Abstract
Tissue perivascular resident macrophages (PVM/Ms), a hybrid cell type with characteristics of both macrophages and melanocytes, are critical for establishing and maintaining the endocochlear potential (EP) required for hearing. The PVM/Ms modulate expression of tight- and adherens-junction proteins in the endothelial barrier of the stria vascularis (intrastrial fluid-blood barrier) through secretion of a signaling molecule, pigment epithelium growth factor (PEDF). Here, we identify a significant link between abnormalities in PVM/Ms and endothelial barrier breakdown from acoustic trauma to the mouse ear. We find that acoustic trauma causes activation of PVM/Ms and physical detachment from capillary walls. Concurrent with the detachment, we find loosened tight junctions between endothelial cells and decreased production of tight- and adherens-junction protein, resulting in leakage of serum proteins from the damaged barrier. A key factor in the intrastrial fluid-blood barrier hyperpermeability exhibited in the mice is down-regulation of PVM/M modulated PEDF production. We demonstrate that delivery of PEDF to the damaged ear ameliorates hearing loss by restoring intrastrial fluid-blood barrier integrity. PEDF up-regulates expression of tight junction-associated proteins (ZO-1 and VE-cadherin) and PVM/M stabilizing neural cell adhesion molecule (NCAM-120). These studies point to the critical role PVM/Ms play in regulating intrastrial fluid-blood barrier integrity in healthy and noise-damaged ears.—Zhang, F., Dai, M., Neng, L., Zhang, J.H., Zhi, Z., Fridberger, A., Shi, X. Perivascular macrophage-like melanocyte responsiveness to acoustic trauma— a salient feature of strial barrier associated hearing loss.
Keywords: mouse cochlea, endothelial cell, instrastrial fluid-blood barrier, paracellular permeability, acoustic trauma
The cochlear endothelial-blood/tissue barrier tightly regulates the cochlear microenvironment for auditory function (1, 2). In particular, the intrastrial fluid-blood barrier in the stria vascularis is critical for generating the ionic gradients and endolymphatic potential (EP) essential for sensory hair cell transduction. Disruption of the intrastrial fluid-blood barrier is closely associated with a number of hearing disorders including autoimmune inner ear disease, noise-induced hearing loss, and several genetically linked hearing diseases (3–11).
Acoustic trauma not only directly damages sensory hair cells, but it also disrupts the intrastrial fluid-blood barrier. Disruption of the intrastrial fluid-blood barrier is an early feature of lesion formation that correlates with noise induced hearing loss, leading to edema and entry of serum proteins and inflammatory cells (12–15). Disruption of the endothelial barrier also produces an intrastrial electric shunt that may be sufficient to account for the absence of an endocochlear potential EP (6). The attenuated EP causes deafness by severely reducing the electromotive gradient for K+ entry through mechanosensitive channels in outer and inner ear hair cells. Thus, breakdown of cell-cell junctions such as the endothelial cell-endothelial cell junction, and subsequent vascular leakage, are suggested to play a causative role in noise-induced edema in the stria vascularis (10, 11, 15, 16). Therapeutic approaches that address and target the separable mechanisms damaged by noise are important for noise-induced hearing protection and recovery.
The vessel wall constitutes the intrastrial fluid-blood barrier of specialized endothelial cells, which are surrounded by accessory cells, including a large number of pericytes and perivascular resident macrophage-like melanocytes (PVM/Ms). Previously, we found that the PVM/Ms are tissue perivascular resident macrophages, a hybrid cell type with characteristics of both macrophage and melanocyte. The PVM/Ms are in close contact with vessels. Like astrocytes and glial cells, which have essential roles in regulation of barrier integrity and tissue-oriented functions (17, 18), PVM/Ms in the intrastrial fluid-blood barrier maintain barrier integrity by controlling tight-junction (TJ) and adhesive junction protein expression, with loss of PVM/Ms associated with blood barrier breakdown and tissue edema (19). We have also identified that PVM/Ms produce pigment epithelium growth factor (PEDF), a 50-kDa glycoprotein first identified in retinal pigment epithelium cells (20). PEDF plays a role in the stria vascularis as an essential signaling molecule essential for stabilizing the intrastrial fluid–blood barrier, maintaining the EP, and establishing a normal hearing threshold (19).
