Abstract
Context:
Intracellular fat within muscle and visceral tissue has been suggested to adversely influence bone development.
Objective:
The aim of the study was to evaluate associations between im fat, as reflected by muscle density as measured by peripheral quantitative computed tomography, and cortical bone parameters in young adults.
Design/Setting/Participants:
We conducted a cross-sectional analysis of 1703 males and 2243 females aged 17.8 years from the Avon Longitudinal Study of Parents and Children.
Outcome Measures:
We measured cortical bone parameters from midtibial peripheral quantitative computed tomography scans.
Results:
Muscle density (inversely related to im fat) was inversely associated with periosteal circumference (PC) (beta = −0.07 [95% confidence interval (CI), −0.1, −0.04]), cortical bone mineral density (BMDC) (beta = −0.21 [95% CI, −0.26, −0.17]), and cortical thickness (CT) (beta = −0.37 [95% CI, −0.42, −0.33]) (males and females combined, adjusted for age, height, gender, and muscle cross-sectional area). In contrast, sc fat area was positively associated with PC (beta = 0.10 [95% CI, 0.07, 0.12]), but no association was seen with BMDC or CT. To examine the role of candidate intermediary metabolic pathways, analyses were repeated after adjustment for insulin, C-reactive protein, and β-C-telopeptides of type I collagen. Whereas similar associations were observed after adjustment for insulin and C-reactive protein, the association between muscle density and BMDC was partially attenuated by adjustment for β-C-telopeptides of type I collagen (beta = −0.14 [95% CI, −0.20, −0.08]).
Conclusion:
Although im and sc fat were both positively associated with cortical bone mass, the nature of these relationships differed in that im fat was predominantly associated with CT and BMDC, whereas sc fat was mainly associated with PC. These relationships were largely independent of candidate metabolic pathways, such as altered bone resorption, insulin resistance, or inflammation.
Although obesity is associated with a range of adverse health outcomes, its impact on the skeleton is unclear. In the Avon Longitudinal Study of Parents and Children (ALSPAC), we observed positive associations between total body fat mass and bone mass (1), reflecting relationships between fat and periosteal expansion and cortical thickness (CT) (2). Despite these associations, which imply that fat improves bone strength, increased body weight is a risk factor for fractures in childhood (3, 4). Although the reason for this apparent discrepancy is currently unclear, one possible explanation is that fracture risk is increased in individuals with a deficient skeletal response to fat (5). Mendelian randomization studies suggest that associations between fat and bone mass involve a causal pathway whereby increases in fat mass lead to greater bone mass (6). However, there is also evidence that actions of bone on fat contribute to this relationship. For example, mouse models suggest that bone turnover directly influences insulin sensitivity and adiposity via a relay involving osteocalcin (an osteoblast-specific protein) and adiponectin (produced by white adipose tissue). Reduced bone turnover, resulting in decreased undercarboxylated osteocalcin, has been associated with lower adiponectin, impaired insulin sensitivity, and increased fat deposition due to reduced energy expenditure (7). Similar relationships between circulating undercarboxylated osteocalcin and adiponectin, plus adiposity measures and insulin sensitivity, are reported in some human populations (8, 9).
Although replicated in some studies (10, 11), an equivalent positive relationship between fat and bone in children and young adults was not observed in others (12–16). Relationships between fat and bone may vary according to skeletal site, as exemplified by the finding that fat mass appears to be related to periosteal circumference (PC) more strongly at weight-bearing sites (11). Associations between fat and bone may also vary according to the region of adiposity. Visceral adipose tissue and im fat have been suggested to contribute to insulin resistance and other components of the metabolic syndrome via a so-called lipotoxic effect on glucose metabolism and proinflammatory pathways (17). These fat deposits may also adversely affect bone. For example, visceral adipose tissue was inversely related with bone structure and strength in adolescent girls and young women, in contrast to sc adipose tissue, which showed a positive relationship (18). Muscle density as measured by peripheral quantitative computed tomography (pQCT) has been used to study the role of im fat, after observations that lower muscle density reflects greater fat content of muscle (19, 20). Using this approach, im fat was also found to be inversely related to bone strength in prepubertal and peripubertal girls, in contrast to evidence of a weak positive association for sc fat (21).
These adverse effects of im fat on bone, as observed in prepubertal and peripubertal girls, may have important implications for subsequent bone health, assuming they persist into later life. We previously observed interactions between fat mass, bone mass, and puberty, with evidence that the relationship between fat mass and subsequent gain in bone mass is attenuated in peripubertal girls (1). In addition, we found that relationships between fat and cortical bone are affected by gender, with stronger associations observed in girls compared to boys (2). In the present study, we aimed to establish whether equivalent relationships between im fat and cortical bone are observed beyond puberty and whether similar associations are also present in males. We used pQCT to characterize relationships of im and sc fat with different cortical bone parameters, based on data obtained in young adult men and women from ALSPAC at age 17.8 years. A further aim was to investigate whether any differences in these relationships according to region of adiposity is explained by metabolic pathways implicated in fat-bone relationships discussed above, ie, bone turnover, insulin resistance, and inflammation.
Subjects and Methods
Study participants
ALSPAC is a geographically based UK cohort that recruited pregnant women residing in Avon (Southwest England) with an expected date of delivery between April 1, 1991, and December 31, 1992. A total of 15 247 pregnancies were enrolled, with 14 775 children born (see www.alspac.bris.ac.uk) (22). Of these births, 14 701 children were alive at 12 months. The present study is based on a research clinic to which the whole cohort was invited, which was held when children were a mean age of 17.8 years and was attended by 5084 participants. Ethical approval was obtained from the ALSPAC Law and Ethics Committee and the Local Research Ethics Committees. Parental consent and the child's assent were obtained for all measurements made.
