Abstract
Human pluripotent stem cells (hPSCs) are self-renewing and have the potential to differentiate into any cell type in the body, making them attractive cell sources for applications in tissue engineering and regenerative medicine. However, in order for hPSCs to find use in the clinic, the mechanisms underlying their self-renewal and lineage commitment must be better understood. Many technologies that have been developed for the maintenance and directed differentiation of hPSCs involve the use of soluble growth factors, but recent studies suggest that other elements of the hPSC microenvironment also influence the growth and differentiation of hPSCs. This includes the influences of cell–cell interactions, substrate mechanics, cellular interactions with extracellular matrix, as well as the nanotopography of the substrate and physical forces such as shear stress, cyclic mechanical strain, and compression. In this review, we highlight the recent progress of this area of research and discuss ways in which the mechanical cues may be incorporated into hPSC culture regimes to improve methods for expanding and differentiating hPSCs.
Introduction
Human pluripotent stem cells (hPSCs) include human embryonic stem cells and induced pluripotent stem cells (hESCs/iPSCs). hESCs are derived from 5–6-day-old blastocysts, whereas hiPSCs are generated by nuclear reprogramming of somatic cells.1,2 They are both self-renewing and could potentially yield a nearly unlimited supply of differentiated cell types for applications in regenerative medicine, tissue engineering, drug discovery, and disease modeling.3–5 They also offer researchers a model for the study of early human embryological development that has been heretofore unavailable due to ethical restrictions.6
However, before hPSCs can be used in the clinic, a deeper understanding of hPSC basic biology is required. Mechanisms underlying the maintenance of their pluripotency and self-renewal must be elucidated in order to allow for their large-scale expansion for downstream applications. Protocols for their directed differentiation necessitate optimization as well for the efficiencies achieved using many current protocols are often quite low and inconsistent. Many differentiation studies have focused on exploring the role of growth factors and small molecules.7–9 Nonetheless, as important as these soluble signaling molecules are, there is accumulating evidence suggesting that they are not the only factors influencing the maintenance and in vitro development of hPSCs.
Physicochemical cues are known to play a critical role in early embryo development, particularly during gastrulation, foregut development, and the emergence of cardiac, hematoendothelial, osteogenic, and chondrogenic lineages.10–16 Cells sense and react to changes in the mechanical properties of their microenvironments by assembling and reassembling focal adhesions, and up- and down-regulating cell adhesion molecules that are associated with cell–cell and cell–extracellular matrix (ECM) interactions. These physicochemical factors have significant implications for stem cell self-renewal, proliferation, and differentiation in vitro. The information gleaned from studies of hPSC mechanobiology can inform the design of more biomimetic in vitro environments for the expansion and directed differentiation of hPSCs as well as the in vitro study of early human embryo development.
In this review, we will discuss the recent progress in this field. hPSCs will be emphasized, but some discussion of mouse embryonic stem cells (mESCs) and other cell types will be included as well for comparison and to highlight areas of interest for which hPSC data does not yet exist in the literature.
Mechanical Properties of hPSCs
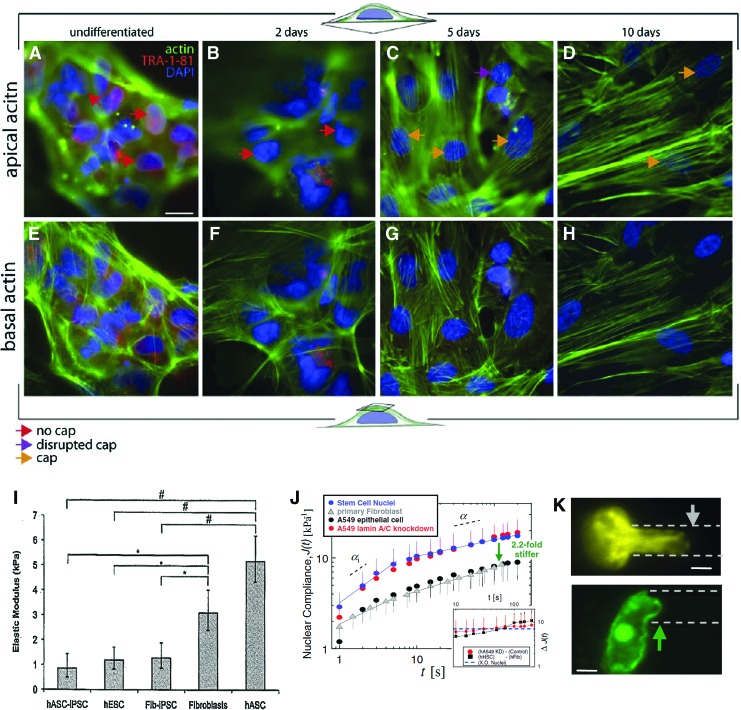
Measurements of the elasticity of undifferentiated hESCs reveal that they have a lower elastic modulus and viscosity than their differentiated counterparts, though the actual measured values vary significantly depending on the methods used for the measurement. Ofek et al. used creep cytocompression to obtain instantaneous moduli values of hESCs (0.53±0.33 kPa), human mesenchymal stem cells (hMSCs) (1.16±0.53 kPa) and chondrocytes (1.33±0.37 kPa).17 Using atomic force microscopy (AFM), another group obtained a much wider range of values of hESC elasticity from 0.05 to10 kPa.18 hiPSCs generated from fibroblasts and adipose-derived stromal cells (ASCs) have elastic moduli that are similar to hESCs (∼1 kPa), again measured with AFM (Fig. 1).19 Using optical tweezers, another group found that hESCs have an elastic modulus of 5.6±1.4 Pa, compared to 14±3.5 Pa for cardiomyocytes derived from hESCs, which is much lower than those obtained in other studies.20 These discrepancies could be due to the differences in the sensitivities of the measurement devices or to differences between cell lines and culture conditions. Measurements made with AFM were performed on cells that had grown into colonies, whereas the creep cytocompression and optical tweezers measurements were made on isolated single cells. Creep cytocompression measurements were made using a 50.8-μm tungsten probe, which applies force to the entire apical surface of the cell. AFM measurements taken by Kiss et al., on the other hand, were made using a pyramidal tip with a 10-nm radius of curvature. Thus, the AFM measures a local elasticity at different points of a cell, whereas creep cytocompression captures more information about the elasticity of the cell as a whole. Moreover, the optical tweezer measurements are often performed on a single-cell suspension, and so the stiffness of the substrate has no influence on the mechanical properties of the cells. Overall, the results for hPSCs are in agreement with similar measurements taken for mESCs, and the evidence points to ES cells being softer than somatic cells.21
FIG. 1.
