Abstract
A clinical isolate of Escherichia coli from a patient in Japan, isolate KU6400, was found to produce a plasmid-encoded β-lactamase that conferred resistance to extended-spectrum cephalosporins and cephamycins. Resistance arising from production of a β-lactamase could be transferred by either conjugation or transformation with plasmid pKU601 into E. coli ML4947. The substrate and inhibition profiles of this enzyme resembled those of the AmpC β-lactamase. The resistance gene of pKU601, which was cloned and expressed in E. coli, proved to contain an open reading frame showing 99.8% DNA sequence identity with the ampC gene of Citrobacter freundii GC3. DNA sequence analysis also identified a gene upstream of ampC whose sequence was 99.0% identical to the ampR gene from C. freundii GC3. In addition, a fumarate operon (frdABCD) and an outer membrane lipoprotein (blc) surrounding the ampR-ampC genes in C. freundii were identified, and insertion sequence (IS26) elements were observed on both sides of the sequences identified (forming an IS26 composite transposon); these results confirm the evidence of the translocation of a β-lactamase-associated gene region from the chromosome to a plasmid. Finally, we describe a novel plasmid-encoded AmpC β-lactamase, CFE-1, with an ampR gene derived from C. freundii.
The AmpC β-lactamase produced by gram-negative bacteria such as Citrobacter spp., Enterobacter spp., Serratia spp., and Morganella spp. can hydrolyze several β-lactam antibiotics, including cephamycins and extended broad-spectrum cephalosporins (30).
The regulation of AmpC β-lactamase expression is intimately linked to cell wall recycling and involves at least three genes: ampR, which encodes a transcriptional regulator of the LysR family; ampG, which encodes a transmembrane permease; and ampD, which encodes a cytosolic N-acetyl-anhydromuramyl-l-alanine amidase that hydrolyzes 1,6-anhydromuropeptides (16, 21, 23, 31). AmpR has been shown to bind to a 38-bp sequence within the intercistronic region between ampR and ampC. In the absence of a β-lactam inducer, AmpR represses the synthesis of β-lactamase 2.5-fold, whereas expression is induced 10- to 200-fold in the presence of a β-lactam inducer (26). Mutations in the specific site of ampR work as an activator of ampC and result in the constitutive hyperproduction of AmpC β-lactamase (3, 4, 24). Deletion mutation of the ampR gene results in a slightly higher level of basal expression of the Citrobacter freundii β-lactamase, but enzyme synthesis can no longer be induced. Knockout mutations in the ampD gene result in constitutive hyperproduction of the AmpC β-lactamase even in the absence of a β-lactam inducer (6, 18).
In recent years ampC genes have been found on conjugative plasmids, mainly among Klebsiella pneumoniae isolates but also occasionally among Escherichia coli isolates. Some of these plasmid-encoded genes have DNA and amino acid sequences very similar to those of the chromosome-encoded AmpC β-lactamases of C. freundii (CMY-2, CMY-4, and LAT-1) (1, 5, 42, 43), Enterobacter cloacae (MIR-1 and ACT-1) (11, 33), and Morganella morganii (DHA-1 and DHA-2) (13, 14), although the phylogenies of the various enzymes (FOX-1, MOX-1, and CMY-9) (12, 15, 19) remain unclear (Fig. 1).
FIG. 1.
Dendrogram for chromosomal and plasmid-encoded AmpC β-lactamases calculated by the Clustal V program by using the neighbor-joining method (38).
Until recently, plasmid-encoded ampC genes were considered noninducible because they lack the regulator gene ampR (35). However, this generalization is no longer valid: three inducible plasmid-encoded AmpC β-lactamases, DHA-1, DHA-2, and ACT-1, have been described; and all of these carry the ampR and the ampC genes (2, 13, 37). The mechanism by which plasmid-encoded AmpC β-lactamase was generated from the chromosomal gene has not yet been discovered.
Compared with plasmid-encoded class A extended-spectrum β-lactamases, these plasmid-encoded AmpC β-lactamases (except for ACC-1) are active against cephamycins and are also effective against oxyimino-cephalosporins, such as cefotaxime, ceftazidime, and aztreonam, a monobactam. The in vitro activities of these AmpC β-lactamases are not inhibited by clavulanic acid. Genes encoding these enzymes are now found on plasmids at increasing frequencies (34).
