Abstract
nfxC-type cells of Pseudomonas aeruginosa that produce the MexEF-OprN efflux pump exhibit resistance to fluoroquinolones and chloramphenicol and hypersusceptibility to most classical β-lactam antibiotics. We investigated the molecular mechanism of how the nfxC mutation causes β-lactam hypersusceptibility. The MexAB-OprM extrusion pump transports and confers resistance to β-lactam antibiotics. Interestingly, expression of the mexAB-oprM operon reached the highest level during the mid-stationary growth phase in both wild-type and nfxC-type mutant strains, suggesting that expression of the mexAB-oprM operon may be controlled by cell density-dependent regulation such as quorum sensing. This assumption was verified by demonstrating that exogenous addition of the quorum-sensing autoinducer N-butyryl-l-homoserine lactone (C4-HSL) enhanced the expression of MexAB-OprM, whereas N-(3-oxododecanoyl)-l-homoserine lactone had only a slight effect. Furthermore, this C4-HSL-mediated enhancement of mexAB-oprM expression was repressed by MexT, a positive regulator of the mexEF-oprN operon. It was concluded that β-lactam hypersusceptibility in nfxC-type mutant cells is caused by MexT-mediated cancellation of C4-HSL-mediated enhancement of MexAB-OprM expression.
Pseudomonas aeruginosa is an opportunistic pathogen that causes infections in immunocompromised hosts and colonizes the lungs of individuals with cystic fibrosis. This organism shows broad resistance to structurally and functionally dissimilar antibiotics. This type of multidrug resistance is attributable mainly to the expression of the xenobiotic extrusion transporter MexAB-OprM coupled with tight outer membrane permeability (21, 23, 29). The mexAB-oprM operon encodes three protein subunits (21, 26, 29): the intrinsic inner membrane protein MexB (9), the inner membrane-associated periplasmic lipoprotein MexA (45), and the outer membrane lipoprotein OprM (13, 22, 44). The mexAB-oprM operon is negatively regulated by the product of the mexR gene, which is located upstream of the mexAB-oprM genes and is divergently transcribed (1, 5, 14, 34, 37). nalB-type mutants caused by the mexR mutation derepress MexAB-OprM production and are highly resistant to fluoroquinolones, chloramphenicol, and most classical β-lactam antibiotics (30, 32, 35, 42).
Recently, it was reported that the MexAB-OprM transporter exports quorum-sensing mediators, acylhomoserine lactones (AHSLs), which induce the production of cell density-dependent virulence factors, including proteases, rhamnolipids, exotoxin A, exoenzyme S, and pyocyanin (24, 25). The AHSLs control at least two quorum-sensing systems in P. aeruginosa, namely, LasR-LasI and RhlR-RhlI. LasI and RhlI catalyze the last steps in the syntheses of N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL) and N-butyryl-l-homoserine lactone (C4-HSL), respectively. LasR and RhlR are specifically activated by the diffusible signaling molecules 3-oxo-C12-HSL and C4-HSL, respectively.
Four resistant-nodulation-division efflux pumps (MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY) have been identified in P. aeruginosa, and they are derepressed in nalB, nfxB, nfxC, and N135 mutant cells, respectively (10, 18-21, 27, 28, 32). Transcription of the mexAB-oprM, mexCD-oprJ, and mexXY operons is derepressed by a mutation of the repressors mexR, nfxB, and an unidentified gene, respectively (2, 3, 28, 40). On the other hand, transcription of the mexEF-oprN operon is dually regulated by a positive regulator, MexT, and a putative negative regulator, MexS (10, 17). Both nfxB and nfxC mutants exhibit resistance to structurally diverse antibiotics and hypersusceptibility to most classical β-lactam antibiotics (12), suggesting the presence of a common mechanism in which pump protein expression is coupled with β-lactam hypersusceptibility.
This paper reports the mechanism of nfxC mutation-mediated β-lactam hypersusceptibility and its connection to quorum sensing and growth phase-dependent expression of MexAB-OprM.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used are listed in Table 1. Escherichia coli DH5α was used as the host in DNA manipulations.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Relevant properties | Source or reference |
|---|---|---|
| Strains | ||
| P. aeruginosa | ||
| PAO4290 | leu-10 argF10 aph-9004; FP− | 18 |
| TNP090 | PAO4290 carrying a chromosomal mexB::xylE fusion; Kmr | This study |
| TNP091 | TNP090 ΔlasI | This study |
| TNP092 | TNP090 ΔrhlI | This study |
| TNP093 | TNP090 ΔlasI ΔrhlI | This study |
| TNP099 | TNP090 ΔmexEF-oprN | This study |
| PAO1S | lacIqlacZΔM15; Tcr | 33 |
| PAO1SC | nfxC-type mutant of PAO1S designated PAO1S-Lac#1 | 16 |
| OCR-Lac4 | nalB-type mutant of PAO1S | 34 |
| E. coli | ||
| DH5α | E. coli strain for transformation | TaKaRa |
| S17-1 | Mobilizer strain | 41 |
| Plasmids | ||
| pMMB67EH | Broad-host-range vector; Ampr IncQ | 8 |
| pMEXEF-OPRN1 | pMMB67EH derivative carrying the mexE, mexF, and oprN genes | 18 |
| pMEXT8380 | pMMB67EH derivative carrying the mexT gene from P. aeruginosa 8380 | 17 |
| X1918 | pUC1918 derivative carrying an xylE cassette; Ampr | 39 |
| pMOB3 | pHSS21 derivative carrying a MOB cassette; Cmr Kmr | 38 |
| pME4510 | Broad-host-range promoter-probe vector carrying a lacZ cassette; Gmr | 33 |
| pME4510-mexOP | pME4510 derivative carrying the mexAB-oprM operator-promoter region | 34 |
| pG19II | pK19mobsac derivative carrying the Gmr gene as a marker | This study |
Recombinant DNA techniques.