Tibial pQCT scans
Tibial pQCT scans of the midtibia were offered to all those attending the age 17.8-year research clinic, using the Stratec XCT2000L (Stratec Inc., Pforzheim, Germany). Scans, which were obtained in 4649 participants, were analyzed with XCT custom software version 6.00B. Thirty-six participants were excluded due to major scanning errors. Cortical bone mineral content (BMCC) and cortical bone mineral density (BMDC) were obtained, and PC and CT were subsequently derived from a circular ring model. A threshold routine was used for defining cortical bone, which specified a voxel with a density >650 mg · cm−3 as cortical bone. Strength strain index (SSI) was derived as described in the Stratec user's manual (23). Muscle cross-sectional area (MCSA), muscle density, and sc fat area (SFA) were obtained after image processing with a set of 4 convoluted image filters (23). Muscle density is strongly affected by movement artifact; streaks within the muscle cross-section caused by movement contain pixels originating from cortical bone, artifactually increasing muscle density. Therefore, movement artifact was quantified by deriving an artifact indicator score, which was used to exclude scans with significant motion artifact, and to adjust muscle density for movement in remaining scans. The artifact indicator variable was derived by calculating the difference in value for cortical bone area obtained after application of high (cortical bone density >650 mg · cm−3; cort mode 1), and low (cortical bone density >200 mg · cm−3; cort mode 1) thresholds for BMDC. This difference in cortical bone area represents a continuous variable, which is directly proportional to the extent of movement artifact. This was confirmed in 100 randomly selected scans in which the artifact indicator was compared with results from qualitative grading based on a scale from 0–5 (beta [increase in artifact indicator (mm2) per increase in qualitative score] = 22.5; 95% confidence interval [CI], 16.7, 28.4; P < .001). Scans with artifact indicator score >250 mm2 were excluded (n = 520), and muscle density from remaining scans adjusted for this variable. Height was measured using a Harpenden Stadiometer (Holtain Ltd, Crymych, United Kingdom).
Metabolic measures
Metabolic measures were based on blood samples obtained when participants attended at mean age 17.8 years. Participants were asked to fast overnight (for those attending in the morning) or for a minimum of 6 hours (for those attending after lunch). Blood samples (EDTA) were immediately spun, plasma was obtained and frozen at −80°C, and measurements were subsequently assayed with no previous freeze-thaw cycles. Insulin was measured by an ultrasensitive ELISA (Mercodia, Uppsala, Sweden) automated microparticle enzyme immunoassay that does not cross-react with proinsulin (sensitivity, 0.07 mU/L). C-reactive protein (CRP) was measured by automated particle-enhanced immunoturbidimetric assay (Roche UK, Welwyn Garden City, United Kingdom) (sensitivity, 0.1 mg/L). Electrochemiluminescence immunoassays (Roche Diagnostics, Lewes, United Kingdom) measured plasma concentrations of β-C-telopeptides of type I collagen (CTX) (sensitivity, 0.01 μg/L) on fasting samples collected at the mean age 15.5 years clinic visit. All assay coefficients of variation were <6% across the working range.
Statistical analysis
Because insulin and CRP were right-skewed, they were log-transformed before statistical analysis. Continuously measured variables were presented as means (standard deviation) and medians (interquartile range). Multivariable linear regression was used to explore the association of SFA and muscle density with cortical bone parameters. Throughout, muscle density was adjusted for (movement) artifact indicator and BMDC for PC to account for the inverse association between these 2 parameters (2). Additional adjustments were as follows: model 1 included age, gender, and height; model 2 also included MCSA to control for confounding by muscle mass; models 3 to 5 also included CTX, insulin, and CRP, respectively, in subsamples with data on these variables. Model 3 also included a time of collection variable to account for diurnal variation. Outcome and exposure variables were standardized; hence, beta coefficients represent a standard deviation change in pQCT parameter per standard deviation increase in SFA/muscle density. Gender differences were assessed by completing analyses separately in males and females and testing for gender*exposure interaction using a likelihood ratio test. All analyses were performed by K.D. in Stata 11.2 (StataCorp, College Station, Texas).
Results
Characteristics of participants
A total of 3946 ALSPAC participants (1703 males, 2243 females) had pQCT and covariate data at age 17.8 years. Body mass index was similar in both sexes (Table 1), as was waist circumference (mean, SD: males, 75.4 cm, 7.2 cm; females, 74.9 cm, 7.6 cm; n = 2519). PC, CT, BMCC, and SSI were greater in males, whereas BMDC was greater in females. MSCA was greater in males, SFA was higher in females, whereas muscle density was similar in both sexes. Insulin concentrations were approximately 30% higher in females, CRP concentrations were approximately 20% higher in females, and CTX concentrations (from mean age 15.5 y) were approximately twice as high in males compared to females.
Table 1.