PEDF is a multifunctional protein exhibiting a wide range of antiangiogenic, antitumorigenic, antioxidant, antiinflammatory, antithrombotic, neurotrophic, and neuroprotective properties (21–24). Most recently, PEDF was shown to be the most potent endogenous inhibitor of vasopermeability (21, 25–27).
In this report, we link PVM/M aberrations with intrastrial fluid–blood barrier breakdown. Administration of pigment epithelium-derived growth factor PEDF ameliorates breaches in the intrastrial fluid-blood barrier and restores normal PVM/M morphology. Normal PVM/Ms, as specialized melanocytes, are known essential for strial development and production of the EP (28–30). These results represent a paradigm shift in our understanding for how noise affects PVM/M biology and leads to breakdown of the fluid–blood barrier in the stria vascularis. The results also suggest that specific targeting of PVM/Ms may open new avenues for protecting against noise-induced hearing loss.
MATERIALS AND METHODS
Animals
Male C57BL/6J mice (age ∼6–8 wk) were purchased from Jackson Laboratory (Bar Harbor, ME, USA). All procedures in this study were reviewed and approved by the Institutional Animal Care and Use Committee at Oregon Health and Science University.
Noise exposure (NE)
Animals were placed in wire mesh cages and exposed to broadband noise at 120 dB SPL in a sound exposure booth for 3 h and for an additional 3 h the following day. The NE regime, routinely used in our laboratory, produces permanent loss of cochlear sensitivity (11).
Auditory testing
An auditory brain-stem response (ABR) audiometry test to pure tones was used to evaluate hearing function. Animals were anesthetized and placed on a heating pad in a sound-isolated chamber. Needle electrodes were placed subcutaneously near the test ear, at the vertex, and on the contralateral ear. Each ear was stimulated separately with a closed tube sound delivery system sealed into the ear canal. The auditory brain-stem response to a 1-ms rise-time tone burst at 4, 8, 12, 16, 24, and 32 kHz was recorded, and thresholds were obtained for each ear. Threshold is defined as an evoked response of 0.2 μV. Auditory brain-stem response was assessed both before and after NE.
Measurement of EP
EP recordings were measured immediately at the end of the NE regime from the left ear of control (n=6), NE (n=6), and PEDF + NE (n=6) mice. The EP measurement method was previously reported (19). In that method, a silver–silver chloride reference electrode is placed under the skin of the dorsum. An incision is made in the inferior portion of the left postauricular sulcus, and the bulla is perforated, exposing the basal turn of the cochlea. Access to the scala media of the basal turn is obtained by thinning the bone over the spiral ligament and making a small opening with a pick. A micropipette electrode filled with 150 mM KCl is advanced through the bony aperture into the spiral ligament. Entry of the electrode tip into the endolymph is characterized by transients in recorded potentials. The electrode is advanced until a stable potential is observed. The signal is amplified through an amplifier (model 3000 AC/DC differential amplifier; A-M Systems, Inc., Carlsbourg, WA, USA). The DC potentials are recorded via an A-D converter (Fluke II multimeter; John Fluke Manufacturing Co., Inc., Everett, WA, USA).
Estimation of PVM/M coverage
Images of the entire length (∼6.25 mm) of the stria vascularis were analyzed. Twenty-seven images, acquired with an ×40 objective, were recorded from the left ear at the apical, middle, and basal regions of control, NE, and PEDF + NE groups. The apical region extended ∼0.8 mm from 0.5 mm of the apex, basal region from 0.5 mm of the base, and middle region from 2 mm of the apex. PVM/Ms were labeled with an antibody for F4/80, and endothelial cells were labeled with isolectin GS-IB4 conjugated to Alexa Fluor 568. Colocation of blood vessels and PVM/Ms was analyzed using ImageJ (V1.38X; U.S. National Institutes of Health, Bethesda, MD, USA). The outer margin of the blood vessel under consideration was outlined with an ImageJ region tool on threshold images of endothelium (red) and PVM/Ms (green). Percentage colocalization was quantified as a ratio of the F4/80-labeled area to the isolectin-labeled area using a method previously reported (11).
