Abstract
Polyoxyethylene (20) sorbitan monolaurate (tween 20) is a non-ionic surfactant that is widely used as an emulsifier and stabilizer in pharmaceutical formulations, food and cosmetic industries. Although a number of studies have showed its non-toxic impacts on target cells, still, it is essential to investigate its effect on target cells. Therefore, in the present study, the anti-cell proliferation and cyto/genotoxicity effects of tween 20 are reported to address the possible mechanism for induction of apoptosis. At 40%–50% confluency, A549 cells and human umbilical vein endothelial cells were exposed to tween 20 at a recommended concentration for 24 h. After 24 h, to detect apoptosis and DNA damage, the treated cells were subjected to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, fluorescein isothiocyanate (FITC)-labeled annexin V flow cytometry, DAPI staining, comet, and DNA ladder assays. Tween 20 decreased the growth of treated cells dose and time dependently, and single-strand DNA cleavage has been confirmed by comet assay. In addition, morphological alteration of DAPI-stained cells showed clear fragmentation in the chromatin and DNA rings within the nucleus of tween 20-treated cells. In addition, flow cytometry and DNA fragmentation assays confirmed DAPI staining assay results and indicated the occurrence of a programmed cell death (apoptosis) in the treated cells. These results demonstrate that, despite consideration of tween 20 as a safe non-ionic surfactant, it can induce apoptosis in target cells.
Tween 20 was shown to induce apoptosis in target cells although it was thought to be a safe non-ionic surfactant.
Introduction
Polyoxyethylene (20) sorbitan monolaurate (tween 20) is a non-ionic polyoxyethylene surfactant that has been used primarily as a water-in-oil emulsifier (Ravindran et al., 2011). However, it has been used as a spreading, penetrating, and an auxiliary emulsifier in paints, human foodstuffs, cosmetic, and pharmaceutical preparations. It is used as a physical stabilizing agent in many topical pharmaceutical formulations such as formulation of various drugs with lipidic nanocarriers in drug delivery systems as well (Sajjadi et al., 2003; Zhang et al., 2013). It is also frequently added to washing buffers such as phosphate-buffered saline (PBS) and tris-buffered saline to prevent non-specific binding in radioimmuno assays and various techniques that employ the enzyme-linked immunosorbent assays (Hoffman and Jump, 1986; Zampieri et al., 2000).
Tween 20 can efficiently reverse the multidrug resistance phenotype by enhancing accumulation of the anticancer drugs and by inhibiting the multidrug-resistant gene expression at a low concentration (Yang et al., 2012). To the best of our knowledge, the cyto/genotoxicity study of tween 20 on various cell lines has not yet been reported in detail. Therefore, to achieve this goal and due to widespread application of tween 20 in pharmaceutical and food industries, more investigation is required.
In the present work, we assessed the cyto/genotoxic effects of tween 20 on the growth/death of A549 lung cancer cells and human umbilical vein endothelial cells (HUVECs) by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), comet, DAPI staining, and DNA fragmentation assays. In addition, flow cytometry analysis was used to determine early and late stages of apoptosis of tween 20-treated and -untreated cells.
Materials and Methods
Materials
HUVEC and A549 lung carcinoma cell lines, cell culture plates, and flasks were obtained from the national cell bank of Iran (Pasteur Institute) and IWAKI, respectively. Tween 20 was purchased from Oleon. RPMI1640 medium, trypsin-ethylenediaminetetraacetic acid (EDTA) (0.02%–0.05%), tri-reagent, and dithiotheritol were purchased from Sigma Aldrich Co. Low and normal melting point agarose and fetal bovine serum (FBS) were supplied by Gibco, Invitrogen. Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit was purchased from Oncogene Research Products.
Cell culture and viability assessment
A549 human lung cancer cells and HUVECs were propagated in RPMI1640 supplemented with 10% FBS, seeded at the density of 4×104 cells/cm2 on 96-well plates, and incubated in a humidified incubator (95% air and 5% CO2) at 37°C. To assess the influence of tween 20 on the cellular viability, HUVECs and A549 cells were seeded and cultured for approximately 40%–50% confluency in the 96-well plates before treatment. The cells were exposed with a range of tween 20 concentrations and were incubated for 24 h at 37°C. Then, cells were washed once with PBS, culture medium was replaced with 150 μL fresh media, and 50 μL MTT reagent (2 mg/mL in PBS) was added to each well. After 4 h incubation with MTT at 37°C, medium was removed and cells were exposed to 200 μL dimethyl sulfoxide (DMSO) plus 25 μL of Sorenson buffer (0.1 M glycine, 0.1 M sodium chloride [NaCl], pH 10.5). The cells were incubated for 30 min at 37°C to ensure the dissolving of formazan crystals, and then, absorbance was measured at 570 nm using a spectrophotometric plate reader, ELx 800 (Biotek). To reach precise results, all experiments were replicated thrice.
