Abstract
Background:
The treatment of various irritant dermatitis involves the elimination of the casual or favoring factor, the control of aggravating factors, and administration of topical agents. Even though corticosteroids are extensively used in these conditions to reduce the inflammation, it can also result in undesirable side effects. Hence, there is a need for a non steroidal topical agent to be used in these conditions.
Aims:
To evaluate the efficacy and tolerance of repairing cream RV 2427B in children and adults in irritant dermatitis care.
Materials and Methods:
In this phase III open labeled, multicenter, non-controlled, non-randomized trial, irritant dermatitis in children and adults either due to diaper rash, pityriasis alba and irritant dermatitis (eczema), perioral dermatitis, perleche or intertrigo were administered; repairing cream RV 2427 B containing a) 4 % zinc oxide, b) 2.5 % dry colloidal oat extract, (c) 0.5 % oat oil, (d) 0.2% copper sulfate, and (e) 0.1 % zinc sulfate to be applied twice-daily in the affected area. The subjects were evaluated on day 7 and day 21 for both efficacy and tolerance and last visit for cosmetic acceptability. The trial was conducted in accordance with the good clinical practices (GCP) after obtaining ethical clearance from respective Institutional Review Boards. Statistical evaluation was by variance analysis and student test for the quantitative variables, chi-square test for the qualitative variables.
Results:
Of the 136 enrolled subjects, 95 completed the study. After 21 days of treatment, 84% of the subjects assessed by the investigator and 76% by the self-assessment for the cream found effective. Investigational product was considered to be safe after 7 and 21 days of use.
Conclusion:
Repairing cream RV 2427 B is effective and safe in the management of irritant dermatitis.
Keywords: Irritant dermatitis, repair cream, zinc oxide
INTRODUCTION
The term “irritant dermatitis” in a broad sense represents response to the physical / toxic effects of a wide range of environmental exposures. Dermatitis arises when the defense or repairing capacity of the skin is exhausted or when the penetration of chemicals excites an inflammatory response. The 3 main factors involved in the pathogeneses of irritant dermatitis are, disturbed barrier function, epidermal cell change, and release of inflammatory mediators and cytokines.[1]
Dermatological conditions like diaper rash, perioral dermatitis, perleche, intertrigo, many a times, are because of the irritant factors. Condition like pityriasis alba although considered as an endogenous eczema, presents as scaly hypopigmented macules on the face, and application of emollient creams helps in its treatment.
The treatment of this irritant dermatitis involves the elimination of the causal or favoring factor, the control of aggravating factors, and administration of topical agents. Even though corticosteroids are extensively used in these conditions to reduce the inflammation, it can also result in undesirable side effects. In cases of diaper dermatitis, the occlusion of the wet diaper raises the absorption of the topical steroid and may lead to purple nodules termed granuloma gluteale infantum.[2] Condition like perioral dermatitis may be precipitated by topical corticosteroids.[3] It has been suggested that topical corticosteroids including hydrocortisone should be discouraged from prolonged usage on the face.[4] So, there is a need for non steroid cream in conditions mentioned above, which helps in correcting the skin barrier function and limit the passage of irritants and allergies.
The repairing cream “RV 2427B” was designed to treat irritated skin, it contains the following components: a) 4 % zinc oxide, b) 2.5 % dry colloidal oat extract, (c) 0.5 % oat oil, (d) 0.2% copper sulfate, and (e) 0.1 % zinc sulfate. Among these, zinc oxide, zinc sulfate, and copper sulfate are known for their soothing, anti-microbial, and re-generating properties. Oat extract and oat oil are natural anti irritants that reduce the inflammatory phenomenon.[5]
This phase III open-labeled, multicenter, non-controlled, non-randomized trial was intended to evaluate the efficacy and tolerance of repairing cream “RV 2427 B” in children and adults either due to diaper rash, pityriasis alba and irritant dermatitis (eczema), perioral dermatitis, perleche or intertrigo. Pityriasis alba was included at the insistence of sponsorers.
MATERIALS AND METHODS
Inclusion criteria:
Male or female adults
Women of child-bearing age practicing effective contraceptive method
Children (age > 1 month) and adults suffering from irritant dermatitis on face and or body (diaper rash, perioral dermatitis, pityriasis alba and irritant dermatitis, perleche, and intertrigo). The inclusions of the patients were at the investigators discretion with relation to extent of involvement.
