Abstract
The global incidence of non-melanoma skin cancer is rising. Significant morbidity leading to unacceptable cosmetic outcomes and/or functional impairment is a major concern. Search for non-surgical, non-invasive and tissue-sparing treatment modalities has led to development of new therapeutic agents. Actinic keratoses (AK) are one part of a continuous spectrum of benign sun damage to squamous cell carcinoma (SCC). Although it is not possible to predict which AK might progress to SCC, the presence of AK is a biomarker of risk for patients and must be treated to avoid possible morbidity and mortality. Ingenol mebutate is a novel topical drug from the latex sap of a plant-Euphorbia peplus that acts by chemoablative and immunostimulatory properties. Clinical studies have proven it to be safe and efficacious, leading to FDA approval of this chemotherapeutic agent for field therapy of AK in 2012. Current topical agents for field therapy of AK must be applied for weeks, whereas ingenol needs to be applied for three days. Ingenol offers a new therapeutic option that is convenient, safe, effective, acceptable and well-tolerated.
Keywords: Actinic keratosis, ingenol mebutate, nonmelanoma skin cancer
INTRODUCTION
Non-melanoma skin cancer (NMSC) is the most common cancer in Caucasians and includes squamous cell carcinoma (SCC) and basal cell carcinoma (BCC).[1] The incidence of NMSC has increased significantly and currently 2-3 million new cases are diagnosed worldwide each year, with the highest rates reported closer to equator, as in the northern territories of Australia.[1] Though NMSC is uncommon in Asians, SCC is the most prevalent skin malignancy reported in India.[2] The exact incidence in India is however unreported in literature. NMSC occupies a small space in the perception of clinical dermatologists in India mainly because of the supposedly protective effects of eumelanin in the brown skin of the Indian population. However, studies show that a large variety of cases, including atypical forms of NMSC do occur in Indians.[2]
Risk factors for NMSC include unprotected and/or excessive exposure to ultraviolet (UV) radiation, older age, male sex, occupational exposure to radium and arsenic, positive family history of atypical moles, smoking tobacco, immunosuppression due to medications and human immunodeficiency virus (HIV) infection, chronic non-healing wounds, previous burns, genetic syndrome such as basal cell nevus syndrome and human papilloma virus (HPV) infection. Important phenotypic features associated with increased risk include fair complexion, red hair, blue eyes, increased number of melanocytic naevi and Fitzpatrick skin type -1.[1]
Up to 60% of SCC begins as actinic keratosis (AK) and there is histologic evidence of contiguous AK in 97% SCC lesions that arise on sun damaged skin.[3] AK is the initial intra-epidermal manifestation of UV radiation induced neoplastic transformation of keratinocytes. UV-B causes thymidine dimer formation in DNA; mutant DNA containing cells resistant to apoptotic death undergo clonal expansion and can potentially progress to SCC. Though presence of AK is a biomarker of risk for patients, it is impossible to predict which AK will advance to skin cancer; therefore early detection and treatment are critical. Although the relative mortality is low (0.1%), NMSCs may cause considerable morbidity, particularly in visible areas, such as the head and neck, with consequent unacceptable cosmetic outcomes and/or functional impairments, causing high direct and indirect costs of management.
Treatment is dependent on location of tumor, age at diagnosis, extent of disease and whether the affected area has been treated before. Field-directed therapy aims to eliminate not only clinically visible lesions, but also subclinical lesions within the treatment area. Current treatment options for NMSC include surgical procedures such as Mohs micrographic surgery, regular excision, cryosurgery, curettage and desiccation, radiotherapy, phototherapy and pharmacological agents like interferon, imiquimod, retinoids, diclofenac and 5 fluorouracil (5FU). These modalities suffer from one or more drawbacks related to therapeutic safety or efficacy, convenience, compliance, cosmetic outcome or cost. Surgical removal is painful and may result in scarring. Treatment of a contiguous area (field treatment) is not feasible using a lesion-directed approach such as cryotherapy. Moreover, cryotherapy can be painful and patients are often left with hypopigmented spot at the site of application of liquid nitrogen. Curettage is done for low risk patients and success is highly dependent on the skill of the practitioner. Long duration of treatment with topical therapies like 5-FU and imiquimod leads to prolonged local reactions such as unsightly skin irritation resulting in non-compliance.[4,5,6] The search for non-surgical and tissue-sparing treatments that offer not only convenience but also superior cosmetic results has led to the development of novel, non-invasive drugs targeting key cellular receptors or immunological responses. Clinical trials evaluating newer treatment options, such as cycloxygenase-2 inhibitors, betulinic acid, vismodegib, resiquimod, afamelanotide, epidermal growth factor receptor (EGFR) inhibitors (gefitinib and erlotinib) and EGFR antibodies (cetuximab and panitumumab) and capecitabine are obtaining promising results.
