Abstract
Background
Seaweed is an important dietary component and a rich source of iodine in several chemical forms in Asian communities. Their high consumption of this element (25 times higher than in Western countries) has been associated with the low incidence of benign and cancerous breast and prostate disease in Japanese people.
Summary
We review evidence showing that, in addition to being a component of the thyroid hormone, iodine can be an antioxidant as well as an antiproliferative and differentiation agent that helps to maintain the integrity of several organs with the ability to take up iodine. In animal and human studies, molecular iodine (I2) supplementation exerts a suppressive effect on the development and size of both benign and cancerous neoplasias. Investigations by several groups have demonstrated that these effects can be mediated by a variety of mechanisms and pathways, including direct actions, in which the oxidized iodine dissipates the mitochondrial membrane potential, thereby triggering mitochondrion-mediated apoptosis, and indirect effects through iodolipid formation and the activation of peroxisome proliferator–activated receptors type gamma, which, in turn, trigger apoptotic or differentiation pathways.
Conclusions
We propose that the International Council for the Control of Iodine Deficient Disorders recommend that iodine intake be increased to at least 3 mg/day of I2 in specific pathologies to obtain the potential extrathyroidal benefits described in the present review.
Introduction
Iodine is a crucial component in the formation of thyroid hormone, and public health policies have been established to supply deficient populations with the necessary amount of this element in order to eradicate the iodine deficiency diseases, that is, endemic goiter and cretinism. The International Council for the Control of Iodine Deficiency Disorders proposed that 150–299 μg/day is adequate to cover the thyroid requirement, and the 10th edition of Recommended Dietary Allowances published in 1989 suggested that the maximal allowable dietary dose of iodine be 1.0 mg/day for children and 2.0 mg/day for adults (1). These limits were established considering that particular individuals with underlying or evident thyroid pathologies (Table 1) can develop hyper- or hypothyroidism if they are exposed to doses higher than 1.5 mg/day (2–4). However, reviews by Baker and Hollowell in 2000 (4), Bürgi in 2010 (5), and Leung and Braverman in 2012 (6) reported that iodine supplements at low (1.5–8 mg/day) and intermediate doses (10–32 mg/day), ingested from a variety of sources (Table 2), are well-tolerated in euthyroid subjects, maintaining levels of thyroid hormones (thyroxine and triiodothyronine) and thyrotropin within normal limits (3–16). Only very high doses (>30 mg/day), mainly as iodide (I−), generate hypothyroidism and goiter, which rapidly revert to normal when these individuals stop taking the high-iodine supplement. On the other hand, considerable evidence indicates that iodine per se can ameliorate physiopathologies of several organs that take up iodine, primarily the thyroid, mammary, and prostate glands and potentially the pancreas, gastric, and nervous systems, and it may act as an antioxidant in the whole organism if this element is ingested at concentrations higher than 3 mg/day (17). Dose-response studies in humans demonstrated that iodine at concentrations of 1.5 mg/day or less had no effect, whereas concentrations of 3, 5, and 6 mg/day, mainly in the form of molecular iodine (I2), exhibited significant beneficial actions in benign pathologies (mastalgia or prostatic hyperplasia) and antineoplastic effects in early and advanced breast cancer (14–16,18,19). These studies included treatments lasting from 5 weeks up to 2 years, and at these concentrations they do not exert any secondary effect. Some of these studies also analyzed higher concentrations of iodine (9 and 12 mg/day) and showed that these doses resulted in the same benefits but caused transient hypothyroidism in 20% of the studied individuals, while also producing an assortment of minor side effects (upper respiratory tract infection [26%], headache [20%], sinusitis [12%], nausea [9.9%], acne [9.0%], back pain [9.0%], diarrhea [9.0%], dyspepsia [8.1%], rash [8.1%], and abdominal pain [6.3%]) which disappeared when the high iodine supplement is stopped (15). Antiproliferative and apoptotic effects have also been observed in preclinical studies, in which rodents (20,21) or tumoral cell lines (3,22) are exposed to micromolar concentrations of I2.
Table 1.
Predisposing Risk Factors Associated with Permanent, Iodine-Induced Thyroid Dysfunction
| 1. Individuals with underlying thyroid disease: |
| • Graves's disease |
| • Hashimoto thyroiditis |
| • Euthyroid with a history of subacute thyroiditis |
| • Euthyroid with a history of postpartum thyroiditis |
| • Euthyroid with a history of type 2 amiodarone–induced thyroiditis |
| • Euthyroid with post-hemithyroidectomy |
| • Euthyroid after interferon-α therapy |
| 2. Individuals with chronic iodine deficiency exposed to iodine at doses higher than 2 mg |
| 3. Fetuses, preterm neonates, and newborn infants exposed to high doses of iodine through the placenta and milk |
| 4. Elderly people with subclinical hypothyroidism |
| 5. Patients with certain nonthyroidal disease such as chronic dialysis and cystic fibrosis, especially those taking sulfisoxazole |
| 6. Patients taking medications such as expectorants or amiodarone that contain high concentrations of iodine |
| 7. Patients taking lithium |
| 8. Individuals with a family history of goiter or thyroiditis |
Table 2.
Sources and Effects of Excess Iodine
| Source of iodine | Iodine dose (mg/day) | Treatment time | Chemical form of iodine | Effects on thyroid function | References |
|---|---|---|---|---|---|
| KI supplements | Days–weeks | Iodide | 3–6 | ||
| Water solution (5–15 mg) | >2 | Transient subclinical hypothyroidism | |||
| 1 tablet (50 mg) | >30 | Thyrotoxicosis (2–10 %) | |||
| Amiodarone | Months–years | Free iodide | 6–7 | ||
| 1 tablet (100 mg) | 3 | Thyrotoxicosis (1.7%); hyperthyroidism (1%) | |||
| 1 tablet (600 mg) | 21 | Hypothyroidism (2–10%) | |||
| Iodinated contrast medium (200 mL/dose) | 7–10 | One dose | Free iodide | Hyperthyroidism or hypothyroidism (1–2%) | 6,8 |
| Seaweed | Weeks–months | Iodide, I2 | 6,9,10 | ||
| Blended brown seaweed (1 bowl, ∼250 mL soup) | 1–3 | Normal values or transient subclinical hypothyroidism (2–10%) | |||
| High level of consumption (>6 g seaweed/day) | >20 | Risk of papillary thyroid cancer (1–10%) | |||
| Iodopovidone (5% solution mouthwash using 2–4 mL) | 14–28 | Days–weeks | I2 | Values remain within normal range | 11,12 |
| Purified water solutions (8 mg/L per tablet) | Months–years | I2 | 4,13 | ||
| 1 tablet | 1–5 | Normal values | |||
| 4 tablets | 10–32 | Transient hypothyroidism and goiter | |||
| Aqueous I2 solution | Months–years | I2, I2/iodide | 14–16 | ||
| I2 water solution, Lugol solution, or 1–2 tablets (3 mg per tablet) | 1–6 | Values remain within normal range | |||
| 3–4 tablets (3 mg per tablet) | 9–12 | Transient subclinical hypothyroidism |
KI, potassium iodide; I2, molecular iodine.