Primary cell culture
Cochleae from 10- or 15-d-old C57BL/6J mice were harvested under sterile conditions. The stria vascularis was pulled gently away from the spiral ligament, placed in ice-cold Hank's calcium- and magnesium-free balanced salt solution, and minced into small pieces (∼0.15–0.20 mm3) with ophthalmic tweezers. To produce PVM/Ms, the minced stria vascularis was cultured on collagen-coated dishes in medium 254 (Invitrogen, Eugene, OR, USA), containing 1% human melanocyte growth factor, 10% FBS, and 0.5% gentamicin/amphotericin. The minced stria vascularis was incubated at 37° in 5% CO2 with the medium changed every 3 d. Cell clones formed and melted at ∼10 d. The cells were detached from the cell colony with a solution of trypsin-EDTA and then purified (for detailed information see refs. 19, 31).
Immunohistochemistry and fluorescence microscopy
The tissues were fixed in 4% formaldehyde overnight, washed in 0.02 M PBS for 30 min, permeabilized in 1% Triton x-100 for 30 min, and immunoblocked with a solution of 10% goat serum and 1% bovine albumin in 0.02 M PBS for 1 h. The specimens were incubated overnight at 4°C with primary antibodies (Table 1) diluted in 1% BSA-PBS. The specimens were washed in 0.02 M PBS for 30 min and incubated with secondary antibody (Table 1) diluted 1:100 in 1% BSA-PBS for 1 h at room temperature. After a 30-min wash in 0.02 M PBS, tissues were visualized under an Olympus IX 81 inverted microscope fitted with an Olympus FV1000 laser-scanning confocal system (Olympus, Tokyo, Japan). 3D rendering of the confocal data was performed using Amira software (Visage Imaging, Inc., San Diego, CA, USA; http://www.amira.com/). Individual channels were split into separate image stacks and filtered by 3D noise reduction to increase the signal to noise. Volume data were displayed as isosurfaces, with blood vessels displayed in red, PVM/Ms in green.
Table 1.
Antibodies employed
| Antibody | Vectors | Identification | Dilution | Source | Specificity |
|---|---|---|---|---|---|
| Ve-cadherin | Abcam, Cambridge, MA, USA | Ab33168 | 1:50 (with 1% BSA-PBS) | Rabbit polyclonal antibody | Reacts with human, mouse |
| F4/80 | eBioscience, San Diego, CA, USA | 14-4801-85 | 1:50 (with 1% BSA-PBS) | Rat monoclonal antibody | React with mouse |
| ZO-1 | Invitrogen, Camarillo, CA, USA | 61-7300 | 1:25 (with 1% BSA-PBS) | Rabbit polyclonal antibody | Reacts with human, mouse |
| Collagen IV | Abcam | Ab6586 | 1:100 (with 1% BSA–PBS) | Rabbit polyclonal antibody | Reacts with mouse, rat, cow, human, pig |
| PEDF | Millipore, Billerica, MA, USA | 07-280 | 1:100 (with 1% BSA-PBS) | Rabbit polyclonal antibody | Reacts with Human, Mouse |
| NCAM | Santa Cruz Biotechnology, Santa Cruz, CA, USA | SC-10735 | 1:50 (with 1% BSA-PBS) | Rabbit polyclonal antibody | Reacts with Mouse, Rat, human, canine, bovine, and avian |
| Alexa Fluor 488-conjugated goat anti-rabbit IgG | Invitrogen, Eugene, Oregon, USA | A11008 | 1:100 (with 1% BSA–PBS) | Goat | Reacts with rabbit |
| Alexa Fluor 568-conjugated goat anti-rabbit IgG | Invitrogen, Eugene, OR, USA | A-21069 | 1:100 (with 1% BSA-PBS) | Goat | Reacts with rabbit |
| Alexa Fluor 488-conjugated goat anti-rat IgG | Invitrogen | A-11006 | 1:100 (with 1% BSA-PBS) | Goat | Reacts with rat |
| Alexa Fluor 568-conjugated goat anti-rat IgG | Invitrogen | A-11077 | 1:100 (with 1% BSA-PBS) | Goat | Reacts with rat |
| Alexa Fluor 647-conjugated goat anti-rat IgG | Invitrogen | A-21247 | 1:100 (with 1% BSA-PBS) | Goat | Reacts with rat |
| Goat anti-rabbit IgG-HRP | Santa Cruz Biotechnology | SC-2004 | 1:2000 (with 5% skim milk) | Goat | Reacts with rabbit |