Participant Characteristics
| Variable | Sex | Mean (SD) | Median | 25th Percentile | 75th Percentile |
|---|---|---|---|---|---|
| Age, y | M | 17.8 (0.4) | 17.8 | 17.6 | 18.0 |
| F | 17.8 (0.4) | 17.8 | 17.6 | 18.0 | |
| All | 17.8 (0.4) | 17.8 | 17.6 | 18.0 | |
| Height, cm | M | 178.3 (6.5) | 178.4 | 174.0 | 182.4 |
| F | 165.0 (6.2) | 165.0 | 161.0 | 169.0 | |
| All | 170.8 (9.1) | 170.1 | 163.9 | 177.6 | |
| Body mass index, kg/m2 | M | 21.9 (3.1) | 21.4 | 19.8 | 23.4 |
| F | 22.2 (3.3) | 21.7 | 19.9 | 23.9 | |
| All | 22.1 (3.2) | 21.6 | 19.9 | 23.7 | |
| PC, mm | M | 77.1 (5.0) | 77.1 | 73.8 | 80.5 |
| F | 68.8 (4.3) | 68.7 | 65.9 | 71.7 | |
| All | 72.4 (6.2) | 72.0 | 67.8 | 76.6 | |
| BMDC, mg · cm−3 | M | 1108.6 (23.5) | 1110.8 | 1095.5 | 1124.8 |
| F | 1136.7 (19.4) | 1138.5 | 1124.9 | 1150.2 | |
| All | 1124.5 (25.4) | 1127.1 | 1108.8 | 1142.5 | |
| CT, mm | M | 5.83 (0.67) | 5.82 | 5.40 | 6.25 |
| F | 5.21 (0.54) | 5.22 | 4.85 | 5.58 | |
| All | 5.48 (0.67) | 5.45 | 5.01 | 5.91 | |
| BMCC, mg | M | 379.7 (51.7) | 378.3 | 346.70 | 412.7 |
| F | 310.3 (39.0) | 309.4 | 284.42 | 336.8 | |
| All | 340.3 (56.5) | 334.6 | 299.33 | 376.4 | |
| SSI | M | 1.2 (0.2) | 1.2 | 1.09 | 1.4 |
| F | 0.9 (0.2) | 0.9 | 0.80 | 1.0 | |
| All | 1.1 (0.3) | 1.0 | 0.86 | 1.2 | |
| SFA, mm2 | M | 1903.6 (674.5) | 1769.5 | 1411.25 | 2294.8 |
| F | 2923.6 (772.6) | 2821.5 | 2371.75 | 3377.8 | |
| All | 2483.4 (889.3) | 2418.6 | 1806.25 | 3031.8 | |
| Muscle density, mg · cm−3 | M | 83.4 (3.1) | 83.0 | 81.25 | 85.0 |
| F | 82.8 (3.1) | 82.3 | 80.69 | 84.4 | |
| All | 83.1 (3.1) | 82.6 | 80.95 | 84.7 | |
| MCSA, mm2 | M | 5809.7 (954.8) | 5751.8 | 5139.3 | 6446.5 |
| F | 4911.4 (799.7) | 4869.5 | 4354.8 | 5419.8 | |
| All | 5299.0 (977.1) | 5217.5 | 4592.0 | 5920.5 | |
| CTX, ng · mLa | M | 1.45 (0.51) | 1.39 | 1.09 | 1.77 |
| F | 0.72 (0.26) | 0.69 | 0.54 | 0.87 | |
| All | 1.05 (0.54) | 0.92 | 0.65 | 1.36 | |
| CRP, mg · Lb | M | 7.29 (5.69) | 5.87 | 4.41 | 8.15 |
| F | 8.25 (5.24) | 7.16 | 5.33 | 9.68 | |
| All | 7.80 (5.48) | 6.55 | 4.85 | 9.10 | |
| Insulin, IU · Lb | M | 1.20 (3.27) | 0.43 | 0.25 | 0.94 |
| F | 1.77 (4.54) | 0.65 | 0.30 | 1.57 | |
| All | 1.50 (4.00) | 0.53 | 0.28 | 1.29 |
Abbreviations: M, male; F, female. Table shows characteristics of 3946 participants (males = 1703, females = 2243) with pQCT scan data obtained at age 17.8 years.
Data from fasting blood samples at age 15.5 years: n = 2085 (941 boys, 1144 girls).
Data from fasting blood samples at age 17.8 years: n = 2529 (1194 males, 1335 females).
Associations of SFA with cortical bone
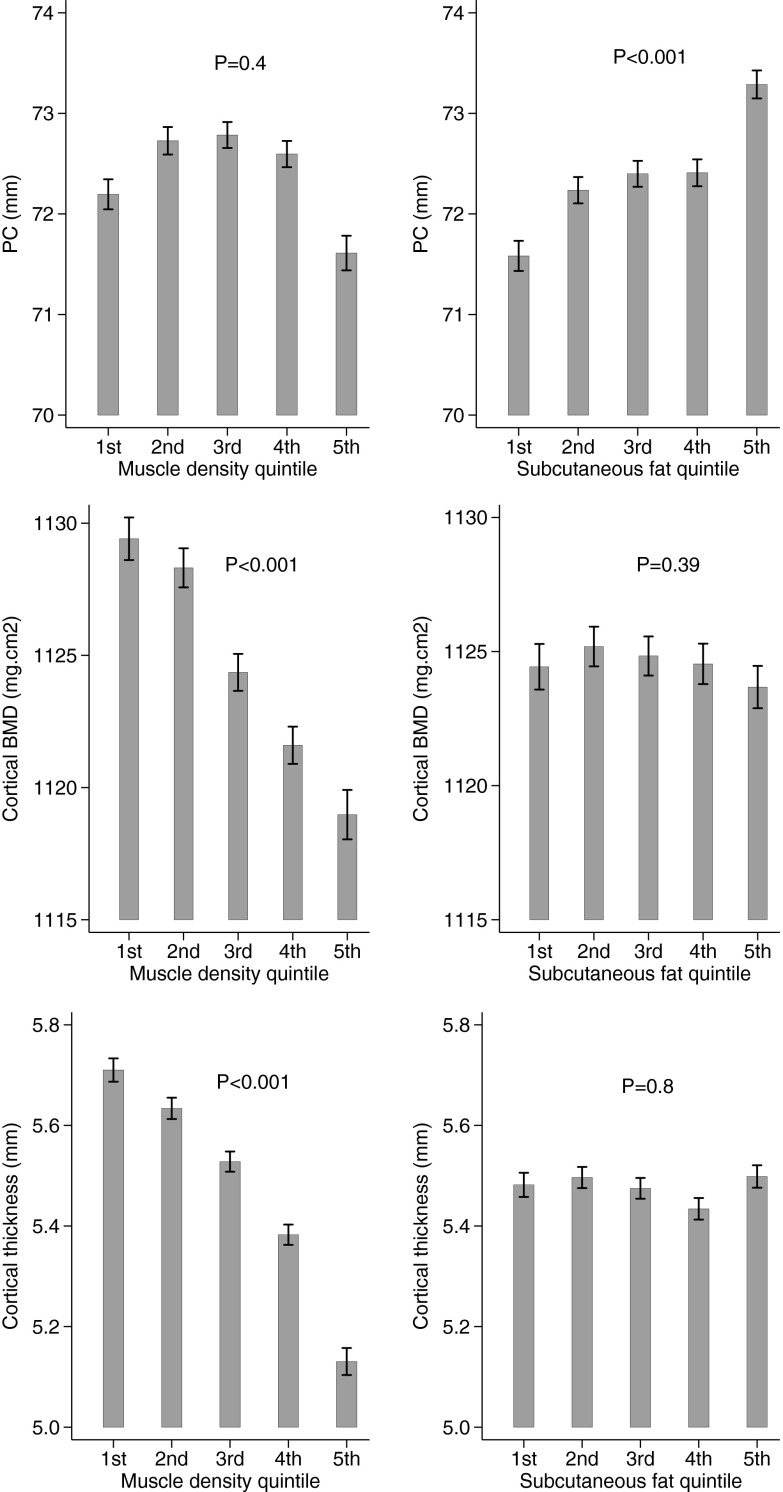
SFA was positively associated with PC in model 1 in males and females combined (β = 0.16 [95% CI, 0.14, 0.19], model 1), with some attenuation after adjustment for MSCA (β = 0.10 [95% CI, 0.07,0.12], model 2) (Table 2). SFA was not associated with BMDC. SFA was positively related to CT in model 1 (β = 0.06 [95% CI, 0.03, 0.10]), but this was fully attenuated after adjustment for MSCA (β = 0.01 [95% CI, −0.02, 0.04]). SFA was positively related to BMCC and SSI, with β coefficients attenuated by approximately 50% after adjustment for MSCA. Relationships were broadly equivalent in males and females, and so we focused on combined results. The main exception was that analyses confined to girls suggested a weak positive association between SFA and CT (β = 0.04 [95% CI, 0.01, 0.08]; P = .05 for gender interaction test, model 2). Cortical bone parameters were also examined according to SFA quintile in males and females combined, with adjustment based on model 2 (because SFA was greater in females, quintiles were assigned using gender-specific ranges). Successive SFA quintiles were associated with greater PC in a dose-responsive manner (Figure 1). In contrast, no clear association was observed between SFA quintile and either BMDC or CT.
