Abstract
Background
The increases in thyroid cancer overall and in the predominant papillary type have been well documented, but trends for follicular thyroid cancer, a less common but more aggressive variant, have not been as well characterized. In this study, we determined the incidence patterns for follicular thyroid cancer and compared trends between the follicular and papillary thyroid cancers in the United States.
Methods
We used the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program to examine incidence in the United States during 1980–2009, stratified by demographic and tumor characteristics. Incidence rates (IR) were calculated, relative risks were expressed as incidence rate ratios (IRR), and temporal trends were expressed as percentage changes and plotted.
Results
Overall we observed a modest increase in age-adjusted follicular thyroid cancer rates among women (31.89%) and men (35.88%). Rates increased most dramatically for regional stage tumors compared to localized tumors in women, whereas the rates for all tumor sizes rose. These findings reveal increases in more aggressive tumors in women in addition to small and localized tumors. The trends for males were different from those among females. Among males, the largest increase was observed for regional and smaller size tumors. The papillary-to-follicular IRR overall was 7.07 [95% confidence interval 6.91–7.24], which varied from 7.37 among Whites to 3.86 among Blacks (SEER race/ethnicity categories), and increased significantly from 3.98 during 1980–1984 to 9.88 during 2005–2009.
Conclusion
The different trends for follicular and papillary types of thyroid cancer illustrate that thyroid cancer is a heterogeneous disease. Our results do not support the hypothesis that increasing thyroid cancer rates are largely due to improvements in detection, and suggest the importance of evaluating thyroid cancer types separately in future studies.
Introduction
The incidence of thyroid cancer has increased worldwide for decades (1–8), and its etiology is not well understood (9). Papillary thyroid cancer is the most common type (comprising ∼80% of thyroid cancer compared to 10–15% for follicular), and it accounts for most of the rise in thyroid cancer overall (1). However, the follicular type is of interest because it is a more aggressive form of thyroid cancer (10) that also seems to be increasing in incidence (1). Follicular thyroid cancer is more aggressive mainly because it can metastasize via vascular invasion, as opposed to papillary thyroid cancer that spreads via the lymphatics (10). The ability to spread through the vasculature means that follicular thyroid cancer more often presents with metastasis outside of the neck (distant metastasis) (11). The most common site of distant metastasis in both papillary and follicular thyroid cancer is the lung. However, ∼11% of patients with follicular thyroid cancer compared to 2–3% of patients with papillary thyroid cancer have metastases beyond the lungs on initial presentation (11–13). Distant metastasis at the time of presentation confers a worse survival for patients with any type of thyroid cancer. Thus, patients with follicular cancer have an overall worse survival compared to patients with papillary cancer, making an improved understanding of follicular cancer an important topic of investigation.
The known risk factors for these two cancer types are different (7,8). Mutation analyses show mutually exclusive molecular pathways lead to each of these tumor types (14). Radiation exposure almost uniformly causes papillary but not follicular thyroid cancer (15,16), and iodine deficiency is associated with follicular but not papillary thyroid cancer risk (9,16). Furthermore, previous research has shown a female/male incidence rate ratio (IRR) of about 3 for the papillary type and about 2 for the follicular type (1,4,5), and the IRRs decrease notably across the lifespan (1,5). Understanding the variation in follicular thyroid cancer incidence according to demographic and tumor characteristics could inform future analytic studies and intervention strategies.
Five groups have recently examined time trends in thyroid cancer incidence using the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) data (1,3–5,8,17). However, most have focused on patterns and trends for the papillary subtype. In this study, we used the SEER program data from 1980–2009 to analyze the incidence patterns for follicular thyroid cancer by sex, SEER race/ethnicity category, age, and tumor characteristics to understand better this rare cancer variant. We also compared the trends for follicular thyroid cancer with those for papillary thyroid cancer to provide additional insight into these cancer types. This investigation of follicular thyroid cancer explores possible factors that could provide insight into etiology and the temporal trends.
Methods
We used the data from the National Cancer Institute's SEER program to analyze female and male thyroid carcinoma incidence rates (IR) from 1980 through 2009 using the nine registries in Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, and Utah, which include approximately 10% of the U.S. population (18). First, primary thyroid cancer cases were coded using the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) (19), and were stratified into the four major histological types (19): follicular carcinoma (ICD-O-3 codes 8290, 8330–8332, 8335) and papillary carcinoma (ICD-O-3 codes 8050, 8260, 8340–8344, 8350, 8450, 8452, 8460). Although the classification for the follicular variant of papillary thyroid cancer (8340) was classified as follicular thyroid cancer prior to 1988, in this analysis, the histological groupings were consistent over the entire time period. As such, we excluded the follicular variant of papillary thyroid cancer cases from the follicular group for the entire time period.
In addition to sex and histologic type, we evaluated demographic and tumor characteristics, including age at diagnosis, race/ethnicity, registry, SEER historic stage A (20), and tumor size. The race/ethnicity categories available in the incidence data over this entire time period, as defined by the U.S. Census Bureau (21), were Whites, Blacks, and Others (American Indian, Alaskan Natives, Asians, and Pacific Islanders combined) or unknown. SEER historic stage A was used to classify thyroid cancers as localized (limited to the thyroid gland), regional (limited to surrounding tissues), and distant or systemic disease (18). Beginning in 1988, SEER systematically collected information on tumor size, the cancer's greatest diameter as recorded on surgical pathology reports, which we categorized as ≤1 cm, >1 cm but ≤2 cm, >2 cm but ≤4 cm, and >4 cm based on the Extent of Disease-10 (EOD-10) codes for 1988–2003 and the Collaborative Staging (CS) codes for 2004–2006 (22).
