [PubMed] [Google Scholar]
- 38.Zhao R-J, Wang H. Chemerin/ChemR23 signaling axis is involved in the endothelial protection by K+ ATP channel opener iptakalim. Acta Pharmacological Sinica. 2011;32:573–580. doi: 10.1038/aps.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao L, Yamaguchi Y, Sharif S, Du X-Y, Lee DM, Recht LD, Robinson WH, Song JJ, Morser J, Leung LLK. Chemerin 158K is the dominant chemerin isoform in synovial and cerebrospinal fluids but not in plasma. J Biol Chem. 2011;286:39520–39527. doi: 10.1074/jbc.M111.258954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du X-Y, Zabel BA, Myles T, Allen SJ, Handel TM, Lee PP, Butcher EC, Leung LL. Regulation of chemerin bioactivity by plasma carboxypeptidase N, carboxypeptidase B (activated thrombin-activable fibrinolysis inhibitor), and platelets. J Biol Chem. 2009;284:751–758. doi: 10.1074/jbc.M805000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.John H, Hierer J, Haas O, Forssmann W-G. Quantification of angiotensin converting enzyme mediated degradation of human chemerin 145-154 in plasma by matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Anal Biochem. 2007;362:117–125. doi: 10.1016/j.ab.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Parlee SD, McNeil JO, Muruganandan S, Sinal CJ, Goralski KB. Elastase and tryptase govern TNFα mediated production of active chemerin by adipocytes. PLoS One. 2012;7(12):e51072. doi: 10.1371/journal.pone.0051072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen W, Tian C, Chen H, Yang Y, Zhu D, Gao P, Liu J. Oxidative stress mediates chemerin-induced autophagy in endothelial cells. Free Radic Biol Med. 2013;55C:73–82. doi: 10.1016/j.freeradbiomed.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 44.Kaur J, Adya R, Tan BK, Chen J, Randeva HS. Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Comm. 2009;391:1762–1768. doi: 10.1016/j.bbrc.2009.12.150. [DOI] [PubMed] [Google Scholar]
- 45.Zhao R-J, Pan Z-Y, Long C-L, Cui W-Y, Zhang Y-F, Wang H. Stimulation of non-neuronal muscarinic receptors enhances chemerin/ChemR23 system in dysfunctional endothelial cells. Life Sci. 2012;92:10–16. doi: 10.1016/j.lfs.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 46.Monnier J, Lewen L, O’Hara E, Huang K, Tu H, Butcher EC, Zabel BA. Expression, regulation, and function of atypical chemerin receptor CCRL2 on endothelial cells. J Immunol. 2012;189:956–967. doi: 10.4049/jimmunol.1102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH. Adpokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb. 2010;17:115–130. doi: 10.5551/jat.1735. [DOI] [PubMed] [Google Scholar]
- 48.Lobato NS, Neves KB, Filgueira FP, Fortes ZB, Carvalho MH, Webb RC, Oliveira AM, Tostes RC. The adipokine chemerin augments vascular reactivity to contractile stimuli via activation of the MEK-ERK1/2 pathway. Life Sci. 2012;91:600–606. doi: 10.1016/j.lfs.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Bozaoglu K, Curan JE, Stocker CJ, et al. Chemerin, a novel adipokine in the regulation of angiogenesis. J Clin Endocrinol Metab. 2010;95:2476–2485. doi: 10.1210/jc.2010-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao X, Mi S, Zhang F, Gong F, Lai Y, Gao F, Zhang X, Wang L, Tao H. Association of chemerin mRNA expression in human epicardial tissue with coronary atherosclerosis. Cardiovasc Diabet. 2011 doi: 10.1186/1475-2840-10-87. doi: 10.1186/1475-2840-10-87 (online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lehrke M, Becker A, Greif M, Stark RN, Laubender RP, von Ziegler F, Lebherz C, Tittus J, Reiser M, Becker C, Goke B, Leber AW, Parhofer KG, Broedl UC. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol. 2009;161:339–344. doi: 10.1530/EJE-09-0380. [DOI] [PubMed] [Google Scholar]
- 52.Hu W, Yu Q, Zhang J, Liu D. Rosiglitazone ameliorates diabetic nephropathy by reducing the expression of chemerin and ChemR23 in the kidney of streptozotocin-induced diabetic rats. Inflammation. 2012;35:1287–1293. doi: 10.1007/s10753-012-9440-y. [DOI] [PubMed] [Google Scholar]