Ingenol mebulate [ingenol 3- angelate, (PEP 005)] is an extract from the sap of a non–invasive weed Eurphorbia peplus (E. peplus) also known as petty spurge, radium weed or milkweed. Chemically, it is a hydrophobic macrocyclic diterpene ester with molecular formula C25H34O6 and molecular weight 430.5 [Figure 1]. Ingenol mebutate is formulated as propylalcohol based gel for topical use.[7] Ingenol mebutate gel is available in two different concentrations: for treatment of the face and scalp, the gel is applied at a concentration of 0.015% once-daily for three consecutive days; whereas for treatment of the body, the gel is applied once-daily for two consecutive days at a concentration of 0.05%. The gel is available in India.
Figure 1.
Ingenol mebutate
Mechanism of action
Topical ingenol mebutate passes the stratum corneum barrier through P-glycoprotein absorptive drug transport and exerts its action in the dermis and hypodermis. Direct effect of the drug along with local production of inflammatory cytokines cause initial tumor ablation which is characterized by rapid disruption of plasma membrane and subsequent mitochondrial swelling followed by cell death via primary necrosis. The second phase is marked by local acute inflammatory response due to neutrophil infiltration. During the third and last phase, tumor-reactive antibodies are induced and relapses are avoided through antibody-dependent neutrophil cytotoxicity that eliminates residual cancer cells.
Ingenol mebutate is a protein kinase C (PKC) pathway activator; consequently it targets and damages the sub-epidermal intrinsic tumor vasculature. PKC delta activation slows cell proliferation, induces cell cycle arrest and enhances differentiation in various undifferentiated cell lines. Additionally, it promotes apoptosis of caspases, increases stability of p53 and promotes phosphorylation of signaling molecules.[8,9]
CLINICAL STUDIES DONE WITH INGENOL MEBUTATE
A Phase IIa study explored effects of three different concentrations of ingenol mebutate gel applied to preselected AK lesions (arm, chest, face, scalp and shoulder) using one of two schedules; day1 and day 2 (arm A) or day 1 and day 8 (arm B). Compared to vehicle, both arms showed statistically significant efficacy difference [Table 1]. However, the histological efficacy assessment showed no dose response; in fact, 42% of lesions treated with vehicle gel were histologically cleared. The reason for this is unclear though spontaneous regression may be responsible.[10]
Table 1.
Phase II and III clinical studies done with ingenol mebutate.