Data analysis
IRs were calculated using SEER*Stat 7.0.9 (23) and expressed per 100,000 person years, man years, or woman years. Age-adjusted rates were standardized to the 2000 U.S. population. Relative risks were expressed as incidence rate ratios (IRR), where a given characteristic was compared to a referent rate with an assigned IRR of 1.0. Statistical significance of rates and IRR were assessed at the p<0.05 alpha level; all hypothesis tests were two-sided.
We used the first and last five-year calendar periods (1980–1984 and 2005–2009) to assess the temporal trends, expressed as the percentage change (%CH=100×[final IR−initial IR]/initial IR) (1990–1994 to 2005–2009 were used for tumor size); 95% confidence intervals (CIs) were calculated with the delta method for men and women separately (24). Temporal trends for IRRs were plotted on a linear x and y scale.
Results
During the period 1980–2009, a total of 6410 follicular thyroid cancer cases (IR=0.88 per 100,000 person years) were diagnosed among residents of the nine SEER registries compared to 45,942 papillary thyroid cancer cases (IR=6.21).
The highest follicular thyroid cancer rates were in the older age groups for both men and women. Overall, follicular carcinoma rates among females were twice those among males (Table 1); the greatest female-to-male disparities occurred at the younger ages of 10–39 (IRR=3.76–4.50). Overall, follicular thyroid cancer IRs were similar among those in the Whites, Blacks, and the “Other” categories, the last of which consisted largely of those of Asian ancestral origin. There was no difference in female-to-male IRRs between the ethnicity categories. When stratified by stage and size, the largest female-to-male IRRs occurred for tumors at the localized stage compared to the regional and distant stages, and in tumors between 1 cm and 2 cm in size. There was also a significant difference between rates in men and women in the largest size (4 cm+group) tumor group (IRR=1.39 [CI 1.29–1.50]). No consistent trends in the female-to-male IRR over time were apparent (IRRs ranged from 1.8 to 2.5 over time; not shown).
Table 1.
Follicular Thyroid Cancer Incidence in SEER's 9-Registry Database (1980–2009), Stratified by Sex
| |
Females |
Males |
|
CI |
|||||
|---|---|---|---|---|---|---|---|---|---|
| n | Rate | SE | n | Rate | SE | Female/male IRR | LL | UL | |
| Total | 4563 | 1.19 | 0.02 | 1847 | 0.55 | 0.01 | 2.15 | 2.05 | 2.26 |
| Age in years | |||||||||
| 0–9 | 1 | – | – | 1 | – | – | – | – | – |
| 10–19 | 96 | 0.18 | 0.02 | 23 | 0.04 | 0.01 | 4.50 | 2.81 | 7.21 |
| 20–29 | 492 | 0.85 | 0.04 | 89 | 0.15 | 0.02 | 5.67 | 4.56 | 7.04 |
| 30–39 | 832 | 1.39 | 0.05 | 218 | 0.37 | 0.03 | 3.76 | 3.24 | 4.36 |
| 40–49 | 931 | 1.77 | 0.06 | 313 | 0.61 | 0.03 | 2.90 | 2.57 | 3.27 |
| 50–59 | 754 | 1.86 | 0.07 | 390 | 1.01 | 0.05 | 1.84 | 1.63 | 2.08 |
| 60–69 | 607 | 2.02 | 0.08 | 420 | 1.59 | 0.08 | 1.27 | 1.12 | 1.44 |
| 70–79 | 549 | 2.46 | 0.10 | 290 | 1.77 | 0.10 | 1.39 | 1.22 | 1.59 |
| +80 years | 301 | 2.06 | 0.12 | 103 | 1.40 | 0.14 | 1.47 | 1.19 | 1.82 |
| Race/ethnicity | |||||||||
| White | 3591 | 1.17 | 0.02 | 1549 | 0.57 | 0.01 | 2.05 | 1.95 | 2.16 |
| Black | 443 | 1.13 | 0.06 | 141 | 0.51 | 0.05 | 2.22 | 1.82 | 2.70 |
| Other | 474 | 1.31 | 0.06 | 145 | 0.48 | 0.04 | 2.73 | 2.30 | 3.24 |
| Unknown | 55 | – | – | 12 | – | – | – | – | – |
| Registry | |||||||||
| Hawaii | 292 | 1.71 | 0.10 | 103 | 0.62 | 0.06 | 2.76 | 2.23 | 3.41 |
| New Mexico | 289 | 1.18 | 0.07 | 95 | 0.44 | 0.05 | 2.68 | 2.14 | 3.36 |