DAPI staining assay
DAPI staining assay was used to determine chromatin fragmentation. HUVECs and A549 cells were seeded in six-well plates (5×104 cells/well) containing 12 mm cover-slips and subsequently treated for various times with tween 20 (4 μL/mL) and with DMSO (200 μL) as positive control. Cells then were fixed with 4% paraformaldehyde, permeabilized in 0.1% (w/v) Triton X-100 for 5 min, washed in PBS, and stained with DAPI.
DNA fragmentation assay
The possible DNA fragmentation in tween 20-treated cells was analyzed by agarose gel electrophoresis. Briefly, treated cells were incubated in the lysis buffer (pH 7.4) containing 50 mM Tris base, 10 mM EDTA, 0.5% sodium dodecyl sulfate, and 5 units RNase for 5 min at 37°C. Then, proteins were denaturized with 500 μL of chloroform/isoamyl alcohol (24:1), and total DNA was separated by centrifugation at 12,000 rpm. Total DNA was precipitated with isopropranol and was finally electrophoresed in 1.2% agarose gel (Gooch and Yee, 1999; Nath et al., 2012).
Single cell-thin layer gel electrophoresis (alkaline comet assay)
Alkaline comet assay was used to show a direct interaction of tween 20 with chromatin and its DNA cleavage properties and probable oxidative stress that can be caused by tween 20 in treated cells. Alkaline comet assay was carried out based on literature (Singh et al., 1988; Eskandani et al., 2010). Briefly, A549 cells were detached from a flask and collected by centrifugation at 800 rpm for 8 min. Then, normal melting point agarose (1.5%) coated slides were used as a supportive surface for the low melting point agarose (%0.5)-embedded cells (1×104), lysed by 4 h incubation at 4°C in a buffer containing 2.5 M NaCl, 100 mM Na2EDTA, 1% triton X-100 (pH 10.5) without third agarose layer (Vandghanooni and Eskandani, 2011), and then placed in an ice-cold electrophoresis chamber containing alkaline electrophoresis solution (300 mM sodium hydroxide, 1 mM Na2EDTA of pH>13) for 30 min to enable DNA unwinding and removal of histones. This step was followed by electrophoresis that was conducted for 20 min at 300 mA and 30 V. After that, the slides were washed with neutralizing buffer (40 mM Tris-HCl, pH 7.5) and stained with a drop of ethidium bromide. Before the coverage of slides 20×20 cover slip, the slides were washed twice to thrice to remove excess background color and immediately, the pictures were taken by a fluorescent microscope using CASP software (Olympus IX81 fluorescence microscope equipped with XM10 monochrome camera; wavelength 546 nm; barrier 580 nm). DNA strand cleavages were expressed as the percentage of total fluorescence DNA migrated in the tail for each nucleus (Eq. I) (Singh et al., 1988; Eskandani et al., 2010).
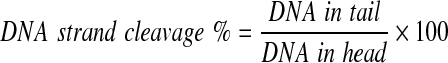 |
(I) |
FITC-labeled annexin V apoptosis assay
To detect the induction of early and late apoptosis, FITC-labeled annexin V assay was carried out. In brief, tween 20-treated cells were gently washed thrice in PBS buffer, detached by tripsinization, and washed thrice with 500 μL 1×binding buffer. Then, 1–4×106 of single unfixed cell suspensions were re-suspended in 100 μL 1×binding buffer containing 5 μL FITC-labeled annexin V in the dark for 14 min at 37°C. After that, the cells were washed again with 500 μL 1×binding buffer and were then exposed to 100 μL 1×binding buffer plus 5 μL propidium iodide (PI). Cells were analyzed by Becton Dickinson FACS Calibur System with emission filters of 515–545 nm for FITC (green) and 600 nm for PI (red) (Kafil and Omidi, 2011).
Statistical analysis
Biological results that were expressed in figures represent the mean of at least two independent experiments (bars represent mean±standard deviation). An independent Student's t-test was used to compare mean differences between two independent groups and a one-way analysis of variance (one-way ANOVA) with multiple comparisons. When the differences between the means were significant, post hoc pairwise comparisons were carried out using Tukey multiple comparison tests (SPSS; version 13.0). The statistical significance was defined as p<0.05.
