Abstract
Injuries to articular cartilage result in significant pain to patients and high medical costs. Unfortunately, cartilage repair strategies have been notoriously unreliable and/or complex. Biomaterial-based tissue-engineering strategies offer great promise, including the use of hydrogels to regenerate articular cartilage. Mechanical integrity is arguably the most important functional outcome of engineered cartilage, although mechanical testing of hydrogel-based constructs to date has focused primarily on deformation rather than failure properties. In addition to deformation testing, as the field of cartilage tissue engineering matures, this community will benefit from the addition of mechanical failure testing to outcome analyses, given the crucial clinical importance of the success of engineered constructs. However, there is a tremendous disparity in the methods used to evaluate mechanical failure of hydrogels and articular cartilage. In an effort to bridge the gap in mechanical testing methods of articular cartilage and hydrogels in cartilage regeneration, this review classifies the different toughness measurements for each. The urgency for identifying the common ground between these two disparate fields is high, as mechanical failure is ready to stand alongside stiffness as a functional design requirement. In comparing toughness measurement methods between hydrogels and cartilage, we recommend that the best option for evaluating mechanical failure of hydrogel-based constructs for cartilage tissue engineering may be tensile testing based on the single edge notch test, in part because specimen preparation is more straightforward and a related American Society for Testing and Materials (ASTM) standard can be adopted in a fracture mechanics context.
Introduction
Articular cartilage injuries and resulting arthritis are one of the leading causes of disability in the United States.1 Biomaterial-based tissue-engineering strategies offer great promise, including the use of hydrogels to regenerate articular cartilage.2–4 Hydrogels are one broad class of biomaterials that have earned widespread interest in cartilage regeneration, with the emphasis primarily being on eliciting desired biological responses. One must appreciate that both the biological response and the mechanical integrity of the hydrogel are very important and both must be considered. Here we focus on the mechanical integrity of hydrogel-based constructs, and highlight failure testing as an important consideration in addition to the standard compression/indentation testing methods.
Hydrogels have been investigated for use in a variety of biomedical applications, such as tissue engineering4–6 and drug delivery.7,8 To replace damaged cartilage tissue, hydrogels will be required to provide appropriate stiffness or deformation properties as well as resistance to fracture. Currently, the most common evaluation of mechanical properties is through compressive modulus measurement.2,4,6,9 However, failure properties, such as the resistance to fracture in the presence of an existing crack, must be evaluated by fracture toughness techniques. In a composite material such as cartilage, apparent fracture toughness reflects how much energy the material will absorb to fracture with an existing defect10 and contributes to the response of materials in crack extension to failure.11 However, virtually all of the hydrogel studies to date in articular cartilage tissue engineering have lacked evaluation of fracture toughness.2 Some studies only consider ultimate compressive stress or strain, which may suffer from issues with reproducibility.10,12
In traditional fracture mechanics, fracture toughness is defined as the quantitative expression of the ability of a brittle material to resist fracture in the presence of an existing sharp crack. The material property of fracture toughness is defined for a given material by measurement using a minimum specimen size that will guarantee dominance of plane strain conditions, therefore allowing for consistent fracture toughness values for the given material. Use of the term “apparent fracture toughness” recognizes that for tough composite materials such as cartilage, the normal rules of traditional fracture toughness measurement are not possible. We suggest that consistent use of apparent fracture toughness be used as a reminder to researchers in the biomaterials community that specimen size does matter and that any comparisons made to other materials should be done with caution and complete understanding of the limitations involved.
Therefore, to establish testing methods for appropriate evaluation of hydrogels for use in cartilage tissue engineering, it is necessary to examine fracture toughness studies of articular cartilage, and juxtapose them with fracture toughness studies of hydrogels used in applications outside of the cartilage tissue-engineering field.13–18 Ultimately, to have an effective hydrogel for cartilage tissue engineering, both the deformation properties and fracture properties should be in the range of those of articular cartilage. Therefore, established methods to test cartilage fracture properties can be used as a guide in testing hydrogels for cartilage tissue engineering. These tests include the single edge notch (SEN) test, trouser tear test, and indentation test.19,20
Based on the apparent fracture toughness measurements of articular cartilage and of hydrogels used in applications outside of cartilage tissue engineering, we will establish the groundwork for linking methodologies between fracture testing of cartilage and hydrogels and provide recommendations for evaluation of fracture properties for hydrogels used in cartilage tissue engineering (Fig. 1).
FIG. 1.
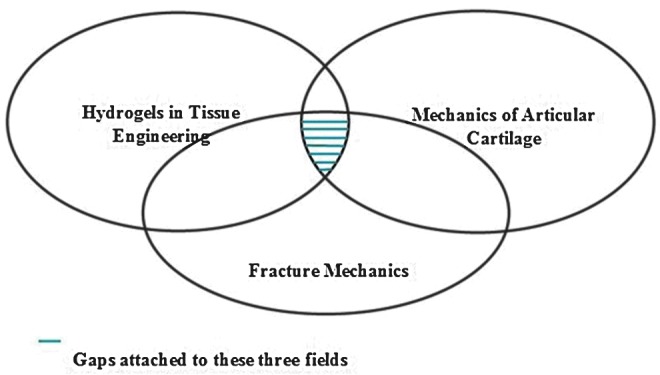
Venn diagram emphasizing the distinct fields of (1) hydrogels in tissue engineering, (2) cartilage biomechanics, and (3) fracture mechanics. The purpose of this review is to identify the common ground for these distinct fields, more specifically, to understand how to best identify fracture mechanics methods most suitable for evaluating both hydrogels and cartilage. The urgency for identifying this common ground is high in light of the advanced state of hydrogels in cartilage regeneration, where fracture is ready to stand alongside stiffness as a functional design requirement. Color images available online at www.liebertpub.com/teb
Fracture Toughness Measurement of Articular Cartilage
The fracture behavior of articular cartilage is intrinsically connected to its structure.21 Articular cartilage is usually divided into four macroscopic layers.22,23 The surface layer, or the superficial zone, is known to be more resistant to shear stress and wear than the underlying layers due to the parallel orientation of the collagen fibers relative to the articular surface.24 Under the superficial zone lie the middle and deep zones, in which fibers turn obliquely to form a radially aligned 3-D mesh.21 The bottom calcified zone bears compressive loads21,25 with collagen fibers distributing load perpendicular to the surface of the articular cartilage.