A plasmid-encoded AmpC β-lactamase which confers resistance to cephamycins and expanded-spectrum cephalosporins was detected in Japan in a clinical isolate of E. coli. In this report, we characterize a novel plasmid-encoded AmpC β-lactamase, CFE-1, and analyze the nucleotide sequences of the ampC, ampR, and surrounding genes to compare with those of chromosome-encoded and plasmid-encoded AmpC β-lactamases.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. Clinical strain E. coli KU6400 was isolated at a hospital in Japan in 1997 and was found to contain plasmid pKU601. E. coli K-12 ML4947 (AmpD wild type) and ML4953 (AmpD mutant) strains were used as recipients of the plasmid (24, 27). Plasmid pHSG398 is a vector plasmid that confers resistance to chloramphenicol.
TABLE 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Characteristicsa |
|---|---|
| Bacterial strains | |
| E. coli | |
| ML4947 | F−galK2 galT22 hsdR hsdM lacY1 metB1 relA supE44 Rifr (AmpD wild type) |
| ML4953 | F−galK2 galT22 hsdR hsdM lacY1 metB1 relA supE44 RiframpD9 (AmpD mutant) |
| KU6400 | Clinical isolate carrying pKU601 |
| C. freundii GC3 | Clinical isolate from Japan which produces AmpC β-lactamase |
| Plasmids | |
| pKU601 | Conjugative plasmid encoding CFE-1 isolated from KU6400 |
| pKU611 | 14-kb fragment containing blaCFE-1 gene and ampR gene from pKU601 cloned into pHSG398 |
| pKU612 | Recombinant plasmid containing ampC gene of pKU611 in pHSG398 |
| pHSG398 | Cloning vector, purchased from Nippon Gene (Tokyo); Chlr |
Characteristics include designation of markers, source, or derivation. Rif, rifampin; Chl, chloramphenicol.
Antibacterial agents.
Reference samples of the antibacterial agents listed below were used in this study and were provided as powders of known potencies by the respective manufacturers. Piperacillin (Toyama Chemical, Toyama, Japan) was used as a representative penicillin, while cephalothin (Shionogi, Osaka, Japan), cefpodoxime (Sankyo, Tokyo, Japan), cefmetazole (Sankyo), cefotaxime (Nippon Hoechst Marion Roussel, Tokyo, Japan), and cefepime (Bristol-Myers Squibb, Tokyo, Japan) were used as representative cephems. Other β-lactam agents, including imipenem (Banyu Pharmaceutical, Tokyo, Japan) as well as chloramphenicol (Sankyo) and rifampin (Sigma Chemical, St. Louis, Mo.), were also used. Clavulanic acid (SmithKline Beecham Pharmaceuticals, Tokyo, Japan) was used as a β-lactamase inhibitor.
Drug susceptibility assay.
The susceptibility profiles were determined by the agar dilution method with sensitivity disk agar (Eiken Chemical, Tokyo, Japan) according to the guidelines of NCCLS (29).
Transconjugation.
Conjugation was carried out by a broth method as described previously (20). Exponential-phase Luria broth cultures of donor strain KU6400 and recipient strain ML4947 were mixed at a ratio of 1:10 (by volume). This mating mixture was incubated for 2 h at 35°C. The transconjugants were selected on sensitivity disk agar containing rifampin at 64 μg/ml and cefpodoxime at 4 μg/ml.
Assay for β-lactamase.
Crude extraction of AmpC β-lactamase was performed as described previously (32). Cells were harvested by centrifugation (1,700 × g, 10 min), resuspended in 3 ml of 50 mM potassium phosphate buffer (pH 7.0), and sonicated. After centrifugation at 14,000 × g for 10 min at 4°C, β-lactamase activity was measured by determination of the protein content of the extract, and the protein contents of the cultures were compared. β-Lactamase activity was determined by spectrophotometry (UV2000; Shimadzu, Tokyo, Japan) at 30°C in 50 mM phosphate buffer (pH 7.0). Protein content was determined by a protein assay (Bio-Rad Laboratories, Hercules, Calif.) (10). One unit of β-lactamase activity was defined as the amount of β-lactamase that hydrolyzed 1 μmol of cephalothin in 1 min at 30°C. Cefoxitin (10 μg/ml) was used as the inducer. Induction was allowed to proceed for 60 min (4).
