Abstract
Stress early in postnatal life may result in long-term memory deficits and selective loss of hippocampal neurons. The mechanisms involved are poorly understood, but they may involve molecules and processes in the immature limbic system that are activated by stressful challenges. We report that administration of corticotropin-releasing hormone (CRH), the key limbic stress modulator, to the brains of immature rats reproduced the consequences of early-life stress, reducing memory functions throughout life. These deficits were associated with progressive loss of hippocampal CA3 neurons and chronic up-regulation of hippocampal CRH expression. Importantly, they did not require the presence of stress levels of glucocorticoids. These findings indicate a critical role for CRH in the mechanisms underlying the long-term effects of early-life stress on hippocampal integrity and function.
Impairment of hippocampal-mediated learning and memory in adults exposed to early-life stress have been well documented (1–4), but the mechanisms involved have remained unclear. Long-term stress in the adult has been shown to result in hippocampal cell loss, promoting the notion that stress early in life might also alter hippocampal neuron structure and function permanently. Likely molecular mechanisms for such long-term effects include signaling processes that have been found to be induced by stressful challenges in the immature central nervous system (3, 5–7).
Established stress-induced molecular cascades in hippocampus include activation of glucocorticoid receptors by adrenal-derived glucocorticoid hormones (8), as well as activation of receptors for the neuropeptide corticotropin-releasing hormone (CRH) (9, 10). Saturation of glucocorticoid receptors by “stress levels” of these hormones can result in hippocampal neuronal injury (11), but these receptors reside primarily in CA1 (12, 13), whereas stress-induced damage involves mainly CA3 (8, 11). In addition, glucocorticoids do not reproduce these effects of stress on hippocampal integrity when administered in a manner that is not stressful to the animal (e.g., in food) (14), suggesting that other factors may be involved (14, 15).
CRH participates in propagation and integration of stress responses in amygdala and hippocampus (9, 10, 16, 17). For example, administration of CRH into the lateral ventricles reproduces the spectrum of behavioral and neuroendocrine responses to stress (16), and enhanced expression of CRH in both adult (18) and immature (10) rat hippocampal interneurons by stress-related neuronal activation has recently been demonstrated. A role for activation of hippocampal CRH receptors in the mechanisms of the effects of early-life stress on hippocampal integrity is supported by several lines of evidence. First, as mentioned, certain stressful situations increase CRH levels in hippocampus (10, 18). In addition, CRH has neurotoxic effects on hippocampal neurons (19–22), and these effects, involving interaction with glutamatergic mechanisms (21, 23) and enhanced calcium entry (21), may be more pronounced in the immature hippocampus (21–23). Indeed, our earlier work has demonstrated that CRH can injure CA3 hippocampal neurons of the immature rat (21, 22), in a pattern highly reminiscent of that found for stress-induced injury. This may be due to the increased numbers of CRH-expressing neurons in developing hippocampus (24) or to increased CRH-receptor density on CA3 pyramidal neurons (25–27).
We reasoned that if the mechanisms by which early-life stress causes long-lasting impairments of hippocampal function and integrity are mediated by CRH, then early-life administration of the peptide should reproduce these deficits. Further, these effects should occur independently of the presence of high plasma glucocorticoid levels. The present study tested these predictions.
Materials and Methods
Animals.
Sprague–Dawley-derived male rats (Zivic–Miller, Zelienople, PA) were born in our vivarium and maintained on a 12-h light/dark cycle with access to unlimited lab chow and water. Delivery was verified at 12-h intervals (date of birth = day 0). Litters were culled to 12 pups and mixed among experimental groups; thus, effects of experimental manipulations were compared among littermates. For technical reasons, animals were reared in several “batches.” However, each batch included both control and experimental groups. When weaned, rats were housed 2–3 per cage.
Surgical and Pharmacological Procedures.
CRH was administered into the lateral ventricle of 10-day-old (P10) freely moving rats kept euthermic on a warming pad, as described (22, 28, 29). Briefly, for acute experiments, CRH was infused via cannulae implanted 24 h earlier under halothane anesthesia (≈10 min/rat). For long-term experiments, 0.75 nmol of CRH were administered by using a semistereotaxic freehand infusion (29). Separation of pups from the dam (<4 h) was equal for all groups. For examination of acute CRH-induced injury, a subgroup of rats was given CRH (0.75 nm) via the cannula twice daily (8 a.m. and 5 p.m.) on P11 and P12 (four times total). Rats were killed 24 h later (P13) by using pentobarbital injection and perfused transcardially with 0.9% saline followed by cold 4% paraformaldehyde.
Adrenalectomy was performed under halothane anesthesia (≈5 min/rat) on P10, 24 h before CRH infusion, via small bilateral dorsal incisions that were closed with acrylic glue (30). The completeness of the adrenalectomy was verified by visual inspection upon death. To permit normal mineralocorticoid function and based on pilot experiments, adrenalectomized rats were given aldosterone (s.c., 2 μg/100 gm body weight per day) during P10–P21 (31). After weaning (P21), corticosterone (10 mg/liter) was added to the drinking solution (0.9% saline) (31, 32). This supplementation (“clamping”) leads to chronic “basal” glucocorticoid levels (31, 32), saturating mineralocorticoid but not glucocorticoid receptors (12, 13).