Table 2.
SFA vs Cortical Bone Parameters
| Outcome (Z) | Sex | Model 1 |
Model 2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Coef | 95% CI | P | p(int) | R2 | Coef | 95% CI | P | p(int) | ||
| PC | M | 0.26 | 0.183 | 0.139, 0.226 | <.001 | 0.38 | 0.107 | 0.066, 0.148 | <.001 | ||
| F | 0.30 | 0.153 | 0.125, 0.181 | <.001 | .236 | 0.41 | 0.088 | 0.062, 0.114 | <.001 | .456 | |
| All | 0.60 | 0.164 | 0.140, 0.188 | <.001 | 0.66 | 0.095 | 0.072, 0.118 | <.001 | |||
| BMDC | M | 0.05 | −0.015 | −0.082, 0.051 | .652 | 0.06 | −0.050 | −0.117, 0.018 | .149 | ||
| F | 0.02 | 0.010 | −0.032, 0.052 | .635 | .638 | 0.02 | 0.015 | −0.028, 0.058 | .490 | .591 | |
| All | 0.08 | 0.000 | −0.036, 0.037 | .987 | 0.09 | −0.014 | −0.051, 0.024 | .475 | |||
| CT | M | 0.02 | 0.024 | −0.038, 0.086 | .446 | 0.08 | −0.044 | −0.106, 0.017 | .156 | ||
| F | 0.03 | 0.086 | 0.048, 0.124 | <.001 | .096 | 0.06 | 0.043 | 0.005, 0.082 | .026 | .046 | |
| All | 0.22 | 0.064 | 0.030, 0.097 | <.001 | 0.26 | 0.010 | −0.024, 0.043 | .562 | |||
| BMCC | M | 0.15 | 0.125 | 0.072, 0.178 | <.001 | 0.27 | 0.037 | −0.013, 0.087 | .150 | ||
| F | 0.18 | 0.131 | 0.101, 0.161 | <.001 | .964 | 0.27 | 0.074 | 0.045, 0.103 | <.001 | .599 | |
| All | 0.47 | 0.129 | 0.101, 0.157 | <.001 | 0.54 | 0.058 | 0.032, 0.085 | <.001 | |||
| SSI | M | 0.24 | 0.184 | 0.134, 0.233 | <.001 | 0.37 | 0.095 | 0.049, 0.141 | <.001 | ||
| F | 0.28 | 0.137 | 0.111, 0.163 | <.001 | .039 | 0.38 | 0.080 | 0.055, 0.104 | <.001 | .088 | |
| All | 0.57 | 0.154 | 0.129, 0.179 | <.001 | 0.64 | 0.083 | 0.059, 0.106 | <.001 | |||
Abbreviations: M, male; F, female. Table shows results of linear regression analysis between SFA and cortical bone parameters, based on pQCT scans from 3946 individuals (males = 1703, females = 2243). Coef represents standard deviation change in cortical bone parameter per standard deviation increase in SFA. Model 1 = adjustment for age at scan, height, and gender; model 2 = model 1 plus MCSA. p(int) represents P value for gender interaction.
Figure 1.
Mean PC, BMDC, and CT, according to quintiles of muscle density and SFA, in 3964 individuals. Error bars represent standard error of the mean. Means are adjusted for age, height, gender, and MCSA. P values show test for trend.
Associations of muscle density with cortical bone
A strong inverse association was evident with PC (β = −0.23 [95% CI, −0.26, −0.21], model 1), which was largely but not completely attenuated after adjustment for MSCA (β = −0.07 [95% CI, −0.1, −0.04], model 2) (Table 3). In contrast, relatively strong inverse associations were observed between muscle density and BMDC and CT, irrespective of adjustment for MCSA (BMDC: β = −0.21 [95% CI, −0.26, −0.17]; CT: β = −0.37 [95% CI, −0.42, −0.33]; model 2). Muscle density was inversely associated with BMCC and SSI, although partial attenuation was observed after adjustment for MSCA. With the exception of PC, associations between muscle density and cortical bone parameters were generally stronger in boys (P ≤ .001 for gender interaction). Based on model 2, successive quintiles for muscle density were associated with reductions in BMDC and CT in a dose-responsive manner (Figure 1). There was only very weak evidence for an inverse association between muscle density quintile and PC.