Willing to give an informed consent in case of adults and from parents in case of children
Exclusion criteria:
Pregnant women
Children below 1 month age
Use of a cosmetic product other than that studied on irritant dermatitis
Known hypersensitivity to one of the ingredients in the product tested
Participation in another clinical trial during present trial
Presence of superadded infection necessitating antibiotic treatment
Antibiotic, anti-viral, anti-fungal treatment or corticosteroids initiated within 3 days of inclusion
This trial was conducted at 5 dermatology departments: Bangalore Medical College (BMC) and St. John's Medical College (SJMC), Bangalore, Jagdguru Shivaratareshwara Medical College (JSS), Mysore, Father Muller Medical College (FMMC) and K. S. Hegde Charitable Hospital (KSHEMA), Mangalore from September 2006 - December 2007.
Ethical consideration: The trial was conducted in accordance with the good clinical practices (GCP) after obtaining permission from the respective institutional review boards (IRB) according the clinical research guidelines established by the basic principles defined in the U.S.21 CFR part 312, 120 ICH – GCP and enunciated in the declaration of Helsinki.
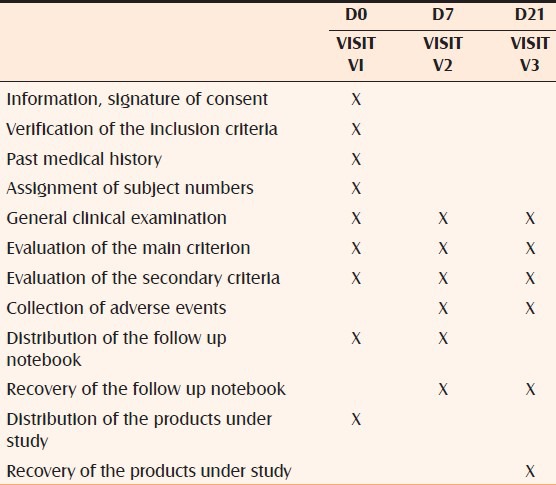
Study design: On day 0, all subjects who met inclusion / exclusion inertia, who consented to participate in this study, were administered 2 tubes of repairing cream to be applied twice-daily (morning and evening) to the affected area. The subjects were evaluated on day 7 (D-7) and days 21 (D21) post-treatment. All subjects were evaluated for both efficacy and tolerance and last visit for cosmetic acceptability. (Overall study design is represented in Table 1). Evaluation criteria was divided as objective (cracks, desquamation, erythema, exudation) and subjective (pruritus, burning, tightness, tingling), this was used to evaluate the efficacy and tolerance. For minors subjective, opinions of a parent were considered. Assent forms were also signed by the appropriate minors. The patients were issued subject dairies, and the tubes were instructed to return after the completion of the study. Patients were instructed not to take any oral medications.
Table 1.
The study design
Efficacy and safety measurements: The primary evaluation criteria were assessed as improvements in clinical data using objective signs (on a 4-point scale: 0 = none, 1 = mild, 2 = moderate, 3 = severe) and subjective signs (with an analogical scale graduated from 0 (absence of symptoms) to 10 (of maximum intensity) by comparing paired measurements from D0 and D7. The secondary evaluation criteria were assessed as improvements in clinical data using objective and subjective signs by comparing paired measurements from D0 and D21. Global efficacy and tolerance were assessed based on the measurements by the investigator (using the following scale: 3 = Very effective, 2 = Effective, 1 = Not very effective, 0 = Not effective at all). All subjects were informed at the beginning of the study to consult the investigator in case they notice any unpleasant reaction during the study period. Global assessment of tolerance was done by the investigator at D7 and D21 (using a scale: 3 = Very good tolerance, 2 = Good tolerance, 1 = Bad tolerance, 0 = Very bad tolerance). The self-assessment of the global efficacy of the tested product by the subject or by the legal guardian in case of a child under 18 years of age, at D7 and D21, was done using a follow up notebook given to the subject at D0. Also, assessment of the cosmetic acceptability of the tested product was done at D21. For safety assessment, any adverse or intercurrent event (were graded by the investigator using a 3-point intensity scale; mild: Awareness of signs or symptoms, but easily tolerated; moderate: Uncomfortable enough to cause interference with usual activity; severe: Incapacity with inability to work or do usual activity), which occurred during the study period was recorded. In infants, response was obtained by parents.