In isotropic, linearly elastic materials, different loading modes result in distinctly different values for apparent fracture toughness.20,26 There are three primary fracture loading modes used in traditional fracture mechanics. Mode I loading opens a crack by inducing tensile stresses normal to the crack plane (Fig. 2A). In contrast, Mode II loading propagates a crack between two surfaces by inducing in-plane shearing loads (Fig. 2B). Finally, Mode III loading extends a crack by transverse (out of plane) shearing (Fig. 2C). Mode I has been used most often for cartilage, as tensile stresses are the primary mode of crack opening and typically the most stringent criteria for evaluation of a material.20 Mode III testing has also been used extensively by the biomechanics community in several forms, including microindentation.
FIG. 2.
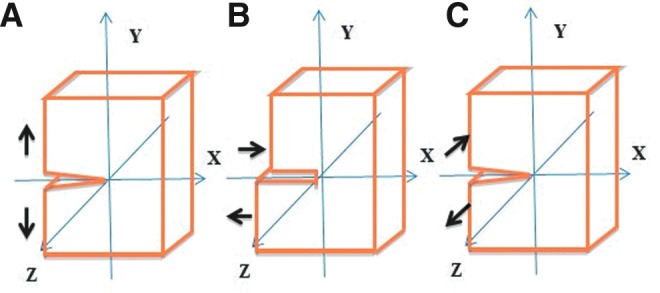
Different modes for testing fracture toughness of cartilage summed up by Ahsan and Sah20: (A) Mode I—Opening mode; (B) Mode II—Shearing mode; (C) Mode III—Tearing mode. Modes I and III have been the preferred methods used for evaluations of cartilage toughness. Color images available online at www.liebertpub.com/teb
It is important to note that fracture mechanics methods were originally developed to evaluate linear elastic materials, so the meaning of the data obtained from tests on other types of materials (such as soft tissues) must be interpreted with care. Since articular cartilage tissue is viscoelastic, anisotropic, and is composed of different layers, even with consistent loading methods, crack extension can vary in magnitude and mode, making it difficult to obtain consistent and understandable apparent fracture toughness measurements. For these types of materials, the energy criterion (versus the stress intensity of the crack tip) is used to represent the apparent fracture toughness instead of the stress intensity factor.20 The energy criterion can be interpreted as the point when sufficient energy is available to overcome the resistance of the material to grow an existing crack per a given unit of crack extension. In addition, the specimen size and shape, loading rates, boundary conditions of gripping, and other factors have an influence on the outcome of the energy release rate, therefore testing must be done in a controlled fashion, and comparisons between tests must be interpreted carefully.20
We are cognizant of the fact that cartilage failure as a biological phenomenon in osteoarthritis is typically considered in the context of an impact injury followed by a cascade of signaling events over an extended period of time that result in the breakdown of the cartilage structure and thus the loss of mechanical integrity. However, for the purposes of this review, we examine cartilage as a material, and we thus review studies that have evaluated its failure properties as a material, which will serve to facilitate the juxtaposition of cartilage failure and hydrogel failure. Therefore, the following subsections will discuss loading Mode I (opening mode) and Mode III (out of plane mode and indentation test). Based on the results of investigations of cartilage failure with these different mechanisms, we will conclude this section with suggestions for selecting a reliable method for fracture toughness measurement of articular cartilage in the context of looking forward to tissue-engineering studies.
Mode I—modified SEN test
Based on Mode I loading, Chin-Purcell and Lewis27 initiated the modified SEN test (MSEN) that was developed from a modification of the compact tension test method recommended in American Society for Testing and Materials (ASTM) E399. In this test, a slice of bone with cartilage attached was prepared by creating a notch from the subchondral bone of the adult mongrel canine patella into the deep and middle zones of articular cartilage (Fig. 3). They equilibrated the sliced specimens for ∼1 h in a temperature-controlled saline bath at 37°C. After equilibration, each specimen was placed in specially designed holders to grip the bone section. The grips were spring loaded with the same spring tension for each test. In this way, the grips grabbed the subchondral bone instead of the articular cartilage, which helped to avoid slippage and deformation of the articular cartilage and provided boundary conditions more similar to that found in the body for a defect extending into the cartilage from the bone. However, free rotation around the grips was not allowed, thus yielding a deviation from compact tension ASTM fracture mechanics tests. Adams et al.28 supplemented the cartilage research of Chin-Purcell and Lewis27 by finding that the thickness (between 0.7 and 2.7 mm) of the cartilage samples used in the MSEN test had no effect on the apparent fracture toughness through a comparison between the crack opening angle and fracture toughness. This finding is important because it provides evidence that plane strain conditions dominated the Mode I fracture toughness measured for cartilage using this specimen type within this range of specimen thickness.
FIG. 3.
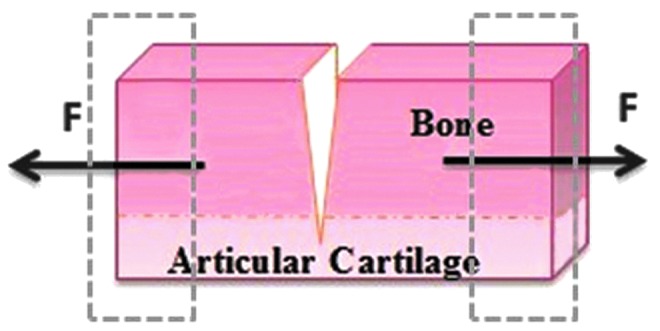
Modified single edge notch (MSEN) test for cartilage (Mode I). Note that the cartilage remains affixed to the bone. The crack made before testing extends through the bone and continues a fixed distance into the cartilage, providing a rigid gripping point with the bone. Color images available online at www.liebertpub.com/teb
Mode I—SEN test
Stok and Oloyede11,21 supplemented these aforementioned studies by testing all four cartilage zones, instead of only the deep zone, in a SEN test that did not incorporate bone. They shaved the cartilage from bovine bone and trimmed the cartilage into strips. A notch was made through the superficial zone into one edge of the cartilage and the tensile loading was applied at both ends of the cartilage (Fig. 4). Furthermore, they analyzed crack extension at varying rates of tensile loading (1.5, 3.0, and 4.5 mm/min) and found that the energy measured during crack extension did not vary significantly with the different loading rates. Therefore, they proposed that it was the structural variations between the diverse zones of the tissue, rather than the speed of loading, that predominately determined the characteristics of fracture in articular cartilage.11
FIG. 4.
