The term adult-onset Atopic Dermatitis (AD) (onset >18 years) was introduced by Bannister and Freeman[1] from Australia, subsequently few reports and series were reported from other parts of the world.[2,3,4] However, there are no clinico-epidemiological studies from India probably due to lack of awareness or non acceptance of the concept of adult onset AD. The diagnosis of this condition is often not straight forward and several other conditions like contact dermatitis, seborrheic dermatitis, endogenous hand or foot eczema, cutaneous lymphoma, drug rash, psoriasis etc. are considered or excluded before making a diagnosis.
Characteristic features of AD differ according to the period of life. In the infantile phase, erythematous and papulovesicular eruptions with oozing and crusting are often observed on the face and extensors. In the childhood phase, atopic dry skin and lichenified flexural eczema of the extremities become more prominent. When it presents as continuation of atopic dermatitis from childhood, the diagnosis is usually easy and the clinical picture is also typical. Difficulty arises when the onset occurs after the adolescence or later as in these cases the disease morphology and pattern is often atypical, although it may still present with flexural dermatitis. The physical and environmental factors involved in adults differ from those in children and this may be responsible for the different patterns of involvement and atypical morphologies like nummular, prurigo-like, follicular and seborrheic dermatitis-like. In the adolescent and adult phases, atopic red face, chronic lichenified eczema on the trunk, subacute or psoriasiform dermatitis [Figure 1] and hand dermatitis often predominate.[1,2,4,5] Clinical features in the elderly subjects (>65 years old) are same, except that flexural lichenification is uncommon and erythroderma [Figure 2] is commonly seen.[6,7]
Figure 1.
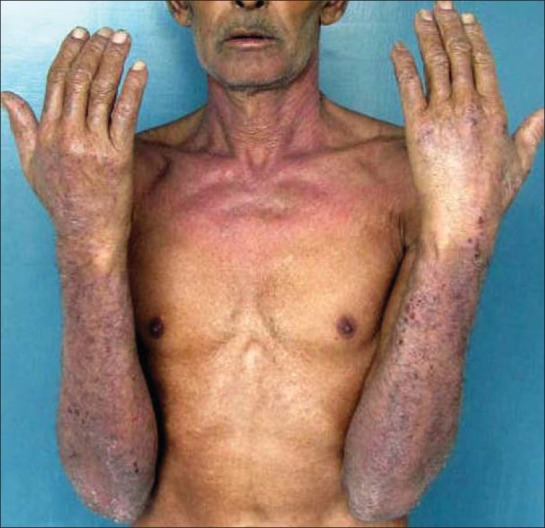
Subacute eczematous plaques on the arms and neck
Figure 2.
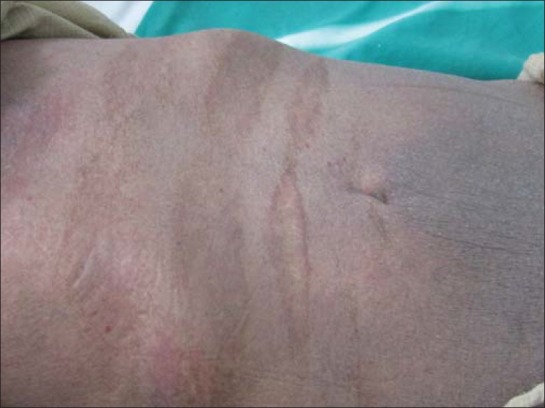
Erythroderma in an elderly male with sparing of skin folds picture resembling mycoses fungoides
With the two- to threefold increase in prevalence of AD over the past few decades,[5] the prevalence of adult-onset AD has also increased and its prevalence ranged from 1-3% in different populations.[2,4] Studies from Singapore, Australia, and Nigeria reported that 13.6%, 9%, and 24.5% of their AD patients had onset after 18 years of age.