Results
Effects of tween 20 on cell physical features and growth
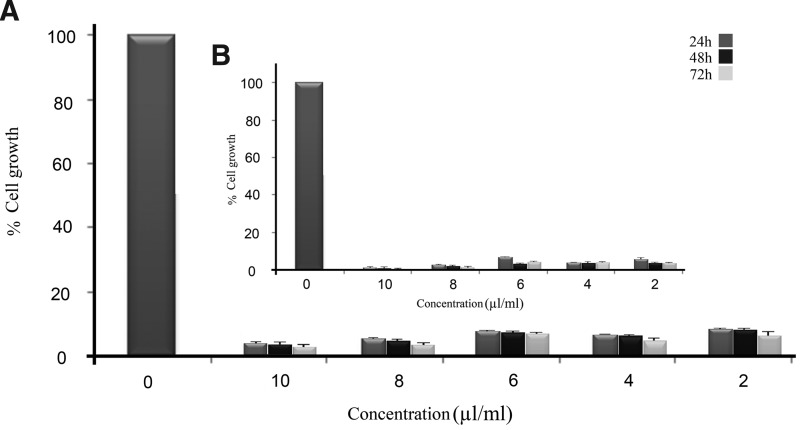
The effect of tween 20 on the growth of HUVECs and A549 cells has been studied by MTT assay. Dose- and time-dependent reduction of cell growth was observed in tween 20-treated HUVECs and A549 cells with an IC50 of approximately 0.3 and 0.4 μL/mL tween 20, respectively. Figure 1 indicates that the growth of treated cells was affected notably. More than 90% cell death was observed after treatment with 2 μL/mL of tween 20, and the highest cell death was observed when the highest concentration of tween 20 was administered.
FIG. 1.
Dose- and time-dependent inhibition properties of polyoxyethylene (20) sorbitan monolaurate (tween 20) on (A) A549 cells and (B) human umbilical vein endothelial cells (HUVECs). The figure shows that tween 20 was able to induce cytotoxicity in both HUVECs and A549 cells in a dose- and time-dependent manner with an IC50 of approximately 0.3 and 0.4 μL/mL tween 20, respectively.
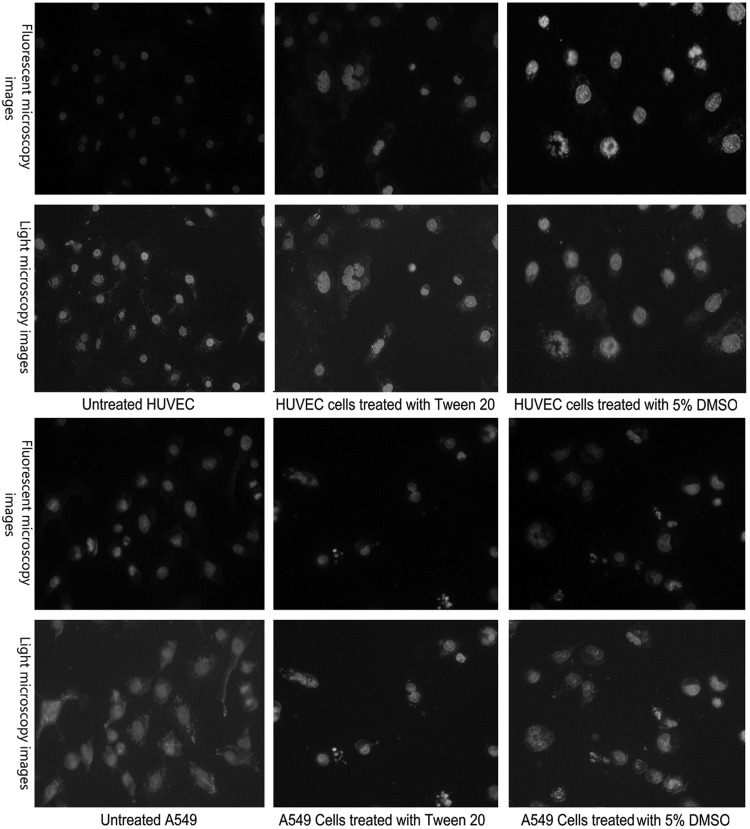
DAPI staining assay
Figure 2 shows DAPI staining in treated and untreated HUVECs and A549 cells. Morphology of DAPI stained cells showed clear morphological changes and fragmentation in the chromatin and DNA rings within the nucleus of treated cells, but their morphology is not altered in untreated normal cells. Tween 20-treated cells showed chromatin and DNA fragmentation as a high positive control (5% DMSO in water v/v).