We manipulated recombinant DNA by standard procedures described previously (36). PCR amplification of chromosomal DNA was carried out by using the LA Taq kit (TaKaRa Shuzo, Osaka, Japan) according to the manufacturer's instructions.
Construction of conjugative plasmid pG19II.
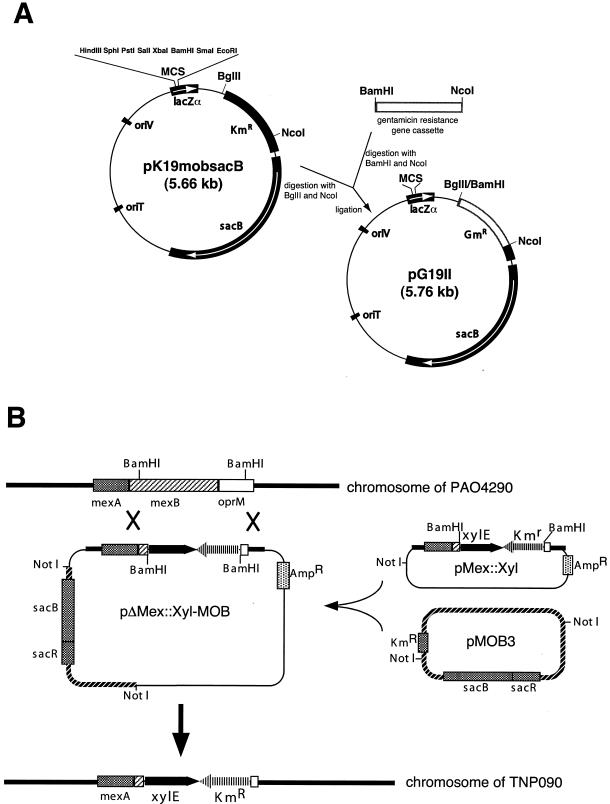
To construct a conjugative plasmid with a gentamicin resistance gene as a marker, pK19mobsac was treated with BglII and NcoI and then ligated with Gmr gene cassette, which was treated by BamHI and NcoI after amplification with Fgm (5′-CGGGATCCCGAATTGACATAAGCCTGTTCG-3′) and Rgm (5′-ACCCATGGACGAATTGTTAGGTGGCGGTACTT-3′), a primer pair containing a newly added cutting site (underlined) for restriction nucleases from pME4510. The resulting plasmid was named pG19II (Fig. 1A).
FIG. 1.
Schematic representation of the procedure for deletion of the chromosome. (A) Construction of a conjugative plasmid with a gentamicin resistance gene. The open box represents the gentamicin resistance gene region amplified by PCR. MCS, multiple cloning site. (B) Procedure used to insert the xylE reporter cassette into the chromosomal mexAB-oprM operon. NotI-treated pMex::Xyl was ligated to the NotI site of MOB3, and the resulting pΔMex::Xyl-MOB was inserted into the chromosomal mexAB-oprM operon by homologous recombination. The transconjugants were Kmr and sucrose sensitive. The unwanted DNA fragment was excised by selecting for sucrose resistance.
Insertion of an xylE gene cassette into the chromosomal mexAB-oprM operon.
The mexAB-oprM reporter with an xylE fusion was constructed as follows (Fig. 1B). The xylE gene from pX1918 was inserted in the EcoRI site of pBluescript SK(+). The resulting plasmid was treated with PstI and ligated with a PstI-treated kanamycin resistance gene (Pharmacia) to yield the pXyl-Km/SK(+) plasmid. The 2.8-kbp xyl-Km cassette from pXyl-Km/SK(+) was inserted into BamHI-treated pMex(sac/Hind)/ΔRI-pNOT19 plasmid, which carries the mexAB-oprM operon. The resulting plasmid, pMex::Xyl, was treated with NotI and ligated with a NotI-treated Mob cassette from pMOB3 to yield the suicide plasmid pMex::Xyl-MOB. This plasmid was introduced into the mobilizer strain E. coli S17-1 and then was transferred to P. aeruginosa PAO4290 by conjugation as reported earlier (18). The insertion of the xyl-Km cassette in the chromosomal mexAB-oprM operon was confirmed by Southern hybridization (36) and PCR analyses. This constructed strain was designated P. aeruginosa TNP090.
Deletion of the chromosomal lasI, rhlI, or mexEF-oprN genes.
To construct a series of isogenic mutants lacking the lasI, rhlI, or lasI and rhlI genes, PCR primers for amplification of regions containing the lasI or rhlI gene were synthesized based on nucleotide sequences from the Pseudomonas genome sequencing project database. After amplification of ca. 2.7-kb DNA fragments on PAO1 chromosomal DNA as a template with Flas1 (5′-CGGGATCCGCTGGAACGCTCAAGTGGAAAATTGGAG-3′) and Rlas2 (5′-CCCAAGCTTTCTGCGAAGGCCTGGAGAACCTTGC-3′) or with Frhl1 (5′-CGAGCTCGCATAACAGATAGGGTTGCCATG-3′) and Rrhl2(5′-GGGAAGCTTGGACCAGGCACCAGGATGG-3′), the amplified DNA fragments were ligated into the BamHI-HindIII site or SacI-HindIII site in a multicloning site of pHSG398 to yield pHSG-las12 or rhl12, respectively. Next, 600 bp of lasI or 67 bp of rhlI in these DNA fragments was deleted by inverted PCR with the primer pair Flas3 (5′-GGAAGATCTCTTCACTTCCTCCAAATAGGAAGCTGAAG-3′) and Rlas4 (5′-GGAAGATCTCGGGGACCTGTCGGCTCGC-3′) or Frhl3 (5′-GGTCTAGACTTCATCGCCAGCTGCGGATCGTCC-3′) and Rrhl4 (5′-GCTCTAGACGGCCATGGAGCGCTATTTCGTTCGC-3′), respectively, and both amplified fragments were self-ligated in the BglII or XbaI site to yield pHSG-dellas or -delrhl, respectively. After reinsertion of the insert fragments of pHSG-dellas into pG19II, the resulting plasmid was named pG19-dellas. After amplification of the 2-kb DNA fragment by using the primer pair delrhl1 (5′-GGGAAGCTTATCCGGCGATCCTCAACGGCCTGC-3′) and delrhl2 (5′-GGGAAGCTTGCCTTGCCGTCGACGATCTGCTGG-3′) from pHSG-delrhl, the amplified DNA fragment was inserted into pG19II to yield pG19-delrhl. For disruption of the chromosomal mexEF-oprN genes, pUC19-mexEFN (18) was treated with SphI and then self-ligated, yielding pUC19-delEFN. An EcoRI-HindIII DNA fragment from pUC19-delEFN was subcloned into pG19II, yielding pG19II-delEFN. pG19-dellas, pG19-delrhl, and pG19-delEFN were mobilized from E. coli S17-1 to P. aeruginosa TNP090 to introduce a deletion of the lasI region, the rhlI region, or the mexEF-oprN region into the recipient chromosomes by allelic exchange as above.