Table 3.
Muscle Density vs Cortical Bone Parameters
| Outcome (Z) | Sex | Model 1 |
Model 2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Coef | 95% CI | P | p(int) | R2 | Coef. | 95% CI | P | p(int) | ||
| PC | M | 0.35 | −0.245 | −0.279, −0.211 | <.001 | 0.38 | −0.103 | −0.150, −0.056 | <.001 | ||
| F | 0.36 | −0.223 | −0.253, −0.194 | <.001 | .359 | 0.41 | −0.039 | −0.079, 0.001 | 0.054 | .356 | |
| All | 0.64 | −0.234 | −0.256, −0.212 | <.001 | 0.66 | −0.071 | −0.101, −0.040 | <.001 | |||
| BMDC | M | 0.13 | −0.288 | −0.342, −0.235 | <.001 | 0.13 | −0.260 | −0.335, −0.185 | <.001 | ||
| F | 0.10 | −0.133 | −0.177, −0.090 | <.001 | .001 | 0.11 | −0.163 | −0.224, −0.101 | <.001 | .001 | |
| All | 0.16 | −0.213 | −0.247, −0.179 | <.001 | 0.16 | −0.213 | −0.261, −0.165 | <.001 | |||
| CT | M | 0.18 | −0.438 | −0.486, −0.390 | <.001 | 0.18 | −0.444 | −0.511, −0.377 | <.001 | ||
| F | 0.12 | −0.318 | −0.357, −0.278 | <.001 | <.001 | 0.12 | −0.313 | −0.369, −0.257 | <.001 | <.001 | |
| All | 0.32 | −0.373 | −0.403, −0.343 | <.001 | 0.32 | −0.374 | −0.417, −0.331 | <.001 | |||
| BMCC | M | 0.32 | −0.425 | −0.465, −0.385 | <.001 | 0.33 | −0.340 | −0.395, −0.284 | <.001 | ||
| F | 0.27 | −0.299 | −0.329, −0.268 | <.001 | <.001 | 0.29 | −0.197 | −0.240, −0.153 | <.001 | <.001 | |
| All | 0.56 | −0.360 | −0.384, −0.335 | <.001 | 0.56 | −0.266 | −0.301, −0.232 | <.001 | |||
| SSI | M | 0.37 | −0.344 | −0.381, −0.306 | <.001 | 0.38 | −0.209 | −0.261, −0.156 | <.001 | ||
| F | 0.35 | −0.233 | −0.260, −0.207 | <.001 | <.001 | 0.38 | −0.091 | −0.128, −0.054 | <.001 | <.001 | |
| All | 0.63 | −0.290 | −0.313, −0.268 | <.001 | 0.64 | −0.152 | −0.183, −0.121 | <.001 | |||
Abbreviations: M, male; F, female. Table shows results of linear regression analysis between muscle density and cortical bone parameters, based on pQCT scans from 3946 individuals (males = 1703, females = 2243). Coef represents standard deviation change in cortical bone parameter per standard deviation increase in muscle density. Model 1 = adjustment for age at scan, height, gender, and artifact indicator; model 2 = model 1 plus MCSA. p(int) represents P value for gender interaction.
Associations of insulin and CRP with SFA and muscle density
We analyzed associations between insulin and CRP concentrations as measured on plasma samples from mean age 17.8 and SFA/muscle density in 2529 participants with complete data (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). In model 1, insulin was positively associated with SFA and inversely associated with muscle density. There was some attenuation of the association between insulin and muscle density after adjustment for MCSA, with β coefficients for the relationship with muscle density approximately 50% that for SFA. Associations between insulin and SFA and muscle density were stronger in boys compared to girls (P ≤ .05 for gender interaction).
CRP was positively associated with SFA and inversely associated with muscle density in model 1, with attenuation of the association with muscle density after adjustment for MSCA. Associations between CRP and SFA/muscle density were equivalent in males and females. We then explored whether these measurements contribute to causal pathways between SFA/muscle density and cortical bone. However, no effect was observed after adjustment of associations between SFA/muscle density and cortical bone parameters for insulin or CRP (Supplemental Tables 2 and 3).
Associations of CTX with SFA and muscle density
We analyzed associations between CTX concentration as measured on plasma samples from mean age 15.5 years and SFA/muscle density as measured by pQCT scans at age 17.8 years in the 2085 participants with matching data. In model 1, CTX was inversely associated with SFA and positively related to muscle density (Table 4). Adjustment for MSCA attenuated the association with muscle density by approximately 50%, after which β coefficients were virtually identical to those seen for SFA. The association between CTX and SFA was considerably stronger in girls compared to boys (P < .001 for gender interaction), and there was also evidence of an equivalent gender difference for the association between CTX and muscle density (P = .06 for gender interaction; model 2).
Table 4.
CTX at Age 15.5 Years vs SFA and Muscle Density at 17.8 Years
| Outcome (Z) | Model | CTX |
||||
|---|---|---|---|---|---|---|
| Sex | Coef | 95% CI | P | p(int) | ||
| SFA | 1 | M | −0.077 | −0.128, −0.025 | .004 | |
| F | −0.324 | −0.423, −0.224 | <.001 | <.001 | ||
| All | −0.137 | −0.184, −0.089 | <.001 | |||
| 2 | M | −0.057 | −0.108, −0.005 | .030 | ||
| F | −0.301 | −0.398, −0.204 | <.001 | <.001 | ||
| All | −0.115 | −0.162, −0.068 | <.001 | |||
| Muscle density | 1 | M | 0.200 | 0.139, 0.262 | <.001 | |
| F | 0.243 | 0.154, 0.333 | <.001 | .229 | ||
| All | 0.207 | 0.158, 0.256 | <.001 | |||
| 2 | M | 0.099 | 0.056, 0.142 | <.001 | ||
| F | 0.162 | 0.098, 0.227 | <.001 | .064 | ||
| All | 0.113 | 0.078, 0.148 | <.001 | |||
Abbreviations: M, male; F, female. Table shows results of linear regression analysis between CTX (measured at age 15.5 y) and muscle density and SFA (measured at age 17.8 y) in 2085 individuals (males = 941, females = 1144). Coef represents standard deviation change in body composition per standard deviation increase in CTX. Model 1 = adjustment for age at scan, height, gender, and artifact indicator (muscle density only); model 2 = model 1 plus MCSA. p(int) represents P value for gender interaction.