Cloning of the ampC and ampR genes.
DNA extraction, restriction enzyme digestion, recombinant DNA manipulation, and transformations of plasmid DNA were performed as described by Sambrook et al. (40). Restriction enzymes and T4 DNA ligase were purchased from Takara Shuzo (Kyoto, Japan) and Nippon Gene (Tokyo, Japan), respectively. Plasmid pKU601 DNA was isolated from E. coli ML4947(pKU601) by the alkaline lysis method (8). The DNA was digested with BamHI and BglII and ligated into the BamHI site of pHSG398. The recombinant plasmid was designated pKU611 and was introduced into E. coli ML4947 by electroporation with a gene pulse controller unit (Bio-Rad Laboratories). Transformants (containing ampC and ampR) were selected on the basis of resistance to cefpodoxime (4 μg/ml) and chloramphenicol (25 μg/ml) after overnight incubation at 37°C and were further characterized by analysis of their antibiotic susceptibility patterns. The size of the insert in the plasmid was estimated by restriction enzyme digestion and electrophoresis in 1.2% agarose gels.
To construct a plasmid containing only the ampC gene, plasmid pKU612, pKU611 was digested with SacI and ligated into the SacI site of pHSG398. The resulting plasmid was used to transform E. coli ML4947, and the plasmid from which the fragment containing the ampR sequence was deleted was identified from the plasmid DNA size and by DNA sequencing.
DNA sequencing and sequence comparisons.
Sequencing of both strands of DNA was carried out as described by Sanger et al. (41) with a BigDye terminator cycle sequencing kit and an ABI 310 DNA sequencer (Applied Biosystems, Foster City, Calif.). Sequence analysis and comparison with other known sequences were performed with the BLAST and FAST programs at the National Center for Biotechnology Information.
Nucleotide sequence accession number.
The nucleotide sequence data presented in this report appear in the DDBJ, EMBL, and GenBank nucleotide databases under accession number AB107899.
RESULTS
Susceptibilities to antibiotics.
The MICs of selected β-lactam antibiotics for clinical isolate E. coli KU6400 and the transconjugants that acquired pKU601 by conjugation at a frequency of 10−5 are given in Table 2. KU6400 was highly resistant to piperacillin, cefotaxime, the combination of cefotaxime and clavulanic acid, aztreonam, and all cephalosporins except cefepime. The MICs of a variety of different cephalosporins were increased for the transconjugants.
TABLE 2.
MICs of selected antibiotics for E. coli strains
| Strain | MIC (μg/ml)a
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PIP | CPD | CEF | CTX | CTX-CLAb | CAZ | CMZ | ATM | FEP | IPM | |
| KU6400 | >256 | >256 | >256 | 64 | 64 | 64 | 64 | 8 | 0.25 | 0.25 |
| ML4947(pKU601) | >256 | >256 | >256 | 256 | 256 | >256 | 256 | 64 | 1 | 0.5 |
| ML4953(pKU601) | >256 | >256 | >256 | 256 | 256 | >256 | 256 | 64 | 1 | 1 |
| ML4947(pKU611) | 64 | >256 | >256 | 16 | 16 | 8 | 16 | 2 | 0.25 | 0.25 |
| ML4947(pKU612) | 8 | 32 | 256 | 4 | 2 | 4 | 2 | 0.5 | <0.063 | 0.25 |
| ML4947 | 2 | 0.5 | 8 | <0.063 | <0.063 | 0.25 | 0.5 | <0.063 | <0.063 | <0.063 |
Antibiotics: PIP, piperacillin; CEF, cephalothin; CPD, cefpodoxime; CTX, cefotaxime; CLA, clavulanic acid; CAZ, ceftazidime; CMZ, cefmetazole; ATM, aztreonam; FEP, cefepime; IPM, imipenem.
MICs were determined in the presence of clavulanic acid (5 μg/ml).
Cloning of the ampC and ampR genes and gene expression in the E. coli recipient.
We selected seven E. coli transformants resistant to β-lactams in a manner similar to that of E. coli ML4947 that acquired pKU601 by conjugation (Table 1). These transformants were resistant to cefpodoxime, cefotaxime, and chloramphenicol.