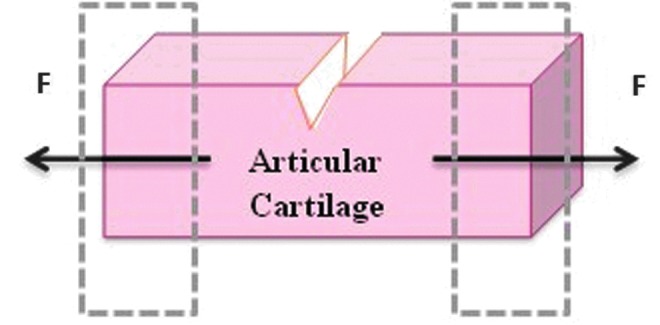
SEN test (Mode I). Note that the cartilage is not affixed to bone, unlike the MSEN test. Here, the cartilage is gripped directly. Color images available online at www.liebertpub.com/teb
The main difference between the MSEN test and SEN test, both classified as Mode I tests, is the geometry of the samples and direction of the defect extension. Specifically, in the MSEN test, the cartilage remains attached to the bone and this single osteochondral unit is sectioned into slices as whole pieces, whereas in the SEN test, the cartilage is removed from the bone. The SEN test does not replicate the boundary conditions of cartilage as it exists in the human body, given that it allows the cartilage to deform freely where it would normally be constrained by the bone. This difference in the geometry affects the shape factor in the data analyzing model and the stresses generated at the crack tip, which will thus influence the final values obtained. The nature of the material in the zone around the tip of the crack is also critical; therefore, the direction of extension of the defect is important to consider. With SEN specimens, crack extension can extend from the deepest or the superficial layer depending on where the defect is made, but with the MSEN notch test, the crack can only extend from the deepest layer of the cartilage into the other zones.
Mode III—trouser tear test
Mode III loading was originally derived from anticlastic plate bending.29 Anticlastic plate bending30 was defined as a rectangular plate undergoing a twisting type of load to deform by two opposite curvatures wherein the plate assumes a saddle-shaped configuration.
As for testing articular cartilage, Chin-Purcell and Lewis27 introduced the trouser tear test based on the mechanics of Mode III loading. In their procedure, the cartilage was cut up the middle with a scalpel through the bone section to the cartilage base, dividing it into two pieces as “trouser legs” (displayed schematically in Fig. 5). The trouser legs were ∼1.5 mm wide. The bone on the legs was carefully placed into the grips so that the length of the leg was parallel to the line of loading. The loading rate was the same as the MSEN test, tearing the cartilage apart along the radial direction of the cartilage. The tear always progressed in this direction. The critical load was determined from the load displacement curve as the first maximum load.
FIG. 5.
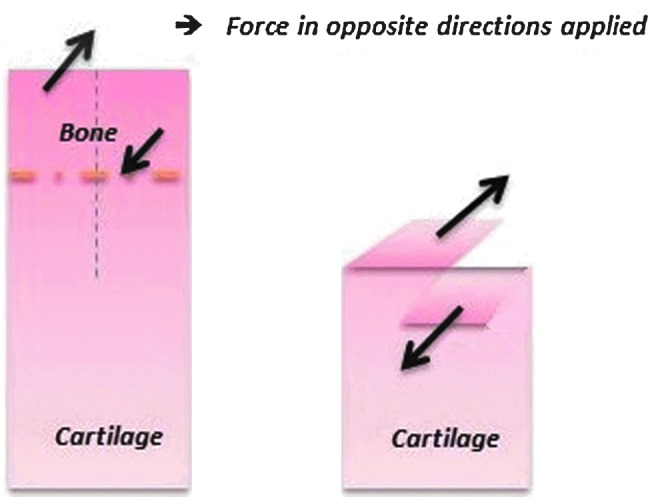
Loading mode III—Trouser tear test: The grips grab the bone parts to tear through the cartilage, where the bone part is the area above the dot-dashed line and the cartilage part is the area below the dot-dashed line. The dotted line indicates the initial crack from the bone into the cartilage. The tearing force is applied only on the bone section. Color images available online at www.liebertpub.com/teb
Mode III—micro-penetration test
Many of the models of the indentation technique focused their attention on the mechanical characteristics of articular cartilage such as surface roughness, wear, and elastic modulus in situ and in vivo.31–36 When combined with visualization techniques such as atomic force microscopy and scanning electron microscopy, we can obtain a much more explicit view of those properties of articular cartilage.19,37,38 However, few indentation methods have focused on measurement of the fracture-related failure properties of cartilage.
In 2004, Simha et al.39 introduced a new method of indentation testing for measuring the apparent fracture toughness of articular cartilage. A penetration or fracture defect in the surface of an intact cartilage, which was left attached to a thin layer of the underlying subchondral bone, was created by a small conical tip (Fig. 6). Unlike tensile and conventional fracture tests, preparation of small or regularly shaped specimens is not required with this method, which is an advantage because this type of specimen preparation is difficult in small animals due to the small volume of cartilage tissue. Due to the small indenting tips they used, the indentation depths were shortened to the order of 100 μm, which were significantly larger than those in conventional nanoindentation methods. Therefore, the name of the described methods above was designated “micro-penetration” by Simha et al.39
FIG. 6.
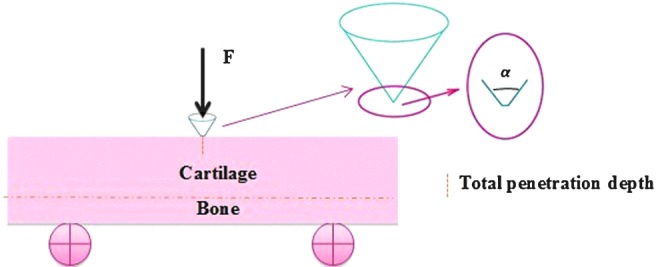
A penetration or fracture defect in the surface of intact cartilage, which is attached to the underlying subchondral bone, is created by a small conical indenter. Color images available online at www.liebertpub.com/teb
To identify whether penetration had occurred, they stained the tested specimens with India ink and examined them under an optical dissecting microscope to identify the penetration by localization of India ink in the created defect. Then, the apparent fracture toughness measurement followed. The apparent fracture toughness, T, was calculated as
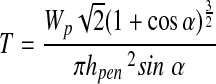 |
(1) |
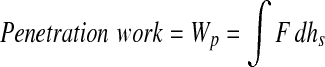 |
(2) |
where Wp was the penetration work, F was the indenting force, hpen was the total penetration depth, hs was the displacement during penetration, and α was the apex angle of the cone.