[3,7,8,9,10] Despite these reports, dermatologists are more comfortable in making a diagnosis of allergic contact dermatitis or air borne contact dermatitis rather than adult onset AD in an adult coming with eczematous condition. This could be because of lack of any specific criteria for adult onset AD as some people are of the opinion that clinical features and diagnostic criteria might vary with age. The Hannifin and Rajka criteria[11] are still the gold standard and can be used to diagnose AD even in adults. In a study on Asian population, the most frequent major criterion observed were typical morphology and distribution and the most frequent minor criterion was disease activity and course influenced by environment or emotion.[3,8] Other studies have found a personal or a family history of atopy in a first degree relative, elevated specific IgE levels or multiple prick test positivity to be more prevalent. Total IgE and aeroallergens IgE levels are highest in AD compared with other atopic diseases and do not decrease with ageing.[2,3]
In our set up, Air-borne contact dermatitis (ABCD) or parthenium dermatitis is sometimes indistinguishable from adult AD. It is very difficult to differentiate between ABCD and adult onset AD because it also involves face, neck, and flexures. Patch testing is helpful in excluding the diagnosis of ABCD. But we should also keep in mind that AD is a risk factor for allergic contact sensitization and contact allergy increases with age in atopics. Moreover, extrinsic AD is more common in adults than children and both immediate and delayed hypersensitivity may play a role in parthenium associated AD.[2,4,8,9] In some of these patients with a positive parthenium contact sensitivity the disease persists despite removal of allergen and it can also be hypothesized that these may by atopics where inhalation of aeroallergens has exacerbated the AD or it may be an apparent superimposed contact dermatitis.
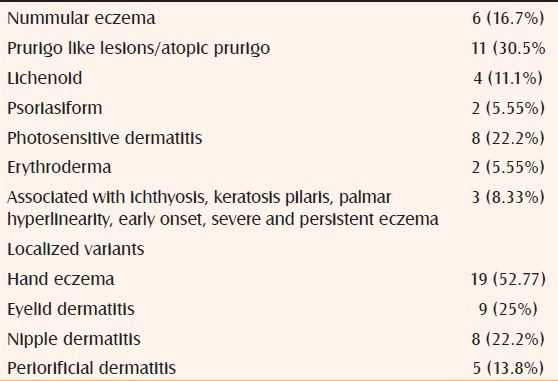
In our experience, 18% of the adult patients referred to contact dermatitis clinic over a period of 1 year fulfilled the Hannifin and Rajka[11] criteria for AD; a total number of 36 cases of adult AD (22 women and 14 men) were identified. Five (13.8%) of them were classified into the intrinsic group (non IgE allergic), and 31 (86.1%) were classified into the extrinsic group (IgE-allergic). All these patients were patch test negative to the Indian standard series, they had a long history (>3 years) of lesions and had elevated serum IgE levels (average IgE levels >1,000 IU/ml). Facial and hand dermatitis were the two most common findings in these patients [Figure 3]. The various patterns observed are given in Table 1.
Figure 3.
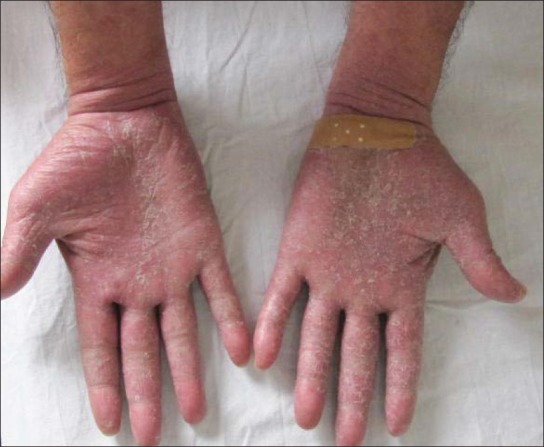
Hand dermatitis in a male
Table 1.