FIG. 2.
Fluorescent and light microscopy images of chromatin fragmentation occurrence in the nucleus of treated cells with tween 20 and dimethyl sulfoxide (DMSO), which have been stained with DAPI.
DNA fragmentation assay
To further clarify the potential of tween 20 in apoptosis induction, we examined DNA fragmentation assay in HUVECs and A549 cells. DNA fragmentation has long been applied to discriminate apoptosis from necrosis, and it is among the most dependable method for detection of apoptotic cells (Nath et al., 2012). We observed that tween 20 has the capability of apoptosis induction in HUVECs and A549 cells by obvious DNA fragmentation after incubation at 37°C for 24 h. Figure 3 clearly indicates the formation of DNA ladder (trailing) in tween 20-treated cells in comparison with untreated cells.
FIG. 3.
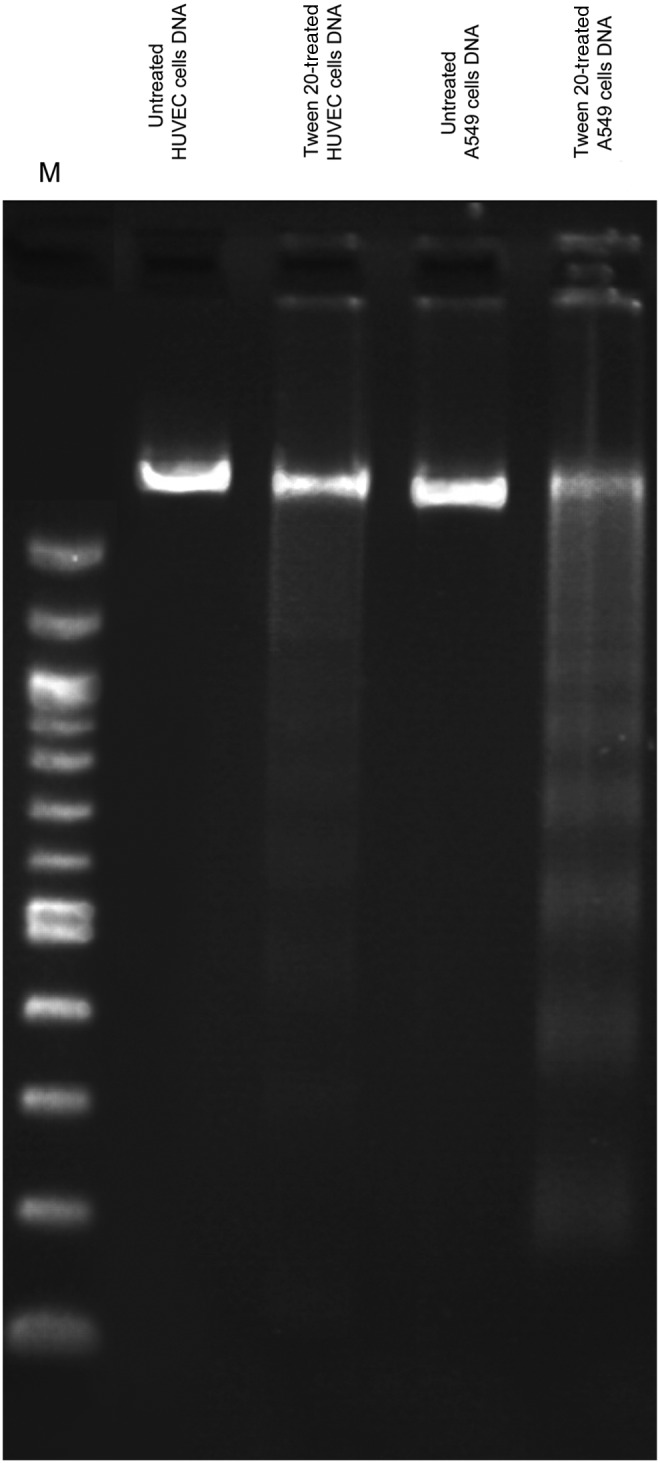
DNA ladder formation in treated HUVECs and A549 cells with 2 μL/mL of tween 20.