Mechanical properties of human pluripotent stem cells (hPSCs). (A–H) Actin stress fibers near the apical (A–D) and basal (E–H) surfaces of human embryonic stem cells (hESCs) at an undifferentiated state or at day 2, 5, and 10 postdifferentiation. Scale bar: 20 μm. (I) Comparison of elasticity among hESCs, hiPSCs, and more differentiated cells. #p<0.05 relative to adipose derived stem cells. *p<0.05 relative to IMR90 fibroblasts. (J) Compliance of stem cell nuclei compared to other cell types. (K) Nuclear deformations of hESCs through microaspiration, control (top), and cation-treated (bottom). Arrows indicate the extent of aspiration into a micropipette (dashed lines). Scale bar: 3 μm. Adapted with permission from Khatau et al.,22 Hammerick et al.,19 and Discher and coworkers.24 Color images available online at www.liebertpub.com/teb
The difference in elasticity between hPSCs and somatic cells has been linked to the development of actin stress fibers. Human ASCs (hASCs) have an elastic modulus of ∼3 kPa as determined by AFM. Treating hASCs with an actin-disrupting agent reduces the elasticity to 1 kPa, which is very close to that of for hPSCs.19 According to Khatau et al., both undifferentiated mESCs and hESCs have actin stress fibers at their basal surfaces (Fig. 1A–H).22 Nonetheless, they both lack perinuclear actin caps, which are actin stress fibers that wrap around the nucleus. hiPSCs derived from lung fibroblasts also lack actin caps, although the lung fibroblasts from which the hiPSCs are derived do have them. Upon differentiation and the localization of nuclear Lamin A/C and linkers of nucleus and cytoskeleton, an actin cap forms. Since Lamin A/C has very low expression in ESCs, it has been suggested as a marker of differentiation.23
The nuclei of hESCs are more deformable than their differentiated counterparts. Pajerowski et al. used microaspiration to determine that the nuclei of hESCs stiffen by as much as sixfold as they reach terminal differentiation.24 Expression of Lamin A/C is linked to the change in mechanical properties of the nucleus. When Lamin A/C is knocked down in epithelial cells using shRNA, their nuclear rheological properties become indistinguishable from those of bone marrow-derived hematopoietic stem cells, which, like ESCs, have no detectable Lamin A/C content (Fig. 1J). However, despite the contribution of Lamin A/C nuclear mechanics, Lamin A/C cannot account for all of the mechanical changes observed. The authors attributed the rest of the stiffening effect to chromatin dynamics. In ESCs, chromatin is highly accessible, that is, it is usually noncondensed. In contrast, many differentiated cell types have highly condensed chromatin. It has been discovered that the treating of hESC nuclei with Ca2+ and Mg2+, divalent cations known to induce chromatin condensation, will result in a significant increase in nuclear stiffness (Fig. 1K). Thus, it is hypothesized that the stiffening observed upon hESC differentiation is at least partially due to changes in chromatin dynamics.
Most data on mechanical properties of hPSCs come from microrheologic studies of hiPSCs and the somatic cell lines from which the hiPSCs are generated.25 One of the general approaches used for these studies is to introduce fluorescent beads into the cytoplasm of fibroblasts and their derived hiPSCs. The migration of the beads through the cytoplasm can be tracked by various means, including confocal laser scanning fluorescence microscopy. It has been found that the mean squared displacement (MSD) of beads moving inside hiPSCs is significantly higher than the MSD of beads in the cytoplasm of the fibroblasts from which the hiPSCs are generated. Beads inside the hiPSCs could move much freely. However, when fibroblasts are treated with an actin-disrupting agent, their mechanical properties mimic those of hiPSCs, indicating that differences in the elasticity of these two cell types are due to differences in the organization of the cells' actin filament networks. Interestingly, while most of the hESCs tested had cytoplasmic properties resembling the hiPSCs, there was a subset of beads within the hESCs (∼26%) that had low MSDs. Compared to hiPSCs, hESCs had a more extensive network of basal actin filaments, and the authors argue that this difference in actin composition accounts for the hESCs increased elasticity.
Moving forward, it will be of critical interest to characterize the development of actin filament networks in hPSC cultures and how these networks inform the self-renewal and differentiation of hPSCs.
The Role of Cell–Cell Interactions and Colony Size in hPSC Self-Renewal and Lineage Commitment
Cell–cell contact in both mPSCs and hPSCs is characterized by E-cadherin expression. E-cadherin is highly expressed in undifferentiated pluripotent cells as well as in the epiblast-like layers of cells in PSC-derived embryoid bodies (EBs).26,27 E-cadherin is coupled to the cell actin–myosin cytoskeleton through a protein complex that includes p120-catenin, β-catenin, α-catenin, vinculin, and nonmuscle myosin IIA (NMMIIA). While both mESCs and hESCs express E-cadherin, they differ from one another in their dependence on intact cell–cell signaling for survival. Unlike mESCs, hESCs are sensitive to dissociation.28 Single-cell suspensions of hESCs have very low plating efficiencies and high rates of apoptosis, and so hESC cultures are usually passaged as multicellular aggregates in order to preserve their cell–cell contact. hPSCs' sensitivity to dissociation has been linked to the loss of E-cadherin signaling and hyperactivation of NMMIIA. Watanabe et al. discovered that the survival of single-cell suspension of hPSCs can be greatly improved if cells are treated with a small molecule inhibitor of Rock, which is a downstream effector of Rho, a key regulator of the actin cytoskeleton.28,29
Cadherins have been implicated as regulators of mechanical signals in multiple cell types.30–32 Vinculin, a mechanical linker protein known to participate in the transduction of mechanical signals through cell–ECM interactions, co-localizes with E-cadherin in response to a shear force.33 This co-localization depends upon the activity of myosin II. hESCs cultured on polyacrylamide coated with an E-cadherin substrate exert higher traction stresses than hESCs treated with blebbistatin, an inhibitor of NMMIIA, suggesting that mechanical signals are indeed transduced through E-cadherin and NMMIIA in hESCs.34 hESCs treated with blebbistatin for extended periods have reduced expression of p120-catenin. This destabilization of p120 leads to reduced E-cadherin expression and ultimately reduced expression of pluripotency markers Oct4, Sox2, and Nanog.
Studies of mESCs divulged that the role of E-cadherin in self-renewal may not be directly related to the transduction of physical forces. Uda et al. determined that a local shear force applied to mESCs via fibronectin or laminin integrin receptors downregulated Oct4 and increased cell spreading and stiffness.35 When the same forces were applied via E-cadherin, cell stiffness increased, but cell spreading and the expression of pluripotency markers were unaffected. It is noteworthy to point out that similar study for hPSCs has not yet been performed, to the best of our knowledge. Given the differences in mPSC and hPSC dependence on E-cadherin, it might be imprudent to assume that hPSC's response to force applied through E-cadherin would be the same.
There is increasing evidence to suggest that E-cadherin signaling affects the lineage commitments of hPSCs. hESCs grown on feeder layers that ectopically expressing E-cadherin differentiate preferentially to neural lineages, for instance.36 More generally, colony size in two-dimensional cultures can have powerful effects on the fate decisions of hESCs. Lee et al. used microcontact printing to deposit Matrigel islands with well-defined diameters.37 hESCs seeded on larger islands ∼1200 μm in diameter expressed significantly higher levels of mesodermal markers, whereas smaller 200 μm colonies differentiated preferentially to definitive endoderm. These results could be attributable to differing patterns of endogenous signaling in the differently sized colonies. However, the authors also suggest the possibility that Rho A expression and cytoskeletal tension might also play a role in the hESC fate decisions. Studies in keratinocytes, an E-cadherin-expressing epithelial cell type, indicate that colonies of different size exert different traction forces on their substrate.38 Furthermore, traction forces exerted by the colonies on their substrate are modulated by cadherin signaling.39 Without E-cadherin signaling, keratinocytes in a colony act independently, but when there is strong E-cadherin signaling, traction forces become localized to the edges of the colony. To the best of our knowledge, knowledge on how the colony size affects traction forces exerted by hPSC colonies to a substrate remains largely elusive.
Mechanical Properties of Substrates Can Regulate hPSC Fate
Some of the first evidence demonstrating the importance of substrate mechanical properties on stem cell fate specification came from studies of MSCs. Engler et al. discovered that the differentiation of hMSCs can be controlled by varying the stiffness of a substrate on which the cells are grown.40 Stiff substrates (34 kPa) promote osteogenic development, moderately stiff substrates (11 kPa) promote myogenic development, and soft substrates (0.1 kPa) promote neuronal development. In each case, the hMSCs differentiated preferentially to a particular lineage when grown on a substrate whose modulus matched that of the tissue where that cell type is typically found.