ELISA and WB analysis
PEDF concentration in the stria vascularis of different groups was measured by ELISA, used with commercially available reagents (E91972Mu; Uscn Life Science Inc., Houston, TX, USA) per the manufacturer's instructions. Western blot was used to assess PEDF and neural cell adhesion molecule (NCAM) protein expression in normal cochlea and brain. Strial and brain tissue was isolated and homogenized in lysis buffer with a protease inhibitor cocktail for 30 s. Total protein (50 μg) from each sample was added to a 10% sodium dodecyl sulfate–polyacrylamide gel to detect PEDF, NCAM, and actin. Proteins were electrophoretically transferred to PVDF membranes and blocked with nonfat milk for 1 h at room temperature. Specific immunodetection was carried out by incubation with primary antibodies, either anti-PEDF antibody diluted 1:1000, or anti-NCAM antibody diluted 1:200 in skim milk overnight at 4°C. After 3 washes with TBST, the membranes were incubated for 1 h with secondary antibody, and antigens were assessed using ECL Plus Western blotting Detection Reagents (Amersham, Arlington Heights, IL, USA).
Reverse transcription-polymerase chain reaction (RT-PCR) and real-time quantitative RT-PCR (qRT-PCR)
Total RNA from the stria vascularis of different groups was separately extracted with RNeasy (Qiagen, Valencia, CA, USA) per the manufacturer's recommendations. Each group of 5 mice was analyzed for Pedf, Ncam, Zo1, Ve-cadherin, and Gapgh mRNA with regular PCR and qRT-PCR. Total RNA sample (1 μg) was reverse transcribed with a RETROscript kit (Invitrogen). Primers for Serpinf1 (Pedf), forward, 5′-GGCAGTGGGTAACCAAGTTTG-3′, reverse, 5′-GCAGCTGGGCAATCTTGCAG-3′, defined an amplicon of 156-bp; Ncam1 forward, 5′-TGTTCAAGCAGACACACCGT-3′, reverse, 5′-CAGATGGCTGCATGATCACG-3′, defined an amplicon of 293-bp; Gaphd forward, 5′-ATGTGTCCGTCGTGGATCTGAC-3′, reverse, 5′-AGACAACCTGGTCCTCAGTGTAG-3′, defined an amplicon of 132-bp. The RT-PCR was cycled at 95°C for 2 min, up to 40 cycles of 95°C for 30 s, 60°C for 30 s, 72°C for 30 s, and a final 4-min extension at 72°C. RT-PCR products were visualized by agarose gel electrophoresis. For qRT-PCR, cDNA synthesized from total RNA was diluted 10-fold with DNase-free water, each cDNA sample independently measured 3 times. Transcripts were quantitated by TaqMan gene expression assay (Invitrogen): Serpinf1 (Mm00441270_m1), Ncam1 (Mm00456812_m1), Zo1 (Mm00493699_m1), and Ve-cadherin (Mm03053719_s1) on a model 7300 real-time PCR system (Applied Biosystems, Foster City, CA, USA). The real-time PCR was cycled at 95°C for 20 s, 40 cycles of 95°C for 1 s, 60°C for 20 s. Mouse Gapdh was the endogenous control. Quantitative PCR and analysis were performed per the guidelines provided by Applied Biosystems using the comparative cycle threshold method.
siRNA transfection
Purified PVM/Ms at passage 3 and at a density of 2.5 × 104/cm2 were seeded and grown on 35-mm collagen I-coated glass-bottom dishes overnight. For the control and siRNA model, PVM/Ms were transfected the next day with scrambled siRNA, Ncam, and Pedf siRNA used according to the manufacturer's guidelines. In brief, 10 μl TransIT-TKO reagent was mixed with 250 μl serum-free medium in a sterile tube, and 25 nM siRNA was added to the mixture. After incubation for 15 min, the TransIT-TKO Reagent/siRNA mixture was added to a dish with 1.25 ml of fresh complete growth medium. For the PEDF treatment model, 1 μg/ml PEDF was added to 1.5 ml fresh complete growth medium. Immunocytochemistry was performed at 48 h.