Experiments were initiated between 8 a.m. and 10 a.m. to minimize diurnal variability, were carried out according to National Institutes of Health guidelines, and approved by the Institutional Animal Care Committee.
In Situ Hybridization (ISH) Histochemistry for CRH and CRF1 mRNA and Data Analysis.
ISH analyses were performed on tissue from 12-mo-old rats that were killed by rapid decapitation. Brains were immediately removed and frozen on dry ice. ISH for CRH mRNA was performed on 20-μm-thick coronal sections as described (10, 26, 30). For CRF1 mRNA, ISH was performed as described for cRNA probes (33, 34). For both methods, hybridized and washed sections were apposed to film (Hyperfilm β-Max, Amersham Pharmacia) for 7–14 days, and selected sections were then dipped in emulsion (NTB-2, Eastman Kodak) and exposed for 3–4 weeks. Semiquantitative analysis of ISH signal was performed on digitized films as described (30, 34). For analysis, three matched dorsal hippocampal sections per rat were sampled by using unbiased methods (34).
BrdUrd Labeling for Detection of Newborn Cells.
BrdUrd (Roche Molecular Biochemicals, 100 mg/kg) was injected into 12-mo-old rats, perfused 48 h later. Brains were sectioned (50 μm), washed in 2 × SSC, immersed in 50% formamide/2 × SSC (2 h, 65°C) to denature DNA, and incubated in 2 M HCl (30 min, 37°C). After neutralization (0.1 M sodium borate), sections were incubated with anti-BrdUrd (1:400, Accurate Chemicals), followed by biotinylated second antibody, and BrdUrd-labeled nuclei were detected by using the avidin–biotin–peroxidase reaction with diaminobenzidine as chromogen (10, 24, 25).
Timm's Stain.
The Chafetz (35) modification for frozen tissue was used. Briefly, thawed, mounted 20-μm coronal sections were dipped six times (1 dip/s) into a 0.37% sulfide solution. Slides were air-dried (3–5 min) and fixed in 4% paraformaldehyde for 20 min. After development (1 h), sections were rinsed in double-distilled water and counterstained with cresyl violet. The extent of aberrant mossy fiber “sprouting” was evaluated by using a semiquantitative scale (0–5) for terminal sprouting in the CA3 hippocampal region (36). Briefly, score criteria were as follows: 0 = no granules in strata pyramidale (SP) or oriens (SO); 1 = occasional discrete granule bundles in SP/SO; 2 = occasional to moderate granules in SP/SO; 3 = prominent granules in SP/SO; 4 = prominent near-continuous granule band in SP/SO along the entire CA3; and 5 = continuous or near-continuous dense granule band in SP/SO along the entire CA3. Scores were determined “blindly” (without knowledge of treatment) on three matched dorsal hippocampal sections/rat per experimental group. Both hippocampi were analyzed and averaged to yield the final score.
Morris Water Maze (MWM).
The procedure established by Morris (37) was followed. Briefly, the MWM, a circular pool (diameter, 2 m; depth, 0.6 m) was filled with water (19–21°C) opacified by powdered milk. A transparent platform (diameter, 13 cm) was placed in a constant position for each set of trials, in the middle of one of the pool's quadrants, 1–2 cm below the water surface to render it invisible. Tested rats likely obtained visual cues from objects in the testing room because, in a probe trial, when the platform was removed they tended to spend more time in the quadrant where the platform had previously been located. Rats were subjected to two consecutive training days (two series of 10 trials) to familiarize them with finding and perching on the hidden platform that was kept in a fixed location. On the test day (day 3), the platform was placed in a novel location, and rats were placed in the water facing the pool wall. Starting positions were randomly rotated in different quadrants, and rats were subjected to six trials. For each trial, latency to reach the platform was recorded. Rats were allowed 60 s to reach the platform and were manually placed on it if they failed.
Object Recognition.
This memory test, relying on spontaneous exploratory behavior, has been described in detail (38). Briefly, adult rats were tested in a quiet room, in a 52 × 27 × 21-cm Plexiglas cage lined with opaque white paper, with the front panel open to observation. Subjects were given five habituation sessions (1 h each in the cage with no objects). Test objects were made of glass and metal (e.g., padlock, light bulb), and duplicate objects were used in sample and test trials to avoid odor cues. During the tests, objects were placed in random locations, ≈6 cm from the cage side. The object recognition memory test consisted of giving each rat one sample trial, during which it was allowed to explore two objects for 5 min. The test trial was given 24 h later and consisted of a 5-min epoch in which the rat was presented with a duplicate of an object from the sample trial and a novel object. In both sample and test trials, the duration of exploration of each object, defined as sniffing with the animal's nose in contact or within 2 cm of the object, was recorded.
Cell Counts.