Substrate mechanics influence the self-renewal and differentiation of PSCs as well. It has been reported that mESCs can remain pluripotent for 15 passages even in the absence of leukemia inhibitory factor (LIF) when they are cultured on soft polyacrylamide substrates (∼0.6 kPa).21,41 Fluorescent beads embedded in the substrate can be displaced by cell traction forces, and this displacement can therefore be used to measure traction forces exerted by mESCs. The studies suggested that mESCs increase cell tractions at their basal surface as substrate stiffness increases, while the projected area of the cells remains the same. The apical surface of the cells does not show any increase in stiffness in response to changing in substrate stiffness. The basal tractions and the apical stiffness appear to be “decoupled,” and this phenomenon has not been seen in MSCs or terminally differentiated cells.
Studies of mESCs plated on poly-L-lysine/hylauronic acid nanofilms show different patterns of adhesion depending upon the amount of crosslinking in the films.42 It was found that cells attach and proliferate more readily on stiffer than on softer films. Cells grown on uncross-linked films tend to form compact spherical colonies similar to those found in suspension cultures. Genes associated with the inner cell mass are downregulated and those associated with the late epiblast stage are upregulated in cells grown on more cross-linked nanofilms, suggesting the importance of soft substrates for the maintenance of mESCs. The elasticity of the native, uncross-linked films was not reported in this study, but other groups reported a Young's modulus of ∼20 kPa for similar uncross-linked PLL/HA films.43
hESCs cultured on Matrigel-coated polyacrylamide substrates (400 Pa) adopt a columnar epithelial morphology, and the organization of actin filaments near the apical surface of the cells more closely resembles that of the epiblast in pregastrulation embryos.44 This contrasts somewhat with results obtained from studies of hESCs cultured on polydimethylsiloxane (PDMS) micropost arrays coated with vitronectin. After 24 h, 39.5%±5.5% of single hESCs grown on rigid arrays (1.2 MPa) expressed Oct4, compared to only 2.8%±2.6% of the cells grown on softer arrays (1.92 kPa),45 suggesting that perhaps rigid substrates, not softer ones, better support hESC pluripotency.45 It is unclear if these differing results reflect differences in cell lines or different experimental conditions. A few differences are worth noting. The hESCs grown on the micropost arrays were cultured without exogenous basic fibroblast growth factor (bFGF), a growth factor included in most hPSC maintenance media that is considered to be very important for maintaining pluripotency. Interestingly, loss of Oct4 expression in hESCs cultured on soft micropost arrays is correlated with the loss of E-cadherin signaling. Oct4+ cells grown on rigid microposts are also E-cadherin+. Treating the hESCs with E-cadherin antibodies led to a significant decrease in Oct4 expression, regardless of the mechanical properties of a substrate. hESCs grown onto the micropost arrays were seeded as a single cell suspension (treated with a ROCK inhibitor), while the hESCs grown on the polyacrylamide substrates produced by Lakins et al. were aggregates with presumably intact E-cadherin signaling. It was found that cell attachment and proliferation rates are higher when they grow on more rigid substrates. Perhaps Oct4+ cells attach more readily to more rigid substrates, whereas Oct4− cells attach to soft or less rigid substrates. More studies are required to determine the role of substrate mechanics in maintaining hPSC pluripotency.
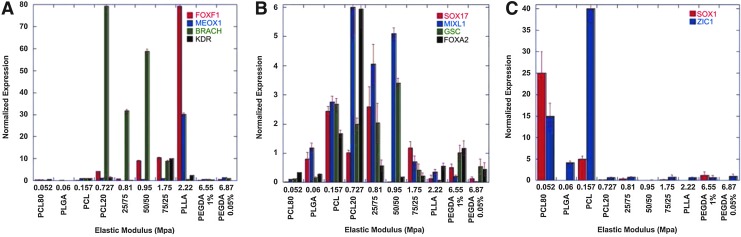
Many studies also suggested that stiff substrates can promote PSC differentiation. For instance, mESCs grown on polyacrylamide show an increase in mesendodermal gene expression with increasing substrate stiffness.46 In particular, osteogenic-related gene expression reaches highest in cells grown on substrates with stiffness greater than 2 MPa. The impact of the stiffness of a 3D scaffold on hESC differentiation has also been investigated. Zoldan et al. fabricated synthetic polymer scaffolds with varied stiffness by mixing differing amounts of PLGA, PLLA, and PCL in a salt leaching process.47 It was discovered that hESC-derived EB cells seeded into the scaffolds with a low elastic modulus (<0.1 MPa) expressed higher levels of ectodermal markers Sox1 and Zic1, whereas cells grown on scaffolds with intermediate moduli (0.1–1 MPa) expressed higher levels of endodermal markers Sox17 and GSC. Intermediate and higher elastic modulus scaffolds promoted more mesodermal differentiation (Fig. 2). Hydrogel scaffolds derived from ECM proteins tend to have lower elastic moduli that more closely match the elasticity of hPSCs.48 We have also found that the elastic modulus of collagen I hydrogels can be modulated by the addition of chitosan. These softer scaffolds may find use in differentiation protocols where undifferentiated cells rather than partially differentiated EB-derived cells are seeded into the scaffolds.49,50
FIG. 2.
Expression of germ layer markers in human embryoid body-derived cells grown on 3D scaffolds with different stiffness. The germ-layer markers were detected using quantitative real-time polymerase chain reaction (qRT-PCR). (A) Mesodermal markers expressed highest in cells grown on scaffolds with intermediate and high modulus, (B) endodermal markers expressed in cells grown on scaffolds with intermediate modulus, and (C) ectodermal markers expressed in cells grown scaffolds with low modulus. Adapted with permission from Zoldan et al.47 Color images available online at www.liebertpub.com/teb
Potential Mechanisms of Substrate–Cell Interactions in hPSC Cultures
Mechanical cues such as substrate stiffness are communicated to cells via their interaction with the ECM. The ECM is a network of fibrillar proteins and proteoglycans secreted by cells themselves.51 Although it was once thought to provide nothing more than structural support and mechanical strength, recent studies revealed that interactions of ECM with cells activate numerous signaling pathways that regulating cell proliferation, differentiation, migration, apoptosis, etc. Thus, many studies of the cellular microenvironment focus on elucidating the cell–ECM interactions.