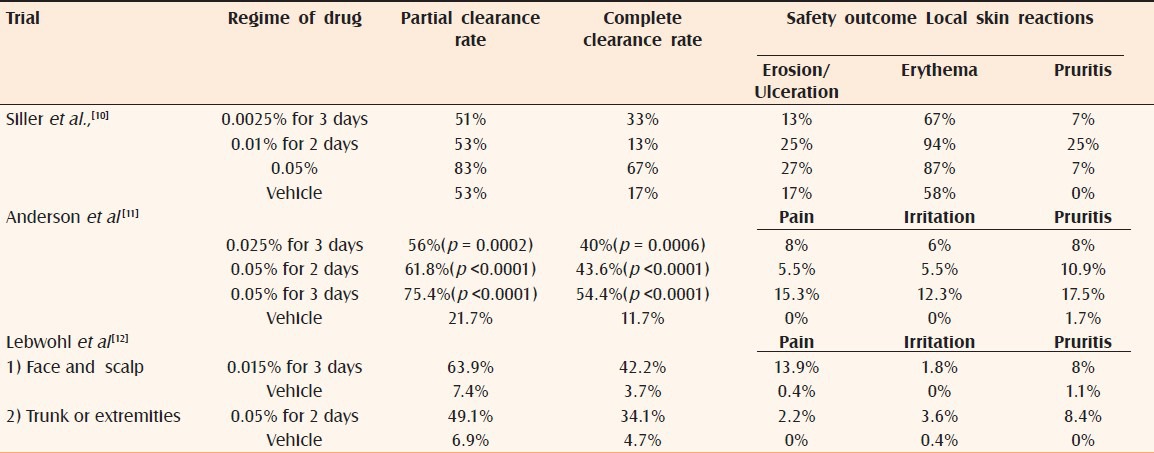
A Phase IIb study investigated short course use of ingenol mebutate for treatment of nonfacial AK. The trial compared ingenol mebutate 0.025% gel applied once-daily for 3 consecutive days or 0.05% gel applied once-daily for 2 or 3 consecutive days as field-directed therapy for an area containing 4–8 clinically typical and discrete AK. The proportion of patients at day 57 with ≥75% reduction in the number of AK lesions (the partial clearance rate) ranged from 56% to 75.4%. The proportion of patients at day 57 with no clinically visible AK lesions in the treatment area (the complete clearance rate) ranged from 40% to 54.4%. A consistent decline in the number of AK lesions from day 3 to day 29 was observed for all three active treatments, and the difference between active treatments and vehicle was highly significant at day 57 (p <0.0001) [Table 1].[11]
The severity of local skin reactions in the active treatment groups followed a dose-dependent pattern, peaked between days 3 and 8 then largely resolved by day 15. There were no serious treatment-related adverse effects during the 8-week follow-up period. Patient approval was high, including satisfaction with respect to tolerability, ease of use and cosmesis.[11]
Four Phase III studies evaluated ingenol mebutate once daily for three consecutive days for treatment of face and scalp (2 studies) and trunk and extremities (2 studies) [Table 1].[12] The median percentage reduction from baseline in number of AK was 83% in the face and scalp studies and 75% in the trunk and extremities group patients compared to 0% in the placebo groups. Local reactions in both study groups peaked at day 4, rapidly decreased by day 8 and approached baseline scores by day 29. Minimal change in pigmentation and minimal scarring was observed.
Apart from its use in clearing AK, ingenol mebutate has shown potential as a unique local chemotherapeutic immunostimulatory debulking agent that could be used in conjunction with CD8 T cell based immunotherapies to promote regression of metastases. Le et al., demonstrated that ingenol mebutate can regress distant pre-existing secondary tumors and subsequently reduce the tumor burden, which improves CD8 T cell-based immunotherapy and additionally, adjuvants the tumor debris to potentiate anti-cancer CD8 T cell activity.[13]
A phase IIb study in 60 patients evaluated ingenol in the treatment of superficial BCC via two applications of ingenol gel at concentrations of 0.0025%, 0.01%, or 0.05%, given either on consecutive days or dosed one week apart. The most convincing efficacy results were reflected in the histological clearance rate of 63% of those randomized to the 0.05% treatment arm as compared to control. Adverse effects were low with no serious events.[14]
SAFETY
The adverse reactions commonly observed in clinical trials involved the application-site reactions such as pain, pruritus, irritation, infection, periorbital edema, nasopharyngitis and headache. The most common local skin responses were dose-related erythema, flaking/scaling/dryness and scabbing/crusting that resolved within one month. Given the dual mechanism of action involving primary necrosis and concurrent inflammation, these adverse effects are not entirely unexpected. Moreover, important safety end points like treatment-related scarring and pigmentary changes were not evident with the topical therapy.
No systemic toxicity was observed in studies. Clinical findings varying from mild epithelial keratoconjunctivitis to severe keratitis have made periocular area unsuitable for application. Safety in pregnant females and children less than 18 years of age has not been established.