Adjustment for CTX caused partial attenuation of the association between muscle density and BMDC, as judged by a 35% reduction in β coefficient (β = −0.14 [95% CI, −0.20, −0.08] and β = −0.22 [95% CI, −0.29, −0.16], with and without adjustment for CTX, respectively) (Table 5). In addition, whereas SFA was initially found to be unrelated to BMDC, after additional adjustment for CTX, there was now evidence of a weak inverse association (Supplemental Table 4). Associations between SFA/muscle density and other cortical bone parameters were unaffected by adjustment for CTX.
Table 5.
Muscle Density vs Cortical Bone Parameters After Adjustment for CTX
| Outcome (Z) | Sex | Model 2 |
Model 3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Coef | 95% CI | P | p(int) | R2 | Coef | 95% CI | P | p(int) | ||
| PC | M | 0.40 | −0.099 | −0.163, −0.035 | .002 | 0.42 | −0.122 | −0.185, −0.058 | <.001 | ||
| F | 0.42 | 0.006 | −0.052,0.064 | .850 | .174 | 0.43 | −0.003 | −0.062, 0.056 | .923 | .078 | |
| All | 0.67 | −0.047 | −0.089, −0.004 | .033 | 0.67 | −0.063 | −0.106, −0.021 | .004 | |||
| BMDC | M | 0.14 | −0.236 | −0.335, −0.137 | <.001 | 0.27 | −0.148 | −0.240, −0.056 | .002 | ||
| F | 0.11 | −0.199 | −0.287, −0.112 | <.001 | .014 | 0.20 | −0.121 | −0.205, −0.036 | .005 | .157 | |
| All | 0.16 | −0.221 | −0.287, −0.156 | <.001 | 0.27 | −0.143 | −0.204, −0.081 | <.001 | |||
| CT | M | 0.19 | −0.455 | −0.546, −0.364 | <.001 | 0.21 | −0.430 | −0.521, −0.338 | <.001 | ||
| F | 0.11 | −0.284 | −0.364, −0.204 | <.001 | .001 | 0.14 | −0.246 | −0.326, −0.166 | <.001 | .005 | |
| All | 0.33 | −0.371 | −0.431, −0.311 | <.001 | 0.34 | −0.343 | −0.403, −0.283 | <.001 | |||
| BMCC | M | 0.35 | −0.339 | −0.414, −0.263 | <.001 | 0.35 | −0.327 | −0.403, −0.251 | <.001 | ||
| F | 0.29 | −0.162 | −0.224, −0.099 | <.001 | <.001 | 0.30 | −0.140 | −0.203, −0.077 | <.001 | <.001 | |
| All | 0.57 | −0.253 | −0.302, −0.205 | <.001 | 0.57 | −0.239 | −0.288, −0.190 | <.001 | |||
| SSI | M | 0.41 | −0.204 | −0.276, −0.133 | <.001 | 0.41 | −0.213 | −0.286, −0.141 | <.001 | ||
| F | 0.39 | −0.055 | −0.109, −0.001 | .046 | .002 | 0.39 | −0.051 | −0.106, 0.004 | .067 | .001 | |
| All | 0.65 | −0.137 | −0.181, −0.092 | <.001 | 0.65 | −0.141 | −0.186, −0.097 | <.001 | |||
Abbreviations: M, male; F, female. Table shows results of linear regression analysis between muscle density and cortical bone parameters, based on pQCT scans from 2085 participants (males = 941, females = 1144). Coef represents standard deviation change in cortical bone parameter per standard deviation increase in muscle density. Model 2 = adjustment for age at scan, height, gender, artifact indicator, and MCSA; model 3 = model 2 plus CTX. p(int) represents P value for gender interaction.
Discussion
We compared associations between SFA, muscle density, and cortical bone in a large mixed cohort of young adults. A dose-response association was observed between SFA and PC, whereas muscle density showed dose-response associations with BMDC and CT. Although these associations were in opposite directions (ie, positive for SFA and negative for muscle density), given the inverse association between muscle density and im fat, our findings suggest that sc and im fat are both positively associated with cortical bone parameters. However, the nature of this association appears to differ according to fat deposit in that sc fat was predominantly associated with PC, whereas im fat was mainly associated with BMDC and CT. MCSA, which is related to SFA and muscle density, was used to adjust for possible confounding by lean mass, given that fat mass is strongly related to lean mass, which is itself strongly related to cortical bone mass (2). Although adjustment for MCSA resulted in some attenuation of associations with PC, this had no discernible effect on associations between muscle density and BMDC and CT. In addition, SFA and muscle density were respectively positively and inversely associated with BMCC and SSI, suggesting that sc and im fat are both positively associated with cortical bone strength. As judged by comparison of standardized β coefficients, these associations were greater for muscle density, suggesting that im fat may be more strongly associated with bone strength than sc fat.
The association between im fat, as reflected by muscle density, and CT was particularly marked, such that a 1 SD decrease in muscle density was associated with a 0.37 SD increase in CT (approximately 250 μm). The explanation for this strong association is currently unclear. Theoretically, a shared pathway involving bone resorption could be involved, suppression of which is expected to increase CT by reducing endosteal expansion. Against this suggestion, the association between muscle density and CT was unaffected by adjustment for systemic levels of bone resorption as represented by plasma CTX levels.