Statistical analysis: Statistical inferential tests for objective and subjective data were assessed by comparing the responses on D7 and D21 from baseline (D0) using paired test procedures. Objective signs were all measured using qualitative scale, and McNemar Bowker test were used for comparisons. Subjective data were quantitative values measured using visual analogue scale (between 0 to 10). For quantitative values, a paired student test was carried out; Wilcoxon signed rank test was also used to check the robustness of distribution assumption in the paired t – test. For all statistical tests, type I error was fixed at 5%. Supportive statistical summaries were presented in the tables and charts for all measurements.
RESULTS
A total of 136 subjects were enrolled into the study, and 95 subjects completed the study. The diagnosis of subjects was as follows; 66 (48%) subjects had eczema (Pityriasis alba and irritant dermatitis), 18 (13%) had perioral dermatitis, 27 (20%) had diaper rash, 8 (5%) had perleche, and 17 subjects had intertrigo (13%).
Among the 95 subjects who completed the study, 82 subjects completed all the 3 visits and the remaining completed only 2 visits and withdrew from study due to completed resolution of lesion.
Among 136 subjects, 61 (44.8%) subjects were males and 75 (55.2%) were females. Subjects in the age group of less than 18 were 54 (40%), and those above 18 were a total of 82 (60%) subjects.
Objective data: Primary comparison- There were marked improvements in clinical sign after 7 days of treatments in all objective sign endpoints, these improvements were all statistically significant when the paired measurements were compared from D0 and D7 using McNemar Bowker test procedure. Secondary comparison – The improvements in clinical signs continued after 21 days of treatment in all objective sign end points; more than 63.0% reduction in erythema symptom was observed at D7 visit and 80.7% of improvement on visit (D21). Similarly, the improvement of desquamation symptoms were observed on D7 (68.9%) and D21 (85.5%) visits. About 50.6% reduction of severity in cracks and 24.1% improvement in sweating was observed. Although less number of subjects (55) was available with paired observation in D0 and D21, this clinical improvement in both groups was statically significant in all objective signs when compared from D0 and D21 using Mc Nemar – Bowker test procedure.
Subjective data: On primary comparison, there was marked improvement in clinical signs after 7 days of treatment in all 4 subjective sign end points (burning sensation, pruritus, tightness, and tingling); these improvements were all statically significant when the paired measurements were compared from D0 and D7 using paired t-test procedure. Wilcoxan and signed rank test procedure also corroborated with the significant results observed using the paired t-test. Secondary comparison showed significant improvements in clinical signs continued after 21 days of treatment in all 4 subjective endpoints. There was marked improvement in clinical signs after 7 days and 21 days of treatment in subjective sign end points, especially the burning sensation and pruritus. The improvement was about 75.38% and 90.0%, respectively, on D21 visit. However, other subjective signs, i.e. tightness and tingling, showed an average improvement of about 60% reduction in severity on D21 visit.
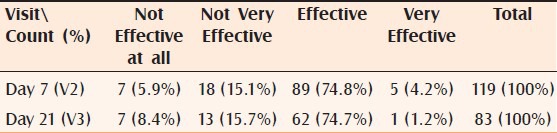
Global efficacy: More than 86% (102) (adding very effective and effective) of the subjects assessed by the investigator as well as by self assessment 79% (94) (adding very effective and effective) found the cream effective or very effective after 7 days of treatment. After 21 days of treatment, 84% (adding very effective and effective) of the subjects assessed by the investigator and 76% by the self-assessment found the cream effective or very effective Tables [Tables 2 and 3].
Table 2.
Distribution of subject by global efficacy: Investigator's opinion
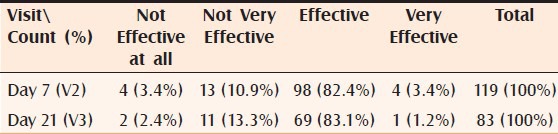
Table 3.