Thus, we constructed mutants lacking the lasI gene, the rhlI gene, the lasI and rhlI genes, and the mexEF-oprN genes from TNP090, and we designated them TNP091, TNP092, TNP093, and TNP099, respectively. The deletions of the lasI, rhlI, and mexEF-oprN genes were confirmed by PCR analyses and phenotypes of the mutants (data not shown).
Experiment to test the effect of C4-HSL on expression of MexAB-OprM.
Cells were grown aerobically in Luria-Bertani (LB) medium and diluted with 4 ml of fresh LB medium to an A600 of ca. 0.025. After incubation for 3 h at 37°C, an appropriate concentration of C4-HSL was added and the cultures were incubated for 3 h at 37°C. At an A600 of 1.3 to 1.8, samples were collected and catechol 2,3-dioxygenase activity was determined as reported previously (39).
Other techniques.
Western blot analysis has been described previously (18). The MICs of antibiotics were determined by the agar dilution method with Mueller-Hinton agar II (Becton Dickinson Microbiology Systems, Cockeysville, Md.). Protein was quantified by the method of Lowry et al. (15). β-Galactosidase activity was assayed by the method of Miller as described by Sambrook et al. (36). For assay of the activity of catechol 2,3-dioxygenase (the xylE gene product), cells were suspended in 990 μl of assay buffer. The reaction was initiated by adding 10 μl of 100 mM catechol dissolved in water, and the A375 was recorded at 25°C. Specific activity was defined as nanomoles of product formed per minute per milligram of protein (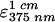 = 4.4 × 104) (39).
= 4.4 × 104) (39).
RESULTS
Linkage between the nfxC mutation and MexAB-OprM expression.
To characterize the antibiotic susceptibility of the nfxC-type cells used in this experiment, we determined MICs of antibiotics. The nfxC-type mutant PAO1SC, which produces the MexEF-OprN efflux pump, exhibited resistance to fluoroquinolones, tetracycline, chloramphenicol, and imipenem and hypersusceptibility to β-lactam antibiotics, confirming previous results (7, 12) (Table 2). In contrast, the nalB mutant OCR-Lac4, which produces a derepressed level of the MexAB-OprM efflux pump, showed resistance to most β-lactam agents. Because the nfxC-type PAO1SC cells exhibited hypersusceptibility to the substrates of the MexAB-OprM efflux pump, a possible linkage between the nfxC mutation and the level of MexAB-OprM expression was suggested. In fact, we and other investigators observed reduced expression of OprM and MexA in the nfxC-type cells compared with the wild-type cells (12, 21).
TABLE 2.
Antibiotic susceptibilities of the strains used to test the effect of mexT
| Strain | Plasmid | Pump component(s) expresseda | MIC (μg/ml)b
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NFLX | CP | CAZ | CPZ | CPR | CZOP | KM | GM | IPM | |||
| PAO1S | ABM | 0.78 | 100 | 1.56 | 6.25 | 3.13 | 0.78 | 100 | 3.13 | 0.78 | |
| OCR-Lac4 | ABM++c | 25 | 800 | 6.25 | 25 | 6.25 | 3.13 | 100 | 3.13 | 0.78 | |
| PAO1SC | ABM, EFN | 25 | ≥1,600 | 0.78 | 3.13 | 0.78 | 0.39 | 50 | 1.56 | 3.13 | |
| PAO1S | pMMB67EH | ABM | 0.78 | 100 | 1.56 | 12.5 | 3.13 | 1.56 | 100 | 3.13 | 0.78 |
| pMEXEF-OPRN1 | ABM, EFN | 25 | ≥1,600 | 1.56 | 12.5 | 1.56 | 0.78 | 50-100 | 3.13 | 0.78 | |
| pMEXT8380 | ABM, EFN | 25 | ≥1,600 | 1.56 | 6.25 | 1.56 | 0.78 | 50 | 1.56 | 3.13 | |
| PAO4290 | pMMB67EH | ABM | 0.78 | 100 | 3.13 | 50 | 6.25 | 1.56 | 6.25 | 3.13 | 1.56 |
| pMEXEF-OPRN1 | ABM, EFN | 25 | ≥1,600 | 1.56 | 25 | 3.13 | 1.56-0.78 | 3.13 | 3.13 | 1.56 | |
| pMEXT8380 | ABM, EFN | 12.5 | ≥1,600 | 1.56 | 12.5 | 3.13 | 0.78 | 3.13 | 1.56 | 6.25 | |
Pump components are shown as combinations of the following subunits: A, MexA; B, MexB; E, MexE; F, MexF; M, OprM; N, OprN.
Abbreviations: NFLX, norfloxacin; CP, chloramphenicol; CAZ, ceftazidime; CPZ, cefoperazone; CPR, cefpirome; CZOP, cefozopram; KM, kanamycin; GM, gentamicin; IPM, imipenem.
++, overexpression.
Linkage between growth phase-dependent quorum sensing and mexAB-oprM expression.