Our observation that im fat has a strong positive association with CT appears to be at odds with previous observations suggesting that so-called pathogenic fat deposits, such as visceral and im fat, have adverse effects on bone development (18, 21). In particular, using an equivalent pQCT-based method in 444 prepubertal and peripubertal girls from the Jump-in: Building Better Bones study, im fat as assessed at the 66% tibial site was found to be inversely related to CT (21). These contrasting findings may reflect changes in the association between fat and bone around the time of puberty, in light of our previous observation that the association between fat mass and subsequent gain in bone mass is attenuated in peripubertal girls (1). Although associations between muscle density and CT were greater in boys, strong relationships were nevertheless observed in girls in the present study, and so restriction of the study by Farr et al (21) to girls is unlikely to explain these differences. In terms of methodological differences, although regression models employed by Farr et al (21) included SFA, our results were unaffected when SFA was also used to adjust associations between muscle density and pQCT parameters (results not shown).
A further potential explanation for the apparent differences in association between im fat and cortical bone reported here as compared with findings from Farr et al (21) is that these associations vary according to age and skeletal maturity, as opposed to puberty per se. Consistent with this suggestion, associations between PC and im fat showed some evidence of an interaction with age because we found weak evidence of a positive association between PC and muscle density at mean age 17.8 years, in contrast to a weak inverse relationship as previously reported in the same cohort at mean age 15.5 years (24). However, any suggestion that associations between PC and muscle density differ according to age appears to be an exception, possibly reflecting the weakness of the underlying association. In contrast, other associations were strong and consistent irrespective of whether these were assessed at age 15.5 or 17.8 years, such as associations between SFA and PC, and between muscle density and CT and BMDC (results not shown). Taken together, these findings suggest that throughout adolescence, and possibly up until the time when peak bone mass is achieved by the third decade, fat mass continues to provide a positive stimulus for bone development, with potentially important long-term implications for future fracture risk. Lack of this stimulus during adolescence may also contribute to deficient bone accrual reported in conditions associated with reduced fat mass during this period, such as anorexia nervosa (25).
It is tempting to speculate that relationships between cortical bone and fat parameters as measured by pQCT at age 17.8 years help to explain associations with total body fat mass as measured by dual-energy x-ray absorptiometry scan at age 15.5 years (2). Specifically, our results suggest that sc fat largely contributes to the positive association we previously reported between total body fat mass and PC, whereas im fat may explain that between total body fat mass and CT. Hence, the overall association between fat and bone may comprise distinct functional relationships between cortical bone and different fat compartments. This conclusion would tend to go against the suggestion that distinct fat compartments influence bone through a common mechanism, for example via increased mechanical strain due to greater body weight. Although fat mass appears to be associated with PC more strongly at weight-bearing sites (11), we previously observed that fat mass is positively related to upper as well as lower limb bone mass (1), consistent with the view that effects of fat on bone are not solely explained by those on body weight. Likewise, these associations did not appear to be explained by co-association with weight-bearing physical activity because our results were unaffected by adjustment for moderate and vigorous physical activity as measured by accelerometry at age 15.5 years (results available from authors on request).
We hypothesized that differences in relationships between sc and im fat and cortical bone outcomes could be mediated by those in metabolic pathways. However, SFA and muscle density showed broadly similar associations with CTX, insulin, and CRP. Nevertheless, there was some evidence that changes in bone resorption contributed to the association between muscle density and BMDC, based on partial attenuation of β coefficients after adjustment for CTX. The suggestion that im and sc fat are inversely related to CTX is consistent with previous observations that bone resorption, as reflected by serum CTX, is inversely related to percentage body fat in 186 children and young people aged 7–19 years (26). That im fat influences BMDC via a pathway involving bone resorption is also consistent with the fact that BMDC largely reflects cortical porosity and secondary mineralization, both of which are closely related to the rate of cortical bone turnover.
CTX, insulin, and CRP levels all differed by gender in this study, but these differences are unlikely to persist into adulthood and may simply reflect differences in growth and maturation between boys and girls at any given age. For example, CRP was higher in girls, but equivalent differences are not seen in adults in whom levels are broadly equivalent in males and females (27).
Limitations
An important caveat to our findings is that because this was a cross-sectional observational study, it is difficult to draw conclusions about causality. However, although biological pathways have been proposed between bone and fat (7), these are thought to involve insulin and bone turnover, adjustment for which had little effect on our overall findings. A further limitation is that it was not possible to measure im fat directly, and instead this was estimated indirectly using pQCT-derived muscle density. This measure is unable to distinguish intramyocellular from extramyocellular fat, which may represent an important limitation; any pathogenic effect of im fat on bone and other tissues, as previously observed for visceral adipose tissue (18), may be limited to intramyocellular fat. In addition, it is conceivable that muscle density is influenced by other factors that have distinct relationships with cortical bone, such as muscle fiber properties. Against this suggestion, muscle density as assessed by pQCT was recently reported to be largely explained by muscle fat content as assessed by concurrent magnetic resonance imaging (28). Our investigation of whether bone resorption mediated any observed associations was limited by the fact that measures of CTX were based on blood samples collected 2 years previously. In addition, because CTX is influenced by puberty, levels measured at 15.5 years are not an ideal proxy for those at 17.8 years. However, we would anticipate that the order in which participants are ranked would be similar at both ages. In terms of other limitations, had a bone formation marker such as osteocalcin been available, this would have provided a more direct measure of bone formation compared to a resorption marker such as CTX, and it may have shown stronger relationships with fat indices. Finally, we were unable to explore the role of other potential pathways linking fat and bone phenotypes due to lack of available data for serum levels, such as 25-hydroxyvitamin D3.
Conclusions
Subcutaneous and im fat were both positively associated with cortical bone mass and strength, suggesting that fat exerts a protective effect on cortical bone irrespective of where it is situated. However, the mechanisms involved appeared to differ in that sc fat was positively associated with PC, whereas im fat was positively associated with CT and BMDC. Moreover, apart from a contribution of altered bone resorption to associations between muscle density and BMDC, these associations appeared to be independent of potential mediation by metabolic pathways previously implicated in fat-bone relationships.