Phenotypes of adult onset AD observed in our study. (n = 36)
Smoking may be another important factor in adult onset AD. In a recent study, Hsin-Su Yu et al., suggested that childhood exposure to passive smoking or environmental tobacco smoke increases the risk for AD in adulthood by three times and the association is cumulative. They found that patients with AD were significantly more likely to be current or ever smokers than individuals without AD, at 53% versus 18%.[12]
Follow-up studies in adult AD show that the eczema may persist but the severity decreases over the years. Head and neck eczema, high values of serum IgE, and a long duration of eczema are poor prognostic factors predicting eczema persisting for longer period.[8,13,14] The increased prevalence of AD in children and the observation that AD in most adults continues for many years suggests that it is very likely we will be seeing more older patients with AD in the future.[8,9]
The treatment in adults is essentially the same as childhood AD and involves appropriate skin care, moisturization, and avoidance of triggers. In adult AD, complex psycho-neuro-immunological interactions assume a major role in initiating and perpetuating AD so stress reduction is very important.[15] It must be stressed upon to the patients that AD is a complex, chronic disorder, and that management should be equated to long-term commitment rather than short-term “quick fix”. Smoking cessation and antifungals for Malassezia sympodialis (esp. head-and-neck eczema)[16] need more evaluation.
The topical therapies in AD can be broadly divided into barrier repair creams and anti inflammatory treatments. Emollients or barrier repair therapies are the cornerstone of management of AD. Barrier skin permeability and antimicrobial function share common structural and biochemical features, and both are co-regulated and interdependent. Barrier repair therapy may reduce secondary colonization of pathogenic S. aureus by targeted correction of lipid biochemical abnormalities. The current recommendations suggest use of emollients in adequate amounts and liberally and frequently, e.g. minimum of 250 g per week. In winter time, more lipid ingredients are preferable. A regular use of emollient has a short- and long-term steroid sparing effect in mild to moderate AE. The rapid progress in knowledge on the predisposing factors on AE has resulted in scientifically designed and better barrier repair creams which are now the cornerstone in aetiological management of AD; rather than mere symptomatic therapy.[17]
The three fundamentals of effective topical management with steroids are sufficient strength, sufficient dosage, and correct application. Topical steroids have immunosuppressive, anti inflammatory, antiproliferative, and vasoconstrictive effects that have been shown to reduce itching, improve the appearance of skin, and improve quality of life. Since their development over 50 years ago, topical corticosteroids have served as the first line of therapy for AD.[17] In cases of severe dermatitis, it is “best to hit hard and hit fast” so high potency topical steroids can be given for short period to control the disease and tapered once the control is achieved. Itch is the key symptom for evaluation of response to treatment, and tapering should not be initiated before the itch has disappeared. There are basically two types of approaches in topical anti inflammatory use the reactive and the proactive approach.[17,18] Reactive approach-anti inflammatory topical therapy is administered to lesional skin only and stopped or tapered down once visible lesions are cleared. Proactive approach-long-term, low dose, anti inflammatory treatment applied to previously affected areas of skin in combination with liberal use of emollients on the entire body. Usually twice weekly moderate potency steroids are started with emollients. The use of topical steroids for acute flares, together with maintenance topical calcineurin inhibitors, is recommended. However, steroid phobia is one of the major problems that need to be addressed. The ISOLATE survey 19 revealed that 58% of patients restricted their use of topical corticosteroids because of concerns about side effects, and 66% said they use these medications “only as a last resort.”[19] Thus, it is evident that adult patients with AD require education from their health providers about the realistic risks and benefits associated with the use of topical medications, including both corticosteroids and non corticosteroid agents such as topical calcineurin inhibitors (TCIs).
TCI are an integral part in management of AD in both adults and children. The potential uses of TCIs are as maintenance therapy to prevent flares of AD, halt the progression of the atopic march, and as corticosteroid “rescue” therapy. They can also be use as first line therapy in cases who are poorly responsive to topical steroids, have steroid phobia, treatment of face and neck dermatitis and extensive body surface area (BSA) involvement. Topical tacrolimus is more effective than pimecrolimus in adults with moderate-to-severe AD, but both agents have a similar safety profile and can be applied on areas with sensitive, thin skin (face, eyelids). In adults with AD, tacrolimus ointment was effective and well-tolerated for up to 2 and 4 years. Adults with refractory AD greatly benefit from 7 days of wet-wrap dressings with diluted steroids. Another therapy, which is underused, is topical tars. Tar inhibits influx of proinflammatory cells and in expression of adhesion molecules in response to epicutaneous allergen challenge. Tar preparation used with topical steroid in chronic AD may reduce need for more potent steroid preparation. Tar shampoos are often beneficial for scalp involvement. One big problem with tar preparations is they are messy and can result in irritant dermatitis or folliculitis, so they should be avoided in inflamed surfaces and are best suited as maintenance therapy.[17,20]
In patients with severe AD, topical therapy may sometimes be insufficient. Phototherapy can be considered because of safety, but may be poorly tolerated if the skin is highly inflamed, and is often alternatively chosen as maintenance therapy. However, the time demands of phototherapy can be a big limitation, However it is a good but underutilized therapeutic modality in our setup.