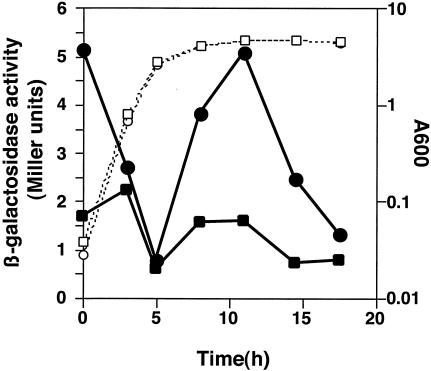
To assess the connection between the overall growth phase of P. aeruginosa cells and the expression of the mexAB-oprM operon, a reporter plasmid, pME4510-mexOP, carrying the mexAB-oprM operator-promoter region, was introduced into the wild-type strain PAO1S. With this strain, the expression of the mexAB-oprM operon was monitored by measuring β-galactosidase activity at several different growth points. The fully grown cells were diluted 200-fold with prewarmed fresh medium, and the β-galactosidase activity dropped dramatically at 3 to 5 h after the dilution. This result was interpreted to mean that fully expressed MexAB-OprM in the high-cell-density preculture was repressed to a low level as the culture was diluted to a low cell density. When the cell growth reached stationary phase and incubation was continued for 3 to 5 h, β-galactosidase activity again reached a high level, and thereafter it gradually declined (Fig. 2). This was interpreted to mean that the cells sensed a high population density in the stationary phase and induced MexAB-OprM expression.
FIG. 2.
Expression of the mexA::lacZ transcriptional fusion at various times during growth of the wild-type strain. Cells were grown at 37°C in LB medium with rotation at 200 rpm and harvested at the time points indicated, and the β-galactosidase activity was determined. Open and closed symbols represent turbidity at A600 and β-galactosidase activity, respectively. Circles, PAO1S(pME4510-mexOP); squares, PAO1S(pME4510). Three independent experiments were done, and representative data are shown.
To verify that the regulation of the chromosomal mexAB-oprM operon is similar to that of the plasmid-borne operon, we constructed a chromosomal fusion of the xylE gene downstream of mexA, generating strain TNP090. With this strain, the transcription of the chromosomal mexAB-oprM operon was monitored. The results showed that the catechol 2,3-dioxygenase activity at several growth points was close to the β-galactosidase activity profile (data not shown). This is consistent with a recent report by Sanchez et al. which demonstrated that mexA expression was triggered at the onset of the stationary growth phase (37).
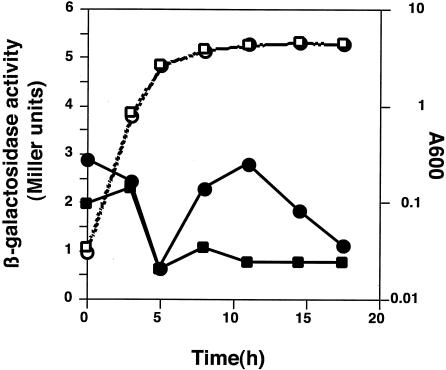
MexT-mediated regulation of mexAB-oprM expression.
To ascertain whether the decreased expression of the MexAB-OprM efflux pump in the nfxC-type mutant was under the control of the MexEF-OprN regulator MexT, pME4510-mexOP, encoding mexA::lacZ, was introduced into PAO1SC, which carries a functional mexT gene. As the cells entered the stationary growth phase, expression of the mexAB-oprM operon increased, and it reached the highest level at mid-stationary phase; this profile was comparable to that of the parent strain, PAO1S (Fig. 3). However, the level of gene expression in PAO1SC at most time points was half as high as that in PAO1S carrying an impaired chromosomal mexT gene (compare Fig. 2 and 3). These data are fully consistent with the antibiotic susceptibility profiles of these strains (Table 2).
FIG. 3.
Expression of the mexA::lacZ transcriptional fusion at various times during growth of the nfxC-type mutant. Experimental procedures and symbols are the same as described in the legend to Fig. 2. Three independent experiments were done, and representative data are shown.
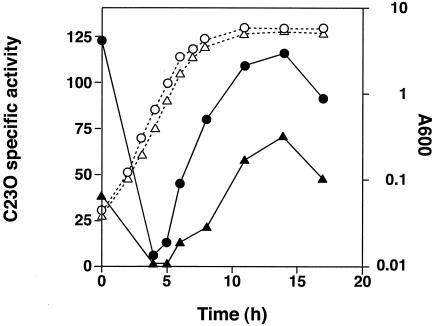
To ascertain whether the decreased transcription of the mexAB-oprM operon in nfxC cells is attributable to the presence of a functional mexT gene, we introduced pMEXT8380, which carries a functional mexT gene, in trans, into the parent cells with the mexB::xylE fusion (TNP090) and investigated whether the transcriptional expression of the mexAB-oprM operon was modulated. As shown in Fig. 4, catechol 2,3-dioxygenase activity in TNP090(pMEXT8380) was about half of that of TNP090 carrying the vector only. The result is fully consistent with the above-mentioned data and firmly establishes that expression of MexAB-OprM in the nfxC-type cells is down regulated in the presence of functional MexT. In fact, both PAO4290 and PAO1S with an intact mexT gene (harboring pMEXT8380) exhibit an elevated level of β-lactam susceptibility compared with cells harboring the vector only (Table 2).
FIG. 4.
Expression of the mexB::xylE chromosomal fusion at various times during growth of TNP090 cells. Open and closed symbols represent A600 and catechol 2,3-dioxygenase (C23O) activity, respectively. Circles and triangles represent TNP090(pMMB67EH) and TNP090(pMEXT8380), respectively. Three independent experiments were done, and representative data are shown.
Growth phase-dependent and C4-HSL-mediated regulation of mexAB-oprM expression.