Cells were counted in paraformaldehyde-fixed, Nissl-stained sections of the hippocampal CA3 pyramidal cell layer (SP). CA3 subdivisions were defined by using an imaginary line connecting the tips of the granule cell layer blades, which separated CA3c (medially) from CA3b (see Fig. 2C). For CA3a, a reticule grid was centered over the lateral tip of CA3, and cells within 300-μm strips were counted in both directions. Cells within 300-μm strips along SP were counted also in CA3b and CA3c. To avoid bias from potential changes in hippocampal volume associated with CRH treatment or neuronal loss, hippocampal volume was estimated according to ref. 39. Briefly, volumes were calculated by summing areas of one in five coronal hippocampal sections, by using a grid reticule at low power, and multiplying this value by the distance between the sections. Profile counts were obtained counting nucleoli in 20-μm sections, thus avoiding stereological confounders (39–41). Every fifth dorsal hippocampal section between −3.8 and −4.3 Bregma (42) was counted (five sections per rat; 5–7 rats per group). Bilateral values were obtained “blindly” and averaged and are reported as absolute number of cells per area counted (0.18 mm2).
Figure 2.
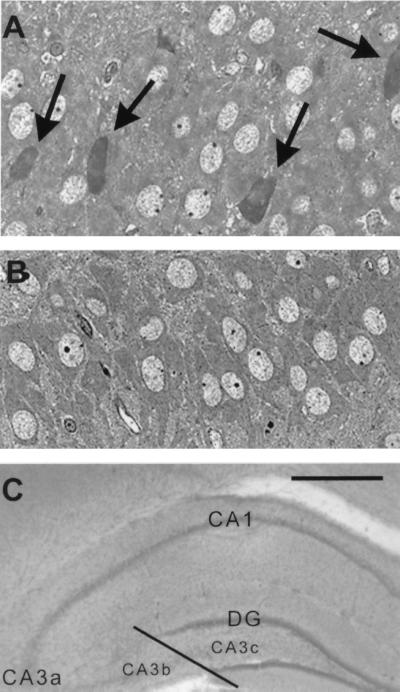
Acute injury of CA3 hippocampal pyramidal cells in P13 rats results from CRH administration. (A) Shrunken, toluidine blue-stained, injured cells (arrows) are visible in 1-μm sections from CRH-treated rats, but not in sections from vehicle-treated controls (B). (C) Subdivisions of CA3 pyramidal cell layer, denoting the CA3b/CA3c border. [Scale bars = 20 μm (A and B) and 200 μm (C).]
Statistical Considerations.
Statistical significance (P < 0.05) was determined by using a one- or two-way ANOVA or Student's t test, as appropriate (Prism GraphPad; San Diego).
Results and Discussion
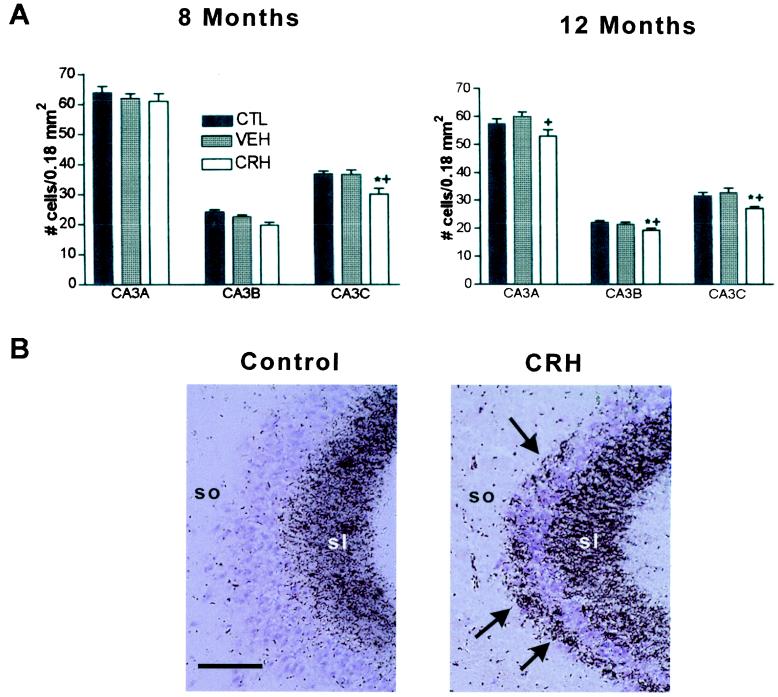
To determine whether neuronal loss in hippocampal CA3 subfields resulted from early-life administration of CRH, we used unbiased stereological cell counts. Indeed, numbers of hippocampal CA3 pyramidal cells were reduced in adult rats that were treated with CRH early in life (P10) (21), compared with vehicle-treated controls (Fig. 1A). A significant, 17% decrease in CA3c pyramidal layer neurons was already evident by age 8 mo (one-way ANOVA with Tukey's posttest, P < 0.05), and by 12 mo, reduced neuronal numbers were observed throughout the CA3 pyramidal cell layer (Fig. 1A). Specifically, at 12 mo, CRH-treated rats (n = 12) lost 12%, 10%, and 18% of cells in CA3a, CA3b, and CA3c, respectively, compared with vehicle-treated controls (n = 7). There was also a slight reduction of CA3 cell numbers of each of the experimental groups at the 12-mo time point compared with the 8-mo counts. This age-related neuronal loss in the “middle-aged” rat hippocampus has been described (ref. 43, but see ref. 44). Hippocampal volumes did not differ between control and experimental groups.