| Atlanta | 455 | 1.22 | 0.06 | 165 | 0.54 | 0.05 | 2.26 | 1.88 | 2.71 |
| Seattle | 663 | 1.19 | 0.05 | 275 | 0.55 | 0.03 | 2.16 | 1.89 | 2.48 |
| Connecticut | 632 | 1.16 | 0.05 | 255 | 0.54 | 0.03 | 2.15 | 1.87 | 2.47 |
| Iowa | 574 | 1.27 | 0.05 | 245 | 0.60 | 0.04 | 2.12 | 1.84 | 2.44 |
| SF–Oakland | 572 | 0.94 | 0.04 | 248 | 0.46 | 0.03 | 2.04 | 1.77 | 2.36 |
| Utah | 297 | 1.14 | 0.07 | 120 | 0.56 | 0.05 | 2.04 | 1.66 | 2.50 |
| Detroit | 789 | 1.26 | 0.05 | 341 | 0.66 | 0.04 | 1.91 | 1.67 | 2.19 |
| SEER stage | |||||||||
| Localized | 2474 | 0.65 | 0.01 | 874 | 0.26 | 0.01 | 2.50 | 2.34 | 2.67 |
| Regional | 1660 | 0.43 | 0.01 | 775 | 0.23 | 0.01 | 1.87 | 1.71 | 2.04 |
| Distant | 271 | 0.07 | 0.00 | 157 | 0.05 | 0.00 | 1.40 | 1.01 | 1.83 |
| Unknown | 155 | 0.04 | 0.00 | 41 | 0.01 | 0.00 | 3.15 | 2.36 | 4.21 |
| Tumor size (1988–2009) | |||||||||
| 0–1 cm | 224 | 0.08 | 0.01 | 72 | 0.03 | 0.00 | 2.67 | 1.82 | 3.92 |
| >1 and ≤2 cm | 703 | 0.24 | 0.01 | 132 | 0.05 | 0.00 | 4.80 | 4.08 | 5.64 |
| >2 and ≤4 cm | 1382 | 0.47 | 0.01 | 457 | 0.17 | 0.01 | 2.76 | 2.52 | 3.04 |
| >4 cm | 1283 | 0.43 | 0.01 | 804 | 0.31 | 0.01 | 1.39 | 1.29 | 1.50 |
| Unknown | 971 | – | – | 382 | – | – | – | – | – |
Rates are per 100,000 person years, man years, or woman years (age-adjusted to the 2000 U.S. standard population). For tumor size, the time period is limited to 1988–2009.
n, number of cases; SE, standard error; IRR, female-to-male incidence rate ratio based on unrounded rates; CI, 95% confidence interval; LL and UL, lower and upper limits of CI; –, not calculated and/or not applicable.
We evaluated trends in follicular thyroid cancer incidence over time in women and men (Table 2). Follicular thyroid cancer incidence rose 31.7% from 1980–4 to 2005–9 (31.89% in females and 35.88% in males). Among females, the most rapid increase was 74.96%, and was found among those aged 50–59 years (Table 2). A nonsignificant decline in follicular thyroid cancer incidence was observed for females in the 80+ age group. Rates rose among White females, but stayed the same or decreased for the other race/ethnicity categories. Rates increased for the smallest follicular tumors but decreased for the localized stage among females, whereas the rate for the largest tumors rose by 11.43%.
Table 2.
Follicular Thyroid Cancer Incidence in SEER's 9-Registry Database (1980–2009): Percentage Change Over Time
| |
1980–1984 |
2005–2009 |
%CH 1980–1984 or 1990–1994a to 2005–2009 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Rate | SE | n | Rate | SE | %CH | LL | UL | |
| A. Females | |||||||||
| Total (N=5136) | 590 | 1.11 | 0.05 | 1087 | 1.46 | 0.05 | 31.89% | 24.87 | 39.13 |
| Age in years | |||||||||
| 0–9 | 0 | – | 0.01 | 0 | – | – | – | – | – |
| 10–19 | 14 | 0.16 | 0.04 | 23 | 0.24 | 0.05 | 50.63% | −16.50 | 117.70 |
| 20–29 | 83 | 0.82 | 0.09 | 100 | 1.02 | 0.10 | 24.15% | 1.11 | 47.09 |
| 30–39 | 122 | 1.48 | 0.14 | 170 | 1.72 | 0.13 | 16.22% | −0.23 | 32.63 |
| 40–49 | 94 | 1.64 | 0.17 | 229 | 2.12 | 0.14 | 29.02% | 12.63 | 45.37 |
| 50–59 | 75 | 1.35 | 0.16 | 224 | 2.36 | 0.16 | 74.96% | 51.63 | 98.37 |
| 60–69 | 103 | 2.19 | 0.22 | 153 | 2.63 | 0.21 | 20.09% | 2.05 | 38.15 |
| 70–79 | 64 | 2.09 | 0.26 | 125 | 3.20 | 0.29 | 52.87% | 26.42 | 79.38 |
| +80 | 35 | 2.06 | 0.35 | 63 | 2.00 | 0.25 | −2.96% | −27.89 | 21.89 |
| Race/ethnicity | |||||||||
| White | 472 | 1.05 | 0.05 | 838 | 1.48 | 0.05 | 41.24% | 32.50 | 49.90 |
| Black | 58 | 1.33 | 0.18 | 114 | 1.32 | 0.13 | −0.60% | −24.76 | 12.76 |