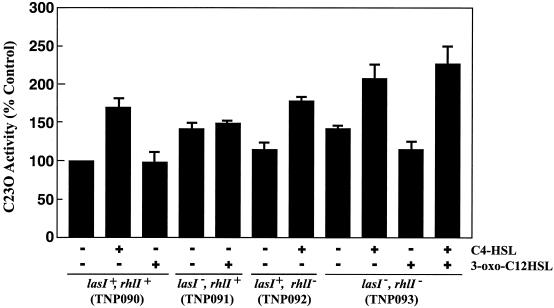
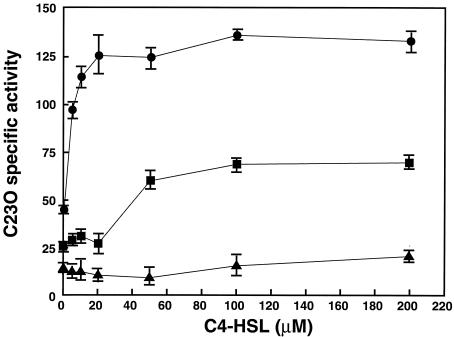
P. aeruginosa produces two major AHSLs, 3-oxo-C12-HSL and C4-HSL (6, 24), one of which, 3-oxo-C12-HSL, was reported to be unrelated to growth phase-dependent regulation of the mexAB-oprM operon (6). An earlier paper reported that production of C4-HSL was nearly undetectable in culture supernatants at cell densities below an A600 of ca. 2 but was quantitatively detectable in cultures with an A600 of over 2 (11). This growth phase-dependent accumulation of C4-HSL appears to be analogous to the mexAB-oprM transcription profile (Fig. 2 and 3). This observation suggests that the growth phase-dependent transcription of mexAB-oprM might be linked to the accumulation of AHSLs in the stationary phase. We therefore tested the effects of AHSLs on the expression of mexAB-oprM by adding 3-oxo-C12-HSL and/or C4-HSL (final concentration, 50 μM) during the logarithmic growth phase to cultures of TNP090 and its derivatives lacking the lasI and/or rhlI genes. Addition of C4-HSL enhanced transcription of mexAB-oprM in TNP090 and derivatives of TNP090. In contrast, addition of 3-oxo-C12-HSL only slightly affected transcription of mexAB-oprM on these strains compared to C4-HSL (Fig. 5), suggesting that especially the enhancement of mexAB-oprM expression in the stationary growth phase can be controlled by the concentration of C4-HSL. Hence, we additionally tested the effects of C4-HSL on the expression of mexAB-oprM by adding various concentrations of C4-HSL to TNP090 harboring vector only, pMEXEF-OPRN1 (carrying the mexEF-oprN genes), or pMEXT8380 (carrying an unimpaired mexT gene). For strain TNP090 with the mexB::xylE reporter, catechol 2,3-dioxygenase activity sharply increased as the extracellular concentration of C4-HSL was raised to approximately 20 μM and thereafter showed a steady expression (Fig. 6). This result clearly indicates that transcription of the mexAB-oprM operon is regulated by C4-HSL and suggests that the increased transcription of mexAB-oprM in stationary phase is most likely dependent on the concentration of C4-HSL. Furthermore, we found that the transcription levels of mexAB-oprM in stationary growth phase in an rhlI mutant (TNP092) were almost lower than those in TNP090 (data not shown). In contrast, transcription of the mexAB-oprM operon in TNP090 harboring pMEXT8380 (carrying an unimpaired mexT gene) was barely affected by C4-HSL (Fig. 6), suggesting that MexT canceled the C4-HSL-mediated enhancement of mexAB-oprM expression. However, the growth phase-dependent expression of mexAB-oprM was observed even in the strains carrying the mexT gene (Fig. 2 and 3). Therefore, it seemed that the expression of mexAB-oprM is regulated by another, unknown mechanism(s) as well as C4-HSL.
FIG. 5.
Effect of exogenous C4-HSL and 3-oxo-C12-HSL on expression of the mexAB-oprM operon. Catechol 2, 3-dioxygenase (C23O) activities are expressed as values relative to the activity (ca. 50) of C23O in P. aeruginosa TNP090 without additions. Cultures were grown for 3 h in the absence of autoinducers; after addition of 50 μM (final concentration) autoinducer(s) (+, addition; −, no addition), cultures were incubated for 3 h. The final optical densities at 600 nm were 1.3 to 1.8. The C23O activities were then determined. Data are means ± standard deviations from three independent experiments.
FIG. 6.
Effect of C4-HSL concentration on expression of the mexB::xylE reporter in TNP090 cells producing MexEF-OprN under different conditions. Experimental procedures are described in Materials and Methods. Circles, TNP090(pMMB67EH); squares, TNP090(pMEXEF-OPRN1); triangles, TNP090(pMEXT8380). C23O, catechol 2,3-dioxygenase. Data are means ± standard deviations from three independent experiments.
One may ask whether MexEF-OprN is involved in extrusion of C4-HSL. This activity would result in a decrease in the cellular concentration of C4-HSL. When the transcription of mexAB-oprM in TNP090 cells harboring pMEXEF-OPRN1 or with the vector only was compared, we observed a lower level of transcription of mexAB-oprM in cells harboring pMEXEF-OPRN1 than in cells with the vector only (Fig. 6). These data suggest but do not prove that the MexEF-OprN efflux pump might participate in export of C4-HSL and consequently decrease the transcription of mexAB-oprM. Needless to say, the activity and expression level of MexEF-OprN were similar in cells carrying pMEXEF-OPRN1 or pMEXT8380 (Table 2 and Fig. 7).
FIG. 7.