Figure 1.
Selective neuronal loss and synaptic reorganization in hippocampal CA3 of adult rats given CRH early in life. (A) Cell numbers in subregions of the CA3 pyramidal cell layer from CRH-treated, vehicle-treated, and naive controls were determined at age 8 and 12 mo. One-way ANOVA with Tukey's analysis indicated significant (P < 0.05; * vs. naive; + vs. vehicle-treated) neuronal loss in CRH-treated rats, which was selective to CA3C at 8 mo but involved all CA3 subregions by 12 mo. (B) Sections of CA3A pyramidal cell regions from vehicle- and CRH-treated rats (killed at 12 mo), subjected to Timm's stain for visualizing the high zinc content of mossy fiber (axons of the CA3-innervating granule cells) terminals. In CRH-treated rats, these terminals were abnormally abundant within CA3 stratum oriens (so). sl, stratum lucidum. (Scale bar = 50 μm.)
The loss of CA3 pyramidal layer cells was reflected by altered growth patterns of the mossy fibers, the axons of the dentate gyrus granule cells that normally innervate these CA3 neurons. Exuberant growth of mossy fibers into the CA3a stratum oriens occurred in CRH-treated animals (n = 7; Timm's score 2.6 ± 0.3) compared with vehicle-treated controls (n = 4, Timm's score 0.3 ± 0.3; Fig. 1B) and naive controls (n = 4, Timm's score 0.2 ± 0.2). This “sprouting” is consistent with, and typical of, a loss of the normal targets of the mossy fibers (22). Importantly, the synapses formed by the aberrant mossy fibers on the remaining CA3 pyramidal cells are excitatory (glutamatergic), which could promote further excitotoxic injury to these neurons.
It should be noted that the observed reduction in neuronal numbers was a true cell loss rather than a suppression of neurogenesis. Stress, and particularly high levels of glucocorticoids, can suppress neurogenesis (45, 46). To determine whether the rate of neurogenesis differed among experimental groups in these studies, BrdUrd was injected to both adrenalectomized, glucocorticoid “clamped” rats and to intact ones to identify newly born cells. Immunohistological analysis 48 h later revealed no evidence of altered numbers of BrdUrd-labeled cells in the dentate gyrus hilus of rats with highly differing glucocorticoid levels (not shown), suggesting that long-term changes in steroid levels were probably not sufficient to account for the significant changes in hippocampal cell numbers observed here.
To investigate the potential mechanisms for CA3 pyramidal cell loss in adult rats treated with CRH early in life, we determined whether acute CRH administration during that developmental period damaged hippocampal neurons. CA3 pyramidal neurons in CRH-treated (Fig. 2A), but not in vehicle-treated (Fig. 2B), rats demonstrated evidence of acute injury (22). This was confined to the CA3 hippocampal field, a region particularly rich in mRNA and protein expression of the CRH receptor subtype (CRF1), which has been shown to mediate the peptide's excitatory effects in immature hippocampus (26, 27).
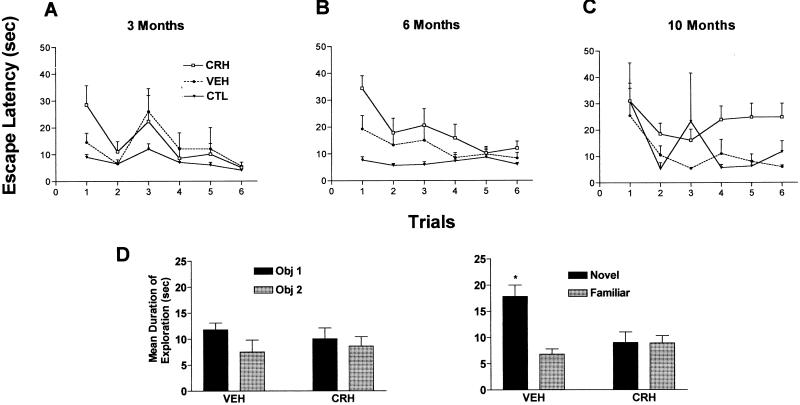
Because neurons damaged by CRH administration comprised only a minority of hippocampal CA3 cells (22), the significance of their loss for hippocampal-mediated memory functions was evaluated. Using the MWM test (37), rats given CRH early in life demonstrated worse and progressively declining memory performance when tested repeatedly at ages 3, 6, and 10 mo. A trend for spatial memory impairment was apparent already by 3 mo in CRH-treated rats (n = 12, F2,132 = 1.95, P = 0.15; Fig. 3A), and was significant both in 6-mo-old (F2,132 = 9.62, P < 0.001; Fig. 3B) and 10-mo-old (F2,132 = 5.53, P < 0.01; Fig. 3C) animals. It should be noted that at all ages examined, the MWM performance of vehicle-treated animals (n = 7) did not differ significantly from that of naive controls (n = 6). Further, based on swim speed calculations and search pattern analyses, there appeared to be no differences in locomotor activity or motivation among the experimental groups. In addition, as shown by others (43, 47), an apparent effect of age was found on the first and second trials; 10-mo-old control animals took longer to reach the platform in these trials compared with younger animals.