Summary of cartilage fracture toughness testing
All of the above methods are tabulated in detail (Table 1), including their advantages, disadvantages, and numerical findings. Among those methods, the MSEN test (a Mode I test) is promising in future studies because it is easy to manipulate and visualize, and it maintains the boundary conditions of cartilage. It is also based on a modification of the ASTM standards designed for the SEN test. In contrast, during the SEN test (also a Mode I test), the sample may be easily over-gripped, which can affect the measurement, as deformation of the sample at the deep zone/bone interface does not conform to that which exists in the body. However, the SEN test is relatively easy to perform and can be approached with defects starting from either the top or bottom side of the cartilage. The tear test (a Mode III test) is limited in that it is difficult to grip the bone close to the cartilage interface and observe the whole measurement process because of the shear mode of crack opening. The indentation test may be promising in the measurement of intrinsic fracture parameters of brittle solids. In particular, basic information on fracture-surface energies and crack-velocity functions may be extracted from experimental observations,40 although additional studies on testing different zones in the cartilage would be required to further support its use.
Table 1.
Comparison of Fracture Toughness Measurements of Articular Cartilage
| Method type | Modified single edge notch test | Single edge notch test | Trouser tear test | Micro-penetration test |
|---|---|---|---|---|
| Sample sources | Cartilage with bone from the patella of adult mongrel dogs* and cows** | Bovine patellae | Cartilage with bone from the patella of adult mongrel canines | Bovine articular cartilage from patellae |
| Geometry of samples | 6 mm width, 0.2 mm thickness in a rectangular shape | 7×25 mm in width×length, 1–4 mm thickness in a rectangular shape | 3 mm width, 0.2 mm thickness in a rectangular shape | 10×10×4 mm in a rectangular shape, including the articular cartilage (1–2 mm thick) |
| Model | From energy balance, a pseudoelastic model | The poroelastic fracture toughness model21 | From energy balance, one-dimensional model | Modified standard protocols for Nanoindenter XP |
| Fracture toughness value | Toughness of canine cartilage range J=0.14–1.2 kN/m (Average 1070±870 Nm/m2)*; Average toughness of bovine cartilage 1030±1019 Nm/m2** | KpIc=1.83±0.8 MPa.mm½ | Finding: T=J/1.7 | Run #1: 1102±136 Nm/m2; Run #2: 825±133 Nm/m2 |
| Crack opening mode | Mode I | Mode I | Mode III | Mode III |
| Advantages | Easily view the whole process and approximate the elastic modulus | The process is simple and fast. Easily view the whole process and approximate the elastic modulus | Straightforward calculation and fewer samples are needed | Significantly smaller standard deviations of toughness value are obtained. |
| Disadvantages | Complicated calculation and more samples are required. | Complicated calculation and sample preparation is difficult to some extent | Difficult to view the fracture process under a microscope. It simulates unrealistic failure mode. | The tip geometry affects the results. |
| References | *27; **28 | 21,11 | 27 | 39 |
KpIc, Apparent fracture toughness; T, Apparent fracture toughness; J, J integral.
Fracture Toughness Measurement of Hydrogels
Hydrogels enable encapsulation of cells and affect their gene expression16 under physiological conditions because of their bio-amenable properties such as their high water content capacity, mild gelation conditions for abundant naturally occurring polymers, and their response under loading.41 For scaffolds in articular cartilage regeneration, the fracture properties of synthetic hydrogels are particularly critical, since cartilage requires a mechanical integrity that can sustain large deformations without fracture.42–44
The deformation of hydrogels in response to applied stress is different from that of cartilage. Cartilage deforms relatively easily at small strains, but stiffens with increasing strain. Hence, the stress–strain response of a cartilage is seen as a J-shaped curve, with an initial toe region. In contrast, typical hydrogels are ideal elastic materials, which means that they fit a neo-Hookean stress–strain model described by the equation:
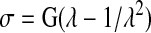 |
(3) |
where G is the shear modulus, σ is the stress, and λ=L/L0, where L is the deformed length and L0 is the undeformed length.45 When plotted as stress versus strain under compression, this function shows a continuously increasing stress with increasing strain, and reduces to Hooke's law only at small strains (below ∼10%). Hence, the stress–strain relationship described by this function differs from the J-shaped curve of cartilage. This is due to the fact that ideal elastic materials have no internal order and their stress–strain response is governed simply by the entropic penalty of shifting randomly oriented chains away from their most probable distribution. However, there are hydrogels that deviate from ideal elastic behavior, and display a response something closer to cartilage. A recent example is that of a double network gel comprised of a methacrylated chondroitin sulfate gel interpenetrated by a polyacrylamide gel. The multicomponent structure of this gel allowed changes in the stress response in different deformation modes, as seen in cartilage.46
In the following subsections, we will introduce the methods that have been used to evaluate the toughness of hydrogels in general. However, in an effort to determine a reliable method for toughness measurement of hydrogels in cartilage regeneration, we will discuss only the methods that can be applied to both hydrogels and cartilage.
Tensile test: with and without notch
For hydrogels, there are two primary methods of tensile failure testing. The first, with notch, is based on the opening Mode I—SEN test, analogous to what was discussed previously with cartilage. The other, without notch, is pure tensile testing. While the word toughness is used to describe the energy to failure parameter for both tests (with and without notch), these two parameters cannot be compared. Fracture toughness is a measure of the energy required to extend a pre-existing defect, while toughness as traditionally measured in tensile testing without a notch refers to the overall ability of the material to resist failure or total energy to failure normalized to specimen volume. This energy includes crack formation as well as extension. A typical tensile test with a notch present would not conform to a standard fracture toughness geometry and would not represent apparent toughness measured from within the cartilage midsubstance.
Kong et al.16 investigated various aspects of gel crosslinking to independently regulate the elastic modulus (E) and toughness in the presence of notches (Wt). After inventing a new type of alginate gel, they assessed the toughness of these hydrogels using the double edge notch test (i.e., the tensile test with notch). They introduced two notches in the rectangular gel strips (10×3×0.1 cm) with a razor blade. The strips were extended at a constant deformation rate of 1 mm/min with the initial notch lengths varying from 1 to 3 mm, and load as a function of displacement was measured. The total work to fracture (Wt) was calculated from the area under the stress versus displacement curve, where stress was defined as the force divided by the cross-sectional area between the notches. Although testing was performed in the presence of defects, the method of determining the toughness parameter was not consistent with fracture toughness approaches. While similar in concept to fracture toughness tests, this test did not use either a standard geometry or typical calculations used in fracture toughness testing and did not measure work to fracture in the presence of notches in the depth direction of the cartilage. The results cannot be directly compared to those from fracture toughness tests.