Distribution of subject by global efficacy: Subject's opinion
Tolerance: Almost everyone in the study had good to very good tolerance of the cream after 7 (97%) and nearly similar after 21 days of treatment (94%).
Safety: The investigational product was considered as safe since most of the subjects had either good or very good tolerance.
Cosmetic acceptability: Majority of the subjects (84%) evaluated after 21 days treatment found ease of application, ease of use, and practicality of packing were satisfying to very satisfying. At visit, three (71.4%) of patients mentioned that they would like to continue to use the product.
DISCUSSION
In this phase III open-labeled, multicenter, uncontrolled, randomized trial repairing cream mainly containing zinc oxide and colloidal oat extract revealed the beneficial effects in the management of irritant dermatitis. The dermatological conditions included in this trial were diaper rash, perioral dermatitis, pityriasis alba, irritant dermatitis (eczema), perleche, and intertrigo. In all these conditions the barrier repair function of the skin is impaired.
In diaper dermatitis, factors like moisture, friction, urine, feces, and sometimes micro organism, result in dermatitis. The barrier function of the skin is affected, with resultant increase in irritant chemical penetration and pathogen replication.[6] Perleche or cheilitis is an irritant dermatitis due to lips licking, cosmetics, and medications.[7] A number of agents have been implicated in the pathogenesis of perioral dermatitis.[3]
Zinc oxide is a relatively weak anti-bacterial agent and is used in the form of powder and ointments. An inflamed skin is usually associated with pain, burning, or itching, and locally applied agents which relieve these uncomfortable sensations are known as soothing preparations. Application of cream containing emollients are useful in these conditions.[8] Zinc oxide is used in the past in conditions like acute eczema, seborrheic dermatitis, neurodermatitis, in which it has the soothing action.[9] In angular stomatitis when applied, zinc oxide ointment helps in preventing maceration due to the constant presence of saliva. Zinc oxide is also a component of many anti pruritic formulations available as lotion, liniments, and ointments.[10]
In this trial, the beneficial effects of RV 2427 B cream are shown in irritant dermatitis. In diaper dermatitis, zinc oxide ointments are considered as the first line therapeutic agent in primary diaper dermatitis.[11] Wananukul S et al., in a multicenter study, indicated that ointment containing dexpathenol and zinc oxide was beneficial in the treatment of irritant diaper dermatitis.[12,13]
This trial repairing cream “RV 2427 B,” containing zinc oxide and colloidal oat extract as main ingredients, demonstrated an overall efficacy rate of more than 82% in the treatment of various irritant dermatitis. More than 90% of the patients demonstrated good tolerance for this product.
Limitations of the study:
Inclusion of pityriasis alba, which is considered as an endogenous eczema. The only justification for using this condition is the requirement of a non-steroidal cream for the remission. These cases also didn’t have oozing from the lesions, and only scaling and erythema, if any, were considered for evaluation.
The statistical analysis was done after compiling the data from all the centers.
CONCLUSION
This study shows that repairing cream “RV 2427 B” is effective, safe, and cosmetically acceptable in the treatment of irritant dermatitis of the skin. As this cream doesn’t include any steroids, there will not be any restrictions in using this product. However, double blind, placebo controlled trials in individual dermatosis may help in further emphasizing the efficacy of this product.
ACKNOWLEDGMENTS
Sponsor: Pierre Fabre Dermocosmetique France
Monitor: Virgine Ribet, Clinical Development,
CRO: - Asiatic Clinical Research Pvt. Ltd
Investigators:- From the Dept of Dermatology
Bangalore Medical College: PI: Dr. S. Sacchidanand Prof and HOD, Co investigator: Dr. G. S. Asha, Lecturer, Sub Investigator: Dr. Eswari L – Resident, Dr. Susheela E – Resident, Co ordinator: Mr. Asad Akhtar, Mr. Diwakar Singh
J.S. S. medical College, Mysore PI: Dr. Jaydev Betkerur, Prof. and HOD, Co investigator: Dr. Veeranna S. Asst. Professor, Co ordinator: Dr. Ashwini Shetty – Resident
Fr. Muller Medical College Mangalore, PI: Dr. Ramesh Bhat M, Prof. Mangalore, Co Investigator: Dr. Jacintha Martis, Assoc. Prof, Co ordinator: Dr. Fiona Sequeira, Resident
St. John's Medical College, PI: Dr. Elizabeth Jayaseelan, Prof and HOD, Bangalore, Co Investigator: Dr. Mary Augustine Joseph, Asst. Prof, Co ordinator: Dr. Mohan K.H. Sr. Resident
Justice K.S. Hegde Charitable Hospital Mangalore, PI: Dr. Manjunath Shenoy, Assoc Professor Co-Investigator: Dr. Girisha B.S., Assoc. Prof.