Here we review information related to the antioxidant, apoptotic, and differentiation effects of iodine that do not include the actions of thyroid hormones. Chemically, the elemental form of iodine (I2) with an atomic weight of 125.9015 is the only substance that should be called “iodine”; however, the term “iodine” is widely used to describe many compounds in which the active principle is iodine per se. These include the different oxidation states of iodine (iodinium [I+], iodine free radical [I0], I2, etc.), iodine-containing salts (KI, NaI), and preparations of I2 together with stabilizing components (povidone–iodine, iodine tincture, or Lugol). In the present work, the term “molecular iodine” or I2 corresponds to any aqueous solution (I2 water solution, povidone–iodine, iodine tincture, or Lugol) that contains I2, “iodide” or I− refers to solutions of KI or NaI, and the generic term “iodine” does not identify the specific chemical form of this element.
Discussion
Iodine in normal tissues
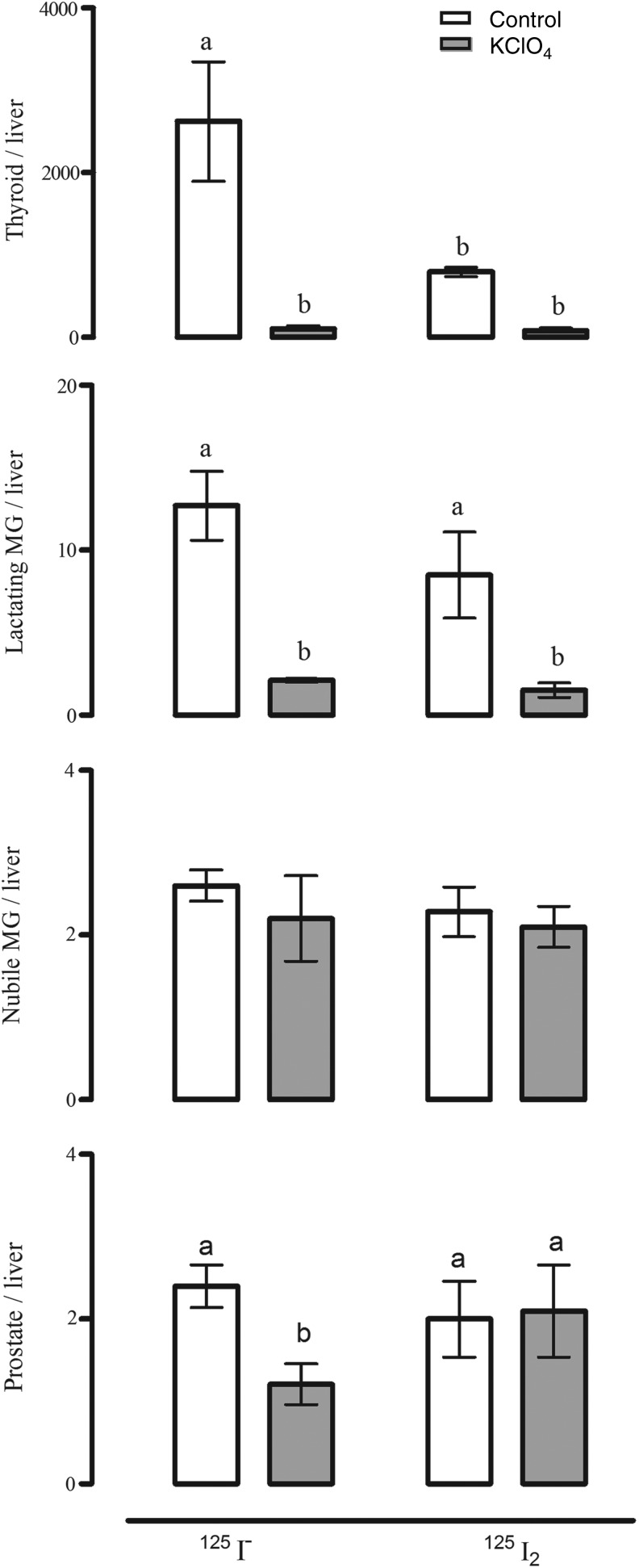
Several tissues share with the thyroid gland the capacity to actively accumulate iodine; these include the salivary glands, gastric mucosa, lactating mammary gland, the choroid plexus, ciliary body of the eye, lacrimal gland, thymus, skin, placenta, ovary, uterus, prostate, and pancreas, and they may either maintain or lose this ability under pathological conditions (23). The I− transport system in these extrathyroidal tissues involves the expression of the specific sodium iodide symporter (NIS) and in some cases also pendrin (PDS/SLC26A4) (3). In previous reports, our group demonstrated that the mammary cancer cell line MCF-7 can accumulate both I− and I2, where I− is internalized by NIS (inhibited by KClO4), whereas I2 uptake is independent of NIS, PDS, Na+, and energy, but it is saturable and dependent on protein synthesis, suggesting a facilitated diffusion system (24). Moreover, as shown in Figure 1, the thyroid gland, the mammary gland, and the prostate can accumulate both types of iodine, and they are captured by different mechanisms. The thyroid gland, the lactating mammary gland, and the prostate exhibit a significant uptake of I−, which is internalized by NIS (inhibited by KClO4). In the thyroid and the lactating mammary gland, I2 uptake is three times lower than I− uptake, and only about half of the I2 capture is inhibited by KClO4. In contrast, in nubile animals, mammary and prostate tissues captured 300 times less I2 than the thyroid and 4 times less than the lactating mammary gland, and NIS did not participate in its internalization (25). These findings suggest the notion that I2 could contribute to maintaining the normal integrity of these organs. Eskin et al. showed that iodine deficiency alters the structure and function of the mammary gland of virgin rats (26), and that I2 is effective in diminishing ductal hyperplasia and perilobular fibrosis secondary to iodine deficiency. Similarly, I2 treatment (3–6 mg/day) of patients with benign breast disease is accompanied by a significant bilateral reduction in breast size and remission of disease symptoms, effects not observed when I− or protein-bound I− is administered (14,15). Moreover, similar benefits have been found in benign prostatic hyperplasia, in animal models with 0.05% I2 supplementation (17), and in human patients with early benign prostatic hyperplasia (stages I and II) where an 8-month Lugol (5 mg/day) supplement was accompanied by diminished symptoms and prostate-specific antigen values, and an increased urine flow rate (16). All these data agree with epidemiological reports showing a direct association in the Japanese population between the low incidence of breast and prostate pathologies and the moderately high dietary intake of iodine (27–29). Seaweeds, which are widely consumed in Asian countries, contain high quantities of iodine in several chemical forms, that is, I−, I2, and iodate (IO3−); the average iodine consumption in the Japanese population is 1200–5280 μg/day versus 166 and 209 μg/day in the United Kingdom and the United States, respectively (27,30–32). Controversial reports related to algae consumption show that only certain types of seaweed correlated with lower breast cancer incidence in the Korean population (33) or with the presence of higher thyroid cancer (papillary) rates in postmenopausal Japanese women who regularly consumed high quantities of seaweed (10). The authors interpreted these findings as indicating that the iodine content of seaweeds is highly variable or that other components present in the algae, such as arsenic, could also be contributing to the correlation between high seaweed intake and cancer. Nevertheless, in spite of the high nutritional iodine consumption, Asia does not differ from the rest of the world in the prevalence of thyroid disorders (1,28).
FIG. 1.