DNA cleavages due to the interaction of tween 20 with A549 cell DNA
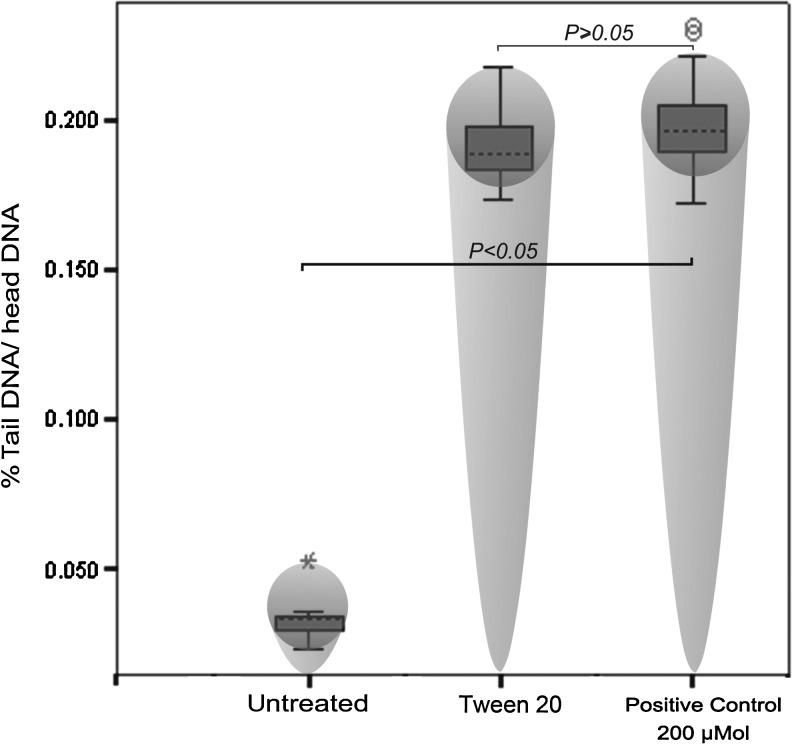
To evaluate the degree of possible DNA cleavage within treated cells, the widely used single cell electrophoresis (comet assay) was exploited. Figure 4 shows the typical results obtained through the comet assay for tween 20-treated cells (2 μL/mL), hydrogen peroxide (H2O2)-treated cells (200 μM) as a positive control, and untreated cells. Finally, the pictures of migrated nucleus were further analyzed with software, and the results of single-cell whole-chromatin gel electrophoresis (% head DNA/tail DNA) were statistically analyzed by one-way ANOVA. These analyses showed considerable DNA cleavages in tween 20-treated cells compared with the untreated cells (p<0.05) (Fig. 5).
FIG. 4.
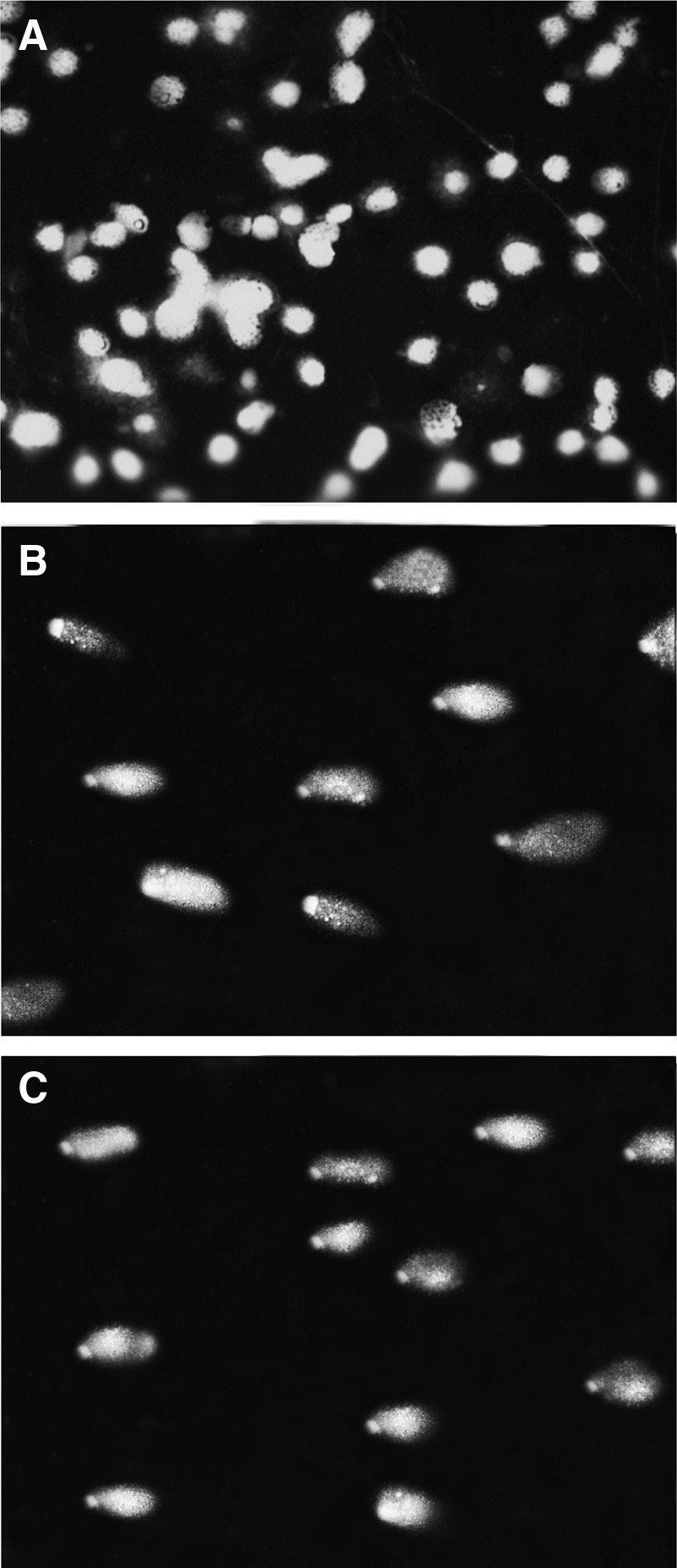