Smith et al.12 studied the toughness of hydrogels in phosphate-buffered saline at different temperatures, conducting failure tests and comparing the elastic modulus and toughness of the specimens. In the test, dog-bone specimens (laser-cut according to dimensions specified in ASTM D 638-03 Type IV or V) were loaded on a universal testing machine (MTS Systems, Insight 2) using a 2 kN load cell with a 1 mm/min strain rate. The elastic modulus was calculated as the slope of the initial linear region of the stress–strain curve, while toughness was calculated as the area under the stress–strain curve up to the fracture stress point in units of MJ/m3. They concluded that the primary factors that influenced the toughness of hydrogels were the test temperature relative to the glass transition temperature, the water content, and the network structure. In relation to cartilage fracture toughness, it must be noted that this toughness measurement approach does not yield values that can be directly compared with fracture mechanics approaches. Toughness measurements (i.e., energy to failure without the presence of a pre-existing crack) can be used as a means of understanding overall energy-absorbing capabilities of materials until failure, but are often difficult to interpret and have relatively large variations, particularly in composite materials, because failure can occur from many different sources throughout the material. Fracture toughness implies testing to failure in the presence of a specifically defined, sharp crack; if performed under specific conditions to account for the size and shape of the sample and material ductility, fracture toughness measurements can result in repeatable test results that can be compared between materials.
Mode III—tear test
Tanaka et al.15 measured the Mode III fracture toughness of poly(2-acrylamido-2-methyl-propanesulfonic acid) (PAMPS)/polyacrylamide (PAAm) double network gels with different cross-linking densities. They first cut the gels into the standardized rectangular shape (30 mm width) with a gel cutting machine (Dumb Bell Co., Ltd.) (Fig. 7A). The notch length was 20 mm, and the two arms of the test sample (Fig. 7B) were placed in the grips. It was not specified whether the test was performed under hydrated or dry conditions. During the test, only the upper grip was pulled upward at a constant velocity Vp. By recording the tearing force F, they calculated fracture energy G using the following equation,
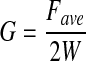 |
(4) |
FIG. 7.
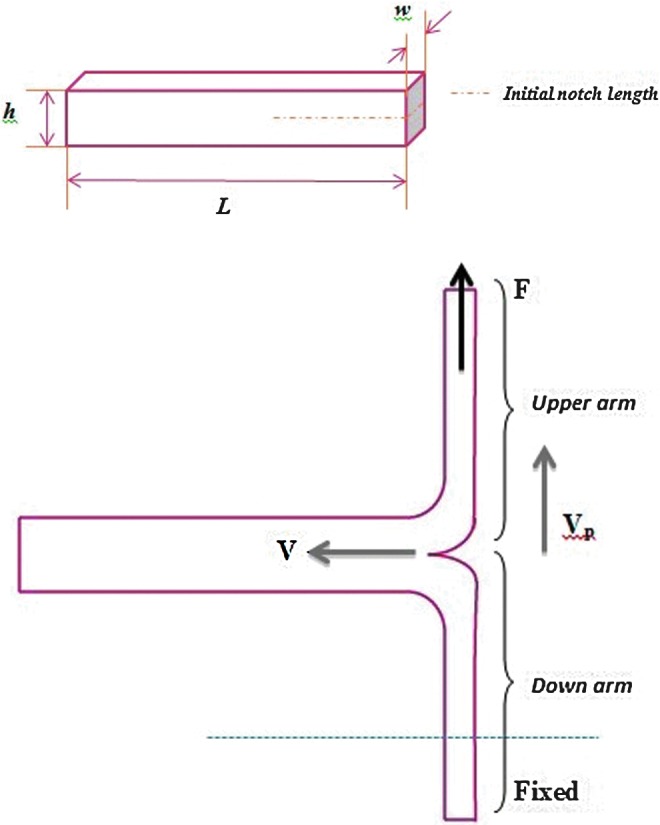
(Top) Standardized rectangular shape for a trouser tear test with a hydrogel: w=5 mm, L=50 mm, h=7.5 mm, the length of the initial notch is 20 mm; (Bottom) trouser tear test: F is the tearing force, Vp is the pulling velocity, and V is the crack velocity. Color images available online at www.liebertpub.com/teb
where Fave was the average of F during tear and w was the width of the gels. G was defined as the energy required to create a unit area of fracture surface in a sample gel. The equation was modified to G=Fave/w by Nakajima et al.47 and in later articles. Since only one arm of the tearing sample was pulled and the other arm was fixed, they assumed that there was no elongation of the arms and that the crack velocity V was equal to Vp/2 for such an asymmetrical loading. The work done to the sample per unit time dW/dt is given by dW/dt=FaveVp, and the newly created fracture area per unit time dA/dt is given by dA/dt=2wV (the numerical number 2 accounts for two surfaces); thus, G≡(dW/dt)/(dA/dt)=Fave/w. Even taking elongation of the arms into account, the tearing velocity V was changed from 0.5×10−5 to 0.5×10−2 m/s, which may be considered negligible. The change of elastic energy stored in the pulled arms resulted in a correction of only a few percent for G and V, which is also insignificant.
Compression test
In transitioning away from toughness in a fracture mechanics context, the most straightforward evaluation of general toughness for hydrogels is through the compression test, through which the elastic modulus and shear modulus can also be obtained. We were the first to develop a new method for encapsulating cells in interpenetrating network (IPN) hydrogels of superior mechanical performance,6 where dynamic mechanical analysis was used to determine the mechanical performance of a new IPN hydrogel based on two biocompatible materials—agarose and poly(ethylene glycol) diacrylate. During these tests, all of the hydrogel samples were prepared in a cylindrical shape and placed between compression platens, which were lubricated with mineral oil. The toughness was then calculated by numerical integration of the stress–strain curve generated by compressing each sample at a rate of 0.0005 mm/s. It should be noted that this approach differs from the toughness parameters measured in tensile testing as well as the fracture toughness evaluated in fracture mechanics approaches, thus the values cannot be directly compared. Compression to failure is highly dependent on artifacts in the gel, and especially considering the nonlinear stress–strain relationship, differences in fracture strain are manifested in larger differences in fracture strength and even larger differences in toughness, resulting in relatively high variability. This is inherent to the method, where there is an extreme limit of 100% strain, and a high degree of variability with materials that fracture at 80%–90% strain under compression. The concern with the relatively large variability in toughness values acquired via compression juxtaposed with the more reproducible methods in fracture mechanics in large part inspired the current review to examine alternatives for evaluating mechanical failure properties of tissue-engineered constructs.