Systemic immunosuppressant therapy is commonly prescribed in adults with severe or intractable disease. Systemic steroids are more frequently prescribed but should be used carefully in elderly who may have hypertension or diabetes. Systemic steroids have an unfavorable risk/benefit ratio and should only be used for short periods to tide over the situation along with other immunosuppressive agents. Most severely affected patients show responses to cyclosporine, azathioprine, mycophenolate mofetil or methotrexate. Among these immunosuppressants, cyclosporine has the most rapid onset of action. Although there are a few comparative trials but guidelines for duration of utilization are not established.
Cyclosporine may be considered as first choice in management of adult AD. The duration of therapy is guided by clinical efficacy and tolerance of the drug. Both short-term and long-term therapy may be useful and continuous administration of cyclosporine, tapered to the lowest therapeutic level, seems to yield approximately the same clinical results as administration of intermittent 12-week courses of therapy. Dose reduction should be considered according to clinical efficacy. Long-term treatment with minimum dose that causes relief of signs and symptoms may be advisable in selected cases. Side effects of cyclosporin argue against a long-term treatment of AE. However there is no consensus regarding cessation of therapy which can be attempted after 1 or 2 years according to different guidelines.[17,21]
Azathioprine is another good option in our set up but it has a delayed onset of efficacy of 4 to 6 weeks, so some clinicians begin concurrent treatment with azathioprine and a corticosteroid. In general, the optimum dosage of azathioprine is 2.5 to 3.5 mg/kg/day of; however, if an intermediate level of the erythrocyte thiopurine methyltransferase (TPMT) enzyme is obtained that dosage can be decreased to 1 mg/kg/day. An effective dose should be maintained for about 3 months, then tapered gradually; azathioprine can be used for 2 years before transitioning to an alternative therapy.[17,18,20,21]
Another option is mycophenolate mofetil, which is the least toxic (although costlier) systemic immunosuppressants. As with azathioprine, mycophenolate mofetil's onset of efficacy is delayed for 4 to 8 weeks. The maximal effect is seen at 8 to 12 weeks after initiation, so, concurrent administration of corticosteroid followed by tapering and then discontinuation, is advisable.[17,21]
Methotrexate (Mtx) is another alternative therapeutic modality in AD. A randomized trial with MTX vs. Azathioprine showed comparable effects in severe atopic eczema.[22] It can be used in doses ranging from 10 mg/week to 25 mg/week. Drug safety data for MTX are largely derived from clinical experience from other low dose indications for MTX, indicating liver toxicity and teratogenicity as main areas of concern. There are no AD specific safety data available for MTX.[17,21]
Patients with AD are likely to develop complications such as bacterial skin infections caused by Staphylococcus or hemolytic Streptococcus and Kaposi's varicelliform eruption caused by Herpes Simplex Virus. If patients are suspected of having these infections during treatment, appropriate antibiotics or antiviral drugs should be administrated immediately.