Photographic illustrations of comet assay after 24 h incubation. (A) Untreated A549 cells, (B) tween 20-treated A549 cells (2 μL/mL), and (C) hydrogen peroxide–treated A549 cells as positive control (200 μM).
FIG. 5.
The DNA cleavage level in tween 20-treated cells was approximately as high as 200 μM hydrogen peroxide–treated cells.
Interestingly, 2 μL/mL tween 20 showed genotoxic and DNA cleavage properties that were approximately the same as 200 μM H2O2.
FITC-labeled annexin V apoptosis assay
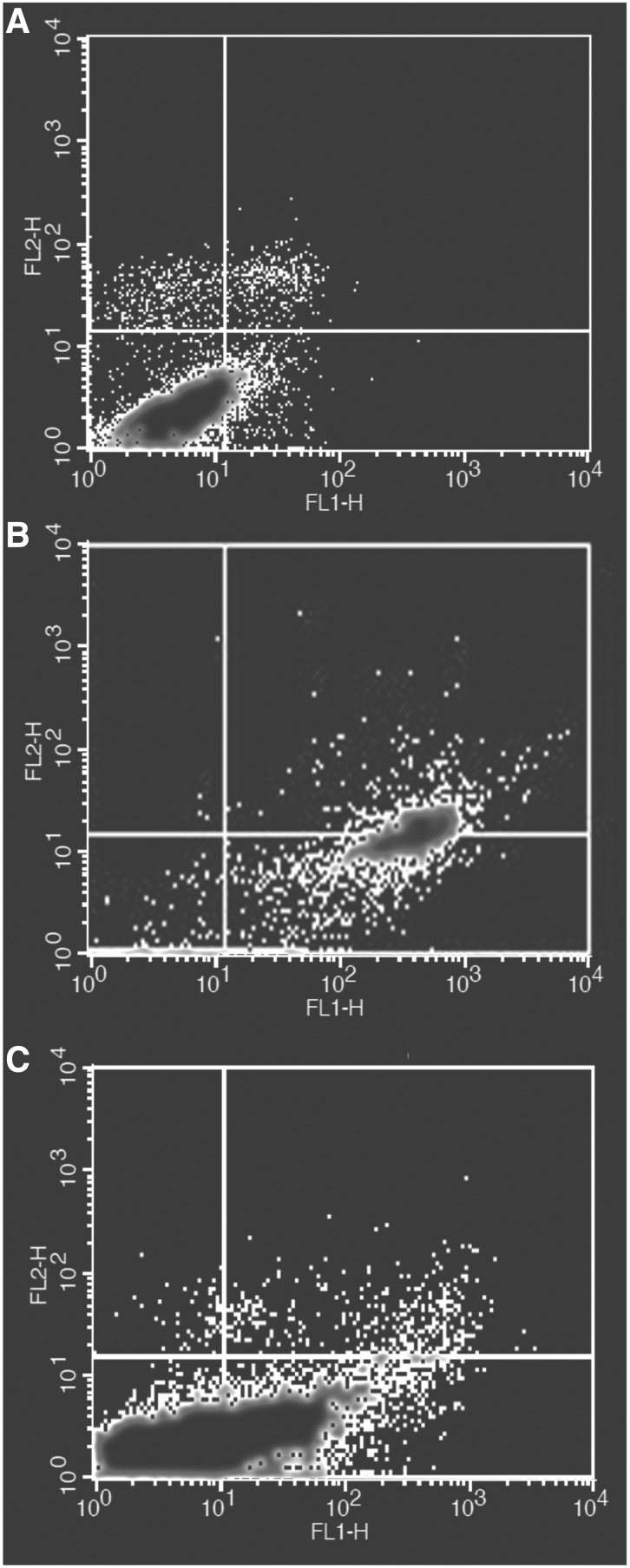
Finally, annexin V assay has been carried out to further elucidate the nature of the chromatin fragmentation and “eat me” signals as well as the alteration of phospholipid phosphatidylserine of plasma membranes, which is the characteristic sign of apoptosis (Kafil and Omidi, 2011; Vandghanooni et al., 2013).