Summary of hydrogel fracture toughness testing
Tests to measure toughness parameters of hydrogels have included tensile tests (with and without notches present), tear testing of a notched specimen, and compression. Only the tests done in the presence of notches represent a fracture mechanics approach and none of these tests are in direct accordance with ASTM standards that were developed for plastic materials. All of the above methods are tabulated in detail (See Table 2), and their advantages and disadvantages are provided. Referring to Table 2, the SEN test based on the tensile testing for polymers from ASTM standards is promising because different types of fracture energy can be calculated, such as the work to fracture dissipated outside the process zone and the essential work at the process zone. Other tests have limitations that may show some unsatisfied aspects. For example, the tensile test without notch can only evaluate the overall toughness. The trouser tear test used for soft hydrogel samples has issues in comparison with cartilage with gripping of the specimen. In cartilage testing, the attached bone is gripped and the cartilage torn. When the grips grab the tear legs of the soft hydrogel samples, the trouser legs will easily be torn before tear loading is applied because of the stress concentrations at the grip faces. In addition, a large amount of sample materials may be needed to meet the standard geometry in the tear test. There are many ASTM standards related to composite polymers and plastic materials, but no specific standards for hydrogels. Similarly, there are no specific standards for toughness measurements for hydrogels used in cartilage regeneration.
Table 2.
Comparison of Fracture Toughness and Toughness Measurements of Hydrogels
| Method type | Single edge notch test | Tensile test | Tear test | Compression test |
|---|---|---|---|---|
| Sample sources | Alginate gels | MMA-co-45% PEGDMA, 2HEMA-co-2% PEGDMA, MA-co-MMA-co-2% PEGDMA, 100% PEGDMA | PAMPS+PAAm double network gels | Agarose, PEG, IPN |
| Geometry of the samples | Rectangular strips | Dog bone shape | Rectangular | Cylindrical |
| Model | Energy balance | Integration of the stress vs. strain curve | One-dimensional model | Integration of the stress vs. strain curve |
| Toughness value | 0.4–80.4 J/m3 | 0.08–200 MJ/m3 | 102–103 J/m2 | ∼0.2–146 kJ/m3 |
| Advantages | Different types of fracture energy can be calculated | Can avoid stress concentrations; This method is applied for many types of hydrogels | Straightforward testing with standardized specimen geometry; It is easy to analyze data | A straightforward test with standardized specimen geometry; It is easy to analyze data |
| Disadvantages | It is likely to crush the gel or lengthen the crack when the gel is loaded | The sample preparation is time-consuming. It can only evaluate the energy to failure (not fracture toughness) for the given specimen shape | It is difficult to load the gels since it is a 3D process | The failure mechanism is different between compression and tension so we cannot compare the value. Reproducibility can be a challenge |
| References | 16 | 12 | 15 | 6 |
MMA, methyl methacrylate; PEGDMA, poly(ethylene glycol) dimethacrylate; PAMPS, poly(2-acrylamido-2-methylpropanesulfonic acid); PAAm, polyacrylamide; PEG, poly(ethylene glycol); IPN, interpenetrating network.
Discussion
In a general view of testing articular cartilage, there are several different methods for testing the apparent fracture toughness. However, diverse cartilage sources may affect the choice of the testing method. For example, in the SEN test, articular cartilage from small animals may be difficult to grip, due to their limited length and thickness. With the articular cartilage from the ankles of even large animals, the trouser tear test (Mode III) may not be practical because the thinness of the cartilage layer and its irregular surface will make it difficult to section into the standard geometry.
Another point of consideration is that cartilage is heterogeneous, which means that different toughness values may be obtained when using different specimen preparation methods. For example, in Chin-Purcell and Lewis's work, they sectioned off the superficial zone of the cartilage to avoid the aberrant crack extension and only tested the fracture resistance in the deep and middle zones. In contrast, in their later micro-penetration test, the fracture resistance was measured in the surface. Experimental data in bovine specimens indicated that the tensile module in the superficial zone was five times greater than in the middle zone. The tensile modulus in the middle zone was in turn four times greater than in the deep zone.
Furthermore, variations in toughness values may occur even with the same testing method for cartilage. Few articles actually mentioned how the crack position was verified. Finding a method to best ensure that cracks are made consistently may help to overcome the problem. Although a number of investigators have supplemented Chin-Purcell and Lewis's work in testing apparent fracture toughness in tension, future research still remains such as changing the depth of the crack in the MSEN test so as to identify the fracture resistance in each zone of cartilage.
Of course, the geometry of hydrogels can be easily controlled. Thus, different testing methods can be applied, such as the tensile test, tear test, or compression test, to one type of hydrogel. Strict attention is necessary to avoid creating any microcracks when loading those hydrogels into the testing machine, especially when using the trouser tear test or SEN test.
However, based on the testing methods of articular cartilage, we may narrow those methods for hydrogels down to fit the purpose of evaluating mechanical failure of hydrogel-based constructs for cartilage tissue engineering. In comparing the articular cartilage and hydrogel-based constructs side by side, the differences in testing systems such as in the micro-penetration method would ideally be eliminated. Although it works well on articular cartilage with straightforward manipulation, it is limited in testing hydrogels. Because the hydrogels in cartilage regeneration are normally softer compared to other types, such as contact lenses,17,48–50 when testing soft samples, a large viscoelastic deformation occurs, the tear closes upon unloading and the equivalence of the indentation of the material with a penetration defect and a flat surface becomes unrealistic.
To consolidate the two distinct fields of cartilage and hydrogel fracture testing, the best method for both cartilage and hydrogels may be tensile testing based on Mode I, thus eliminating the Mode III trouser tear test. The testing system setup for micro-penetration is not suitable for hydrogels. Moreover, compression testing does not lend itself well to yielding reproducible data, nor does it provide fracture toughness in a strict fracture mechanics sense. Thus, the test in Mode I may be the most appropriate approach for testing both hydrogels and articular cartilage, thus allowing for more relevant comparisons. In addition, Mode I has a solid data analytical method based on ASTM standards.