A number of case reports, pilot studies, and retrospective analyses on the effect of biologics in patients with moderate to severe AD refractory to topical and/or systemic therapy have been published recently. However, representative, randomized, placebo-controlled studies evaluating the efficacy and safety of biologics in AD are still not available. In patients with severe AD refractory to topical and systemic treatment, biologics (omalizumab, rituximab or alefacept) can be considered.[15,19]
The efficacy of specific immunotherapy in AD has been shown in a number of case reports and smaller cohort studies,[23] and, more recently, in a larger multicenter trial with subcutaneous house dust mite immunotherapy.[24] Allergen Specific Immunotherapy (ASIT) may have positive effects in selected, highly sensitized patients with AE. The best evidence so far is available for ASIT with house dust mite allergens.[17]
A number of complementary and alternative therapies have been tried for AD but there is not sufficient evidence to support their role. However, psychosomatic counseling can be a helpful adjuvant procedure in the management of patients with AD including psychotherapeutical approaches and behavioral therapy techniques. Individual psychotherapeutic approaches can be helpful.[17]
In conclusion, adult onset AD is an important subgroup of AD with a broad range of age of onset (1871 years) and has a significant impact on quality of life in adults. The quality of life impairment in severe AD, was equal to that seen in angina, chronic anxiety, rheumatoid arthritis, multiple sclerosis, or esophageal cancer.[25]
Jean Martin Charcot aptly said “We see only what we are ready to see, what we have been taught to see”. For ages, the classic teaching has been that AD is synonymous with childhood and it resolves with age so accepting adult onset AD may not be easy. However, we think that it can be considered as a diagnosis in an adult with long standing flexural or truncal lichenified dermatitis or atypical morphology (nummular, follicular, prurigo-like, or seborrheic dermatitis-like). More clinico-epidemiologic studies will help us in understanding disease burden of AD among adult populations and may help to develop efficient interventions and even early preventions.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
REFERENCES
- 1.Bannister MJ, Freeman S. Adult-onset atopic dermatitis. Australas J Dermatol. 2000;41:225–8. doi: 10.1046/j.1440-0960.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- 2.Katsarou A, Armenaka M. Atopic dermatitis in older patients: Particular points. J Eur Acad Dermatol Venereol. 2011;25:12–8. doi: 10.1111/j.1468-3083.2010.03737.x. [DOI] [PubMed] [Google Scholar]
- 3.Tay YK, Khoo BP, Goh CL. The profile of atopic dermatitis in a tertiary dermatology outpatient clinic in Singapore. Int J Dermatol. 1999;38:689–92. doi: 10.1046/j.1365-4362.1999.00667.x. [DOI] [PubMed] [Google Scholar]
- 4.Ozkaya E. Adult-onset atopic dermatitis. J Am Acad Dermatol. 2005;52:579–82. doi: 10.1016/j.jaad.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 5.Williams HC. Epidemiology of atopic dermatitis. Clin Exp Dermatol. 2000;25:522–9. doi: 10.1046/j.1365-2230.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 6.Tanei R. Atopic dermatitis in the elderly. Inflamm Allergy Drug Targets. 2009;8:398–404. doi: 10.2174/1871528110908050398. [DOI] [PubMed] [Google Scholar]
- 7.Wolkewitz M, Rothenbacher D, Löw M, Stegmaier C, Ziegler H, Radulescu M, et al. Lifetime prevalence of self-reported atopic diseases in a population-based sample of elderly subjects: Results of the ESTHER study. Br J Dermatol. 2007;156:693–7. doi: 10.1111/j.1365-2133.2006.07659.x. [DOI] [PubMed] [Google Scholar]
- 8.Kulthanan K, Samutrapong P, Jiamton S, Tuchinda P. Adult-onset atopic dermatitis: A cross-sectional study of natural history and clinical manifestation. Asian Pac J Allergy Immunol. 2007;25:207–14. [PubMed] [Google Scholar]