Figure 3.
Deficient short-term memory skills in adult rats given CRH centrally early in life. (A) CRH-treated rats show a trend toward impaired performance (increased escape latency) using the MWM at age 3 mo. By 6 (B) and 10 (C) mo, rats treated with CRH early in life take significantly longer to locate the hidden platform (two-way ANOVA, treatment effect at 6 mo: F2,132 = 9.62, P < 0.001; at 10 mo: F2,132 = 5.53, P < 0.01). This deficit is not attributable to injection procedures because latencies of vehicle controls were significantly shorter than those of the CRH-treated group and not significantly different from those of naive controls. Note the progression of the spatial memory acquisition impairment in CRH-treated rats. (D) CRH-treated rats suffer from hippocampus-dependent memory dysfunction also in the nonaversive, nonstressful object recognition test. On day 1, pattern and duration of exploration of two novel objects were indistinguishable in CRH- and vehicle-treated rats. However, 24 h later (day 2), vehicle-treated rats discriminated between familiar and novel objects [remembered the familiar object and explored it for a significantly (*) shorter time; paired t test; P < 0.05], whereas CRH-treated rats did not discern the novel from the familiar object, indicating impairment of short-term recognition memory. n = 6–12 rats per group.
These data demonstrate that functional hippocampal impairment in rats given CRH early in life arose already at age 3 mo. Because the same animals were tested repeatedly, and because behavioral and histological studies were conducted on the same rats, cell counts could only be obtained upon death (12 mo). Thus, it is not possible to determine whether the early (3 mo) cognitive impairment required actual cell death or reflected cellular/molecular dysfunction that eventually led to cell loss. Indeed, similar MWM spatial memory deficits in the absence of hippocampal cell loss (44), or in association with altered pyramidal cell morphology (48), have been described.
Because the MWM test entails stressful elements, deficits in this paradigm might reflect potential consequences of early-life CRH treatment on the rats' ability to cope with stress or on their motivation. Therefore, to further test for hippocampus-dependent learning deficits, 10-mo-old rats were subjected to the nonaversive object recognition test, which relies on the fact that rats with intact hippocampi will spend more time exploring a novel object compared with one encountered on the previous day (38). CRH treatment early in life did not influence the duration of exploration of the two objects on the sample trial day (day 1, Fig. 3D). However, whereas vehicle controls (n = 7) remembered the familiar object on the test day (day 2) and spent significantly more time exploring the novel one (paired t test, P < 0.05; Fig. 3D), CRH-treated animals (n = 10) did not distinguish between the novel and familiar objects and explored both equally. Again, the total duration of object exploration did not differ between the groups on the test day (day 2), indicating that the motivation of CRH-treated rats was not affected. Taken together, the results of the object recognition and MWM tests indicate that CRH-treated rats were deficient in short-term memory functions, and this dysfunction was not attributable to poor motivation or abnormal responses to stressful situations.
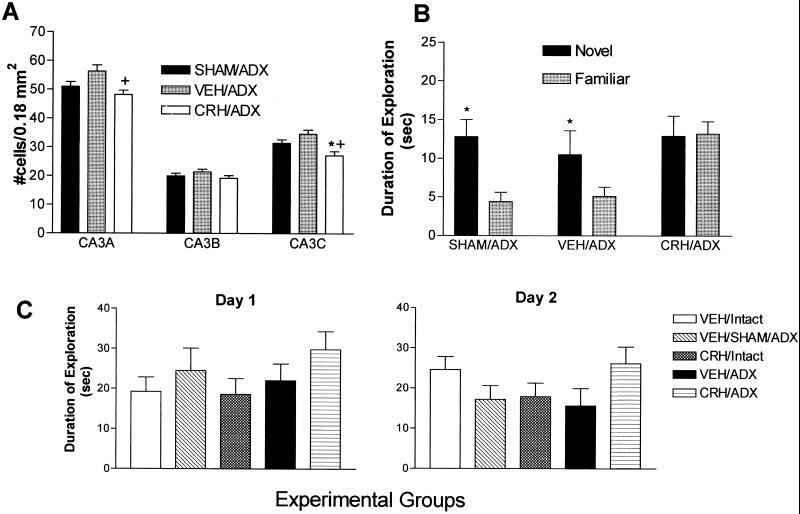
High (“stress”) levels of glucocorticoids, saturating hippocampal glucocorticoid receptors, may damage hippocampal neurons (8, 11). Therefore, we determined whether such high glucocorticoid levels were required for the hippocampal injury and deficits caused by early-life administration of CRH. Thus, CRH was given to immature rats rendered devoid of endogenous steroids (adrenalectomized), in which glucocorticoid levels were thereafter maintained (by supplementing the drinking water) at levels much lower than those seen during stress (“clamped”) (30–32). These rats were tested (at the age of 10 mo) for hippocampal-mediated memory function and analyzed for hippocampal neuronal cell loss (at 12 mo). As observed with intact animals, CRH given early in life to animals with clamped levels of glucocorticoids (n = 5) led to significant loss (≈13–21%) of hippocampal CA3 pyramidal cells during adulthood as compared with vehicle-treated animals with similar, clamped glucocorticoid levels (n = 4) and sham-operated controls (n = 4, one-way ANOVA with Tukey's analysis, P < 0.05; Fig. 4A). In addition, cognitive impairment in the object recognition test was still produced by early-life CRH administration even in animals with low, constant glucocorticoid levels (n = 10; Fig. 4B), whereas vehicle-treated adrenalectomized (n = 7) and sham-operated rats (n = 5) did not demonstrate impairments. This deficit was specific and most likely not associated with decreased motivation as determined by similar total exploration time on both the sample trial and testing day (Fig. 4C). Although there was a discrepancy in exploration behavior on day 2 between CRH-treated intact animals (which treated the novel object as familiar) and CRH-treated glucocorticoid clamped animals (which treated the familiar object as novel), both sets of results importantly suggest that the animals given CRH early in life could not discriminate between the two objects. Thus, these data clearly indicate that high plasma glucocorticoid levels were not required for the anatomical and cognitive effects of early-life CRH administration.