| Other | 60 | 1.63 | 0.22 | 107 | 1.21 | 0.12 | −25.77% | −39.98 | −11.62 |
| Unknown | 0 | – | – | 21 | – | – | – | – | – |
| SEER stage | |||||||||
| Localized | 387 | 0.73 | 0.04 | 638 | 0.86 | 0.03 | 17.81% | 9.98 | 25.64 |
| Regional | 142 | 0.26 | 0.02 | 387 | 0.52 | 0.03 | 100.00% | 93.37 | 106.62 |
| Distant | 42 | 0.08 | 0.01 | 51 | 0.06 | 0.01 | −25.00% | −47.60 | −2.44 |
| Unknown | 19 | – | – | 27 | – | – | – | – | – |
| Tumor size (1990–1994)a | |||||||||
| 0–1 cm | 39 | 0.07 | 0.01 | 68 | 0.09 | 0.01 | 31.43% | 1.05 | 61.75 |
| >1 but ≤2 cm | 112 | 0.19 | 0.02 | 225 | 0.30 | 0.02 | 60.00% | 39.71 | 80.29 |
| >2 but ≤4 cm | 224 | 0.36 | 0.02 | 445 | 0.60 | 0.03 | 66.94% | 52.76 | 81.04 |
| +4 cm | 259 | 0.42 | 0.03 | 349 | 0.47 | 0.03 | 11.43% | 0.64 | 22.16 |
| B. Males | |||||||||
| Total (N=2071) | 223 | 0.51 | 0.04 | 476 | 0.69 | 0.03 | 35.88% | 20.53 | 43.47 |
| Age in years | |||||||||
| 0–9 | 0 | – | – | 0 | – | – | – | – | – |
| 10–19 | 6 | – | – | 0 | – | – | – | – | – |
| 20–29 | 11 | 0.11 | 0.03 | 22 | 0.21 | 0.05 | 90.91% | −0.03 | 181.83 |
| 30–39 | 27 | 0.34 | 0.07 | 42 | 0.42 | 0.07 | 22.94% | −17.24 | 63.04 |
| 40–49 | 37 | 0.67 | 0.11 | 70 | 0.65 | 0.08 | −2.84% | −26.33 | 20.73 |
| 50–59 | 46 | 0.88 | 0.13 | 121 | 1.33 | 0.12 | 50.80% | 22.68 | 78.92 |
| 60–69 | 51 | 1.27 | 0.18 | 113 | 2.10 | 0.20 | 64.96% | 33.81 | 96.19 |
| 70–79 | 33 | 1.55 | 0.27 | 72 | 2.33 | 0.27 | 50.19% | 13.51 | 86.89 |
| +80 | 12 | 1.59 | 0.46 | 31 | 1.72 | 0.31 | 8.05% | −38.89 | 55.09 |
| Race/ethnicity | |||||||||
| White | 181 | 0.50 | 0.04 | 393 | 0.72 | 0.04 | 43.40% | 29.68 | 57.12 |
| Black | 22 | 0.55 | 0.12 | 37 | 0.60 | 0.11 | 8.55% | −31.83 | 48.83 |
| Other | 20 | 0.60 | 0.14 | 38 | 0.51 | 0.08 | −15.33% | −46.01 | 15.41 |
| Unknown | 0 | – | – | 7 | – | – | – | – | – |
| SEER stage | |||||||||
| Localized | 128 | 0.29 | 0.03 | 230 | 0.33 | 0.02 | 14.14% | −0.43 | 28.63 |
| Regional | 71 | 0.16 | 0.02 | 208 | 0.30 | 0.02 | 87.50% | 61.01 | 113.99 |
| Distant | 16 | 0.05 | 0.01 | 36 | 0.06 | 0.01 | 18.00% | −22.79 | 58.79 |
| Unknown | 8 | – | – | 2 | – | – | – | – | – |
| Tumor size (1990–1994)a | |||||||||
| 0–1 cm | 14 | 0.02 | 0.01 | 27 | 0.04 | 0.01 | 95.00% | −51.44 | 241.44 |
| >1 but ≤2 cm | 24 | 0.04 | 0.01 | 48 | 0.07 | 0.01 | 75.00% | 15.70 | 134.30 |
| >2 but ≤4 cm | 91 | 0.17 | 0.02 | 146 | 0.21 | 0.02 | 22.35% | 3.11 | 41.69 |
| +4 cm | 161 | 0.32 | 0.03 | 255 | 0.38 | 0.02 | 17.19% | 3.13 | 31.27 |
Rates are per 100,000 person years, man years, or woman years (age-adjusted to the 2000 U.S. standard population).
The comparison for percentage change for tumor size is based on data from 1990 to 1994 rather than 1980 to 1984, as tumor size was not available until 1988.
%CH, percentage change in rates; LL and UL, lower and upper limits of %CH; –, not calculated, not applicable, or <10 cases.
The trends for males by age group were somewhat different from those among females. The trends by race/ethnicity category among males were also only slightly different to those among females, with rates rising among Whites and Blacks and decreasing for the “other” racial category in males (comprised largely of those who identified themselves as Asian). However, although the greatest increase by stage was observed for regional tumors (87.50%), an even larger increase (95.00%) was observed for the smallest size tumors. Rates among males for the localized and distant tumor groups increased by 14.14% and 18.00% respectively, whereas a 87.50% increase was observed for regional stage tumors.