Effect of perchlorate (KClO4) on 125I− and 125I2 uptake. Rats were first injected intraperitoneally with KClO4 (20 mg/kg) or saline (sham injected), and 2 hours later, with 50 μCi of either 125I− or 125I2. Animals were sacrificed 1.0 hour after the radioactivity administration. Radioactivity uptake (cpm) was measured in several tissues and normalized to that in the liver (a nonuptake organ); the results are expressed as cpm tissue/cpm liver. Thyroid tissues were obtained from female and male rats. Results are expressed as mean±SD, n=7 rats/group. Data were analyzed with a one-way analysis of variance, and differences between means were evaluated by the Tuckey test. a,bDifferent letters indicate significant differences between groups, p<0.05. MG, mammary gland. [Data adapted from Aceves et al. (17).]
Antioxidative effects
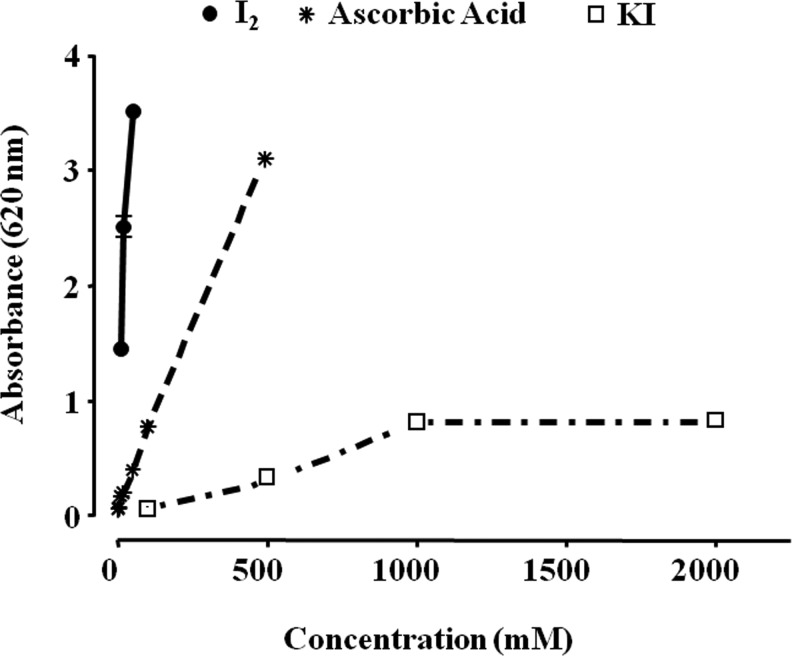
Several studies have shown iodine to be a potent antioxidant (34). In the brown algae Laminaria, which contains a 300,000-fold greater iodine concentration than any other living organism, the inorganic iodine acts as an antioxidant, neutralizing hydrogen peroxide in a two-step process, by converting it first to hypoiodous acid and then to water, thereby preventing formation of a hydroxyl radical (35). Similar antioxidant effects have been described in other photosynthetic organisms, and it has been suggested that in some invertebrates a diet of these iodinated organic molecules serves as a “primitive” thyroid gland (36). Berking et al. (37) demonstrated the antioxidant action of I− in polyps of the jellyfish Aurelia aurita, whereas Elstner and co-workers found this antioxidant effect in the rabbit eye (38). Micromolar amounts of I− decrease damage by free oxygen radicals, increase the total antioxidant status in human serum (39,40), and defend brain cells in rats from lipid peroxidation (41). Thyroxine and other iodothyronines act as antioxidants and inhibitors of lipid peroxidation after they are oxidized by hemoglobin and their iodine is released (42,43). I2 supplements decrease lipid peroxidation in normal and tumoral mammary tissues from rats with methylnitrosourea (MNU)-induced mammary cancer (44), and prevent the cardiac damage induced by the antineoplastic agent doxorubicin when I2 (0.05% in drinking water) is administered 2 days before starting the antineoplastic treatment (45). Although the specific mechanisms involved in the antioxidant effect of iodine have not been analyzed in depth, several studies show that I− could be acting directly as an electron donor that quenches free radicals such as OH• or H2O2; alternatively, it may act as a free radical that readily iodinates tyrosine, histidine, and double bonds of some polyunsaturated fatty acids in cellular membranes, making them less reactive with oxygen radicals (46). Figure 2 illustrates the reductive capacity of different chemical forms of iodine in comparison with ascorbic acid, using the in vitro ferric reducing/antioxidant power assay; it shows that I2 exerts a 10- or 50-fold greater antioxidant action than ascorbic acid or KI, respectively.
FIG. 2.
Iodine antioxidant power. The antioxidant capacity of molecular iodine (I2) and potassium iodide (KI) was analyzed with the ferric reducing/antioxidant power assay (FRAP). Ascorbic acid was used as positive control. Three reagents were used: sodium acetate and acetic acid buffer (300 mM, pH 3.6); 10 mM 2,4,6-tripyridyl-s-triazine in a 40 mM solution of hydrochloric acid; and a 20 mM solution of ferric chloride hexahydrate prepared with high-performance liquid chromatography–grade water. FRAP reagent was prepared fresh before each analysis by combining these three reagent solutions in the proportions 10:1:1. Ascorbic acid standards were freshly prepared before analysis. The FRAP assay was carried out in a microplate, with sodium acetate buffer (60 μL), ascorbic acid standards (60 μL) or samples (60 μL), and 240 μL of the FRAP reagent; after a 4-minute reaction, the microplate was read at 620 nm.
Iodine also has well-known anti-inflammatory effects. It is well established that povidone–iodine, besides its excellent antibacterial effect (47), also exerts an anti-inflammatory action by neutralizing radical oxygen species (48). Moreover, I2 inhibits the generation of nitric oxide in murine macrophages and tumor necrosis factor-α expression in human monocytes/macrophages (49). These specific actions of iodine agree with reports describing the anti-inflammatory effects of marine algae, which, as noted above, contain the highest iodine concentration of living organism, and suppress the levels of proinflammatory messengers such as nitric oxide, prostaglandine-E2, and proinflammatory cytokines (tumor necrosis factor-α, interleukin-6, and interleukin-1β) (50).
Effects on apoptosis
Iodine is not only the main substrate for the synthesis of thyronines by the thyroid, but it also participates directly in the regulation of thyroid function and thyrocyte proliferation. This phenomenon is called “autoregulation,” which has been defined as the ability of the thyroid to regulate its own function and growth, depending on the intrathyroidal availability of iodine (51). An excess of I− causes inhibitory actions that include a decreased organification of iodine, hormone secretion, decreasing thyroid blood flow, thyroglobulin proteolysis, glucose and aminoacid transport, protein and RNA biosynthesis, and thyroid follicular cell growth in vivo (0.05% in drinking water) and thyrocyte proliferation in vitro (200–500 μM) (52). With the exception of blood flow and vascularization, all other inhibitory actions of iodine can be reversed by drugs that block the enzyme thyroid peroxidase (TPO), such as methylmercaptoimidazole or propylthiouracil (53). These results are in agreement with data showing that I− exerts antiproliferative and apoptotic effects in thyrocytes, but that the rate-limiting step for these effects depends on TPO activity. In the presence of H2O2, this enzyme oxidizes I− and covalently links it to proteins or lipids. The specific iodine species generated by TPO has not been identified, but several candidates exist, such as iodinium (I+), iodine free radical (I0), hypoiodite (IO−), and I2 (46). Vitale et al. (54) showed that an excess of KI (10–50 mM) induces apoptosis in primary thyrocytes cells, but if TPO activity is blocked with propylthiouracil, the apoptotic effect of I− is eliminated. Moreover, comparing lung cancer cells (without natural iodine uptake) transfected with NIS or NIS/TPO, Zhang et al. (55) observed that KI (30 mM) induced apoptosis only in NIS/TPO transfected cells.