Extensive studies suggest that cells bind to sites within ECM through cellular membrane proteins called integrins.52 Integrins are glycoproteins consisting of noncovalently bound α and β subunits. In mammals, 18 α and 8 β subunits have been identified. Cells of different phenotypes present differing patterns of integrin expression, and different integrins bind to distinct ligands or groups of ligands. For example, the integrin αVβ5, which binds to vitronectin, is highly expressed in definitive endoderm as well as in hPSCs.53 αVβ5 is required for initial attachment of hiPSCs to a vitronectin substrate, but once attachment is achieved, the proliferation of these cells is not affected by blocking just αVβ5 with antibodies.54 The inhibition of these cells' proliferation necessitates the blocking of both αVβ5 and β1.54,55 Similarly, αVβ3, α6β1, and α2β1 play a decisive role in the adhesion and proliferation of hESCs on Matrigel substrates.56 Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of integrin expression in mESCs indicated that integrins α5β1, αvβ5, α6β1, and α9β1 are involved in the maintenance of pluripotency, indicating that there is significant overlap in patterns of integrin expression between mPSCs and hPSCs.57
Integrins are linked to the actin cytoskeleton of a cell through a series of proteins that include talin, vinculin, α-actinin, F-actin, etc.58 Together, this group of proteins is called the focal adhesion complex. When the stiffness of a substrate increases, integrins cluster around the site of adhesion, and the density of stress fibers (e.g., F-actin) increases. As substrate tension increases, the stress fibers align parallel to the applied stress.59 The reorganization of the actin cytoskeleton gives a cell the mechanical properties required in order for it to hold its shape in response to mechanical stress. Some proteins involved in the focal adhesion complex have cryptic binding sites in their sequences. Talin is a prominent example. Applying a force to talin can cause it to partially unfold, thus leading to a conformation change that exposes binding sites that had been hidden in the interior of the protein. One model proposes that the conformational change that is necessary to make a cryptic binding site accessible is dependent on the magnitude of the force applied.59 According to this model, a cell adhering to a very soft matrix would experience a very low strain, perhaps too low to expose the mechanically sensitive binding site of the protein. Similarly, if a cell adheres to a matrix with a high elastic modulus, the force on the protein near the focal adhesions would be so great that the binding site unfolds to such an extent that it is rendered nonfunctional. Thus, a binding site will become available to its molecular ligands in response to externally applied force, but only a force that is not too weak and not too strong. del Rio et al. showed that the forced extension of talin reveals cryptic sequences that then become available for binding to vinculin.60 Once talin is bound to vinculin, those proteins are activated for F-actin assembly. This biochemical mechanism can potentially account for the increased stress fiber density that is observed in cells cultured on more stiff substrates. Vinculin has also been shown to play a role in the differentiation of cell types known to be mechanically sensitive. For instance, a murine mesenchymal chondrogenic cell line expressing a truncated, nonfunctional form of vinculin shows reduced expression of chondrogenesis-associated genes, including Col2a1, aggrecan, and Col10a1.61 Also, mESCs with a disrupted vinculin gene adopt a round morphology and adhere less readily to fibronectin.62
In addition, changes in substrate mechanics can induce distinct signaling pathways, thereby coupling mechanotransduction to intracellular events. ERK, Rho, and Src kinases are examples of intracellular signaling molecules that are sensitive to changes in cytoskeletal tension.63 Src family kinases are more highly activated in mammary epithelial cells grown on soft basement membrane substrates (∼400 Pa) compared to more stiff substrates (∼5 kPa). hPSCs treated with Src kinase inhibitors rapidly differentiate, indicating that high Src family kinase activity is important for the self-renewal of the hPSCs.64 Increased Src activation could help explain the improved self-renewal some groups report for PSCs grown on softer substrates.
The Effect of Nanotopography of a Substrate on hPSC Lineage-Specific Commitment
The nanotopography of a surface on which hESCs are cultured could have a significant impact on the self-renewal of hESCs. hESCs seeded onto glass slides that have been roughened using photolithography and reactive ion etching techniques show reduced expression of Oct4 and have lower proliferation rates than controls.65 Substrate topography also affects hPSC morphology. For example, hESCs grown on fibronectin coated PDMS substrates that include 600-nm ridges exhibit very distinct morphology and proliferation rates.66 These cells align with the ridges and show increased elongation and reduced proliferation. However, when cells are treated with actin-disrupting agents, they adopt a more round morphology and show decreased alignment with the gratings, and the nanotopology-induced reduction in cell proliferation is somewhat mitigated.
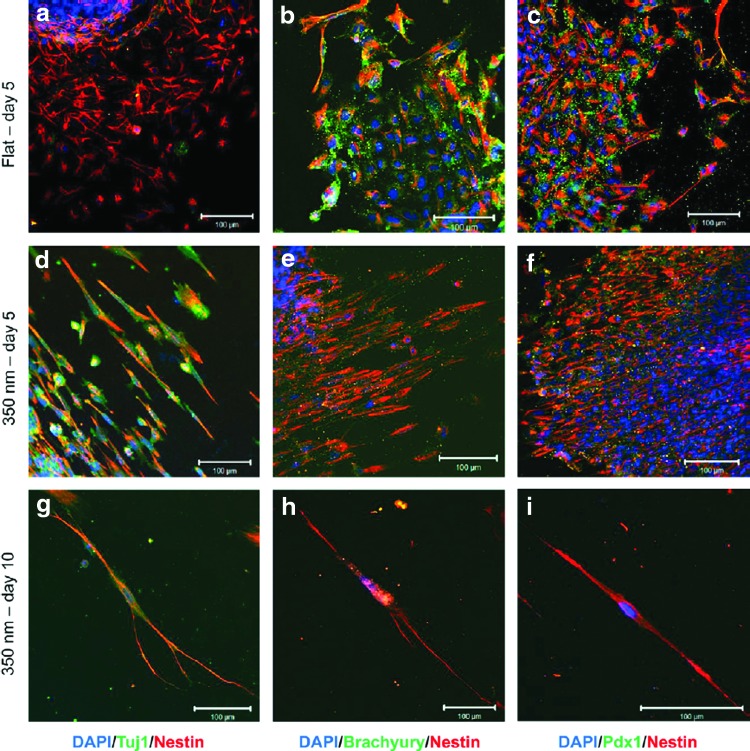
Surface topology has been used to direct the differentiation of hESCs to neural progenitors. Lee et al. seeded hESCs onto polyurethane acrylate groove/ridge-patterned arrays.67 The grooves were 350 nm wide and deep, and the cells aligned with the grooves and adopted an elongated morphology after growing for 5 days. qRT-PCR revealed an increased expression of neuronal lineage marker genes, neurod1 and nestin, in these cells as compared to those grown on smooth glass slides (Fig. 3). In another study, the growth of hESCs onto a collagen 1–carbon nanotube (CNT) matrix promoted their neuronal lineage specification.68 More than 90% of the differentiated cells stained positive for nestin after 3 days of culture on the matrix. Remarkably, no exogenous cytokines or small molecules like retinoic acid were included in the cell culture medium. AFM revealed that the collagen 1-CNT produced ∼70-nm-thick one-dimensional fibrils that hESCs aligned with during the culture. This experiment clearly suggested that the topographic cues of a scaffold play critical roles in inducing lineage-specific commitment of hESCs.
FIG. 3.
hPSCs grown on grooved surfaces (d–i) align well with the direction of the groove and exhibit a change in morphology and proliferation, as compared to control hPSCs grown on flat surfaces (a–c). Neuroepithelium markers are upregulated, while mesendodermal markers are downregulated, as compared to controls. Adapted with permission from Lee et al.67 Color images available online at www.liebertpub.com/teb
Using nanotopography to direct hPSCs to neural lineages can be advantageous. It can reduce the need for expensive exogenous growth factors and small molecule. Furthermore, retinoic acid, a common inducer of neuronal differentiation, is a known teratogen.69 Hence, developing ways to generate neural lineages from hPSCs could potentially allow researchers to produce cell products that are safer. Nanotopography can also be used to guide hPSCs to lineages other than neural lineages. It has been found that hESCs aligned parallel to the wrinkled topography within hours of seeding when they were seeded onto polyethylene shrink films that included aligned wrinkles with depths that ranged from 150 to 300 nm.70 Their nucleus became deformed and showed a decreased surface area. The cardiomyocytes differentiated from hESCs had contractions that were more synchronized than unaligned controls and the conduction velocity of the aligned cell action potentials was higher as well.