Co ordinator at Pierre Fabre Dr. Rajeev Chavda, Country Manager, PF-India
Biostatistician Dr. Saurabh mukhpadhyay, Kolkata
Abbreviations-PI Principal Investigator
Footnotes
Source of Support: The clinical trial was sponsored by Pierre Fabre, France
Conflict of Interest: Dr. Ramesh Bhat M. is one of the Principal Investigators and was financially supported by the sponsorer through the Institution as per the Clinical Trial Agreement in conducting this trial
REFERENCES
- 1.Wilkinson SM, Back MH. Contact dermatitis: Irritant. In: Burns T, Breathnach S, Cox N, Griffiths SC, editors. Rooks Text Book of Dermatology. 7th ed. Oxford: Blackwell Science; 2004. p. 19.1. 19.3, 19.18. [Google Scholar]
- 2.Boiko S. Treatment of diaper dermatitis. Dermatol Clin. 1999;17:235–40. doi: 10.1016/s0733-8635(05)70079-6. [DOI] [PubMed] [Google Scholar]
- 3.Hafeez ZH. Perioral dermatitis: An update. Int J Dermatol. 2003;42:514–7. doi: 10.1046/j.1365-4362.2003.01852.x. [DOI] [PubMed] [Google Scholar]
- 4.Guin JD. Complication of topical hydrocortisone. J Am Acad Dermatol. 1981;4:417–22. doi: 10.1016/s0190-9622(81)70040-9. [DOI] [PubMed] [Google Scholar]
- 5.Gilman GA, Goodman LS, Rall TW, Murad F, editors. Goodman and Gilman's The pharmacologic basis and therapentics. 7th ed. United Kingdom: Macmillan Publishing Co; 1985. Antispetics and disinfectants: Fungicides: Ectoparasiticides; pp. 959–79. [Google Scholar]
- 6.Scheinfeld N. Diaper dermatitis A review and brief survey of eruptions of the diaper area. Am J Clin Dermatol. 2005;6:273–81. doi: 10.2165/00128071-200506050-00001. [DOI] [PubMed] [Google Scholar]
- 7.Arndt KA. Boston, Toranto: Little, Brown and Co; 1989. In manual of dermatologic Therapeutics; pp. 49–50. [Google Scholar]
- 8.Pasricha JS. Treatment of skin diseases. 4th ed. Oxford: IBH Publications Co. Pvt. Ltd; 1996. General Principles; pp. 48–50. [Google Scholar]
- 9.Dobson LR, Eczema . In: Con FH, editor. Philadelphia and London: WB Saunders and Co; 2000. p. 423. [Google Scholar]
- 10.Perry HO, Lovestedt SA. Mouth disorders. In: Conn FH, editor. Current Therapy. Philadelphia and London: WB Saunders and Co; 2000. pp. 447–8. [Google Scholar]
- 11.Rhealba. [Last accessed on 2009 Mar 6]. Available from: http://www.dermaweb.com/English/dermato/aderma/index2.html .
- 12.Wananukul S, Limpongsanuruk W, Singalavanija S, Wisuthsarewong W. Comparison of dexpanthemol and zincoxide ointment with wointmetn base in the treatment of irritant diaper dermatitis from diarrhea: A multicenter study. J Med Assoc Thai. 2006;89:1654–8. [PubMed] [Google Scholar]
- 13.Patrizi A, Neri L, Varotti E, Raone B. Clinical evaluation of the efficacy and tolerability of the “No all Bimbi Pasta Trattante” barrier cream in napkin dermatitis. Minerv Pediatr. 2007;59:23–8. [PubMed] [Google Scholar]