- 9.Sandström Falk MH, Faergemann J. Atopic dermatitis in adults: Does it disappear with age? Acta Derm Venereol. 2006;86:135–9. doi: 10.2340/00015555-0040. [DOI] [PubMed] [Google Scholar]
- 10.Nnoruka EN. Current epidemiology of atopic dermatitis in southeastern Nigeria. Int J Dermatol. 2004;43:739–44. doi: 10.1111/j.1365-4632.2004.02360.x. [DOI] [PubMed] [Google Scholar]
- 11.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1980;92:44–7. [Google Scholar]
- 12.Lee CH, Chuang HY, Hong CH, Huang SK, Chang YC, Ko YC, et al. Lifetime exposure to cigarette smoking and the development of adult-onset atopic dermatitis. Br J Dermatol. 2011;164:483–9. doi: 10.1111/j.1365-2133.2010.10116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandström MH, Faergemann J. Prognosis and prognostic factors in adult patients with atopic dermatitis: A long-term follow-up questionnaire study. Br J Dermatol. 2004;150:103–10. doi: 10.1111/j.1365-2133.2004.05711.x. [DOI] [PubMed] [Google Scholar]
- 14.Katoh N, Hirano S, Kishimoto S. Prognostic factor of adult patients with atopic dermatitis. J Dermatol. 2008;35:477–83. doi: 10.1111/j.1346-8138.2008.00507.x. [DOI] [PubMed] [Google Scholar]
- 15.Arndt J, Smith N, Tausk F. Stress and atopic dermatitis. Curr Allergy Asthma Rep. 2008;8:312–7. doi: 10.1007/s11882-008-0050-6. [DOI] [PubMed] [Google Scholar]
- 16.Casagrande BF, Fluckiger S, Linder MT, Johansson C, Scheynius A, Crameri R, et al. Sensitization to the yeast Malassezia sympodialis is specific for extrinsic and intrinsic atopic eczema. J Invest Dermatol. 2006;126:2414–21. doi: 10.1038/sj.jid.5700431. [DOI] [PubMed] [Google Scholar]
- 17.Ring J, Alomar A, Bieber T, Deleuran M, Fink-Wagner A, Gelmetti C, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J Eur Acad Dermatol Venereol. 2012;26:1045–60. doi: 10.1111/j.1468-3083.2012.04635.x. [DOI] [PubMed] [Google Scholar]
- 18.Ellis CN, Mancini AJ, Paller AS, Simpson EL, Eichenfield LF. Understanding and managing atopic dermatitis in adult patients. Semin Cutan Med Surg. 2012;31(3 Suppl):S18–22. doi: 10.1016/j.sder.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Zuberbier T, Orlow SJ, Paller AS, Taïeb A, Allen R, Hernanz-Hermosa JM, et al. Patient perspectives on the management of atopic dermatitis. J Allergy Clin Immunol. 2006;118:226–32. doi: 10.1016/j.jaci.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 20.Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132–40. doi: 10.1159/000331916. [DOI] [PubMed] [Google Scholar]
- 21.Saeki H, Furue M, Furukawa F, Hide M, Ohtsuki M, Katayama I, et al. Guidelines for management of atopic dermatitis. J Dermatol. 2009;36:563–77. doi: 10.1111/j.1346-8138.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- 22.Schram ME, Roekevisch E, Leeflang MM, Boos JD, Schmitt J, Spuls PI. A randomized trial of methotrexate versus azathioprine for severe atopic eczema. J Allergy Clin Immunol. 2011;128:353–9. doi: 10.1016/j.jaci.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Darsow U, Forer I, Ring J. Allergen-specific immunotherapy in atopic eczema. Curr Allergy Asthma Rep. 2011;11:277–83. doi: 10.1007/s11882-011-0194-7. [DOI] [PubMed] [Google Scholar]
- 24.Werfel T, Breuer K, Ruéff F, Przybilla B, Worm M, Grewe M, et al. Usefulness of specific immunotherapy in patients with atopic dermatitis and allergic sensitization to house dust mites: A multi-centre, randomized, dose-response study. Allergy. 2006;61:202–5. doi: 10.1111/j.1398-9995.2006.00974.x. [DOI] [PubMed] [Google Scholar]
- 25.Evers AW, Lu Y, Duller P, van der Valk PG, Kraaimaat FW, van de Kerkhof PC. Common burden of chronic skin diseases? Contributors to psychological distress in adults with psoriasis and atopic dermatitis. Br J Dermatol. 2005;152:1275–81. doi: 10.1111/j.1365-2133.2005.06565.x. [DOI] [PubMed] [Google Scholar]