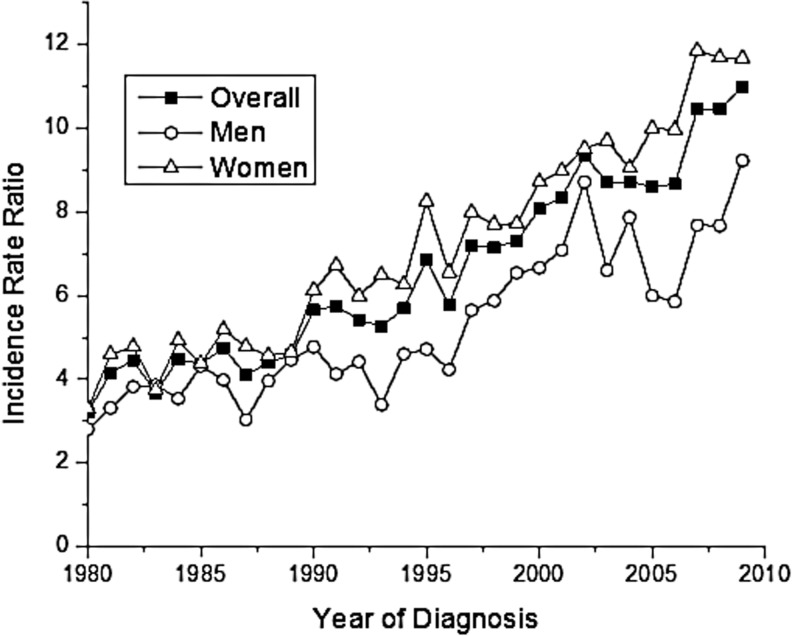
When we compared the incidence of papillary and follicular types (Table 3), we found a papillary-to-follicular IRR overall of 7.07 [CI 6.91–7.24]. The papillary-to-follicular IRR was greater among females than males (7.77 vs. 5.63). The largest IRRs were observed during reproductive years, ages 20–29 (IRR=9.65) and 30–39 (IRR=9.36), and then gradually decreased with age to 2.72 among those age 80+. There was a notable difference in IRRs by race/ethnicity, with papillary-to-follicular IRRs among the White and Other race/ethnicity categories of 7.37 and 7.96 respectively, compared to an IRR among Blacks of 3.86. The papillary-to-follicular IRRs range from 9.48 in Utah to 5.93 in Detroit. The papillary-to-follicular IRR was highest for localized stage (IRR=8.39), and then decreased to 6.06 at the regional stage and 3.67 at the distant stage. Similarly, the papillary-to-follicular IRR decreased as tumor size increased from 48.6 for the smallest size tumors (0–1 cm) to 12.60 (>1–2 cm), 4.66 (>2–4 cm), and to 3.14 for tumors >4 cm. When we plotted the temporal trends for the papillary-to-follicular IRR, an increase was observed over time that was most pronounced among women (Fig. 1).
Table 3.
Comparison of Papillary and Follicular Thyroid Cancer in SEER's 9-Registry Database (1980–2009)
| |
Papillary |
Follicular |
|
CI |
|||||
|---|---|---|---|---|---|---|---|---|---|
| n | Rate | SE | n | Rate | SE | Pap/FollIRR | LL | UL | |
| Total | 45,942 | 6.21 | 0.03 | 6410 | 0.88 | 0.01 | 7.07 | 6.91 | 7.24 |
| Sex | |||||||||
| Female | 35,131 | 9.21 | 0.05 | 4563 | 1.19 | 0.02 | 7.77 | 7.56 | 7.97 |
| Male | 10,811 | 3.10 | 0.03 | 1847 | 0.55 | 0.01 | 5.63 | 5.39 | 5.87 |
| Age in years | |||||||||
| 0–9 | 32 | 0.03 | 0.01 | 2 | – | – | – | – | – |
| 10–19 | 1014 | 0.94 | 0.03 | 119 | 0.11 | 0.01 | 8.55 | 7.28 | 10.03 |
| 20–29 | 5572 | 4.73 | 0.06 | 581 | 0.49 | 0.02 | 9.65 | 9.03 | 10.31 |
| 30–39 | 9878 | 8.24 | 0.08 | 1050 | 0.88 | 0.03 | 9.36 | 8.90 | 9.85 |
| 40–49 | 10,586 | 10.17 | 0.10 | 1244 | 1.20 | 0.03 | 8.48 | 8.07 | 8.90 |
| 50–59 | 8827 | 11.14 | 0.12 | 1144 | 1.44 | 0.04 | 7.74 | 7.34 | 8.15 |
| 60–69 | 5761 | 10.18 | 0.13 | 1027 | 1.82 | 0.06 | 5.59 | 5.28 | 5.93 |
| 70–79 | 3171 | 8.15 | 0.14 | 839 | 2.16 | 0.07 | 3.77 | 3.53 | 4.04 |
| +80 years | 1101 | 4.97 | 0.15 | 404 | 1.83 | 0.09 | 2.72 | 2.44 | 3.03 |
| Race/ethnicity | |||||||||
| White | 38,023 | 6.41 | 0.03 | 5140 | 0.87 | 0.01 | 7.37 | 7.21 | 7.53 |
| Black | 2385 | 3.28 | 0.07 | 584 | 0.85 | 0.04 | 3.86 | 3.53 | 4.21 |
| Other | 5091 | 7.32 | 0.1 | 619 | 0.92 | 0.04 | 7.96 | 7.43 | 8.52 |
| Unknown | 443 | – | – | 67 | – | – | – | – | – |
| Registry | |||||||||
| Utah | 4112 | 8.06 | 0.13 | 417 | 0.85 | 0.04 | 9.48 | 8.73 | 10.30 |
| New Mexico | 3472 | 7.34 | 0.13 | 384 | 0.82 | 0.04 | 8.95 | 8.18 | 9.79 |
| Connecticut | 6761 | 6.68 | 0.08 | 887 | 0.87 | 0.03 | 7.68 | 7.24 | 8.14 |
| SF–Oakland | 6135 | 5.14 | 0.07 | 820 | 0.7 | 0.02 | 7.34 | 6.89 | 7.83 |
| Seattle | 6975 | 6.31 | 0.08 | 938 | 0.87 | 0.03 | 7.25 | 6.82 | 7.71 |
| Hawaii | 2779 | 8.16 | 0.16 | 395 | 1.16 | 0.06 | 7.03 | 6.41 | 7.72 |
| Iowa | 4996 | 5.92 | 0.08 | 819 | 0.94 | 0.03 | 6.30 | 5.92 | 6.70 |
| Atlanta | 3946 | 5.38 | 0.09 | 620 | 0.9 | 0.04 | 5.98 | 5.53 | 6.46 |
| Detroit | 6766 | 5.75 | 0.07 | 1130 | 0.97 | 0.03 | 5.93 | 5.61 | 6.27 |
| SEER stage | |||||||||
| Localized | 28,482 | 3.86 | 0.02 | 3348 | 0.46 | 0.01 | 8.39 | 8.16 | 8.62 |
| Regional | 14,939 | 2.00 | 0.02 | 2435 | 0.33 | 0.01 | 6.06 | 5.78 | 6.35 |
| Distant | 1598 | 0.22 | 0.01 | 428 | 0.06 | 0.00 | 3.67 | 3.13 | 4.30 |
| Other/Unknown | 923 | 0.12 | 0.00 | 199 | 0.03 | 0.00 | 4.29 | 4.03 | 4.56 |
| Tumor size (1988–2009) | |||||||||
| 0–1 cm | 13,887 | 2.43 | 0.02 | 296 | 0.05 | 0.00 | 48.60 | 45.59 | 51.81 |
| >1 but ≤2 cm | 10,883 | 1.89 | 0.02 | 835 | 0.15 | 0.01 | 12.60 | 11.82 | 13.43 |
| >2 but ≤4 cm | 8618 | 1.49 | 0.02 | 1839 | 0.32 | 0.01 | 4.66 | 4.40 | 4.93 |