- 53.Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AH, Pande R, Creager MA, Serhan CN, Conte MS. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol. 2010;177:2116–2123. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest. 2011;121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimamura K, Matsuda M, Miyamoto Y, Yoshimoto R, Seo T, Tokita S. Identification of a stable chemerin analog with potent activity toward ChemR23. Peptides. 2009;30:1529–1538. doi: 10.1016/j.peptides.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 56.Russell A, Watts SW. Vascular reactivity of isolated thoracic aorta of the C57BL/6J mouse. J Pharmacol Exp Ther. 2000;294:598–604. [PubMed] [Google Scholar]
- 57.Douglas SA, Sulpizio AC, Piercy V, Sarau HM, Ames RS, Aiyar NV, Ohlstein EH, Willette RN. Differential vasoconstrictor activity of human urotensin –II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. Br J Pharmacol. 2000;131:1262–1274. doi: 10.1038/sj.bjp.0703690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Widmer CC, Mundy AL, Kretz M, Barton M. Marked heterogeneity of endothelin-mediated contractility and contraction dynamics in mouse renal and femoral arteries. Exp Biol Med. 2006;231:777–781. [PubMed] [Google Scholar]
- 59.Zhou Y, Dirksen WP, Zweier JL, Periasamy M. Endothelin-1 induced responses in isolated mouse vessels: the expression and function of receptor types. Am J Physiol Heart Circ Physiol. 2004;287:H573–H578. doi: 10.1152/ajpheart.01170.2003. [DOI] [PubMed] [Google Scholar]
- [1].Ni W, Geddes TJ, Priestley JR, Szasz T, Kuhn DM, Watts SW. The existence of a local 5-hydroxytryptaminergic system in peripheral arteries. Br J Pharmacol. 2008;154:663–674. doi: 10.1038/bjp.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure SI. Extracellular Calcium and Agonist-driven tone is Necessary for Potentiation of Chemerin-9-induced contraction. Nifedipine reduces KCl-induced half-maximal contraction and reduces the potentiation of chemerin-9-induced contraction in isolated rat thoracic aorta +E without PVAT. Points represent means±SEM for number of animals in parentheses. * significant differences compared to responses of vehicle-incubated tissues.
Figure SII. Chemerin-9-induced Arterial Contraction in Disease Models. a, Concentration-dependent response to chemerin from baseline and above PE-induced contraction in the endothelium intact (+E) isolated thoracic aorta from control and obese rats. b, Concentration-dependent response to chemerin from baseline and above PE-induced contraction in the endothelium intact (+E) isolated thoracic aorta from normotensive WKY and hypertensive SHRSP rats. Points represent means±SEM for number of animals in parentheses.
Figure SIII. Chemerin-9 vs Chemerin-15 Contraction. Comparison of chemerin-9 vs chemerin-15 induced contraction in normal male Sprague-Dawely rats from baseline (a) and above a PE-induced contraction (b). Points represent means±SEM for number of animals in parentheses.
Figure SIV. Lack of Chemerin-9 Induced Contraction in Mouse Thoracic Aorta. a, Lack of ability of chemerin-9 to cause baseline contraction in the endothelium intact, cleaned thoracic aorta +/− LNNA (100 μM) from the wild type or chemerin R23 receptor mouse knockout. Points represent means±SEM for number of animals in parentheses. Parenthetic values in key report the magnitude of contraction to PE (10 μM), validating these tissues were living. b, Immunohistochemical localization of ChemR23 in the wild type receptor knockout thoracic aorta. Arrows point to medial staining. Representative of three individual mice.
Figure SV. ChemR23 expression in Sham and DOCA-salt arteries. Full sized blot for expression of ChemR23 in SMA (superior mesenteric artery) and RA (rat aorta) from sham and DOCA-salt rats. Each lane represents an individual animal (N=4 total). Arrows point to those bands recognized in the positive control of the JAR cell lysate (figure 1) and which were used in quantitative analysis/densitometry. Stripped blot was reprobed for smooth muscle α-actin expression and densitometry values were normalized to this measure.
Supplemental Table I. Activity of CCX832 against a panel of chemokine receptors.