Mechanical Stimulation Can Alter hPSC Fate
As described above, the fate of hPSCs is influenced considerably by mechanical properties of their surrounding environment. Nonetheless, mechanisms underlying these regulations in HPSCs remain largely elusive. Our knowledge on these effects has been mostly limited to mESCs. Only very few works on hPSCs have been reported, indicating the need for further study. Let us examine how shear stress affects ESC differentiation. In vertebrates, endothelial cells are constantly exposed to shear stress stemming from blood flow. It had previously been shown that Flk1 tyrosine phosphorylation in endothelial cells increases in response to shear stress.71 Flk1 is a receptor for vascular endothelial growth factor (VEGF), a potent inducer of angiogenesis. Yamamoto et al. discovered that exposing Flk1+ mESCs to shear stress promotes endothelial differentiation.72 In their experiment, the shear stress was applied to cells grown inside a parallel plate chamber through which a medium was pumped. The flow was laminar, with Reynold's numbers never rising above about 40. When placed in a collagen I gel, the cells formed tube-like structures reminiscent of blood vessels. A later study on mESCs by another group obtained similar results.73 Shear stress appears to encourage endothelial differentiation in hESCs as well. CD34+CD43− cells derived from hESCs become elongated and align parallel to applied shear stress when cultured in a flow chamber, and a number of endothelial marker genes are upregulated.74
Fluid shear stress also promotes hematopoietic differentiation of mESCs, but in this case, the effect of shear stress was mediated by nitric oxide.75 it was found that inhibition of nitric oxide production in cells subjected to shear stress reduces the hematopoietic colony forming units by 50%. Another study found that mouse EBs undergoing vasculogenesis or cardiomyogenesis as a result of mechanical strain exhibited increased levels of reactive oxygen species (ROS) as well as higher level expression of ROS-producing NADPH subunits.76 It was hypothesized that ROS acts as a transducer of mechanical strain. They observed reduction in the size of PECAM-1 capillary areas, an indicator of cardiomyogenesis, when free radical scavengers such as vitamin E or N-(2-mercapto-propionyl)-glycine were added to the culture medium. Moreover, mRNA levels of MEF2C, a transcription factor involved with cardiomyogenesis, were reduced in cultures that included free radical scavengers.
Mechanical strain can be applied to cells indirectly by seeding them onto flexible membranes and then stretching the membrane. For example, Flk1+ mESCs showed increased proliferation rate and vascular smooth muscle cell marker gene expression level after being exposed to cyclic mechanical strains of 8 or 12%.77 Interestingly, the strain-induced differentiation could be abolished by treating the cells with an inhibitor of platelet-derived growth factor receptor kinase, indicating that PDGFRβ plays a role in the strain-mediated mESC differentiation. However, there are relatively few studies detailing the effect of cyclic mechanical strain on the hPSC differentiation. One study suggested that applying uniaxial mechanical strain to hiPSC-derived neural crest cells results in increased mRNA expression of calponin-1, a marker associated with smooth muscle cells, as well as increased ERK2 phosphorylation.78 Their differentiation toward smooth muscle cells can be further enhanced by treating the stretched cells with transforming growth factor beta 1 (TGFβ1). TGFβ1 pretreatment also increases chondrogenic marker expression in hEB cells subjected to mechanical strain.79 Based on these results, it appears that combining growth factors with physical stimulation may yield synergistic effects and increase differentiation efficiency for some cell types.
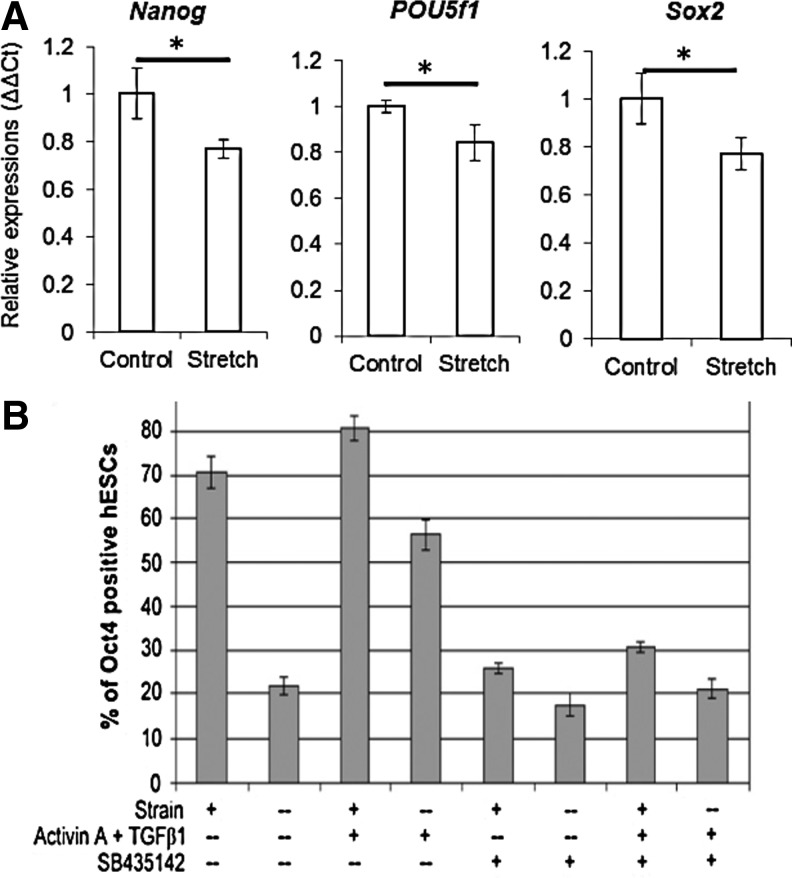
The experiments discussed above were performed on mESCs and hPSCs that were already partially differentiated. What are the effects of externally applied forces on undifferentiated cells? Studies on mESCs revealed that their pluripotency marker Oct3/4 expression is significantly reduced when a local stress is applied to undifferentiated mESCs using magnetic twisting cytometry.80 Also, the spreading response in mESCs is greater than in airway smooth muscle cells or mESC-derived cells. Based on these observations, it can be hypothesized that a threshold cell deformation is crucial for initiating a cell spreading response. Similar phenomena were observed when cyclic mechanical stimulation was applied to hiPSCs. Pluripotency markers Oct4, Nanog, and Sox2 were all significantly downregulated over the course of a 12-h experiment (Fig. 4A).81 The reduction in pluripotency marker expression was accompanied by a decrease in AKT phosphorylation. This effect could be negated by treating the cells with an inhibitor of Rho-associated kinase ROCK during cyclic mechanical stretching, indicating a possible role for actin myosin cytoskeleton in the regulation of hiPSC pluripotency.