All transformants were found to produce an AmpC β-lactamase; they harbored a recombinant plasmid (pKU611) with an insert of 14 kb from pKU601. E. coli isolates harboring pKU611 showed a resistance profile similar to that of pKU601 (Table 2). Addition of clavulanic acid did not modify the resistance pattern in any transformant.
Characterization of the blaCFE-1 gene.
Both strands of the entire 14-kb insert from recombinant plasmid pKU611 were sequenced. Analysis of this insert for coding regions revealed two open reading frames (ORFs) (Fig. 2). The first consisted of 1,137 bp encoding a putative protein of 378 amino acids (Fig. 3). This ORF had an ATG start codon at position 1008 and a stop codon at position 2151. Database searches with this ORF identified similarities with several chromosome- and plasmid-encoded AmpC β-lactamases, particularly the chromosome-encoded AmpC β-lactamase of C. freundii GC3 (99.8% sequence identity) (17). The deduced amino acid sequence carried catalytic residues S-X-X-K, with the initial serine at position 64 (which is typical of AmpC β-lactamases); the motif Y-S-N at position 150; and the K-T-G motif at position 315.
FIG. 2.
Nucleotide sequence of the 2,153-bp fragment of pKU611 containing the ampC- and ampR-coding regions. Deduced amino acid sequences are designated in single-letter code. Putative promoter sequences are represented by the −35 and −10 regions (boxed). The start and stop codons of these genes are underlined. Additionally, conserved residues among class C β-lactamases are shown in shaded boxes.
FIG. 3.
Derived amino acid sequence of the CFE-1 β-lactamase compared to the sequences of other selected class C β-lactamases. The alignments of the deduced amino acid sequence of the CFE-1 β-lactamase with the AmpC β-lactamases of C. freundii GC3 (AmpC Cit GC3), C. freundii OS60 (AmpC Cit OS60), and E. cloacae P99 (AmpC Ent P99) and with the CMY-2 β-lactamase are shown. Dots indicate identical amino acids at that residue.
The second ORF, which contained 876 nucleotides was transcribed in the opposite orientation and was located in the 5′ direction from the ampC structural gene. It began with an ATG start codon at nucleotide 876 and had a stop codon at nucleotide 3. By analogy with the ampC and ampR genes of the AmpC β-lactamase, this ORF may correspond to the regulatory gene ampR. A sequence corresponding to transcriptional regulators of the LysR family, particularly the AmpR proteins of the family Enterobacteriaceae, was deduced. The DNA sequence of the corresponding gene from C. freundii GC3 was 99.0% identical to the sequence of this ORF. The deduced protein sequence showed only one difference, at position 135 (Ala for Asp), compared to the sequence of C. freundii GC3.
The 131-bp region between the ampR and ampC start codons contained overlapping putative promoters. This region was 97% identical to the corresponding region of C. freundii GC3.
Sequences surrounding ampR and ampC regions.
In addition to identifying the sequences in the regions surrounding the ampR and ampC sequences, sequence analysis indicated that the frdABCD operon of C. freundii (7) was located upstream from the ampC gene; and a part of the ORF contained the sequence for the outer membrane lipoprotein encoded by the blc gene of C. freundii (GenBank accession nos. D85910 and U21727), which was located immediately downstream of the ampC gene (Fig. 4). Furthermore, two IS26 elements were observed to surround the ampR and ampC genes and were directed in the same orientation, forming an IS26 composite transposon. One was inserted in the frdA gene (as detected by PCR), and another was inserted in the blc gene.
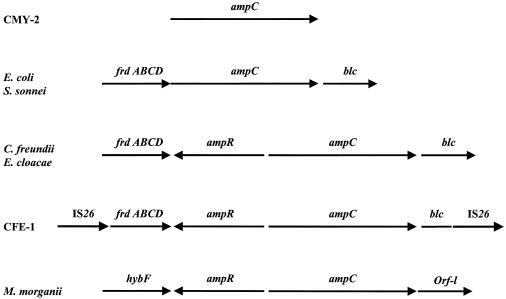
FIG. 4.
Organization of the sequences surrounding ampC in various enterobacterial species. The positions of the fumarate operon (frdABCD), blc, hybF, orf-1, IS26, ampC, and ampR genes are shown, with directions indicated by arrows.
β-Lactamase activities.