Figure 4.
Hippocampal cell loss and short-term memory deficits in 12-mo-old rats given CRH early in life do not require glucocorticoid receptor saturation. (A) Cells were counted in CA3 pyramidal cell layer subregions of vehicle-treated sham-adrenalectomized (SHAM/ADX), of vehicle-treated (VEH/ADX), and of CRH-treated and ADX rats (CRH/ADX), which were maintained with chronic low-level glucocorticoids. Significant neuronal loss (P < 0.05; *, vs. SHAM/ADX; +, vs. VEH/ADX; one-way ANOVA with Tukey's multiple comparison analysis) occurred in CRH-treated rats, confined to CA3A and CA3C. (B) On the second day (day 2, see also Fig. 3D) of the object recognition test, SHAM/ADX controls and those with chronic low-level glucocorticoids after early-life adrenalectomy (VEH/ADX) explored the novel object significantly longer (*, P < 0.05; paired t test) than the familiar one, a behavior requiring intact hippocampal function. In contrast, the CRH-treated group (CRH/ADX, also maintained with low plasma glucocorticoid levels) explored both objects for a similar amount of time (i.e., did not remember the familiar object). This indicates that memory impairment after early-life CRH administration did not require the presence of stress levels of plasma glucocorticoids. (C) Neither ADX with low-level steroid supplementation nor early-life CRH altered the overall exploration skills and patterns of adult rat. Total exploration time on both day 1 and day 2 demonstrated the expected individual variability and did not differ significantly among experimental groups (see also Fig. 3D).
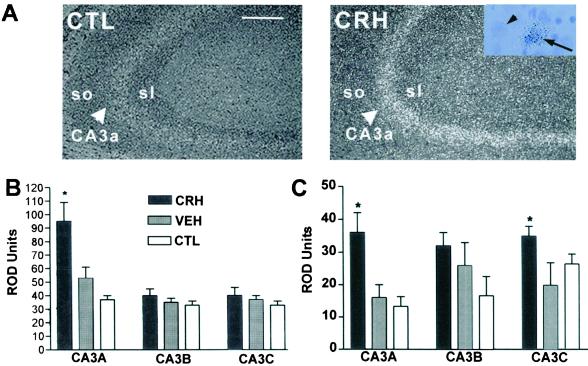
Both the structural (hippocampal cell loss) and functional consequences of early-life CRH administration appeared to progress with age. Comparing 8-mo-old to 12-mo-old rats, the loss of hippocampal CA3 neurons spread from CA3c to other CA3 subfields (Fig. 1A), and memory performance of CRH-treated rats increasingly diverged from that of vehicle-treated controls (Fig. 3, compare A vs. C). In considering potential mechanisms for this progression, we speculated that prolonged up-regulation of CRH expression in interneurons residing in the CA3 pyramidal cell layer may promote ongoing neuronal injury and associated memory impairment (21, 24). Using ISH to determine CRH expression in hippocampus (10, 30), steady-state CRH mRNA levels in the CA3 hippocampal field were significantly higher in adult animals treated with CRH early in life (n = 7), compared with vehicle-treated (n = 4) or naive (n = 4) controls (Fig. 5 A and B). Our previous studies have demonstrated up-regulation of CRH mRNA levels in CA3 hippocampal interneurons upon neuronal activation induced by some (but not all) early-life stresses (10). It is therefore suggested that the neuronal stimulation induced by CRH administration to immature rats (21, 27), which generally reproduces the pattern of neuronal activation provoked by stress (28, 49), led to chronic elevation of CRH synthesis in CRH-expressing basket cells (24). Increased release of the peptide from terminals innervating pyramidal cells throughout CA3 would promote excessive activation of CRH receptors on CA3 pyramidal neurons (25–27). Interestingly, these receptors were also up-regulated in adult animals given CRH early in life (Fig. 5C). Increased activation of CRH receptors, known to enhance glutamatergic neurotransmission (21, 23), coupled with the aberrant mossy-fiber excitatory synapses (Fig. 1B), may thus contribute to progressive vulnerability of CA3 pyramidal neurons to excitotoxic injury (8, 19–22) and progressive loss of these neurons, with consequent functional deficits.