The first report demonstrating that iodine exhibited an antineoplastic effect in extrathyroidal tissues was by Kato et al. (20) in the rat mammary cancer model induced by the carcinogen 7,12-dimethylbenz[α]anthrancene. This suppressive effect was exerted by 0.05% Lugol. Recently, we reported that in this model, KI, I2, or a mixture of both (1:2 I2/I−) can induce antineoplastic actions and that the protective effect of 0.1% KI is lost when the enzyme lactoperoxidase (LPO), which is present in these mammary cancers, was inhibited by methylmercaptoimidazole, indicating that I− from KI needs to be oxidized to have the apoptotic effect (21). In this study our group also demonstrated that a 0.5% mix prevents 7,12-dimethylbenz[α]anthrancene–induced DNA adduct formation in premalignant and cancer tissues. These findings are particularly relevant since LPO can oxidize natural or synthetic estrogens to catechol estrogens (56,57). The resulting estrogenic quinones have been shown to react with DNA to form mutagenic adducts that can initiate or promote cancer (56). This notion agrees with the report of Cavalieri's group showing that higher levels of E2-DNA adducts are present in the urine of breast cancer patients and women at high risk for this disease (58).
Various groups have described effects of iodine in several cancer cell lines and have proposed different mechanisms and pathways that mediate the antiproliferative effect of I2, including a direct action, where the oxidized iodine dissipates the mitochondrial membrane potential, thereby triggering mitochondrion-mediated apoptosis, or an indirect effect through iodolipid formation and the activation of peroxisome proliferator–activated receptors type gamma (PPARγ), which, in turn, trigger the BAX-caspase apoptotic pathway (3,59,60).
It is well known that the mitochondrial transmembrane potential (MMP) is required for a variety of mitochondrial functions, including protein import, ATP production, and regulation of metabolite transport. The mitochondrial intermembrane space contains several proteins that can either induce apoptosis involving caspases (e.g., cytochrome c) or can execute a caspase-independent apoptotic death program through the apoptosis-inducing factor. Release of these factors requires abatement of the MMP, and thiol depletion is a powerful trigger (61). I2 treatment (10–40 μM) is accompanied by depletion of cellular thiol content and dissipation of the MMP in estrogen-responsive (MCF-7) and nonresponsive (MDA-MB231) human cell lines (60), and the preincubation of MCF-7 cells with N-acetyl-cysteine (NAC), a thiol-containing agent, prevented the apoptotic effect of I2 (62). Moreover, comparative studies of mitochondria isolated from tumoral versus extra-tumoral human breast tissue showed that the I2 treatment increased mitochondrial permeability in tumoral tissue and decreased it in extra-tumoral tissue, suggesting a differential sensitivity toward iodine in the two physiological conditions (63).
The indirect action of I2 could be exerted by its formation of covalent bonds to specific lipids such as arachidonic acid (AA) or eicosapentaenoic acid, forming 6-iodo-5-hydroxy-8,11,14-eicosatrienoic acid (also called 6-iodolactone; 6-IL) or alpha-iodohexadecanal, respectively (51). These iodocompounds have been detected in thyroid tissue of rat, pig, horse, and human origin, and they mimic some of the inhibitory effects of excess I− on several thyroid parameters (64,65). Although the specific iodinated components have not yet been characterized in other tissues, several studies have reported elevated prostaglandin levels in cancerous compared with normal tissues (66,67). Prostaglandins are produced from AA by the enzyme cyclooxygenase, indicating the presence of high levels of AA in several tumors (68,69). In relation to the mammary gland, we reported that MNU-induced tumors contain four times higher basal concentrations of AA, and after 0.05% I2 treatment, 6-IL levels were 15-fold higher than in normal mammary tissue, suggesting a role for 6-IL in the antiproliferative effect of I2 (70). These findings have been corroborated in human cancer cell lines in which lipids similar to 6-IL were identified after I2 treatment (71), or the addition of I2 or 6-IL triggered apoptosis (22,62). In this regard, the consistent observation that cancer cells are more sensitive than normal cells to I2 (22,62,71) led us to propose that the high concentration of AA in tumoral cells is the crucial component that, when iodinated, is responsible for the antiproliferative effect of I2 (71).
In the search for cellular mechanisms associated with iodine effects, studies from our laboratory demonstrated that both I2 and 6-IL supplementation significantly modified the expression of PPARs (72). These receptors, originally associated with regulating lipid metabolism, are widely expressed, and they form part of the nuclear receptor family that binds thyroid hormones, steroids, and vitamins. To date, three isotypes, PPARα, PPARβ/δ, and PPARγ, have been identified. These three subtypes display differential tissue distribution, and each is involved in specific functions such as early development, cell proliferation, differentiation, apoptosis, and metabolic homeostasis (73,74). In our experiments, 20–200 μM I2 increases the expression of PPARγ mRNA and protein, decreases expression of mRNA for PPARα, and has no effect on PPARβ/δ expression in MCF-7 cells. We also showed that 6-IL is a specific agonist of PPARγ with an in vitro affinity six times higher than AA (72). These findings agree with the observation that the affinity and selectivity of the PPARγ isoform for some fatty acids is increased by the conformational changes resulting from the incorporation of halogens (phenyl acetate<phenyl butyrate<p-chlorophenyl acetate<p-iodophenyl butyrate) (75). Moreover, recent reports have shown that antineoplastic effects of iodine or iodolipids are exerted on different types of cells that, first, can take up I2 and, second, exhibit apoptotic induction by PPARγ agonists. Such cells include prostate, lung carcinoma, pancreas carcinoma, melanoma, glioblastoma, and neuroblastoma cells (22,62).
In analyzing iodine/PPARγ interactions, our laboratory has obtained results showing that treatment with I2 or 6-IL induced apoptosis accompanied by increases in p53, p21, BAX, and caspases 3 and 7 (22,71,72). All these effects have been reported in cancer cells in response to specific agonists of PPARγ (73). Moreover, in an early phase clinical study conducted in 22 patients with mammary cancer, we found that 2–5 weeks of 5 mg/day I2 treatment caused significant increases of apoptotic rate and PPARγ expression, whereas there was a significant decrease in proliferation and diminished translocation of the estrogen receptor alpha to the nucleus, suggesting that the antineoplastic effect of iodine involves PPARγ activation (18). In accordance with these findings, a recent report of Eskin's group showed that in MCF-7 cells, iodine treatment may inhibit the estrogen response through upregulating proteins such as CYP1A1 and BRCA1 (76), both of which are also modulated by PPARγ (77,78).