The β-lactamase activities encoded by the plasmids are shown in Table 3. E. coli ML4947 (pKU601) and ML4953(pKU601) produced large amounts of β-lactamase (10.9 and 14.4 U/mg of protein, respectively). When E. coli ML4953 (AmpD mutant) was used as the host, the β-lactamase activities encoded by pKU601 were slightly higher than those detected when E. coli ML4947 (AmpD wild type) was used as the host. The β-lactamase activities of these strains in the presence of cefoxitin were slightly increased compared with the basal level, but the increases were not significantly different. This suggests that the blaCFE-1 gene may produce the enzyme constitutively.
TABLE 3.
β-Lactamase activities of E. coli strains
| Strain | Relative β-lactamase activity (U/mg of protein)a
|
|
|---|---|---|
| Noninduced | Inducedb | |
| KU6400 | 7.7 | 7.9 |
| ML4947(pKU601) | 10.9 | 11.1 |
| ML4953(pKU601) | 14.4 | 16.3 |
| ML4947(pKU611) | 1.4 | 1.7 |
| ML4947(pKU612) | 0.2 | NDc |
β-Lactamase activities are the geometric mean determinations for three independent cultures. The standard deviations were within 10%.
Cefoxitin (10 μg/ml); was used as the inducer.
ND, not done.
The activity of CFE-1 was not inhibited by clavulanic acid, a characteristic confirming the close resemblance of CFE-1 to the AmpC β-lactamase, as mentioned in the description of the nucleotide sequence.
pKU611, which encoded the ampR and ampC genes, and recombinant plasmid pKU612, which encoded only the ampC gene (i.e., it lacked the ampR gene), were introduced into E. coli ML4947; and the β-lactamase activities of each construct were analyzed (Table 3). The specific enzyme activity of E. coli ML4947(pKU611) was 1.4 U/mg of protein, while that of E. coli ML4947(pKU612) was 0.2 U/mg of protein, a markedly lower level of expression.
DISCUSSION
In addition to previous reports concerning the MOX-1 and CMY-9 plasmid-encoded AmpC β-lactamases, which were detected in an E. coli clinical isolate in Japan (12, 19), in the present study we characterized a novel AmpC β-lactamase gene, blaCFE-1, in a Japanese E. coli clinical isolate. This is the first report in East Asia of a plasmid-encoded AmpC β-lactamase, CFE-1, carrying an ampR gene derived from the C. freundii chromosome.
Our findings depict the organization of sequences surrounding the ampR-ampC region, including blaCFE-1, which is seen in various enterobacterial species. Most plasmid-encoded AmpC β-lactamases, like CMY-2, CMY-4, and LAT-1, lack the ampR gene. Citrobacter spp. and Enterobacter spp. possess ampR and ampC genes, the fumarate operon frdABCD immediately downstream of the ampR gene, and also outer membrane lipoprotein blc immediately downstream of the ampC gene (7, 9). In contrast, M. morganii possesses the ampR and ampC genes but not the fumarate operon (36); hybF is substituted for the fumarate operon upstream from the ampC gene (Fig. 4).
Analysis of the blaCFE-1 gene revealed that it has a very close relationship to the chromosomal gene that encodes the AmpC β-lactamase in C. freundii (1) (Table 4). The amino acid sequence of CFE-1 showed 99.5% identity with that of C. freundii GC3 isolated from clinical specimens in Japan (17), differing only at position 221 (Leu for Trp) and position 298 (Val for Leu) (Fig. 3). This identity was greater than that with C. freundii OS60 (95.0%) (26). The amino acid sequence of AmpR showed 99.0% identity with that of C. freundii GC3, differing only at position 135 (Ala for Asp). This similarity strongly suggests that the blaCFE-1 gene is derived from the chromosomal ampC gene of C. freundii GC3 (1). This hypothesis is supported by the finding that blaCFE-1 has both the ampR gene and the ampC gene, as well as the frdABCD gene operon and the blc gene, which were found to surround the ampR and ampC genes, as in C. freundii.
TABLE 4.