| +4 cm | 6636 | 1.16 | 0.01 | 2087 | 0.37 | 0.01 | 3.14 | 3.01 | 3.26 |
| Other/unknown | 5918 | – | – | 1353 | – | – | – | – | – |
Rates are per 100,000 person years (age-adjusted to the 2000 U.S. standard population). For tumor size, the time period is limited to 1988–2009.
FIG. 1.
Papillary-to-follicular thyroid cancer incidence rate ratios overall and for men and women, 1980–2009.
Discussion
Overall we observed a small but consistent increase in follicular thyroid cancer IRs between 1980 and 2009. For the same time period, we also found that follicular thyroid cancer is increasing in both sexes, more rapidly in Whites, and inconsistently for different tumor characteristics. We also found that despite improvements in our diagnostic capabilities (i.e., the introduction of ultrasound and fine-needle aspiration), a greater proportion of newly diagnosed follicular thyroid cancers are larger in size and have more regional metastasis than in the past. For women, follicular thyroid cancer has increased in incidence at a steady rate among all age groups except 80+, while more women were diagnosed with more aggressive or later stage cancers. In contrast, in men, follicular thyroid cancer rates have decreased or increased modestly in the younger age groups while increasing significantly in older age groups. The overall effect of these changes shows that the sex disparity in follicular thyroid cancer is decreasing, and at the same time these cancers are becoming more aggressive.
The reason for the change in pathology from the follicular to papillary type over time is not clear, but one possible explanation could have to do with changes in iodine sufficiency at a population level. In the past, follicular thyroid cancer was known to be associated with iodine deficiency (8). In general, as a population becomes iodine sufficient, the number of follicular thyroid cancer cases decreases. It is possible that iodine supplementation of salt has not decreased the rate of follicular cancers, or alternatively, that the United States was largely iodine sufficient prior to the study period. In the United States, salt manufacturers have been adding iodine to table salt since the 1920s (25). Urinary iodine measurements from NHANES have been used since 1971 to monitor the iodine status of the U.S. population (26). Since the inception of the NHANES monitoring program, urinary iodine measurements have shown that the general U.S. population is iodine sufficient (26). Studies comparing follicular thyroid cancer incidence rate changes in iodine replete versus iodine deficient parts of the world would help to clarify this phenomenon.
Our findings show that the overall incidence of follicular thyroid cancer is increasing at a much slower rate than papillary thyroid cancer (1–4). Changes in the papillary-to-follicular thyroid cancer IRRs by sex and over time provide evidence of diverging incidence patterns for these two types of thyroid cancer. Compared to follicular thyroid cancer rates, the papillary type has shown a much greater increase in incidence in females. In addition, the highest IRR between the two types occurs during reproductive years and IRRs decreased with age. We also found the highest IRR in Utah, which is not surprising, as ionizing radiation is a risk factor for the papillary type (8), and Utah is suspected to have experienced radioactive fallout (27). Overall these findings suggest that female sex and young age play important roles in the etiology and diagnosis of the papillary type, particularly during the reproductive years. In contrast, these factors seem to be reversed for the follicular type, where older age and male sex seem to have more influence on disease incidence. Other factors such as ethnicity category and tumor characteristics also differ significantly when comparing papillary and follicular thyroid cancer incidence. Interestingly, the papillary-to-follicular IRR is much lower in Blacks than in Whites or for those in the Other race/ethnicity category.