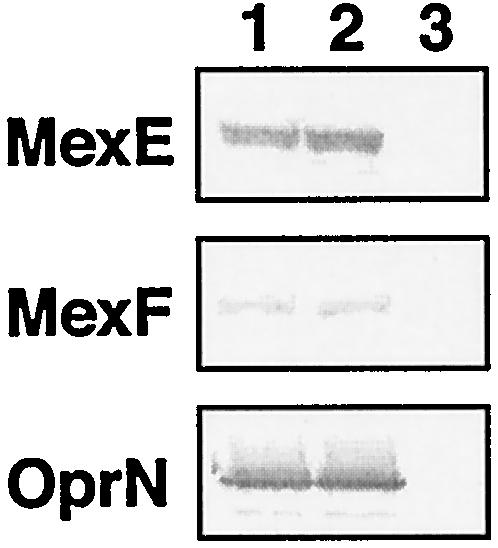
Western blot analysis of the production of MexE, MexF, and OprN in TNP090 cells producing MexEF-OprN under different conditions. Total cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the protein bands were visualized by immunoblotting with antibodies against MexE, MexF, or OprN. The amounts of protein applied per lane were 10, 40, and 20 μg for MexE, MexF, and OprN, respectively. Lanes: 1, TNP090(pMEXEF-OPRN1); 2, TNP090(pMEXT8380); 3, TNP090(pMMB67EH).
DISCUSSION
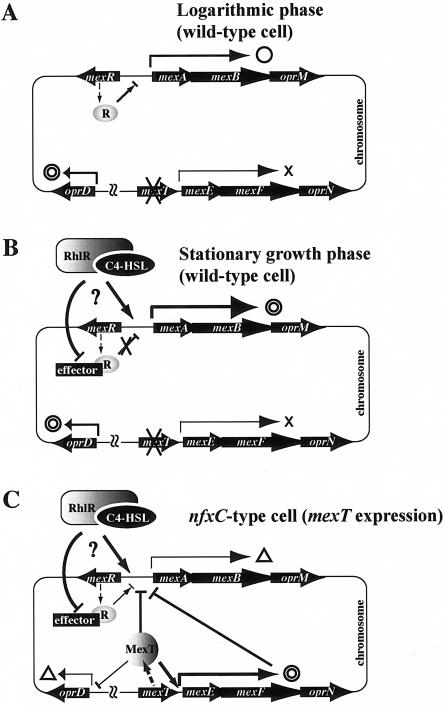
We demonstrated in this investigation that the mexAB-oprM operon was actively transcribed in the stationary growth phase and that this observation could be linked with the report that the quorum-sensing system was switched on in the stationary phase (4, 31, 43). In fact, transcription of the mexAB-oprM operon in wild-type cells was increased by the extracellular addition of C4-HSL (Fig. 5 and 6). These results suggest that the enhanced transcription of mexAB-oprM in the stationary phase depends on quorum-sensing autoinducer accumulation in the medium. How would one summarize the complicated regulation of the mexAB-oprM expression? We reported recently that the MexR protein negatively regulates transcription of mexAB-oprM operon (34), and we interpret the available data as follows. In the logarithmic growth phase, the MexR repressor negatively regulates mexAB-oprM expression by binding at the MexR-MexAB-OprM operator-promoter region, as reported recently (Fig. 8A) (5, 34). As the cells enter the stationary growth phase, they sense a high population density and turn on a quorum-sensing switch producing an autoinducer, C4-HSL (Fig. 8B). We considered two mechanisms: (i) C4-HSL induces the expression of mexAB-oprM operon directly and not through participation of the mexR gene, or (ii) C4-HSL inactivates the MexR repressor or represses the expression of the mexR gene by an unknown mechanism and consequently enhances the transcription of mexAB-oprM operon. We explain in the final paragraph of Discussion that probably C4-HSL directly induces the expression of mexAB-oprM.
FIG. 8.
Schematic representation of mexAB-oprM operon regulation by C4-HSL, MexR (R), and MexT. Symbols: double circles, high-level expression; open circles, low-level expression; open triangles, very low-level expression; ×, no expression or no function; ⊢, repression. Thick and thin arrows represent high and low expression or effect, respectively. Arrows with question marks indicate deduced regulatory pathways.
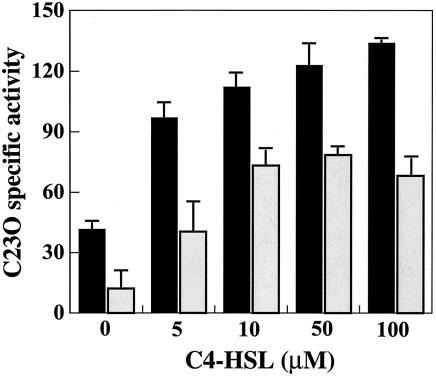
Wild-type cells of P. aeruginosa have the mexEF-oprN operon, but it is not transcribed under normal growth conditions due to the mutation in the positive regulator gene mexT (17). The nfxC-type mutant produces active MexT that promotes transcription of the mexEF-oprN operon, simultaneously lowering the growth phase-dependent enhancement of mexAB-oprM transcription (Fig. 3 and 4) and rendering the cells hypersusceptible to β-lactam antibiotics (Table 2). Both the growth phase-dependent and C4-HSL-mediated increases in the production of MexAB-OprM were totally repressed by overdose of the plasmid-borne MexT (Fig. 6). It is likely, therefore, that β-lactam hypersusceptibility in the nfxC-type mutant is caused by the activation of MexT, consequently decreasing the C4-HSL effect. Thus, all the data presented in this report suggest that MexT may regulate the expression of the mexAB-oprM operon in mediating the quorum-sensing system. We carried out a further experiment to determine whether it is MexT itself or MexEF-OprN which affects the expression of mexAB-oprM. We constructed a mexEF-oprN disruption mutant of TNP090, named TNP099, and then observed the induction level of mexAB-oprM in TNP099 with or without MexT (pMEXT8380) after adding exogenous C4-HSL (Fig. 9). The expression of MexAB-OprM in the mexEF-oprN disruption mutant (TNP099) was further decreased in the presence of MexT (pMEXT8380) compared to the level in the absence of MexT, indicating that MexT also possesses the regulatory function required for the decrease of the expression of mexAB-oprM not through expression of MexEF-OprN. Moreover, MexT is involved in expression of various genes, including mexEF-oprN, oprD, lasB (encoding elastase), rhlAB, and swarming and pyocyanin genes (10, 11). Hence, the MexT protein may function as a global regulator. On the other hand, we observed a lower level of transcription of mexAB-oprM in TNP090 cells harboring pMEXEF-OPRN1 than that of vector only (Fig. 6). Therefore, it is concluded that the MexT-mediated down regulation of the transcription of the mexAB-oprM operon is caused by the regulatory function of MexT itself and the MexEF-OprN efflux pump expressed by MexT (Fig. 8C).