Identity of the CFE-1 amino acid sequence to those of other AmpC β-lactamases
| β-Lactamase | % Identity with:
|
||||
|---|---|---|---|---|---|
| CFE-1 | GC3 | OS60 | CMY-2 | P99 | |
| CFE-1 | 100 | 99.5 | 95.0 | 95.3 | 74.5 |
| C. freundii GC3 AmpC | 100 | 95.5 | 95.8 | 74.5 | |
| C. freundii OS60 AmpC | 100 | 95.8 | 73.2 | ||
| CMY-2 | 100 | 75.1 | |||
| E. cloacae P99 AmpC | 100 | ||||
In addition, two IS26 elements were detected on plasmid pKU601; one was located immediately upstream from the frdA gene, while the other was located immediately downstream of the ampC gene and was inserted in the blc gene (Fig. 4). These were directed in the same orientation as that seen for the IS26 composite transposon (25). Some plasmid-encoded β-lactamase genes form part of transposons frequently flanked by insertion sequence elements, such as IS26 (SHV-2a and ACC-1) (22, 28) and ISEcp1 (CTX-M5) (39). These results present direct evidence that the blaCFE-1 gene translocated to a plasmid from the chromosome of C. freundii strain GC3 by using the IS26 function(s). Further studies are continuing to determine whether the blaCFE-1 gene is capable of translocation.
As shown in Tables 2 and 3, an E. coli strain harboring pKU601 encoding blaCFE-1 expressed β-lactamase constitutively in the presence or absence of a β-lactam inducer and in the AmpD wild type (ML4947) or AmpD mutant (ML4953). E. coli ML4947(pKU612), which lacks the ampR gene, showed a decrease in β-lactamase activity compared with that of E. coli ML4947(pKU611); nevertheless, the AmpC β-lactamases of C. freundii, E. cloacae, and M. morganii with an ampR deletion showed increased levels of β-lactamase expression. This result indicates that AmpR of pKU601 seems to function as regulator of the constitutive expression of blaCFE-1. Bartowsky and Normark (3, 4) have reported that the activation of ampC transcription in C. freundii is dependent on the conversion of AmpR into a transcriptional activator. The AmpR mutants of C. freundii, which have Glu instead of Gly at position 102 or Tyl instead of Asp at position 135, express β-lactamase at high levels. Increased levels of β-lactamase expression have been reported (24) when the Arg-86 and Asp-135 mutations are present in E. cloacae AmpR. In the ampR gene of pKU601, the amino acid at position 135 was Ala, whereas it was Asp in the wild type. These results may indicate that overexpression of β-lactamase is dependent on mutation of the ampR gene.
In summary, E. coli plasmid pKU601 was characterized as harboring a novel plasmid-encoded AmpC β-lactamase, CFE-1, with an ampR gene. The high level of constitutive CFE-1 expression in E. coli is presumably caused by the mutation in the ampR gene, in which the Asp at position 135 is changed to Ala. These results indicate the dissemination of a resistance gene to different enterobacterial species through mobilization of a plasmid and transposable event-mediated events.
Acknowledgments
This work was supported in part by grants (12670264 and 10008336) from the Japanese Ministry of Education, Culture, Sports, Science and Technology; the COE program of the Japanese Ministry of Education, Culture, Sports, Science and Technology; the Japanese Ministry of Health, Labor, and Welfare (the Research Project of Emerging and Establishment of Rapid Identification Methods); and the Charitable Trust Clinical Pathology Research Foundation of Japan.