Perhaps one of the most significant differences in the papillary to follicular IRR across these demographic and tumor characteristics is the large papillary-to-follicular IRR observed for small (<2 cm) and localized tumors. In prior analyses of the 1976–2005 SEER 9 data, we found that the greatest increase in papillary thyroid cancer was for small, localized tumors, while for follicular thyroid cancer in women, the greatest increase is in larger tumors with regional metastasis (1). This finding has an important implication regarding the rise in papillary thyroid cancer rates. It has been previously suggested that improved surveillance and changes in diagnostic techniques has caused the large rise in papillary thyroid cancer by increasing the detection of small, previously hidden, subclinical tumors (3). Our data show increases in both small (<1 cm) and larger tumors, suggesting that improved detection techniques are not solely responsible for the significant rise in follicular thyroid cancer. Our data also illustrate increases at younger ages in both men and women, which is particularly important because undergoing cancer treatment early in life puts people at risk for a myriad of late and long-term effects, including second cancers and psychosocial issues.
There is a source of potential bias in any study that compares papillary and follicular thyroid cancer incidence over time that we have attempted to avoid. A change in thyroid tumor classification occurred in 1988 when a new WHO classification system was introduced, which recommended reclassifying tumors with follicular architecture but nuclear features characteristic of papillary carcinomas as papillary (ICD-O-3 morphology code=8340) (28). It is a concern that the change in classification may have artificially inflated papillary thyroid cancer rates but, at the same time, may have masked a real increase in follicular thyroid cancer rates. As observed in Supplementary Table S1 (Supplementary Data are available online at www.liebert.com/thy), an increase in the rate of these cancers has occurred over the course of the study period. However, we attempted to avoid this bias in our study by using the most current thyroid cancer subtype classification system throughout the entire time period. As such, we excluded the follicular variant of papillary thyroid cancer cases from the follicular group for the entire time period.
Our study is also limited by the usual concerns related to analyses of registry data: nonreview of histopathologic diagnoses, potential incomplete data collection, and inconsistencies in tumor classification over time due to changing classification and staging systems. However, our results are consistent with other population-based studies (4,5).
The illumination of the differences between papillary and follicular thyroid cancer rates here is important for a number of reasons. First, it shows that thyroid cancer is a heterogeneous disease and that future studies seeking to evaluate the etiology of thyroid cancer should be designed to look at the papillary type and other types independently. Second, it casts doubt on the notion that changing thyroid cancer rates are largely a reflection of improvements in diagnostic technology, as the ratio of papillary:follicular type has changed over time. A better understanding of the population trends in follicular thyroid cancer may point to opportunities for improvements in diagnosis, treatment, survival, or even prevention.
Supplementary Material
Acknowledgments
This research was supported in part by the University of Chicago Comprehensive Cancer Center. This research was also supported in part by the Intramural Research Program of the National Cancer Institute (NCI).
Author Disclosure Statement
There are no financial or other interests with regard to the submitted article that might be construed as a conflict of interest.
References
- 1.Kilfoy BA. Devesa SS. Ward MH. Zhang Y. Rosenberg PS. Holford TR. Anderson WF. Gender is an age-specific effect modifier for papillary cancers of the thyroid gland. Cancer Epidemiol Biomarkers Prev. 2009;18:1092–1100. doi: 10.1158/1055-9965.EPI-08-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kilfoy BA. Zheng T. Holford TR. Han X. Ward MH. Sjodin A. Zhang Y. Bai Y. Zhu C. Guo GL. Rothman N. Zhang Y. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2009;20:525–531. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 4.Enewold L. Zhu K. Ron E. Marrogi AJ. Stojadinovic A. Peoples GE. Devesa SS. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18:784–791. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aschebrook-Kilfoy B. Ward MH. Sabra MM. Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid. 2011;21:125–134. doi: 10.1089/thy.2010.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu S. Semenciw R. Ugnat AM. Mao Y. Increasing thyroid cancer incidence in Canada, 1970–1996: time trends and age-period-cohort effects. Br J Cancer. 2001;85:1335–1339. doi: 10.1054/bjoc.2001.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds RM. Weir J. Stockton DL. Brewster DH. Sandeep TC. Strachan MW. Changing trends in incidence and mortality of thyroid cancer in Scotland. Clin Endocrinol (Oxf ) 2005;62:156–162. doi: 10.1111/j.1365-2265.2004.02187.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhu C. Zheng T. Kilfoy BA. Han X. Ma S. Ba Y. Bai Y. Wang R. Zhu Y. Zhang Y. A birth cohort analysis of the incidence of papillary thyroid cancer in the United States, 1973–2004. Thyroid. 2009;19:1061–1066. doi: 10.1089/thy.2008.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ron E. Thyroid cancer. In: Schottenfeld D, editor; Fraumeni JF Jr, editor. Cancer Epidemiology and Prevention. Third. Oxford University Press; New York: 2006. pp. 975–994. [Google Scholar]