Conclusion
Biomaterial-based tissue-engineering strategies offer great promise, including the use of hydrogels to regenerate articular cartilage. Cartilage and hydrogels have different fracture properties and mechanisms, and because of these differences, the cartilage tissue-engineering research community would benefit from the development of a uniform method that can be applied to both materials. Based on fracture mechanics literature from both the cartilage and hydrogel fields, a leading candidate for a fracture toughness testing method for hydrogels in cartilage regeneration may be the MSEN test. This recommendation is primarily based on the ability to test identical geometries of cartilage and hydrogels (and therefore reduce related boundary condition inconsistencies that will influence measured values), measurement in Mode I, the ability to use minimal materials during testing, and the ability to measure and compare fracture properties in the depth direction of various zones. Providing standards and testing methods that accommodate for both hydrogel and cartilage will allow us to improve the failure properties of hydrogels and will ultimately lead to better tissue replacements for damaged articular cartilage.
Acknowledgments
The authors would like to acknowledge funding from the NIH/NIBIB (R21 EB008783).
Disclosure Statement
No competing financial interests exist.
References
- 1.Knecht S. Vanwanseele B. Stussi E. A review on the mechanical quality of articular cartilage—implications for the diagnosis of osteoarthritis. Clin Biomech. 2006;21:999. doi: 10.1016/j.clinbiomech.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Azuma C. Yasuda K. Tanabe Y. Taniguro H. Kanaya F. Nakayama A. Chen Y.M. Gong J.P. Osada Y. Biodegradation of high-toughness double network hydrogels as potential materials for artificial cartilage. J Biomed Mater Res Part A. 2007;81A:373. doi: 10.1002/jbm.a.31043. [DOI] [PubMed] [Google Scholar]
- 3.Huang A.H. Yeger-McKeever M. Stein A. Mauck R.L. Tensile properties of engineered cartilage formed from chondrocyte- and MSC-laden hydrogels. Osteoarthritis Cartilage. 2008;16:1074. doi: 10.1016/j.joca.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sant S. Hancock M.J. Donnelly J.P. Iyer D. Khademhosseini A. Biomimetic gradient hydrogels for tissue engineering. Can J Chem Eng. 2010;88:899. doi: 10.1002/cjce.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu J.M. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeKosky B.J. Dormer N.H. Ingavle G.C. Roatch C.H. Lomakin J. Detamore M.S. Gehrke S.H. Hierarchically designed agarose and poly(ethylene glycol) interpenetrating network hydrogels for cartilage tissue engineering. Tissue Eng Part C Methods. 2010;16:1533. doi: 10.1089/ten.tec.2009.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oss-Ronen L. Seliktar D. Polymer-conjugated albumin and fibrinogen composite hydrogels as cell scaffolds designed for affinity-based drug delivery. Acta Biomater. 2011;7:163. doi: 10.1016/j.actbio.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Hegedus C. Bako J. Hydrogels as a drug delivery system in dentistry. Acta Physiol Hung. 2010;97:84. [Google Scholar]
- 9.Cha C. Kohmon R.E. Kong H. Biodegradable polymer crosslinker: independent control of stiffness, toughness, and hydrogel degradation rate. Adv Funct Mater. 2009;19:3056. [Google Scholar]
- 10.Abdurrahmanoglu S. Can V. Okay O. Design of high-toughness polyacrylamide hydrogels by hydrophobic modification. Polymer. 2009;50:5449. [Google Scholar]
- 11.Stok K. Oloyede A. Conceptual fracture parameters for articular cartilage. Clin Biomech. 2007;22:725. doi: 10.1016/j.clinbiomech.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Smith K.E. Temenoff J.S. Gall K. On the toughness of photopolymerizable (meth)acrylate networks for biomedical applications. J Appl Polym Sci. 2009;114:2711. [Google Scholar]
- 13.Nakajima T. Kurokawa T. Furukawa H. Yu Q.M. Tanaka Y. Osada Y. Gong J.P. Super tough gels with a double network structure. Chinese J Polym Sci. 2009;27:1. [Google Scholar]
- 14.Kundu S. Crosby A.J. Cavitation and fracture behavior of polyacrylamide hydrogels. Soft Matter. 2009;5:3963. [Google Scholar]
- 15.Tanaka Y. Kuwabara R. Na Y.H. Kurokawa T. Gong J.P. Osada Y. Determination of fracture energy of high strength double network hydrogels. J Phys Chem B. 2005;109:11559. doi: 10.1021/jp0500790. [DOI] [PubMed] [Google Scholar]
- 16.Kong H.J. Wong E. Mooney D.J. Independent control of rigidity and toughness of polymeric hydrogels. Macromolecules. 2003;36:4582. [Google Scholar]
- 17.Jackson A.P. Measurement of the fracture toughness of some contact lens hydrogels. Biomaterials. 1990;11:403. doi: 10.1016/0142-9612(90)90095-8. [DOI] [PubMed] [Google Scholar]
- 18.Gong J.P. Why are double network hydrogels so tough? Soft Matter. 2010;6:2583. [Google Scholar]
- 19.Loparic M. Wirz D. Daniels A.U. Raiteri R. VanLandingham M.R. Guex G. Martin I. Aebi U. Stolz M. Micro- and nanomechanical analysis of articular cartilage by indentation-type atomic force microscopy: validation with a gel-microfiber composite. Biophys J. 2010;98:2731. doi: 10.1016/j.bpj.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahsan T. Sah R.L. Biomechanics of integrative cartilage repair. Osteoarthritis Cartilage. 1999;7:29. doi: 10.1053/joca.1998.0160. [DOI] [PubMed] [Google Scholar]
- 21.Stok K. Oloyede A. A qualitative analysis of crack propagation in articular cartilage at varying rates of tensile loading. Connect Tissue Res. 2003;44:109. [PubMed] [Google Scholar]
- 22.Glenister T.W. Embryological view of cartilage. J Anat. 1976;122:323. [PMC free article] [PubMed] [Google Scholar]