REFERENCES
- 1.Barlow, M., and B. G. Hall. 2002. Origin and evolution of the AmpC β-lactamases of Citrobacter freundii. Antimicrob. Agents Chemother. 46:1190-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnaud, G., G. Arlet, C. Verdet, O. Gaillot, P. H. Lagrange, and A. Philippon. 1998. Salmonella enteritidis: AmpC plasmid-mediated inducible β-lactamase (DHA-1) with an ampR gene from Morganella morganii. Antimicrob. Agents Chemother. 42:2352-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartowsky, E., and S. Normark. 1991. Purification and mutant analysis of Citrobacter freundii AmpR, the regulator for chromosomal AmpC β-lactamase. Mol. Microbiol. 5:1715-1725. [DOI] [PubMed] [Google Scholar]
- 4.Bartowsky, E., and S. Normark. 1993. Interactions of wild-type and mutant AmpR of Citrobacter freundii with target DNA. Mol. Microbiol. 10:555-565. [DOI] [PubMed] [Google Scholar]
- 5.Bauernfeind, A., I. Stemplinger, R. Jungwirth, and H. Giamarellou. 1996. Characterization of the plasmidic β-lactamase CMY-2, which is responsible for cephamycin resistance. Antimicrob. Agents Chemother. 40:221-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett, P. M., and I. Chopra. 1993. Molecular basis of β-lactamase induction in bacteria. Antimicrob. Agents Chemother. 37:153-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergstrom, S., F. P. Lindberg, O. Olsson, and S. Normark. 1983. Comparison of the overlapping frd and ampC operons of Escherichia coli with the corresponding DNA sequences in other gram-negative bacteria. J. Bacteriol. 155:1297-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop, R. E., S. S. Penfold, L. S. Frost, J. V. Holtje, and J. H. Weiner. 1995. Stationary phase expression of a novel Escherichia coli outer membrane lipoprotein and its relationship with mammalian apolipoprotein D. Implications for the origin of lipocalins. J. Biol. Chem. 270:23097-23103. [DOI] [PubMed] [Google Scholar]
- 10.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 11.Bradford, P. A., C. Urban, N. Mariano, S. J. Projan, J. J. Rahal, and K. Bush. 1997. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the foss of an outer membrane protein. Antimicrob. Agents Chemother. 41:563-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doi, Y., N. Shibata, K. Shibayama, K. Kamachi, H. Kurokawa, K. Yokoyama, T. Yagi, and Y. Arakawa. 2002. Characterization of a novel plasmid-mediated cephalosporinase (CMY-9) and its genetic environment in an Escherichia coli clinical isolate. Antimicrob. Agents Chemother. 46:2427-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortineau, N., L. Poirel, and P. Nordmann. 2001. Plasmid-mediated and inducible cephalosporinase DHA-2 from Klebsiella pneumoniae. J. Antimicrob. Chemother. 47:207-210. [DOI] [PubMed] [Google Scholar]
- 14.Gaillo, O., C. Clement, M. Simonet, and A. Philippon. 1997. Novel transferable β-lactam resistance with cephalosporinase characteristics in Salmonella enteritidis. J. Antimicrob. Chemother. 39:85-87. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez Leiza, M., J. C. Perez-Diaz, J. Ayala, J. M. Casellas, J. Martinez-Beltran, K. Bush, and F. Baquero. 1994. Gene sequence and biochemical characterization of FOX-1 from Klebsiella pneumoniae, a new AmpC-type plasmid-mediated β-lactamase with two molecular variants. Antimicrob. Agents Chemother. 38:2150-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson, N. D., and C. C. Sanders. 1999. Regulation of inducible AmpC beta-lactamase expression among Enterobactericeae. Curr. Pharm. Des. 5:881-894. [PubMed] [Google Scholar]
- 17.Haruta, S., M. Nukaga, and T. Sawai. 2001. Characterization of an extended-spectrum class C β-lactamase of Citrobacter freundii. Microbiol. Immunol. 45:277-283. [DOI] [PubMed] [Google Scholar]
- 18.Honore, N., M. H. Nicolas, and S. T. Cole. 1989. Regulation of enterobacterial cephalosporinase production: the role of a membrane-bound sensory transducer. Mol. Microbiol. 3:1121-1130. [DOI] [PubMed] [Google Scholar]
- 19.Horii, T., Y. Arakawa, M. Ohta, T. Sugiyama, R. Wacharotayankun, H. Ito, and N. Kato. 1994. Characterization of a plasmid-borne and constitutively expressed blaMOX-1 gene encoding AmpC-type β-lactamase. Gene 139:93-98. [DOI] [PubMed] [Google Scholar]