- 10.Zanotti-Fregonara P. Hindié E. Faugeron I. Moretti JL. Ravasi L. Rubello D. Toubert ME. Update on the diagnosis and therapy of distant metastases of differentiated thyroid carcinoma. Minerva Endocrinol. 2008;33:313–327. [PubMed] [Google Scholar]
- 11.Muresan MM. Olivier P. Leclère J. Sirveaux F. Brunaud L. Klein M. Zarnegar R. Weryha G. Bone metastases from differentiated thyroid carcinoma. Endocr Relat Cancer. 2008;15:37–49. doi: 10.1677/ERC-07-0229. [DOI] [PubMed] [Google Scholar]
- 12.Rahbar K. Hutzenlaub V. Fischer RJ. Schober O. Riemann B. Risk-profile and outcome of small papillary and follicular thyroid carcinomas (< or =1 cm) Nuklearmedizin. 2008;47:188–193. doi: 10.3413/nukmed-0147. [DOI] [PubMed] [Google Scholar]
- 13.Zanotti-Fregonara P. Hindié E. Faugeron I. Moretti JL. Ravasi L. Rubello D. Toubert ME. Update on the diagnosis and therapy of distant metastases of differentiated thyroid carcinoma. Minerva Endocrinol. 2008;33:313–327. [PubMed] [Google Scholar]
- 14.Grogan RH. Mitmaker EJ. Clark OH. The evolution of biomarkers in thyroid cancer—from mass screening to a personalized biosignature. Cancers. 2010;2:885–912. doi: 10.3390/cancers2020885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikiforov YE. Radiation-induced thyroid cancer: what we have learned from Chernobyl. Endocr Pathol. 2006;17:307–317. doi: 10.1007/s12022-006-0001-5. [DOI] [PubMed] [Google Scholar]
- 16.Galanti MR. Sparén P. Karlsson A. Grimelius L. Ekbom A. Is residence in areas of endemic goiter a risk factor for thyroid cancer? Int J Cancer. 1995;61:615–621. doi: 10.1002/ijc.2910610506. [DOI] [PubMed] [Google Scholar]
- 17.Albores-Saavedra J. Henson DE. Glazer E. Schwartz AM. Changing patterns in the incidence and survival of thyroid cancer with follicular phenotype–papillary, follicular, and anaplastic: a morphological and epidemiological study. Endocr Pathol. 2007;18:1–7. doi: 10.1007/s12022-007-0002-z. [DOI] [PubMed] [Google Scholar]
- 18.Surveillance, Epidemiology, End Results (SEER) Program. National Cancer Institute DCCPS, Surveillance Research Program, Cancer Statistics Branch; Public-Use Database (1973–2009) Released April 2012, based on the November 2011 submission. [Google Scholar]
- 19.Fritz A. Percy C. Jack A. Shanmugaratnam K. Sobin L. Parkin DM, et al. Third. World Health Organization; Geneva, Switzerland: 2000. International classification of diseases for oncology. [Google Scholar]
- 20.Egevad L. Heanue M. Berney D. Fleming K. Ferlay J. Histological groups. In: Curado MP, editor; Edwards B, editor; Shin HR, editor; Storm H, editor; Ferlay J, editor; Heanue M, editor; Boyle P, editor. Cancer Incidence in Five Continents. IX. IARC Press; Lyon, France: 2007. pp. 61–66. [Google Scholar]
- 21.Surveillance, Epidemiology, End Results (SEER) Program. Cancer Statistics Review Technical Notes. http://seer.cancer.gov/csr/1975_2009_pops09/results_figure/sect_01_intro2_24pgs.pdf. [Dec 12;2012 ]. http://seer.cancer.gov/csr/1975_2009_pops09/results_figure/sect_01_intro2_24pgs.pdf
- 22.Johnson CH. SEER Program Coding and Staging Manual 2007. In: Adamo M, editor. National Cancer Institute; Bethesda, MD: 2007. NIH Publication number 07–5581. [Google Scholar]
- 23.Surveillance Research Program. National Cancer Institute SEER*Stat software version 7.0.9. www.seer.cancer.gov/seerstat www.seer.cancer.gov/seerstat
- 24.Oehlert GW. A note on the δ method. Am Stat. 1992;46:27–29. [Google Scholar]
- 25.Dasgupta PK. Liu Y. Dyke JV. Iodine nutrition: iodine content of iodized salt in the United States. Environ Sci Technol. 2008;42:1315–1323. doi: 10.1021/es0719071. [DOI] [PubMed] [Google Scholar]
- 26.Caldwell KL. Miller GA. Wang RY. Jain RB. Jones RL. Iodine status of the U.S. population, National Health and Nutrition Examination Survey 2003–2004. Thyroid. 2008;18:1207–1214. doi: 10.1089/thy.2008.0161. [DOI] [PubMed] [Google Scholar]
- 27.Kerber RA. Till JE. Simon SL. Lyon JL. Thomas DC. Preston-Martin S, et al. A cohort study of thyroid disease in relation to fallout from nuclear weapons testing. JAMA. 1993;270:2076–2082. [PubMed] [Google Scholar]
- 28.Hedinger C. Williams ED. Sobin LH. Histological typing of thyroid tumours. Second. World Health Organization; Springer Verlag; Berlin: 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.