Effects on cellular differentiation
Another possible effect of iodine is the induction of cellular differentiation. It is known that iodine plays a central role in thyroid physiology by maintaining the normalcy of thyroid tissue (79). Epidemiological studies associating iodine intake and thyroid cancer led to controversy; chronic iodine deficiency is firmly established as a risk factor for follicular thyroid cancer, whereas some studies suggested that iodine supplementation programs could increase the incidence of papillary thyroid cancer in chronic iodine-deficient populations (80,81). In relation to differentiation induction, cancer studies have shown that sufficient iodine prevents the transformation from differentiated to anaplastic thyroid cancer, the most aggressive type of thyroid cancer with a median survival of 4–12 months from the time of diagnosis (79). Both in vivo (MNU-induced model, human patients, xenotransplants in the nu/nu Foxn1 mouse) and in vitro (MCF-7, MDA-B231) models of mammary cancer have shown that I2 treatment induces, in addition to an important apoptotic effect, increased expression of NIS, PDS, LPO, and/or estrogen alpha receptors as well as a reduction of invasive and metastatic inducers such as vascular endothelial growth factor, urokinase-type plasminogen activator, Bcl2, and survivin, indicating a consistent effect on differentiation (18,19,70,82). Although the mechanism of these effects has not been elucidated, it is possible that this action again involves PPARγ activation. Indeed, some authors believe that PPARγ activation is antiproliferative by virtue of its differentiation-promoting effects. Treatment with PPARγ agonists inhibits cancer cell growth in various cancer types both in vitro and in vivo by inducing G0-G1 cell-cycle arrest, promoting differentiation, and reverting the epithelial–mesenchymal transition. The epithelial–mesenchymal transition, which plays an important role in the physiological processes of embryo development (gastrulation, neural crest migration, mesoderm establishment, etc.), has also been shown to participate in chronic fibrotic disorders and cancer progression (83,84). At the molecular level, epithelial–mesenchymal transition is defined by the loss of cell–cell adhesion molecules (e.g., E-cadherin), induction of mesenchymal markers such as vimentin and N-cadherin, and acquisition of chemoresistance by upregulation of ATP-binding cassette transporters and antiapoptotic markers such as Bcl2, Bcl-xl, or survivin (85). In functional studies we demonstrated that a 5 μM 6-IL supplement induces neutral lipid accumulation in MCF7 cells accompanied by decreases in Bcl2 and vascular endothelial growth factor (72). Similar results were found in an early phase clinical study conducted in 21 patients with advanced mammary cancer treated with 5 mg/day of I2 and chemotherapy (fluorouracil–epirubicin–cyclophosphamide or docetaxel–epirubicin [FEC/DE]); the FEC/DE-I2 group showed higher levels of PPARγ and lower levels of Bcl2 and survivin expression, as well as a total absence of the chemoresistance observed in 30% of the patients treated with the chemotherapy alone (FEC/DE-placebo) (19,86).
Summary and Comments
Animal and human studies have shown that (a) oral administration of I− and I2 exhibits distinct pharmacological and toxicological profiles, where I− is more thyrotoxic than I2, and (b) I2, but not I−, has beneficial effects in both benign and cancer pathologies of organs that are capable of iodine uptake, but only if this element is ingested in milligram amounts. We propose that the International Council for the Control of Iodine Deficiency Disorders considers the importance of these studies and recommend, for pathologies of tissues that take up iodine (primarily thyroid, mammary, and prostate glands and potentially pancreas, gastric, and nervous systems) and under the care of a physician, an iodine intake of at least 3 mg/day in the form of I2.
Acknowledgments
The authors are grateful to Francisco Javier Valles and Rafael Silva for bibliographic assistance; Leonor Casanova and Lourdes Lara for academic support; Alberto Lara, Omar Gonzalez, Ramon Martinez, and Lorena Ortiz for computer assistance; and Dr. Dorothy Pless for proofreading. Iodine was analyzed in the Laboratory of Environmental Geochemistry, Geosciences Center, UNAM–Juriquilla (Dr. Maria Teresa Orozco Esquivel). The work of our group was partially supported by grants UNAM/DGAPA-PAPIIT IN201210, IN202513, IN200813, and CONACYT 174439, 176911, and 127368.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.World Health Organization, United Nations Children's Fund, International Council for Control of Iodine Deficiency Disorders 1999 Progress Towards the Elimination of Iodine Deficiency Disorders (IDD) WHO Booklet. World Health Organization; Geneva: pp. 1–33. [Google Scholar]
- 2.Venturi S. Venturi A. Cimini D. Arduini C. Venturi M. Guidi A. A new hypothesis: iodine and gastric cancer. Eur J Cancer Prev. 1993;2:17–23. [PubMed] [Google Scholar]
- 3.Torremante PE. Rosner H. Antiproliferative effects of iodine in cancers. Curr Chem Biol. 2011;5:171–176. [Google Scholar]
- 4.Baker H. Hollowell J. Use of iodine for water disinfection: iodine toxicity and maximum recommended dose. Environ Health Persp. 2000;108:679–684. doi: 10.1289/ehp.00108679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bürgi H. Iodine excess. Best Pract Res Clin Endocrinol Metab. 2010;24:107–115. doi: 10.1016/j.beem.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Leung A. Braverman LE. Iodine-induced thyroid dysfunction. Curr Opin Endocrinol Obes. 2012;19:414–419. doi: 10.1097/MED.0b013e3283565bb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogazzi F. Tomisti L. Bartalena L. Aghini-Lombardi F. Martino E. Amiodarone and the thyroid: a 2012 update. J Endocrinol Invest. 2012;35:340–348. doi: 10.3275/8298. [DOI] [PubMed] [Google Scholar]
- 8.Rhee CM. Bhan I. Alexander EK. Brunelli SM. Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism. Arch Intern Med. 2012;172:153–159. doi: 10.1001/archinternmed.2011.677. [DOI] [PubMed] [Google Scholar]
- 9.Rhee SS. Braverman LE. Pino S. He X. Pearce EN. High iodine content of Korean seaweed soup: a health risk for lactating women and their infants? Thyroid. 2011;21:927–928. doi: 10.1089/thy.2011.0084. [DOI] [PubMed] [Google Scholar]