Figure 5.
Chronic up-regulation CRH and its receptor CRF1 mRNAs in hippocampal CA3 pyramidal cells of 12-mo-old rats results from early-life administration of CRH. (A) Enhanced CRH mRNA expression in CA3 pyramidal cell layer (large arrowheads) in dark-field micrographs of emulsion-dipped sections from CRH-treated rats and controls. (Inset) Silver grains over an eccentrically located neuron (arrow) suggest CRH expression in nonpyramidal cells (interneurons). Small arrowhead points to an adjacent cell devoid of silver grains. (B) Semiquantitative analysis shows enhanced CRH mRNA levels in CA3A of CRH-treated rats. (C) CRF1 mRNA is significantly up-regulated in CA3A and CA3C of CRH-treated rats. *, significant (P < 0.05) difference from vehicle and naive controls (one-way ANOVA with Tukey's analysis). CTL, control; so and sl, strata oriens and lucidum, respectively. [Scale bars = 100 μm (A) and 25 μm (Inset).]
In summary, these studies demonstrate that progressive hippocampal memory dysfunction and cell loss found after early-life stress result from early-life administration of the stress-neurohormone, CRH. Loss of CA3 pyramidal cells, as well as deficits in spatial memory acquisition and in object recognition, both dependent on hippocampal integrity, were observed. Importantly, cell loss and memory impairment in the object recognition test were also detected in CRH-treated animals in which plasma glucocorticoids were clamped at low levels, suggesting that stress levels of these hormones and saturation of glucocorticoid receptors were not required for these effects. Thus, the data presented here support the notion that CRH may be a critical contributor to the processes by which early-life stress compromises hippocampal structure and function and provide a rationale for targeting early modulation of hippocampal CRH for amelioration of certain human stress-related disorders.
Acknowledgments
We thank Drs. C. M. Gall and F. E. Bloom for critical comments and U. Staubli for input on behavioral testing. Technical assistance of B. Mouradi and G. Hanna, and excellent editorial support of M. Hinojosa, are appreciated. This work was supported by National Institutes of Health Grants NS 28912 and HD34975 (to T.Z.B.) and AG00096 (to K.L.B.).
Abbreviations
- CRH
corticotropin-releasing hormone
- ISH
in situ hybridization
- MWM
Morris water maze
References
- 1.Trickett P K, McBride-Chang C. Dev Rev. 1995;15:311–337. [Google Scholar]
- 2.Ammerman R T, Cassisi J E, Hersen M, van Hasselt V B. Clin Psychol Rev. 1986;6:291–310. [Google Scholar]
- 3.Heim C, Owens M J, Plotsky P M, Nemeroff C B. Psychopharmacol Bull. 1997;33:185–192. [PubMed] [Google Scholar]
- 4.Sanchez M M, Hearn E F, Do D, Rilling J K, Herndon J G. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- 5.Plotsky P M, Meaney M J. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 6.Coplan J D, Andrews M W, Rosenblum L A, Owens M J, Friedman S, Gorman J M, Nemeroff C B. Proc Natl Acad Sci USA. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ladd C O, Huot R L, Thrivikraman K V, Nemeroff C B, Meaney M J, Plotsky P M. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- 8.McEwen B S, Magarinos A M. Ann NY Acad Sci. 1997;821:271–284. doi: 10.1111/j.1749-6632.1997.tb48286.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee E H, Huang A M, Tsuei K S, Lee W Y. Chin J Physiol. 1996;39:197–203. [PubMed] [Google Scholar]
- 10.Hatalski C G, Brunson K L, Tantayanubutr B, Chen Y, Baram T Z. Neuroscience. 2000;101:571–580. doi: 10.1016/s0306-4522(00)00386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sapolsky R M, Krey L C, McEwen B S. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reul J M, de Kloet E R. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 13.Herman J P. Cell Mol Neurobiol. 1993;13:349–372. doi: 10.1007/BF00711577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leverenz J B, Wilkinson C W, Wamble M, Corbin S, Grabber J E, Raskind M A, Peskind E R. J Neurosci. 1999;19:2356–2361. doi: 10.1523/JNEUROSCI.19-06-02356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez M M, Young L J, Plotsky P M, Insel T R. J Neurosci. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koob G F, Heinrichs S C, Pich E M, Menzaghi F, Baldwin H, Miczek K, Britton K T. Ciba Found Symp. 1993;172:277–289. doi: 10.1002/9780470514368.ch14. [DOI] [PubMed] [Google Scholar]