Supplementary Material
Acknowledgments
We are grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
The UK Medical Research Council (Grant ref 74882), the Wellcome Trust (Grant ref 076467), and the University of Bristol provide core support for ALSPAC. Salary support for K.D. was provided by the Wellcome Trust (Grant ref 084632), which also funded the bone measures along with Wellcome Trust grant ref 079960. J.P.K. is funded by a Wellcome Trust 4-year PhD studentship in molecular, genetic, and life course epidemiology (WT083431MA), and this grant also funded the CTX assays. H.T.V. is funded by the Academy of Finland. This publication is the work of the authors, and J.H.T. will serve as guarantor for the contents of this paper.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMCC
- cortical bone mineral content
- BMDC
- cortical bone mineral density
- CI
- confidence interval
- CRP
- C-reactive protein
- CT
- cortical thickness
- CTX
- β-C-telopeptides of type I collagen
- MCSA
- muscle cross-sectional area
- PC
- periosteal circumference
- pQCT
- peripheral quantitative computed tomography
- SFA
- sc fat area
- SSI
- strength strain index.
References
- 1. Clark EM, Ness AR, Tobias JH. Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab. 2006;91:2534–2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sayers A, Tobias JH. Fat mass exerts a greater effect on cortical bone mass in girls than boys. J Clin Endocrinol Metab. 2010;95:699–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goulding A, Grant AM, Williams SM. Bone and body composition of children and adolescents with repeated forearm fractures. J Bone Miner Res. 2005;20:2090–2096 [DOI] [PubMed] [Google Scholar]
- 4. Manias K, McCabe D, Bishop NJ. Fractures and recurrent fractures in children; varying effects of environmental factors as well as bone size and mass. Bone. 2006;39:652–657 [DOI] [PubMed] [Google Scholar]
- 5. Tobias JH. Fat mass and bone development. Expert Rev Endocrinol Metab. 2010;5:323–325 [DOI] [PubMed] [Google Scholar]
- 6. Timpson NJ, Sayers A, Davey-Smith G, Tobias JH. How does body fat influence bone mass in childhood? A Mendelian randomization approach. J Bone Miner Res. 2009;24:522–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kindblom JM, Ohlsson C, Ljunggren O, et al. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res. 2009;24:785–791 [DOI] [PubMed] [Google Scholar]
- 9. Wu N, Wang QP, Li H, Wu XP, Sun ZQ, Luo XH. Relationships between serum adiponectin, leptin concentrations and bone mineral density, and bone biochemical markers in Chinese women. Clin Chim Acta. 2010;411:771–775 [DOI] [PubMed] [Google Scholar]
- 10. Leonard MB, Shults J, Wilson BA, Tershakovec AM, Zemel BS. Obesity during childhood and adolescence augments bone mass and bone dimensions. Am J Clin Nutr. 2004;80:514–523 [DOI] [PubMed] [Google Scholar]
- 11. Lorentzon M, Landin K, Mellstrom D, Ohlsson C. Leptin is a negative independent predictor of areal BMD and cortical bone size in young adult Swedish men. J Bone Miner Res. 2006;21:1871–1878 [DOI] [PubMed] [Google Scholar]
- 12. Petit MA, Beck TJ, Shults J, Zemel BS, Foster BJ, Leonard MB. Proximal femur bone geometry is appropriately adapted to lean mass in overweight children and adolescents. Bone. 2005;36:568–576 [DOI] [PubMed] [Google Scholar]
- 13. Janicka A, Wren TA, Sanchez MM, et al. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab. 2007;92:143–147 [DOI] [PubMed] [Google Scholar]
- 14. Pollock NK, Laing EM, Baile CA, Hamrick MW, Hall DB, Lewis RD. Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am J Clin Nutr. 2007;86:1530–1538 [DOI] [PubMed] [Google Scholar]
- 15. Ducher G, Bass SL, Naughton GA, Eser P, Telford RD, Daly RM. Overweight children have a greater proportion of fat mass relative to muscle mass in the upper limbs than in the lower limbs: implications for bone strength at the distal forearm. Am J Clin Nutr. 2009;90:1104–1111 [DOI] [PubMed] [Google Scholar]
- 16. Farr JN, Chen Z, Lisse JR, Lohman TG, Going SB. Relationship of total body fat mass to weight-bearing bone volumetric density, geometry, and strength in young girls. Bone. 2010;46:977–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muoio DM. Intramuscular triacylglycerol and insulin resistance: guilty as charged or wrongly accused? Biochim Biophys Acta. 2010;1801:281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009;94:3387–3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89:104–110 [DOI] [PubMed] [Google Scholar]
- 20. Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54:509–515 [DOI] [PubMed] [Google Scholar]
- 21. Farr JN, Funk JL, Chen Z, et al. Skeletal muscle fat content is inversely associated with bone strength in young girls. J Bone Miner Res. 2011;26:2217–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boyd A, Golding J, Macleod J, et al. Cohort profile: the 'Children of the 90s'–the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42:111–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stratec Medizintechnik GmbH. 2005 XCT 2000 Manual Version 6.66. [Google Scholar]
- 24. Sayers A, Lawlor DA, Sattar N, Tobias JH. The association between insulin levels and cortical bone: findings from a cross-sectional analysis of pQCT parameters in adolescents. J Bone Miner Res. 2012;27:610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Misra M, Klibanski A. Bone health in anorexia nervosa. Curr Opin Endocrinol Diabetes Obes. 2011;18:376–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Viljakainen HT, Pekkinen M, Saarnio E, Karp H, Lamberg-Allardt C, Makitie O. Dual effect of adipose tissue on bone health during growth. Bone. 2011;48:212–217 [DOI] [PubMed] [Google Scholar]
- 27. Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, Lowe G, et al. C-Reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wong AK, Beattie K, Bhargava A, et al. Inter and intramuscular adiposity explains only a proportion of the association between muscle density and fractures. J Bone Miner Res. 2012;27(suppl 1):SA0013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.