- 10.Michikawa T. Inoue M. Shimazu T. Sawada N. Iwasaki M. Sasazuki S. Yamaji T. Tsugane S. Seaweed consumption and the risk of thyroid cancer in women: the Japan Public Health Center-based Prospective Study. Eur J Cancer Prev. 2012;21:254–260. doi: 10.1097/CEJ.0b013e32834a8042. [DOI] [PubMed] [Google Scholar]
- 11.Sato K. Ohmori T. Shiratori K. Yamazaki K. Yamada E. Kimura H. Takano K. Povidone iodine-induced overt hypothyroidism in a patient with prolonged habitual gargling: urinary excretion of iodine after gargling in normal subjects. Intern Med. 2007;46:391–395. doi: 10.2169/internalmedicine.46.1899. [DOI] [PubMed] [Google Scholar]
- 12.Yeginsu A. Karamustafaoglu A. Ozugurlu F. Etikan I. Iodopovidone pleurodesis does not effect thyroid function in normal adults. Interact Cardiovasc Thorac Surg. 2007;6:563–564. doi: 10.1510/icvts.2007.154914. [DOI] [PubMed] [Google Scholar]
- 13.Smith SM. Zwart SR. McMonigal KA. Huntoon CL. Thyroid status of Space Shuttle crewmembers: effects of iodine removal. Aviat Space Environ Med. 2011;82:49–51. doi: 10.3357/asem.2926.2011. [DOI] [PubMed] [Google Scholar]
- 14.Ghent WR. Eskin BA. Low DA. Hill LP. Iodine replacement in fibrocystic disease of the breast. Cancer J Surg. 1993;36:453–460. [PubMed] [Google Scholar]
- 15.Kessler J. Are there side effects when using supraphysiological levels of iodine in treatment regimens. In: Preedy VR, editor; Burrow GN, editor; Watson RR, editor. Comprehensive Handbook of Iodine., Nutritional, Endocrine and Pathological Aspects. Academic Press; San Diego, CA: 2009. pp. 801–810. [Google Scholar]
- 16.Anguiano B. Ledezma O. Juárez MA. Nuñez F. Aceves C. Therapeutic effect of iodine on human benign prostatic hyperplasia.. 14th International Thyroid Congress; Paris, France. Sep 11–16;; 2010. Abstract ITC2010-2585. [Google Scholar]
- 17.Aceves C. Anguiano B. Is iodine an antioxidant, antiproliferative agent for the mammary, prostate glands? In: Preedy VR, editor; Burrow GN, editor; Watson RR, editor. Comprehensive Handbook of Iodine., Nutritional, Endocrine and Pathological Aspects. Academic Press; San Diego, CA: 2009. pp. 249–257. [Google Scholar]
- 18.Vega-Riveroll L. Mondragón-Angeles P. Rojas J. Delgado G. González-Cedillo F. Romero J. Hernández-Pando R. Aceves C. Impaired nuclear translocation of estrogen receptor alfa could be associated with the antineoplastic effect of iodine in premenopausal breast cancer. Abstract presented at the 33rd Annual CRTC-AARC San Antonio Breast Cancer Symposium (SABCS); San Antonio, Texas. Dec 8–12;; 2010. Abstract P6-14-15. [Google Scholar]
- 19.Peralta G. Torres JM. Delgado G. Domínguez A. De Obaldía R. Duarte L. Paredes E. Avecilla C. Hernández S. Vega-Riveroll L. Aceves C. Iodine exhibits dual effects on breast cancer as a co-treatment with anthracyclines: antineoplastic synergy, cardioprotector. Abstract presented at the 101th Annual Meeting, AACR; Orlando, FL. Apr 2–6;; 2011. Abstract 3509. [Google Scholar]
- 20.Kato N. Funahashi H. Ando K. Takagi H. Suppressive effect of iodine preparations on proliferation of DMBA-induced breast cancer in rat. J Jpn Soc Cancer Ther. 1994;29:582–588. [Google Scholar]
- 21.Soriano O. Delgado G. Anguiano B. Petrosyan P. Molina-Servín ED. Gonsebatt ME. Aceves C. Antineoplastic effect of iodine and iodide in DMBA-induced mammary tumors. Association between lactoperoxidase and estrogen-adduct production. Endocr Relat Cancer. 2011;18:529–539. doi: 10.1530/ERC-11-0065. [DOI] [PubMed] [Google Scholar]
- 22.Aranda N. Sosa S. Delgado G. Aceves C. Anguiano B. Uptake and antitumoral effects of iodine and 6-iodolactone in differentiated and undifferentiated human prostate cancer cell lines. Prostate. 2013;73:31–41. doi: 10.1002/pros.22536. [DOI] [PubMed] [Google Scholar]
- 23.Riesco-Eizaguirre G. Santisteban P. A perspective view of sodium iodide symporter research and its clinical implications. Eur J Endocrinol. 2006;155:495–512. doi: 10.1530/eje.1.02257. [DOI] [PubMed] [Google Scholar]
- 24.Arroyo-Helguera O. Delgado G. Anguiano B. Aceves C. Uptake and antiproliferative effect of molecular iodine in the MCF-7 breast cancer cell line. Endocr Relat Cancer. 2006;13:1147–1158. doi: 10.1677/erc.1.01250. [DOI] [PubMed] [Google Scholar]
- 25.Anguiano B. García-Solís P. Delgado G. Aceves C. Uptake and gene expression of antitumoral doses of iodine in thyroid and mammary gland: evidence that chronic administration has no harmful effects. Thyroid. 2007;17:851–859. doi: 10.1089/thy.2007.0122. [DOI] [PubMed] [Google Scholar]
- 26.Eskin BA. Grotkowski CE. Connolly CP. Ghent WR. Different tissue responses for iodine and iodide in rat thyroid and mammary glands. Biol Trace Elem Res. 1995;49:9–18. doi: 10.1007/BF02788999. [DOI] [PubMed] [Google Scholar]
- 27.Cann SA. Van Netten JP. Van Netten C. Hypothesis: iodine, selenium and the development of breast Cancer. Cancer Causes Control. 2000;11:121–127. doi: 10.1023/a:1008925301459. [DOI] [PubMed] [Google Scholar]
- 28.Kamangar F. Dores GM. Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 29.Miller DW. Extrathyroidal benefits of iodine. J Am Phys Surg. 2006;11:106–110. [Google Scholar]
- 30.Teas J. Pino S. Critchley A. Braverman LE. Variability of iodine content in common commercially available edible seaweeds. Thyroid. 2004;14:836–841. doi: 10.1089/thy.2004.14.836. [DOI] [PubMed] [Google Scholar]
- 31.Nagataki S. The average of dietary iodine intake due to the ingestion of seaweeds is 1.2 mg/day in Japan. Thyroid. 2008;18:667–668. doi: 10.1089/thy.2007.0379. [DOI] [PubMed] [Google Scholar]
- 32.Zava TT. Zava DT. Assessment of Japanese iodine intake based on seaweed consumption in japan: a literature-based analysis. Thyroid Res. 2011;4:14–21. doi: 10.1186/1756-6614-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang YJ. Nam SJ. Kong G. Kim MK. A case–control study on seaweed consumption and the risk of breast cancer. Br J Nutr. 2010;103:1345–1353. doi: 10.1017/S0007114509993242. [DOI] [PubMed] [Google Scholar]
- 34.Venturi S. Evolutionary significance of iodine. Curr Chem Biol. 2011;5:155–176. [Google Scholar]
- 35.Kupper FC. Carpenter LJ. McFiggans GB. Carl J. Palmer CJ. Waite TJ. Boneberg EM. Woitsch S. Weiller M. Abela R. Grolimund D. Potin P. Butler A. Luther GW., III Kroneck PMH. Meyer-Klauck W. Feiters MC. Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proc Natl Acad Sci USA. 2008;105:6954–6958. doi: 10.1073/pnas.0709959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson LG. Thyroxine's evolutionary roots. Perspect Biol Med. 1997;140:529–536. doi: 10.1353/pbm.1997.0076. [DOI] [PubMed] [Google Scholar]