- 17.Swiergel A H, Takahashi L K, Kalin N H. Brain Res. 1993;623:229–234. doi: 10.1016/0006-8993(93)91432-r. [DOI] [PubMed] [Google Scholar]
- 18.Smith M A, Weiss S R, Berry R L, Zhang L X, Clark M, Massenburg G, Post R M. Brain Res. 1997;745:248–256. doi: 10.1016/s0006-8993(96)01157-2. [DOI] [PubMed] [Google Scholar]
- 19.Maecker H, Desai A, Dash R, Rivier J, Vale W, Sapolsky R. Brain Res. 1997;744:166–170. doi: 10.1016/s0006-8993(96)01207-3. [DOI] [PubMed] [Google Scholar]
- 20.Strijbos P J, Relton J K, Rothwell N J. Brain Res. 1994;656:405–408. doi: 10.1016/0006-8993(94)91485-0. [DOI] [PubMed] [Google Scholar]
- 21.Baram T Z, Hatalski C G. Trends Neurosci. 1998;21:471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baram T Z, Ribak C E. NeuroReport. 1995;6:277–280. doi: 10.1097/00001756-199501000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollrigel G S, Chen K, Baram T Z, Soltesz I. Neuroscience. 1998;84:71–79. doi: 10.1016/s0306-4522(97)00499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan X X, Toth Z, Schultz L, Ribak C E, Baram T Z. Hippocampus. 1998;8:231–243. doi: 10.1002/(SICI)1098-1063(1998)8:3<231::AID-HIPO6>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Brunson K L, Muller M B, Cariaga W, Baram T Z. J Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avishai-Eliner S, Yi S J, Baram T Z. Dev Brain Res. 1996;91:159–163. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baram T Z, Chalmers D T, Chen C, Koutsoukos Y, De Souza E B. Brain Res. 1997;770:89–95. doi: 10.1016/s0006-8993(97)00759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubé C, Brunson K L, Nehlig A, Baram T Z. J Cereb Blood Flow Metab. 2000;20:1414–1424. doi: 10.1097/00004647-200010000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baram T Z, Schultz L. Dev Brain Res. 1991;61:97–101. doi: 10.1016/0165-3806(91)90118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunson K L, Khan N, Eghbal-Ahmadi M, Baram T Z. Ann Neurol. 2001;49:304–312. [PMC free article] [PubMed] [Google Scholar]
- 31.Walker C-D, Akana S F, Cascio C S, Dallman M F. Endocrinology. 1990;127:832–842. doi: 10.1210/endo-127-2-832. [DOI] [PubMed] [Google Scholar]
- 32.Akana S F, Dallman M F. Endocrinology. 1997;138:3249–3258. doi: 10.1210/endo.138.8.5291. [DOI] [PubMed] [Google Scholar]
- 33.Chalmers D T, Lovenberg T W, De Souza E B. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eghbal-Ahmadi M, Avishai-Eliner S, Hatalski C G, Baram T Z. J Neurosci. 1999;19:3982–3991. doi: 10.1523/JNEUROSCI.19-10-03982.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chafetz M D. Brain Res Bull. 1985;16:19–24. doi: 10.1016/0361-9230(86)90007-9. [DOI] [PubMed] [Google Scholar]
- 36.Holmes G L, Sarkisian M, Ben-Ari Y, Chevassus-Au-Louis N. J Comp Neurol. 1999;404:537–553. doi: 10.1002/(sici)1096-9861(19990222)404:4<537::aid-cne9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Morris R. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 38.Clark R E, Zola S M, Squire L R. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunderson H J, Bendtsen T F, Korbo L, Marcussen N, Moller A, Nielsen K, Nyeengaard J R, Pakkenberg B, Sorensen F B, Versterby A. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 40.Coggeshall R E, Lekan H A. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 41.Popken G J, Farel P B. J Comp Neurol. 1997;386:8–15. [PubMed] [Google Scholar]
- 42.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic; 1982. [DOI] [PubMed] [Google Scholar]
- 43.Meaney M J, Aitken D H, Van Berkel C, Bhatnagar S, Sapolsky R M. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- 44.Rasmussen T, Schliemann T, Sørensen J C, Zimmer J, West M J. Neurobiol Aging. 1996;17:143–147. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- 45.Gould E, Tanapat P, McEwen B S, Flügge G, Fuchs E. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gould E, Tanapat P. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 47.Aitken D H, Meaney M J. Neurobiol Aging. 1989;10:273–276. doi: 10.1016/0197-4580(89)90062-6. [DOI] [PubMed] [Google Scholar]
- 48.Landfield P W, Braun L D, Pitler T A, Lindsey J D, Lynch G. Neurobiol Aging. 1981;2:265–275. doi: 10.1016/0197-4580(81)90034-8. [DOI] [PubMed] [Google Scholar]
- 49.Imaki T, Shibasaki M, Hotta M, Demura H. Brain Res. 1993;616:114–125. doi: 10.1016/0006-8993(93)90199-w. [DOI] [PubMed] [Google Scholar]