- 20.Inoue, M., J. Itoh, and S. Mitsuhashi. 1983. pMS76, a plasmid capable of amplification by treatment with chloramphenicol. Plasmid 9:86-97. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs, C., L. J. Huang, E. Bartowsky, S. Normark, and J. T. Park. 1994. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for β-lactamase induction. EMBO J. 13:4684-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, J., H. S. Shin, S. Y. Seol, and D. T. Cho. 2002. Relationship between blaSHV-12 and blaSHV-2a in Korea. J. Antimicrob. Chemother. 49:261-267. [DOI] [PubMed] [Google Scholar]
- 23.Korfmann, G., and C. C. Sanders. 1989. ampG is essential for high-level expression of AmpC β-lactamase in Enterobacter cloacae. Antimicrob. Agents Chemother. 33:1946-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuga, A., R. Okamoto, and M. Inoue. 2000. ampR gene mutations that greatly increase class C β-lactamase activity in Enterobacter cloacae. Antimicrob. Agents Chemother. 44:561-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, K. Y., J. D. Hopkins, and M. Syvanen. 1990. Direct involvement of IS26 in an antibiotic resistance operon. J. Bacteriol. 172:3229-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindberg, F., L. Westman, and S. Normark. 1985. Regulatory components in Citrobacter freundii ampC β-lactamase induction. Proc. Natl. Acad. Sci. USA 82:4620-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maejima, T., Y. Ohya, S. Mitsuhashi, and M. Inoue. 1987. Cloning and expression of the gene(s) for chromosome-mediated β-lactamase production of Proteus vulgaris in Escherichia coli. Plasmid 18:120-126. [DOI] [PubMed] [Google Scholar]
- 28.Nadjar, D., M. Rouveau, C. Verdet, L. Donay, J. Herrmann, P. H. Lagrange, A. Philippon, and G. Arlet. 2000. Outbreak of Klebsiella pneumoniae producing transferable AmpC-type β-lactamase (ACC-1) originating from Hafnia alvei. FEMS Microbiol. Lett. 187:35-40. [DOI] [PubMed] [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed., vol. 17, no. 2. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 30.Nordmann, P. 1998. Trends in β-lactam resistance among Enterobacteriaceae. Clin. Infect. Dis. 27(Suppl. 1):S100-S106. [DOI] [PubMed] [Google Scholar]
- 31.Normark, S. 1995. β-Lactamase induction in gram-negative bacteria is intimately linked to peptidoglycan recycling. Microb. Drug Resist. 1:111-114. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto, R., T. Okubo, and M. Inoue. 1996. Detection of genes regulating β-lactamase production in Enterococcus faecalis and Staphylococcus aureus. Antimicrob. Agents Chemother. 40:2550-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papanicolaou, G. A., A. A. Medeiros, and G. A. Jacoby. 1990. Novel plasmid-mediated β-lactamase (MIR-1) conferring resistance to oxyimino- and α- methoxy β-lactams in clinical isolates of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 34:2200-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez-Perez, F. J., and N. D. Hanson. 2002. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Philippon, A., G. Arlet, and G. A. Jacoby. 2002. Plasmid-determined AmpC-type β-lactamases. Antimicrob. Agents Chemother. 46:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poirel, L., M. Guibert, D. Girlich, T. Naas, and P. Nordmann. 1999. Cloning, sequence analyses, expression, and distribution of ampC-ampR from Morganella morganii clinical isolates. Antimicrob. Agents Chemother. 43:769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reisbig, M. D., and N. D. Hanson. 2002. The ACT-1 plasmid-encoded AmpC β-lactamase is inducible: detection in a complex β-lactamase background. J. Antimicrob. Chemother. 49:557-560. [DOI] [PubMed] [Google Scholar]
- 38.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 39.Saladin, M., V. T. Cao, T. Lambert, J. L. Donay, J. L. Herrmann, Z. Ould-Hocine, C. Verdet, F. Delisle, A. Philippon, and G. Arlet. 2002. Diversity of CTX-M β-lactamases and their promoter regions from Enterobacteriaceae isolated in three Parisian hospitals. FEMS Microbiol. Lett. 209:161-168. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., vol. 1. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 41.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tzouvelekis, L. S., E. Tzelepi, A. F. Mentis, and A. Tsakaris. 1993. Identification of a novel plasmid-mediated β-lactamase with chromosomal cephalosporinase characteristics from Klebsiella pneumoniae. J. Antimicrob. Chemother. 31:645-654. [DOI] [PubMed] [Google Scholar]
- 43.Verdet, C., G. Arlet, S. Ben Redjeb, A. Ben Hassen, P. H. Lagrange, and A. Philippon. 1998. Characterisation of CMY-4, an AmpC-type plasmid-mediated β-lactamase in a Tunisian clinical isolate of Proteus mirabilis. FEMS Microbiol. Lett. 169:235-240. [DOI] [PubMed] [Google Scholar]