- 37.Berking S. Czech N. Gerharz M. Herrmann K. Hoffmann U. Raifer H. Sekul G. Siefker B. Sommerei A. Vedder F. A newly discovered oxidant defence system and its involvement in the development of Aurelia aurita (Scyphozoa, Cnidaria): reactive oxygen species and elemental iodine control medusa formation. Int J Dev Biol. 2005;49:969–976. doi: 10.1387/ijdb.052024sb. [DOI] [PubMed] [Google Scholar]
- 38.Elstner EF. Adamczyk R. Kromer R. Furch A. The uptake of potassium iodide and its effects as an antioxidant in isolated rabbit eyes. Ophthalmologica. 1985;191:122–126. doi: 10.1159/000309572. [DOI] [PubMed] [Google Scholar]
- 39.Winkler R. Griebenow S. Wonisch W. Effect of iodide on total antioxidant status of human serum. Cell Biochem Funct. 2000;18:143–146. doi: 10.1002/(SICI)1099-0844(200006)18:2<143::AID-CBF857>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 40.Soriguer F. Gutierrez-Repiso C. Rubio-Martin E. Linares F. Cardona I. Lopez-Ojeda J. Pacheco M. Gonzalez-Romero S. Garriga MJ. Velasco I. Santiago P. García-Fuentes E. Iodine intakes of 100–300 mg/d do not modify thyroid function and have modest anti-inflammatory effects. Br J Nutr. 2011;105:1783–1790. doi: 10.1017/S0007114510005568. [DOI] [PubMed] [Google Scholar]
- 41.Cocchi M. Venturi S. Iodide, antioxidant function and omega-6 and omega-3 fatty acids: a new hypothesis of biochemical cooperation? Prog Nutr. 2000;2:15–19. [Google Scholar]
- 42.Tseng YL. Latham KR. Iodothyronines: oxidative deiodination by hemoglobin and inhibition of lipid peroxidation. Lipids. 1984;19:96–102. doi: 10.1007/BF02534498. [DOI] [PubMed] [Google Scholar]
- 43.Turner KB. Khayat GB. Studies on the prevention of cholesterol atherosclerosis in rabbits. The influence of thyroidectomy upon the protective action of potassium iodide. J Exp Med. 1993;58:127–132. doi: 10.1084/jem.58.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.García-Solís P. Alfaro Y. Anguiano B. Delgado G. Guzman R. Nandi S. Díaz-Muñoz M. Vázquez-Martínez O. Aceves C. Inhibition of N-methyl-N-nitrosourea-induced mammary carcinogenesis by molecular iodine (I2) but not by iodide (I−) treatment. Evidence that I2 prevents cancer promotion. Mol Cell Endocrinol. 2005;236:49–57. doi: 10.1016/j.mce.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Alfaro-Hernández Y. Delgado G. Aceves C. Iodine/anthracyclines is the best combination against mammary cancer. Antineoplastic synergism and cardioprotection; Abstract presented at the 33rd Annual CRTC-AARC San Antonio Breast Cancer Symposium (SABCS); San Antonio, TX. Dec 8–12;; 2010. Abstract P5-10-16. [Google Scholar]
- 46.Smyth PP. Role of iodine in antioxidant defense in thyroid and breast disease. Biofactors. 2003;19:121–130. doi: 10.1002/biof.5520190304. [DOI] [PubMed] [Google Scholar]
- 47.Zhao D. Lim CP. Miyanaga K. Tanji Y. Iodine from bacterial iodide oxidization by Roseovarius spp. inhibits the growth of other bacteria. Appl Microbiol Biotechnol. 2012;97:2173–2182. doi: 10.1007/s00253-012-4043-y. [DOI] [PubMed] [Google Scholar]
- 48.Beukelman CJ. van den Berg AJ. Hoekstra MJ. Uhl R. Reimer K. Mueller S. Anti-inflammatory properties of a liposomal hydrogel with povidone-iodine (Repithel) for wound healing in vitro. Burns. 2008;34:845–855. doi: 10.1016/j.burns.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Moore K. Thomas A. Harding KG. Iodine released from the wound dressing Iodosorb modulates the secretion of cytokines by human macrophages responding to bacterial lipopolysaccharide. Int J Biochem Cell Biol. 1997;29:163–171. doi: 10.1016/s1357-2725(96)00128-8. [DOI] [PubMed] [Google Scholar]
- 50.Pangestuti R. Kim SK. Neuroprotective effects of marine algae. Mar Drugs. 2011;9:803–818. doi: 10.3390/md9050803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Juvenal GJ. Thomasz L. Oglio R. Perona M. Pisarev MA. Rossich L. Salvarredi L. Thyroid: iodine beyond the thyronines. Curr Chem Biol. 2011;5:163–167. [Google Scholar]
- 52.Pisarev M. Gartner R. Autoregulatory actions of iodine. In: Braverman E, editor; Utiger R, editor. Werner and Ingbar's. The Thyroid. A Fundamental and Clinical Text. Lippincott-Williams & Williams; Philadelphia, PA: 2000. pp. 85–90. [Google Scholar]
- 53.Van Sande J. Grenier G. Willems C. Dumont JE. Inhibition by iodide of the activation of the thyroid cyclic 3′,5′-AMP system. Endocrinology. 1975;96:781–786. doi: 10.1210/endo-96-3-781. [DOI] [PubMed] [Google Scholar]
- 54.Vitale M. Di Matola T. D'Ascoli F. Salzano S. Bogazzi F. Fenzi G. Martino E. Rossi G. Iodide excess induces apoptosis in thyroid cells through a p53-independent mechanism involving oxidative stress. Endocrinology. 2000;141:598–605. doi: 10.1210/endo.141.2.7291. [DOI] [PubMed] [Google Scholar]
- 55.Zhang L. Sharma S. Zhu LX. Kogai T. Hershman JM. Brent GA. Dubinett SM. Huang M. Nonradioactive iodide effectively induces apoptosis in genetically modified lung cancer cells. Cancer Res. 2003;63:5065–5072. [PubMed] [Google Scholar]
- 56.Cavalieri EL. Stack DE. Devanesan PD. Todorovic R. Dwivedy I. Higginbotham S. Johansson SL. Patil KD. Gross ML. Gooden JK. Ramanathan R. Cerny RL. Rogan EG. Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumour initiators. Proc Natl Acad Sci USA. 1997;94:10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lovstad RA. A kinetic study on the lactoperoxidase catalyzed oxidation of estrogens. Biometals. 2006;19:587–592. doi: 10.1007/s10534-006-0002-3. [DOI] [PubMed] [Google Scholar]
- 58.Cavalieri EL. Rogan EG. Depurinating estrogen-DNA adducts in the etiology and prevention of breast and other human cancers. Future Oncol. 2010;6:7–10. doi: 10.2217/fon.09.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anguiano B. Aceves C. Iodine in mammary and prostate pathologies. Curr Chem Biol. 2011;5:177–182. [Google Scholar]
- 60.Singh P. Godbole M. Rao G. Annarao S. Mitra K. Roy R. Ingle A. Agarwal G. Tiwari S. Inhibition of autophagy stimulate molecular iodine-induced apoptosis in hormone independent breast tumors. Biochem Biophys Res Commun. 2011;415:181–186. doi: 10.1016/j.bbrc.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 61.Chen FY. Han MS. Choon NO. Intracellular thiol depletion causes mitochondrial permeability transition in ebselen-induced apoptosis. Arch Biochem Biophys. 2000;380:319–330. doi: 10.1006/abbi.2000.1939. [DOI] [PubMed] [Google Scholar]
- 62.Rosner H. Torremante P. Moller W. Gartner R. Antiproliferative/cytotoxic activity of molecular iodine and iodolactones in various human carcinoma cell lines. No interfering with EGF-signaling, but evidence for apoptosis. Exp Clin Endocrinol Diab. 2010;118:410–419. doi: 10.1055/s-0029-1225615. [DOI] [PubMed] [Google Scholar]