FIG. 4.
hPSC fate can be altered by mechanical strain. (A) qRT-PCR results for hiPSCs subjected to mechanical strain for 12 h. p<0.05. (B) Flow cytometrical analysis data showing Oct4 expression in hESCs subjected to mechanical strain, Activin A and TGFβ1 treatment, and inhibition of TGFβ signaling for 7 days. Adapted with permission from Teramura et al.81 and Saha et al.83
Interestingly, another group has reported that hESCs do not differentiate after exposure to a 10%, 0.6 Hz equibiaxial cyclic mechanical strain, even after 12 days (Fig. 4B).82 On the contrary, the strain contributed to hESC pluripotency, leading to increases in TGFβ1, Activin A, and Nodal mRNA levels as well as phosphorylation of SMAD2/3.83 Each of these signaling molecules has been known to be intimately involved in hESC self-renewal. However, mechanical strain does not obviate the requirement for conditioned medium. It was observed that stretched cells that are fed with unconditioned medium differentiate at the same rate as unstretched controls. However, stretched hESCs grown in an MEF-conditioned medium not only maintained their pluripotency, but also expressed higher levels of Oct4 and SSEA-4. Saha et al. suggested that incorporating a regime of mechanical strain may allow for the culturing of hESCs to a higher density with less passaging than traditional culture methods.82
Two separate experiments involving cyclic mechanical strain yielded highly dissimilar results. One suggests that a biaxial mechanical strain promotes or at least does not adversely affect the self-renewal of hESCs, while the other indicates that mechanical strain antagonizes hiPSC self-renewal. The different outcomes could be attributable to differing experimental conditions. The hiPSCs were subjected to a strain leading to a 15% increase in surface area, while the hESCs only experienced a 10% increase. Perhaps cyclic mechanical strain promotes pluripotency only below a certain threshold, and 15% exceeded this threshold. In addition, the different results could reflect intrinsic differences between cell lines or between hESCs and hiPSCs.
The studies in hiPSC are in agreement with similar studies of mESCs. However, mESCs require different culture conditions than hPSCs do. hPSCs dependence on bFGF and Activin/Nodal signaling for self-renewal make them more similar to mouse epiblast stem cells than mESCs.84 Thus, an increase in TGFβ1 and Activin/Nodal signaling as a result of mechanical strain would not be expected to promote pluripotency in mESCs, but such an increase could have an effect on hPSC pluripotency.
Conclusion and Future Directions
Pluripotent stem cells are an invaluable resource for tissue engineering and regenerative medicine. Various protocols have been developed to direct or control the differentiation of these cells into clinically relevant cell lineages for cell-based therapy. However, many issues must be resolved before these cells find use in a clinical setting. One of the crucial problems that must be resolved is the immaturity of hPSC-derived cells, which hampers their clinical applications due to their rapid loss of biological function after trans- or implantation. A better understanding of the biology of hPSCs is required so that optimized differentiation protocols can be established to guide or direct their differentiation into mature or biologically functional cell lineages for cell-based therapy. It has long been known that cell differentiation can be controlled using a set of soluble signaling molecules such as growth factors, cytokines, or small molecules. Increasingly, it is becoming clear recently that other factors, such as mechanical stimulation, topographical structure of a substrate or a scaffold, and other physicochemical factors, also play crucial roles in regulating their fate. Among these factors, mechanical stimulation has been extensively investigated. Much of the work so far has been focused on adult stem cells. The effects of substrate stiffness, cell–ECM interactions, cell–cell interactions, and externally applied physical forces are just beginning to be explored in the context of hPSCs. The mechanisms of transduction within the cell are being elucidated, and the information gained from these studies can help tissue engineers devise culture protocols in order to maximize their maintenance and differentiation efficiency. The effects of mechanical stimulation also have implications for the scale-up of hPSC cultures. For example, microcarriers have been widely used for expanding hPSCs in a bioreactor. Cells grown on these carriers will suffer from shear stress due to the agitation inside the bioreactor. Such effects vary among different cell lines. Some cells are sensitive to mechanical stress; some are not. For instance, HES-2 cells grown on microcarriers in agitated 6-well plates showed no signs of differentiation after seven passages, while HES-3 cells and IMR90 hiPSCs began showing reduced pluripotency marker expression and increased germ layer marker expression after just one passage.85 These observations call for more extensive studies in order to elucidate how exactly hPSCs respond to mechanical stimuli and alter their fate. However, it is important to note that the study of hPSC mechanobiology is still in its early stages, and it is too soon to predict which cues in cell microenvironment will be most crucial for directing their lineage-specific differentiation or large-scale expansion.
Acknowledgment
This work is in part supported by NSF Grant CBET 0756455, JDRF 5-2009-381, and ABI 0152 27504-23-0029.
Disclosure Statement
No competing financial interests exist.
References
- 1.Thomson J.A. Itskovitz-Eldor J. Shapiro S.S. Waknitz M.A. Swiergiel J.J. Marshall V.S., et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Greenhough S. Medine C.N. Hay D.C. Pluripotent stem cell derived hepatocyte like cells and their potential in toxicity screening. Toxicology. 2010;278:250. doi: 10.1016/j.tox.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Pouton C.W. Haynes J.M. Pharmaceutical applications of embryonic stem cells. Adv Drug Deliv Rev. 2005;57:1918. doi: 10.1016/j.addr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Pera M.F. Trounson A.O. Human embryonic stem cells: prospects for development. Development. 2004;131:5515. doi: 10.1242/dev.01451. [DOI] [PubMed] [Google Scholar]
- 6.Dvash T. Benvenisty N. Human embryonic stem cells as a model for early human development. Best Pract Res Clin Obstet Gynaecol. 2004;18:929. doi: 10.1016/j.bpobgyn.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Chadwick K. Wang L.S. Li L. Menendez P. Murdoch B. Rouleau A., et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 8.D'Amour K.A. Agulnick A.D. Eliazer S. Kelly O.G. Kroon E. Baetge E.E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 9.Koch P. Opitz T. Steinbeck J.A. Ladewig J. Brustle O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc Natl Acad Sci U S A. 2009;106:3225. doi: 10.1073/pnas.0808387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krieg M. Arboleda-Estudillo Y. Puech P.H. Kafer J. Graner F. Muller D.J., et al. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10:429–U122. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 11.Farge E. Mechanical induction of twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- 12.Hove J.R. Koster R.W. Forouhar A.S. Acevedo-Bolton G. Fraser S.E. Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 13.North T.E. Goessling W. Peeters M. Li P.L. Ceol C. Lord A.M., et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frangos J.A. Eskin S.G. McIntire L.V. Ives C.L. Flow effects on prostacyclin production by cultured human-endothelial cells. Science. 1985;227:1477. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 15.Burger E.H. Klein-Nulend J. Mechanotransduction in bone—role of the lacuno-canalicular network. FASEB J. 1999;13:S101. [PubMed] [Google Scholar]