- 23.Little C.J. Bawolin N.K. Chen X. Mechanical properties of natural cartilage and tissue-engineered constructs. Tissue Eng Part B. 2011;17:213. doi: 10.1089/ten.TEB.2010.0572. [DOI] [PubMed] [Google Scholar]
- 24.Clarke J.C. Surface characteristics of human articular cartilage—scanning electron microscope study. J Anat. 1971;108:23. [PMC free article] [PubMed] [Google Scholar]
- 25.Madry H. van Dijk C.N. Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010;18:419. doi: 10.1007/s00167-010-1054-z. [DOI] [PubMed] [Google Scholar]
- 26.Lewis J.L. Johnson S.L. Collagen architecture and failure processes in bovine patellar cartilage. J Anat. 2001;199:483. doi: 10.1046/j.1469-7580.2001.19940483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ChinPurcell M.V. Lewis J.L. Fracture of articular cartilage. J Biomech Eng. 1996;118:545. doi: 10.1115/1.2796042. [DOI] [PubMed] [Google Scholar]
- 28.Adams D.J. Brosche K.M. Lewis J.L. Effect of specimen thickness on fracture toughness of bovine patellar cartilage. J Biomech Eng. 2003;125:927. doi: 10.1115/1.1635405. [DOI] [PubMed] [Google Scholar]
- 29.Podczeck F. The determination of fracture mechanics properties of pharmaceutical materials in mode III loading using an anti-elastic plate bending method. Int J Pharmaceut. 2001;227:39. doi: 10.1016/s0378-5173(01)00783-9. [DOI] [PubMed] [Google Scholar]
- 30.Farshad M. Flueler P. Investigation of mode III fracture toughness using an anti-clastic plate bending method. Eng Fract Mech. 1998;60:597. [Google Scholar]
- 31.Bae W.C. Temple M.A. Amiel D. Coutts R.D. Niederauer G.G. Sah R.L. Indentation testing of human cartilage—sensitivity to articular surface degeneration. Arthritis Rheum. 2003;48:3382. doi: 10.1002/art.11347. [DOI] [PubMed] [Google Scholar]
- 32.Julkunen P. Korhonen R.K. Herzog W. Jurvelin J.S. Uncertainties in indentation testing of articular cartilage: a fibril-reinforced poroviscoelastic study. Med Eng Phys. 2008;30:506. doi: 10.1016/j.medengphy.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Simha N.K. Jin H. Hall M.L. Chiravarambath S. Lewis J.L. Effect of indenter size on elastic modulus of cartilage measured by indentation. J Biomech Eng. 2007;129:767. doi: 10.1115/1.2768110. [DOI] [PubMed] [Google Scholar]
- 34.Miller G.J. Morgan E.F. Use of microindentation to characterize the mechanical properties of articular cartilage: comparison of biphasic material properties across length scales. Osteoarthritis Cartilage. 2010;18:1051. doi: 10.1016/j.joca.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korhonen R.K. Wong M. Arokoski J. Lindgren R. Helminen H.J. Hunziker E.B. Jurvelin J.S. Importance of the superficial tissue layer for the indentation stiffness of articular cartilage. Med Eng Phys. 2002;24:99. doi: 10.1016/s1350-4533(01)00123-0. [DOI] [PubMed] [Google Scholar]
- 36.Korhonen R.K. Laasanen M.S. Toyras J. Rieppo J. Hirvonen J. Helminen H.J. Jurvelin J.S. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J Biomech. 2002;35:903. doi: 10.1016/s0021-9290(02)00052-0. [DOI] [PubMed] [Google Scholar]
- 37.Park S. Costa K.D. Ateshian G.A. Hong K.S. Mechanical properties of bovine articular cartilage under microscale indentation loading from atomic force microscopy. Proc Inst Mech Eng H. 2009;223:339. doi: 10.1243/09544119JEIM516. [DOI] [PubMed] [Google Scholar]
- 38.Monclus M.A. Young T.J. Di Maio D. AFM indentation method used for elastic modulus characterization of interfaces and thin layers. J Mater Sci. 2010;45:3190. [Google Scholar]
- 39.Simha N.K. Carlson C.S. Lewis J.L. Evaluation of fracture toughness of cartilage by micropenetration. J Mater Sci Mater Med. 2004;15:631. doi: 10.1023/b:jmsm.0000026104.30607.c7. [DOI] [PubMed] [Google Scholar]
- 40.Lawn B. Wilshaw R. Indentation fracture—principles and applications. J Mater Sci. 1975;10:1049. doi: 10.1007/s10853-020-04991-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaramillo-Botero A. Blanco M. Li Y.Y. McGuinness G. Goddard W.A. First-principles based approaches to nano-mechanical and biomimetic characterization of polymer-based hydrogel networks for cartilage scaffold-supported therapies. J Comput Theor Nanosci. 2010;7:1238. [Google Scholar]
- 42.Gong J.P. Osada Y. Soft and wet materials: from hydrogels to biotissues. High Solid Dispersions. 2010;236:203. [Google Scholar]
- 43.Gong J.P. Liang S.M. Wu Z.L. Hu J. Kurokawa T. Yu Q.M. Direct observation on the surface fracture of ultrathin film double-network hydrogels. Macromolecules. 2011;44:3016. [Google Scholar]
- 44.Gong J.P. Katsuyama Y. Kurokawa T. Osada Y. Double-network hydrogels with extremely high mechanical strength. Adv Mater. 2003;15:1155. [Google Scholar]
- 45.Erman B. Mark J. Structures and Properties of Rubberlike Networks. New York, NY: Oxford University Press; 1997. [Google Scholar]
- 46.Suekama T.C. Hu J. Kurokawa T. Gong J.P. Gehrke S.H. Double-network strategy improves fracture properties of chondroitin sulfate networks. ACS Macro Lett. 2013;2:137. doi: 10.1021/mz3006318. [DOI] [PubMed] [Google Scholar]
- 47.Nakajima T. Furukawa H. Tanaka Y. Kurokawa T. Osada Y. Gong J.P. True chemical structure of double network hydrogels. Macromolecules. 2009;42:2184. [Google Scholar]
- 48.Bruce A.S. Mainstone J.C. Golding T.R. Analysis of tear film breakup on Etafilcon A hydrogel lenses. Biomaterials. 2001;22:3249. doi: 10.1016/s0142-9612(01)00162-4. [DOI] [PubMed] [Google Scholar]
- 49.Miller K.L. Polse K.A. Radke C.J. Fenestrations enhance tear mixing under silicone-hydrogel contact lenses. Invest Ophth Vis Sci. 2003;44:60. doi: 10.1167/iovs.02-0348. [DOI] [PubMed] [Google Scholar]
- 50.Paugh J.R. Stapleton F. Keay L. Ho A. Tear exchange under hydrogel contact lenses: methodological considerations. Invest Ophth Vis Sci. 2001;42:2813. [PubMed] [Google Scholar]