- 63.Upadhyay G. Singh R. Ramesh Sharma R. Anil K. Balapure AK. Godbole MM. Differential action of iodine on mitochondria from human tumoral and extra-tumoral tissue in inducing the release of apoptogenic proteins. Mitochondrion. 2002;2:199–210. doi: 10.1016/s1567-7249(02)00068-5. [DOI] [PubMed] [Google Scholar]
- 64.Boeynaems JM. Hubbard WC. Transformation of arachidonic acid into an iodolactone by the rat thyroid. J Biol Chem. 1980;255:9001–9004. [PubMed] [Google Scholar]
- 65.Dugrillon A. Uedelhoven W. Pisarev M. Bechtner G. Gartner R. Identification of delta-iodolactone in iodide treated human goiter and its inhibitory effect on proliferation of human thyroid follicles. Horm Metab Res. 1994;26:465–469. doi: 10.1055/s-2007-1001734. [DOI] [PubMed] [Google Scholar]
- 66.Tan WC. Privett OS. Goldyne ME. Studies of prostaglandins in rat mammary tumors induced by 7,12-dymethyl-benz(a)anthracene. Cancer Res. 1974;34:3229–3231. [PubMed] [Google Scholar]
- 67.Bennett A. Charlier EM. McDonald AM. Simpson JS. Stamford IF. Zebro T. Prostaglandins and breast cancer. Lancet. 1977;2:624–626. doi: 10.1016/s0140-6736(77)92496-5. [DOI] [PubMed] [Google Scholar]
- 68.Howe LR. Subbaramaiah K. Brown AMC. Dannenberg AJ. Cyclooxygenase-2: a target for the prevention and treatment of breast cancer. Endocr Relat Cancer. 2001;8:97–114. doi: 10.1677/erc.0.0080097. [DOI] [PubMed] [Google Scholar]
- 69.Pham H. Banerjee T. Ziboh VA. Suppression of cyclooxygenase-2 overexpression by 15S-hydroxyeicosatrienoic acid in androgen-dependent prostatic adenocarcinoma cells. Int J Cancer. 2004;111:192–197. doi: 10.1002/ijc.20245. [DOI] [PubMed] [Google Scholar]
- 70.Aceves C. García-Solís P. Arroyo-Helguera O. Vega-Riveroll L. Delgado G. Anguiano B. Antineoplastic effect of iodine in mammary cancer. Participation of 6-iodolactone (6-IL) and peroxisome proliferator-activated receptors (PPAR) Mol Cancer. 2009;8:33–36. doi: 10.1186/1476-4598-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arroyo-Helguera O. Rojas E. Delgado G. Aceves C. Signaling pathways involved in the antiproliferative effect of molecular iodine in normal and tumoral breast cells: evidence that 6-iodolactone mediates apoptotic effects. Endocr Relat Cancer. 2008;15:1003–1011. doi: 10.1677/ERC-08-0125. [DOI] [PubMed] [Google Scholar]
- 72.Nuñez-Anita RE. Arroyo-Helguera O. Cajero-Juárez M. López-Bojorquez L. Aceves C. A complex between 6-iodolactone and the peroxisome proliferator-activated receptor type gamma may mediate the antineoplastic effect of iodine in mammary cancer. Prostaglandins Other Lipid Mediat. 2009;89:34–42. doi: 10.1016/j.prostaglandins.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 73.Elrod HA. Sun SY. PPARgamma and apoptosis in cancer. PPAR Res. 2008;70:41–65. doi: 10.1155/2008/704165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nuñez-Anita RE. Cajero-Juárez M. Aceves C. Peroxisome proliferator-activated receptors. Role of isoform gamma in the antineoplasic effect of iodine in mammary cancer. Curr Cancer Drug Targets. 2011;11:775–786. doi: 10.2174/156800911796798931. [DOI] [PubMed] [Google Scholar]
- 75.Samid D. Wells M. Greene ME. Shen W. Palmer CN. Thibault A. Peroxisome proliferator–activated receptor γ as a novel target in cancer therapy: binding and activation by aromatic fatty acid with clinical antitumoral activity. Clin Cancer Res. 2000;6:933–941. [PubMed] [Google Scholar]
- 76.Stoddard FN. Brooks A. Eskin B. Johannes G. Iodine alters gene expression in the MCF7 breast cancer cell line: evidence for an anti-estrogen effect of iodine. Int J Med Sci. 2008;5:189–196. doi: 10.7150/ijms.5.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim HG. Han EH. Jeong HG. Effect of troglitazone on CYP1A1 induction. Toxicology. 2008;246:166–171. doi: 10.1016/j.tox.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Yuan H. Kopelovich L. Yin Y. Lu J. Glazer RI. Drug-targeted inhibition of peroxisome proliferator-activated receptor-gamma enhances the chemopreventive effect of anti-estrogen therapy. Oncotarget. 2012;3:345–356. doi: 10.18632/oncotarget.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dijkstra B. Prichard RS. Lee A. Kelly LM. Smyth PP. Crotty T. McDermott EW. Hill AD. O'Higgins N. Changing patterns of thyroid carcinoma. Ir J Med Sci. 2007;176:87–90. doi: 10.1007/s11845-007-0041-y. [DOI] [PubMed] [Google Scholar]
- 80.Knobel M. Medeiros-Nieto G. Relevance of iodine intake as a reputed predisposing factor for thyroid cancer. Arq Bras Endocrinol Metabol. 2007;51:701–712. doi: 10.1590/s0004-27302007000500007. [DOI] [PubMed] [Google Scholar]
- 81.Dal Maso L. Bosetti C. La Vecchia C. Franceschi S. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control. 2009;20:75–86. doi: 10.1007/s10552-008-9219-5. [DOI] [PubMed] [Google Scholar]
- 82.Mendieta I. Nuñez-Anita RE. Delgado G. Aceves C. Differential effect of iodine on the implantation, metastasis potential of xenografts from two different human breast cancer cell lines. Abstract presented at the 101th Annual Meeting, AACR; Orlando, FL. Apr 2–6;; 2011. Abstract 4224. [Google Scholar]
- 83.Reka AK. Kurapati H. Narala VR. Bommer G. Chen J. Standiford TJ. Keshamouni VG. Peroxisome proliferator-activated receptor-gamma activation inhibits tumor metastasis by antagonizing Smad3-mediated epithelial-mesenchymal transition. Mol Cancer Ther. 2010;12:3221–3232. doi: 10.1158/1535-7163.MCT-10-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iseri OD. Kars MD. Arpaci F. Atalay C. Pak I. Gunduz U. Drug resistant MCF-7 cells exhibit epithelial-mesenchymal transition gene expression pattern. Biomed Pharmacother. 2011;65:40–45. doi: 10.1016/j.biopha.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 85.Reka AK. Goswami MT. Krishnapuram R. Standiford TJ. Keshamouni VG. Molecular cross-regulation between PPARγ and other signaling pathways: implications for lung cancer therapy. Lung Cancer. 2011;72:154–159. doi: 10.1016/j.lungcan.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aceves C. Peralta G. Torres JM. Delgado G. Dominguez A. De Obaldia R. Duarte LF. Paredes E. Avecilla C. Iodine-supplemented diet prevents the development of resistance in breast cancer chemotherapy: participation of proliferative peroxisome-activated receptor gamma (PPAR(). Abstract presented at AACR Special Conference: Advances in Breast Cancer Research; San Francisco, CA. Oct 12–15;; 2011. Abstract B62. [Google Scholar]