- 16.Hung C.T. Mauck R.L. Wang C.C.B. Lima E.G. Ateshian G.A. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 17.Ofek G. Willard V.P. Koay E.J. Hu J.C. Lin P. Athanasiou K.A. Mechanical characterization of differentiated human embryonic stem cells. J Biomech Eng Trans ASME. 2009;131:061011. doi: 10.1115/1.3127262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiss R. Bock H. Pells S. Canetta E. Adya A.K. Moore A.J., et al. Elasticity of human embryonic stem cells as determined by atomic force microscopy. J Biomech Eng-Trans ASME. 2011;133:101009. doi: 10.1115/1.4005286. [DOI] [PubMed] [Google Scholar]
- 19.Hammerick K.E. Huang Z.B. Sun N. Lam M.T. Prinz F.B. Wu J.C., et al. Elastic properties of induced pluripotent stem cells. Tissue Eng Part A. 2011;17:495. doi: 10.1089/ten.tea.2010.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan Y.H. Kong C.W. Chen S.X. Cheng S.H. Li R.A. Sun D. Probing the mechanobiological properties of human embryonic stem cells in cardiac differentiation by optical tweezers. J Biomech. 2012;45:123. doi: 10.1016/j.jbiomech.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury F. Li Y.Z. Poh Y.C. Yokohama-Tamaki T. Wang N. Tanaka T.S. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS One. 2010;5:e15655. doi: 10.1371/journal.pone.0015655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khatau S.B. Kusuma S. Hanjaya-Putra D. Mali P. Cheng L.Z. Lee J.S.H., et al. The differential formation of the LINC-mediated perinuclear actin cap in pluripotent and somatic cells. PLoS One. 2012;7:e33689. doi: 10.1371/journal.pone.0036689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melcer S. Hezroni H. Rand E. Nissim-Rafinia M. Skoultchi A. Stewart C.L., et al. Histone modifications and lamin A regulate chromatin protein dynamics in early embryonic stem cell differentiation. Nat Commun. 2012;3:910. doi: 10.1038/ncomms1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pajerowski J.D. Dahl K.N. Zhong F.L. Sammak P.J. Discher D.E. Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci U S A. 2007;104:15619. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniels B.R. Hale C.M. Khatau S.B. Kusuma S. Dobrowsky T.M. Gerecht S., et al. Differences in the microrheology of human embryonic stem cells and human induced pluripotent stem cells. Biophys J. 2010;99:3563. doi: 10.1016/j.bpj.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L. Bennett S.A.L. Wang L.S. Role of E-cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cells. Cell Adhes Migr. 2012;6:59. doi: 10.4161/cam.19583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachlos E. Auguste D.T. Embryoid body morphology influences diffusive transport of inductive biochemicals: a strategy for stem cell differentiation. Biomaterials. 2008;29:4471. doi: 10.1016/j.biomaterials.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Ohgushi M. Matsumura M. Eiraku M. Murakami K. Aramaki T. Nishiyama A., et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 2010;7:225. doi: 10.1016/j.stem.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe K. Ueno M. Kamiya D. Nishiyama A. Matsumura M. Wataya T., et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 30.Borghi N. Sorokina M. Shcherbakova O.G. Weis W.I. Pruitt B.L. Nelson W.J., et al. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proc Natl Acad Sci U S A. 2012;109:12568. doi: 10.1073/pnas.1204390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McBeath R. Pirone D.M. Nelson C.M. Bhadriraju K. Chen C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z.J. Tan J.L. Cohen D.M. Yang M.T. Sniadecki N.J. Ruiz S.A., et al. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A. 2010;107:9944. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.le Duc Q. Shi Q.M. Blonk I. Sonnenberg A. Wang N. Leckband D., et al. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189:1107. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li D. Zhou J.X. Wang L. Shin M.E. Su P. Lei X.H., et al. Integrated biochemical and mechanical signals regulate multifaceted human embryonic stem cell functions. J Cell Biol. 2010;191:631. doi: 10.1083/jcb.201006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uda Y. Poh Y.C. Chowdhury F. Wu D.C. Tanaka T.S. Sato M., et al. Force via integrins but not E-cadherin decreases Oct3/4 expression in embryonic stem cells. Biochem Biophys Res Commun. 2011;415:396. doi: 10.1016/j.bbrc.2011.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore R.N. Cherry J.F. Mathur V. Cohen R. Grumet M. Moghe P.V. E-Cadherin-expressing feeder cells promote neural lineage restriction of human embryonic stem cells. Stem Cells Dev. 2012;21:30. doi: 10.1089/scd.2010.0434. [DOI] [PubMed] [Google Scholar]
- 37.Lee L.H. Peerani R. Ungrin M. Joshi C. Kumacheva E. Zandstra P.W. Micropatterning of human embryonic stem cells dissects the mesoderm and endoderm lineages. Stem Cell Res. 2009;2:155. doi: 10.1016/j.scr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Mertz A.F. Banerjee S. Che Y.L. German G.K. Xu Y. Hyland C., et al. Scaling of traction forces with the size of cohesive cell colonies. Phys Rev Lett. 2012;108:198101. doi: 10.1103/PhysRevLett.108.198101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mertz AF. Che YL. Banerjee S. Goldstein JM. Rosowski KA. Revilla SF, et al. Cadherin-based intercellular adhesions organize epithelial cell-matrix traction forces. Proc Natl Acad Sci U S A. 2013;110:842. doi: 10.1073/pnas.1217279110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engler A.J. Sen S. Sweeney H.L. Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 41.Poh Y.C. Chowdhury F. Tanaka T.S. Wang N. Embryonic stem cells do not stiffen on rigid substrates. Biophys J. 2010;99:L19. doi: 10.1016/j.bpj.2010.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blin G. Lablack N. Louis-Tisserand M. Nicolas C. Picart C. Puceat M. Nano-scale control of cellular environment to drive embryonic stem cells selfrenewal and fate. Biomaterials. 2010;31:1742. doi: 10.1016/j.biomaterials.2009.11.055. [DOI] [PubMed] [Google Scholar]
- 43.Richert L. Engler A.J. Discher D.E. Picart C. Elasticity of native and cross-linked polyelectrolyte multilayer films. Biomacromolecules. 2004;5:1908. doi: 10.1021/bm0498023. [DOI] [PubMed] [Google Scholar]
- 44.Lakins J.N. Chin A.R. Weaver V.M. Methods in molecular biology. Vol. 916. Clifton, NJ: Springer; 2012. Exploring the link between human embryonic stem cell organization and fate using tension-calibrated extracellular matrix functionalized polyacrylamide gels; pp. 317–50. [DOI] [PubMed] [Google Scholar]
- 45.Sun Y.B. Villa-Diaz L.G. Lam R.H.W. Chen W.Q. Krebsbach P.H. Fu J.P. Mechanics regulates fate decisions of human embryonic stem cells. PLoS One. 2012;7:e37178. doi: 10.1371/journal.pone.0037178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans N.D. Minelli C. Gentleman E. LaPointe V. Patankar S.N. Kallivretaki M., et al. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur Cells Mater. 2009;18:1. doi: 10.22203/ecm.v018a01. [DOI] [PubMed] [Google Scholar]
- 47.Zoldan J. Karagiannis E.D. Lee C.Y. Anderson D.G. Langer R. Levenberg S. The influence of scaffold elasticity on germ layer specification of human embryonic stem cells. Biomaterials. 2011;32:9612. doi: 10.1016/j.biomaterials.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu Y.X. Dong Z.X. Wejinya U.C. Jin S. Ye K.M. Determination of mechanical properties of soft tissue scaffolds by atomic force microscopy nanoindentation. J Biomech. 2011;44:2356. doi: 10.1016/j.jbiomech.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Wang X.L. Ye K.M. Three-dimensional differentiation of embryonic stem cells into islet-like insulin-producing clusters. Tissue Eng Part A. 2009;15:1941. doi: 10.1089/ten.tea.2008.0181. [DOI] [PubMed] [Google Scholar]
- 50.Liu T. Zhang S.C. Chen X. Li G.Q. Wang Y.J. Hepatic differentiation of mouse embryonic stem cells in three-dimensional polymer scaffolds. Tissue Eng Part A. 2010;16:1115. doi: 10.1089/ten.TEA.2009.0391. [DOI] [PubMed] [Google Scholar]