Flow cytometry analysis was used to estimate early and late apoptosis in the treated and untreated cells. The results confirmed the findings obtained from DAPI staining assay. As shown in Figure 6, almost all of the tween 20-treated cells were in early and late stages of apoptosis after 24 h. Meanwhile, less than half of DMSO-treated cells were in early and late stages of apoptosis for the same period of exposure.
FIG. 6.
Fluorescein isothiocyanate (FITC)-labeled annexin V flow cytometric detection of apoptosis in A549 cells. (A) Untreated control cells, (B) tween 20-treated cells (2 μL/mL), and (C) positive control (DMSO-treated cells).
Discussion
Non-ionic surfactants have long been considered the least toxic materials with lower irritant potential. Tween 20, as a member of these types of materials, has been introduced in different parts of human life by various industries (Hoffman and Jump, 1986; Dikici et al., 2013). Some in vivo studies showed low toxicity of tween 20, proposing it as a good candidate for application in drug delivery systems as a potential stabilizing agent (Lu et al., 2011; Weiszhár et al., 2012). The widespread usage of tween 20 in food and pharmaceutical industries motivated us to analyze the probable cyto/genotoxicity of tween 20 in HUVEC and A549 human lung epithelial carcinoma cell line as a model for cognitive understanding of cell response by various quantitative and qualitative techniques. There are no obvious data in the literature indicating tween 20 effects on cell growth inhibition. To the best of our knowledge, this article is the first in vitro study related to the possible geno/cytotoxicity of tween 20.
In the current study, we observed incidence of cell growth inhibition by tween 20 in HUVEC and A549 cell line as indicated by MTT assay results. DAPI staining and DNA fragmentation assays showed that the DNA cleavage and chromatin fragmentation have occurred in a regular 200 bp fragment, and this can only happen when cell apoptosis occurs. In addition, early/late stages of apoptosis and even necrosis within tween 20-treated cells have been shown by FITC-labeled annexin V flow cytometry. In addition, to evaluate the mechanism behind such cyto/genotoxicity, comet assay was used and significant fragmentation of chromatin was observed in the treated cells. It seems that both increase of free toxic radicals and direct interactions of tween 20 with chromatin may be two significant possible reasons for this genotoxicity effect, which results in apoptosis induction (Singh et al., 1988; Eskandani et al., 2010). Therefore, we speculated that the cyto/genotoxic effect of the tween 20 is mediated via the induction of apoptosis (Nath et al., 2012).
In summary, tween 20 has the capability of interaction with DNA in treated cells and results in DNA damage and fragmentation. Therefore, it can be concluded that tween 20 inhibits the growth of both normal and cancer cell lines by inducing apoptosis via chromatin and DNA fragmentation; and considering the results of this study, tween 20 should be used with some precautions as an emulsifier in food and pharmaceutical industries. Therefore, combining our results and those of the other researchers, we believe that it is worthwhile to conduct thorough studies on the widespread usage of tween 20 in food and pharmaceutical industries, for instance, in terms of its carcinogenic potential.
Acknowledgment
This project is financially supported by the Drug Applied Research Center (grant no. 91/118), Tabriz University of Medical Sciences.
Author Disclosure Statement
No competing financial interests exist.