FIG. 9.
Effect of C4-HSL concentration on expression of the mexB::xylE reporter in TNP099 cells with a defect in the mexEF-oprN operon under different conditions. Experimental procedures are described in Materials and Methods. Black bars, TNP099(pMMB67EH); gray bars, TNP099(pMEXT8380). Data are means ± standard deviations from three independent experiments.
MexT may bind either the MexR binding site or a putative second repressor binding site (34) at the mexR-mexAB-oprM operator-promoter region and repress the transcription of the mexAB-oprM operon in a MexR-independent manner (Fig. 8C). If this was the case, the nfxC-type cells would lower the transcription of mexAB-oprM even in the absence of MexR. In fact, the mexR nfxC double mutant showed a 50% lower MIC of aztreonam, which is one of the β-lactam agents and is extruded effectively by the MexAB-OprM efflux pump, than the cells with a mexR single mutation. Needless to say, the former cells showed a MIC of nolfloxacin that is eight times higher than that for the latter cells (H. Maseda, unpublished results). To substantiate such a possibility, the transcriptional expression of mexR and mexT at different growth phases needs to be tested. Such experiments are in progress in our laboratory.
Acknowledgments
This study was partially supported by a grant to N.N. from Industrial Technology Research Grant Program '01 of the New Energy and Industrial Technology Development Organization (NEDO) of Japan.
REFERENCES
- 1.Adewoye, L., A. Sutherland, R. Srikumar, and K. Poole. 2002. The mexR repressor of the mexAB-oprM multidrug efflux operon in Pseudomonas aeruginosa: characterization of mutations compromising activity. J. Bacteriol. 184:4308-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aires, J. R., T. Köhler, H. Nikaido, and P. Plesiat. 1999. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob. Agents Chemother. 43:2624-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beinlich, K. L., R. Chuanchuen, and H. P. Schweizer. 2001. Contribution of multidrug efflux pumps to multiple antibiotic resistance in veterinary clinical isolates of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 198:129-134. [DOI] [PubMed] [Google Scholar]
- 4.de Kievit, T. R., and B. H. Iglewski. 2000. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68:4839-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans, K., L. Adewoye, and K. Poole. 2001. MexR repressor of the mexAB-oprM multidrug efflux operon of Pseudomonas aeruginosa: identification of MexR binding sites in the mexA-mexR intergenic region. J. Bacteriol. 183:807-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans, K., and K. Poole. 1999. The MexA-MexB-OprM multidrug efflux system of Pseudomonas aeruginosa is growth-phase regulated. FEMS Microbiol. Lett. 173:35-39. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda, H., M. Hosaka, K. Hirai, and S. Iyobe. 1990. New norfloxacin resistance gene in Pseudomonas aeruginosa PAO. Antimicrob. Agents Chemother. 34:1757-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fürste, J. P., W. Pansegrau, R. Frank, H. Blocker, P. Scholz, M. Bagdasarian, and E. Lanka. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119-131. [DOI] [PubMed] [Google Scholar]
- 9.Guan, L., M. Ehrmann, H. Yoneyama, and T. Nakae. 1999. Membrane topology of the xenobiotic-exporting subunit, MexB, of the MexA, B-OprM extrusion pump in Pseudomonas aeruginosa. J. Biol. Chem. 274:10517-10522. [DOI] [PubMed] [Google Scholar]
- 10.Köhler, T., S. F. Epp, L. K. Curty, and J. C. Pechére. 1999. Characterization of MexT, the regulator of the MexE-MexF-OprN multidrug efflux system of Pseudomonas aeruginosa. J. Bacteriol. 181:6300-6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Köhler, T., C. van Delden, L. K. Curty, M. M. Hamzehpour, and J. C. Pechére. 2001. Overexpression of the MexEF-OprN multidrug efflux system affects cell-to-cell signaling in Pseudomonas aeruginosa. J. Bacteriol. 183:5213-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, X. Z., N. Barre, and K. Poole. 2000. Influence of the MexA-MexB-OprM multidrug efflux system on expression of the MexC-MexD-OprJ and MexE-MexF-OprN multidrug efflux systems in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 46:885-893. [DOI] [PubMed] [Google Scholar]
- 13.Li, X. Z., and K. Poole. 2001. Mutational analysis of the OprM outer membrane component of the MexA-MexB-OprM multidrug efflux system of Pseudomonas aeruginosa. J. Bacteriol. 183:12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim, D., K. Poole, and N. C. Strynadka. 2002. Crystal structure of the MexR repressor of the mexRAB-oprM multidrug efflux operon of Pseudomonas aeruginosa. J. Biol. Chem. 277:29253-29259. [DOI] [PubMed] [Google Scholar]
- 15.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 16.Maseda, H., M. Kitao, S. Eda, E. Yoshihara, and T. Nakae. 2002. A novel assembly process of the multicomponent xenobiotic efflux pump in Pseudomonas aeruginosa. Mol. Microbiol. 46:677-686. [DOI] [PubMed] [Google Scholar]
- 17.Maseda, H., K. Saito, A. Nakajima, and T. Nakae. 2000. Variation of the mexT gene, a regulator of the MexEF-oprN efflux pump expression in wild-type strains of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 192:107-112. [DOI] [PubMed] [Google Scholar]