CONCLUSION
AK is a premalignant condition and a precursor to sun-related SCC. Ingenol mebutate is a FDA approved novel topical drug for AK. Clinical studies have established its efficacy and safety in treating AK and ongoing studies are evaluating it in NMSC. Short course treatment with ingenol ensures adherence to treatment and gives the physician confidence to eradicate AK lesions early in their evaluation.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
REFERENCES
- 1.Samarasinghe V, Madan V. Nonmelanoma skin cancer. J Cutan Aesthet Surg. 2012;5:3–10. doi: 10.4103/0974-2077.94323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panda S. Non melanoma skin cancer in India: Current scenario. Indian J Dermatol. 2010;55:373–8. doi: 10.4103/0019-5154.74551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramalingam VS, Sinnakirouchenan R, Thappa DM. Malignant transformation of actinic keratoses to squamous cell carcinoma in an albino. Indian J Dermatol. 2009;54:46–8. doi: 10.4103/0019-5154.48986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebwohl M, Sohn A. Ingenol mebutate (ingenol 3- angelate; Pep 005): Focus on its uses in the treatment of non melanoma skin cancer. Expert Rev Dermatol. 2012;7:121–8. [Google Scholar]
- 5.Amini S, Viera MH, Valins W, Berman B. Non surgical innovations in the treatment of non melanoma skin cancer. J Clin Aesthet Dermatol. 2010;3:20–34. [PMC free article] [PubMed] [Google Scholar]
- 6.Patel RV, Frankel A, Goldenberg G. An update on nonmelanoma skin cancer. J Clin Aesthet Dermatol. 2011;4:20–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Fallen RS, Gooderham M. Ingenol mebutate:an introduction. Skin Therapy Lett. 2012;17:1–3. [PubMed] [Google Scholar]
- 8.Rosen RH, Gupta AK, Tyring SK. Dual mechanism of action of Ingenol mebutate gel for topical treatement of acinic keratosis: rapid lesion necrosis followed by lesion - specific immune response. J Am Acad Dermatol. 2012;66:486–93. doi: 10.1016/j.jaad.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 9.Ersvaer E, Kittang AO, Hampson P, Sand K, Gjertsen BT, Lord JM, et al. The protein kinase C agonist PEP 005 (ingenol 3- angelate) in the treatment of human cancer: A balance between efficacy and toxicity. Toxins (Basel) 2010;2:174–94. doi: 10.3390/toxins2010174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siller G, Gebauer K, Welburn P, Katsamas J, Ogbourne SM. PEP 005 (ingenol mebutate) gel, a novel agent for the treatment of actinic keratosis: results of a randomized, double- blind, vehicle- controlled, multicentre, phase IIa study. Australas J Dermatol. 2009;50:16–22. doi: 10.1111/j.1440-0960.2008.00497.x. [DOI] [PubMed] [Google Scholar]
- 11.Anderson L, Schmieder GJ, Werschler WP, Tschen EH, Ling MR, Stough DB, et al. Randomized, double- blind, double- dummy, vehicle-controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. J Am Acad Dermatol. 2009;60:934–43. doi: 10.1016/j.jaad.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Lebwohl M, Swanson N, Anderson LL, Melgaard A, Xu Z, Berman B. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010–9. doi: 10.1056/NEJMoa1111170. [DOI] [PubMed] [Google Scholar]
- 13.Le TT, Gardner J, Hoang-Le D, Schmidt CW, MacDonald KP, Lambley E, et al. Immunostimulatory cancer chemotherapy using local ingenol 3- angelate and synergy with immunotherapies. Vaccine. 2009;27:3053–62. doi: 10.1016/j.vaccine.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Siller G, Rosen R, Freeman M, Welburn P, Katsamas J, Ogbourne SM. PEP 005 (ingenol mebutate) gel for the topical treatment of superficial basal cell carcinoma: Results of a randomized phase IIa trial. Australas J Dermatol. 2010;51:99–105. doi: 10.1111/j.1440-0960.2010.00626.x. [DOI] [PubMed] [Google Scholar]