- 51.Daley W.P. Peters S.B. Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 52.Hynes R.O. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 53.Wong J.C.Y. Gao S.Y. Lees J.G. Best M.B. Wang R.N.A. Tuch B.E. Definitive endoderm derived from human embryonic stem cells highly express the integrin receptors alpha V and beta 5. Cell Adhes Migr. 2010;4:39. doi: 10.4161/cam.4.1.10627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rowland T.J. Miller L.M. Blaschke A.J. Doss E.L. Bonham A.J. Hikita S.T., et al. Roles of integrins in human induced pluripotent stem cell growth on Matrigel and vitronectin. Stem Cells Dev. 2010;19:1231. doi: 10.1089/scd.2009.0328. [DOI] [PubMed] [Google Scholar]
- 55.Braam S.R. Zeinstra L. Litjens S. Ward-van Oostwaard D. van den Brink S. van Laake L., et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alpha V beta 5 integrin. Stem Cells. 2008;26:2257. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- 56.Meng Y. Eshghi S. Li Y.J. Schmidt R. Schaffer D.V. Healy K.E. Characterization of integrin engagement during defined human embryonic stem cell culture. FASEB J. 2010;24:1056. doi: 10.1096/fj.08-126821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee S.T. Yun J.I. Jo Y.S. Mochizuki M. van der Vlies A.J. Kontos S., et al. Engineering integrin signaling for promoting embryonic stem cell self-renewal in a precisely defined niche. Biomaterials. 2010;31:1219. doi: 10.1016/j.biomaterials.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 58.Reilly G.C. Engler A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech. 2010;43:55. doi: 10.1016/j.jbiomech.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Vogel V. Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 60.del Rio A. Perez-Jimenez R. Liu R.C. Roca-Cusachs P. Fernandez J.M. Sheetz M.P. Stretching Single Talin Rod Molecules Activates Vinculin Binding. Science. 2009;323:638. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koshimizu T. Kawai M. Kondou H. Tachikawa K. Sakai N. Ozono K., et al. Vinculin functions as regulator of chondrogenesis. J Biol Chem. 2012;287:15760. doi: 10.1074/jbc.M111.308072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coll J.L. Benzeev A. Ezzell R.M. Fernandez J.L.R. Baribault H. Oshima R.G., et al. Targeted disruption of vinculin genes in F9 and embryonic stem-cells changes cell morphology, adhesion, and locomotion. Proc Natl Acad Sci U S A. 1995;92:9161. doi: 10.1073/pnas.92.20.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paszek M.J. Zahir N. Johnson K.R. Lakins J.N. Rozenberg G.I. Gefen A., et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 64.Ding V.M.Y. Boersema P.J. Foong L.Y. Preisinger C. Koh G. Natarajan S., et al. Tyrosine phosphorylation profiling in FGF-2 stimulated human embryonic stem cells. PLoS One. 2011;6:e17538. doi: 10.1371/journal.pone.0017538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen W.Q. Villa-Diaz L.G. Sun Y.B. Weng S.N. Kim J.K. Lam R.H.W., et al. Nanotopography influences adhesion, spreading, and self-renewal of human embryonic stem cells. ACS Nano. 2012;6:4094. doi: 10.1021/nn3004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gerecht S. Bettinger C.J. Zhang Z. Borenstein J.T. Vuniak-Novakovic G. Langer R. The effect of actin disrupting agents on contact guidance of human embryonic stem cells. Biomaterials. 2007;28:4068. doi: 10.1016/j.biomaterials.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee M.R. Kwon K.W. Jung H. Kim H.N. Suh K.Y. Kim K., et al. Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. Biomaterials. 2010;31:4360. doi: 10.1016/j.biomaterials.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 68.Sridharan I. Kim T. Wang R. Adapting collagen/CNT matrix in directing hESC differentiation. Biochem Biophys Res Commun. 2009;381:508. doi: 10.1016/j.bbrc.2009.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams S.S. Mear J.P. Liang H.C. Potter S.S. Aronow B.J. Colbert M.C. Large-scale reprogramming of cranial neural crest gene expression by retinoic acid exposure. Physiol Genomics. 2004;19:184. doi: 10.1152/physiolgenomics.00136.2004. [DOI] [PubMed] [Google Scholar]
- 70.Chen A. Lieu D.K. Freschauf L. Lew V. Sharma H. Wang J.X., et al. Shrink-film configurable multiscale wrinkles for functional alignment of human embryonic stem cells, their cardiac derivatives. Adv Mater. 2011;23:5785. doi: 10.1002/adma.201103463. [DOI] [PubMed] [Google Scholar]
- 71.Chen K.D. Li Y.S. Kim M. Li S. Yuan S. Chien S., et al. Mechanotransduction in response to shear stress - Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem. 1999;274:18393. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto K. Sokabe T. Watabe T. Miyazono K. Yamashita J.K. Obi S., et al. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol. 2005;288:H1915. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 73.Ahsan T. Nerem R.M. Fluid shear stress promotes an endothelial-like phenotype during the early differentiation of embryonic stem cells. Tissue Eng Part A. 2010;16:3547. doi: 10.1089/ten.tea.2010.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Metallo C.M. Vodyanik M.A. de Pablo J.J. Slukvin II. Palecek S.P. The response of human embryonic stem cell-derived endothelial cells to shear stress. Biotechnol Bioeng. 2008;100:830. doi: 10.1002/bit.21809. [DOI] [PubMed] [Google Scholar]
- 75.Adamo L. Naveiras O. Wenzel P.L. McKinney-Freeman S. Mack P.J. Gracia-Sancho J., et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–U120. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmelter M. Ateghang B. Helmig S. Wartenberg M. Sauer H. Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain-induced cardiovascular differentiation. FASEB J. 2006;20:1182. doi: 10.1096/fj.05-4723fje. [DOI] [PubMed] [Google Scholar]
- 77.Shimizu N. Yamamoto K. Obi S. Kumagaya S. Masumura T. Shimano Y., et al. Cyclic strain induces mouse embryonic stem cell differentiation into vascular smooth muscle cells by activating PDGF receptor beta. J Appl Physiol. 2008;104:766. doi: 10.1152/japplphysiol.00870.2007. [DOI] [PubMed] [Google Scholar]
- 78.Li X. Chu J.L. Wang A.J. Zhu Y.Q. Chu W.K. Yang L., et al. Uniaxial mechanical strain modulates the differentiation of neural crest stem cells into smooth muscle lineage on micropatterned surfaces. PLoS One. 2011;6:e26029. doi: 10.1371/journal.pone.0026029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Terraciano V. Hwang N. Moroni L. Park H.B. Zhang Z. Mizrahi J., et al. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells. 2007;25:2730. doi: 10.1634/stemcells.2007-0228. [DOI] [PubMed] [Google Scholar]
- 80.Chowdhury F. Na S. Li D. Poh Y.C. Tanaka T.S. Wang F., et al. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater. 2010;9:82. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Teramura T. Takehara T. Onodera Y. Nakagawa K. Hamanishi C. Fukuda K. Mechanical stimulation of cyclic tensile strain induces reduction of pluripotent related gene expressions via activation of Rho/ROCK and subsequent decreasing of AKT phosphorylation in human induced pluripotent stem cells. Biochem Biophys Res Commun. 2012;417:836. doi: 10.1016/j.bbrc.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 82.Saha S. Lin J. De Pablo J.J. Palecek S.P. Inhibition of human embryonic stem cell differentiation by mechanical strain. J Cell Physiol. 2006;206:126. doi: 10.1002/jcp.20441. [DOI] [PubMed] [Google Scholar]
- 83.Saha S. Ji L. de Pablo J.J. Palecek S.P. TGF beta/Activin/Nodal pathway in inhibition of human embryonic stem cell differentiation by mechanical strain. Biophys J. 2008;94:4123. doi: 10.1529/biophysj.107.119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tesar P.J. Chenoweth J.G. Brook F.A. Davies T.J. Evans E.P. Mack D.L., et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–U10. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 85.Leung H.W. Chen A. Choo A.B.H. Reuveny S. Oh S.K.W. Agitation can induce differentiation of human pluripotent stem cells in microcarrier cultures. Tissue Eng Part C Methods. 2011;17:165. doi: 10.1089/ten.TEC.2010.0320. [DOI] [PubMed] [Google Scholar]