References
- Dikici A. Arslan A. Yalcin H. Ozdemir P. Aydin I. Calicioglu M. Effect of Tween 20 on antibacterial effects of acidic, neutral and alkaline decontaminants on viability of Salmonella on chicken carcasses and survival in waste decontamination fluids. Food Control. 2013;30:365–369. [Google Scholar]
- Eskandani M. Golchai J. Pirooznia N. Hasannia S. Oxidative stress level and tyrosinase activity in vitiligo patients. Indian J Dermatol. 2010;55:15–19. doi: 10.4103/0019-5154.60344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooch J.L. Yee D. Strain-specific differences in formation of apoptotic DNA ladders in MCF-7 breast cancer cells. Cancer Lett. 1999;144:31–37. doi: 10.1016/s0304-3835(99)00208-6. [DOI] [PubMed] [Google Scholar]
- Hoffman W.L. Jump A.A. Tween 20 removes antibodies and other proteins from nitrocellulose. J Immunol Methods. 1986;94:191–196. doi: 10.1016/0022-1759(86)90232-2. [DOI] [PubMed] [Google Scholar]
- Kafil V. Omidi Y. Cytotoxic impacts of linear and branched polyethylenimine nanostructures in A431 cells. BioImpacts. 2011;1:23–30. doi: 10.5681/bi.2011.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W. Ning R. Qin X. Zhang Y. Chang G. Liu S. Luo Y. Sun X. Synthesis of Au nanoparticles decorated graphene oxide nanosheets: noncovalent functionalization by TWEEN 20 in situ reduction of aqueous chloroaurate ions for hydrazine detection and catalytic reduction of 4-nitrophenol. J Hazard Mater. 2011;197:320–326. doi: 10.1016/j.jhazmat.2011.09.092. [DOI] [PubMed] [Google Scholar]
- Nath M. Vats M. Roy P. Tri- and diorganotin(IV) complexes of biologically important orotic acid: synthesis, spectroscopic studies, in vitro anti-cancer, DNA fragmentation, enzyme assays and in vivo anti-inflammatory activities. Eur J Med Chem. 2012;59:310–321. doi: 10.1016/j.ejmech.2012.11.023. [DOI] [PubMed] [Google Scholar]
- Ravindran R. Juliet S. Gopalan A. Kavalimakkil A. Ramankutty S. Nair S. Narayanan P. Ghosh S. Toxicity of DMSO, Triton × 100 and Tween 20 against Rhipicephalus (Boophilus) annulatus. J Parasit Dis. 2011;35:237–239. doi: 10.1007/s12639-011-0054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjadi S. Zerfa M. Brooks B.W. Phase inversion in p-xylene/water emulsions with the non-ionic surfactant pair sorbitan monolaurate/polyoxyethylene sorbitan monolaurate (Span 20/Tween 20) Colloid Surface A. 2003;218:241–254. [Google Scholar]
- Singh N.P. McCoy M.T. Tice R.R. Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Vandghanooni S. Eskandani M. Comet assay: a method to evaluate genotoxicity of nano-drug delivery system. BioImpacts. 2011;1:87–97. doi: 10.5681/bi.2011.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandghanooni S. Forouharmehr A. Eskandani M. Barzegari A. Kafil V. Kashanian S. Ezzati Nazhad Dolatabadi J. Cytotoxicity and DNA fragmentation properties of butylated hydroxyanisole. DNA Cell Biol. 2013;32:98–103. doi: 10.1089/dna.2012.1946. [DOI] [PubMed] [Google Scholar]
- Weiszhár Z. Czúcz J. Révész C. Rosivall L. Szebeni J. Rozsnyay Z. Complement activation by polyethoxylated pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20. Eur J Pharm Sci. 2012;45:492–498. doi: 10.1016/j.ejps.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Yang S. Liu J. Chen Y. Jiang J. Reversal effect of Tween-20 on multidrug resistance in tumor cells in vitro. Biomed Pharmacother. 2012;66:187–194. doi: 10.1016/j.biopha.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Zampieri S. Ghirardello A. Doria A. Tonello M. Bendo R. Rossini K. Gambari P.F. The use of Tween 20 in immunoblotting assays for the detection of autoantibodies in connective tissue diseases. J Immunol Methods. 2000;239:1–11. doi: 10.1016/s0022-1759(00)00168-x. [DOI] [PubMed] [Google Scholar]
- Zhang H. Xu G. Liu T. Xu L. Zhou Y. Foam and interfacial properties of Tween 20–bovine serum albumin systems. Colloid Surface A. 2013;416:23–31. [Google Scholar]