- 18.Maseda, H., H. Yoneyama, and T. Nakae. 2000. Assignment of the substrate-selective subunits of the MexEF-OprN multidrug efflux pump of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:658-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masuda, N., E. Sakagawa, S. Ohya, N. Gotoh, H. Tsujimoto, and T. Nishino. 2000. Contribution of the MexX-MexY-OprM efflux system to intrinsic resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:2242-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mine, T., Y. Morita, A. Kataoka, T. Mizushima, and T. Tsuchiya. 1999. Expression in Escherichia coli of a new multidrug efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:415-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morshed, S. R., Y. Lei, H. Yoneyama, and T. Nakae. 1995. Expression of genes associated with antibiotic extrusion in Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 210:356-362. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima, A., Y. Sugimoto, H. Yoneyama, and T. Nakae. 2000. Localization of the outer membrane subunit OprM of resistance-nodulation-cell division family multicomponent efflux pump in Pseudomonas aeruginosa. J. Biol. Chem. 275:30064-30068. [DOI] [PubMed] [Google Scholar]
- 23.Nikaido, H. 1989. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob. Agents Chemother. 33:1831-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsek, M. R., and E. P. Greenberg. 2000. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 97:8789-8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearson, J. P., C. Van Delden, and B. H. Iglewski. 1999. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 181:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poole, K. 2000. Efflux-mediated resistance to fluoroquinolones in gram-negative bacteria. Antimicrob. Agents Chemother. 44:2233-22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poole, K. 2001. Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J. Mol. Microbiol. Biotechnol. 3:255-264. [PubMed] [Google Scholar]
- 28.Poole, K., N. Gotoh, H. Tsujimoto, Q. Zhao, A. Wada, T. Yamasaki, S. Neshat, J. Yamagishi, X. Z. Li, and T. Nishino. 1996. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol. Microbiol. 21:713-724. [DOI] [PubMed] [Google Scholar]
- 29.Poole, K., K. Krebes, C. McNally, and S. Neshat. 1993. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J. Bacteriol. 175:7363-7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poole, K., K. Tetro, Q. Zhao, S. Neshat, D. E. Heinrichs, and N. Bianco. 1996. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa: mexR encodes a regulator of operon expression. Antimicrob. Agents Chemother. 40:2021-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahmati, S., S. Yang, A. L. Davidson, and E. L. Zechiedrich. 2002. Control of the AcrAB multidrug efflux pump by quorum-sensing regulator SdiA. Mol. Microbiol. 43:677-685. [DOI] [PubMed] [Google Scholar]
- 32.Rella, M., and D. Haas. 1982. Resistance of Pseudomonas aeruginosa PAO to nalidixic acid and low levels of beta-lactam antibiotics: mapping of chromosomal genes. Antimicrob. Agents Chemother. 22:242-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rist, M., and M. A. Kertesz. 1998. Construction of improved plasmid vectors for promoter characterization in Pseudomonas aeruginosa and other gram-negative bacteria. FEMS Microbiol. Lett. 169:179-183. [DOI] [PubMed] [Google Scholar]
- 34.Saito, K., S. Eda, H. Maseda, and T. Nakae. 2001. Molecular mechanism of MexR-mediated regulation of MexAB-OprM efflux pump expression in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 195:23-28. [DOI] [PubMed] [Google Scholar]
- 35.Saito, K., H. Yoneyama, and T. Nakae. 1999. nalB-type mutations causing the overexpression of the MexAB-OprM efflux pump are located in the mexR gene of the Pseudomonas aeruginosa chromosome. FEMS Microbiol. Lett. 179:67-72. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook, J., E. Fritsch, F., and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Sanchez, P., F. Rojo, and J. L. Martinez. 2002. Transcriptional regulation of mexR, the repressor of Pseudomonas aeruginosa mexAB-oprM multidrug efflux pump. FEMS Microbiol. Lett. 207:63-68. [DOI] [PubMed] [Google Scholar]
- 38.Schweizer, H. P. 1992. Allelic exchange in Pseudomonas aeruginosa using novel ColE1-type vectors and a family of cassettes containing a portable oriT and the counter-selectable Bacillus subtilis sacB marker. Mol. Microbiol. 6:1195-1204. [DOI] [PubMed] [Google Scholar]
- 39.Schweizer, H. P. 1993. Two plasmids, X1918 and Z1918, for easy recovery of the xylE and lacZ reporter genes. Gene 134:89-91. [DOI] [PubMed] [Google Scholar]
- 40.Shiba, T., K. Ishiguro, N. Takemoto, H. Koibuchi, and K. Sugimoto. 1995. Purification and characterization of the Pseudomonas aeruginosa NfxB protein, the negative regulator of the nfxB gene. J. Bacteriol. 177:5872-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon, R., M. O'Connell, M. Labes, and A. Pühler. 1986. Plasmid vector for the genetic analysis and manipulation of rhizobia and other gram-negative bacteria. Methods Enzymol. 118:640-659. [DOI] [PubMed] [Google Scholar]
- 42.Srikumar, R., C. J. Paul, and K. Poole. 2000. Influence of mutations in the mexR repressor gene on expression of the MexA-MexB-OprM multidrug efflux system of Pseudomonas aeruginosa. J. Bacteriol. 182:1410-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitehead, N. A., A. M. Barnard, H. Slater, N. J. Simpson, and G. P. Salmond. 2001. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol. Rev. 25:365-404. [DOI] [PubMed] [Google Scholar]
- 44.Wong, K. K., F. S. Brinkman, R. S. Benz, and R. E. Hancock. 2001. Evaluation of a structural model of Pseudomonas aeruginosa outer membrane protein OprM, an efflux component involved in intrinsic antibiotic resistance. J. Bacteriol. 183:367-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoneyama, H., H. Maseda, H. Kamiguchi, and T. Nakae. 2000. Function of the membrane fusion protein, MexA, of the MexA, B-OprM efflux pump in Pseudomonas aeruginosa without an anchoring membrane. J. Biol. Chem. 275:4628-4634. [DOI] [PubMed] [Google Scholar]