Assessment of vascular permeability
Vascular permeability in control, NE, and PEDF + NE cohorts was assessed using a FITC conjugated bovine albumin tracer (FITC-albumin, 66 kDa, A-9771; Sigma, Cream Ridge, NJ, USA) immediately at the cessation of the NE regime. The tracer was intravenously administered to the tail vein of anesthetized animals 30 min prior to harvesting. Anesthetized animals were perfused intravascularly through the left ventricle with HBSS. The mice were decapitated, and whole mounts of the stria vascularis were imaged on a fluorescent microscope (DM2500; Leica, Wetzlar, Germany).
Vascular leakage in the 3 groups receiving FITC-albumin was analyzed quantitatively. The mice were anesthetized and perfused intravascularly for 5 min with HBSS. The stria vascularis was removed and homogenized in 1% Triton X-100 in PBS, and the lysate was centrifuged at 16,000 rpm for 20 min. Relative fluorescence of the supernatant was measured on a Tecan GENios Plus microplate reader (Tecan Group Ltd, San Jose, CA, USA), with samples for each group run in quintuplicate.
Vascular permeability in vivo was assessed by recording tracer movement through a specially prepared vessel window. Animals were anesthetized and wrapped in a heating pad, with rectal temperature maintained at ∼38.5°C (32). A lateral and ventral approach was used to open the left bulla and open a rectangular fenestration (0.1×01 mm) in the cochlea at the basal turn. FITC conjugated to bovine albumin in saline was administered at a dose of 40 mg/ml intravenously infused for 10 min prior to recording.
For in situ detection, Alexa Fluor-568 conjugated goat anti-human IgG (molecular mass, 200 kDa; A-21090; Invitrogen) was administered i.v. for 2 h. Anesthetized animals were perfused intravascularly through the left ventricle for 2 min with HBSS, followed by 5 min of 4% PFA in PBS. The stria vascularis from each group was removed and postfixed overnight with 4% PFA in PBS (pH 7.2) at 4°C. Whole-mounted stria vascularis was immunolabeled with anti-collagen IV antibody (Table 1). Tissue samples were imaged with an FV1000 Olympus laser-scanning confocal microscope.
Isolation of strial capillaries and analysis of fluorescence intensity
A previously reported “sandwich-dissociation” method of capillary isolation was used (15). Isolated capillaries were immunolabeled with antibodies for ZO-1 and VE-cadherin. Change in immunfluorescence was calculated as (FT − FB)/(FC − FB) × 100%, where FT is the average fluorescence intensity in treated groups, FC is the average fluorescence intensity in control groups, and FB is the background fluorescence.
In-cell Western blotting and transfection with siRNA
PVM/Ms (200 μl) suspended at 11,500 cells/well in a 96-well microplate were incubated for 2 d or until the cells consistently adhered to the bottom of the plate. For the control and siRNA groups, PVM/Ms were transfected with scrambled and siRNA Pedf for 72 h (Applied Biosystems) per the manufacturer's guidelines. In brief, 1.5 μl TransIT-TKO reagent was mixed with 27 μl serum-free medium containing 25 nM siRNA. The TransIT-TKO Reagent/siRNA mixture was added to the 96-well plate for 72 h. For the PEDF-treated group, PEDF diluted 0.01 μg/ml to 1 μg/ml was dispensed separately to microplate wells 72 h prior to analysis. The cells were fixed in 4% formaldehyde for 20 min and washed 5 times with PBS containing 0.1% Triton X-100 for permeabilization, blocked with LI-COR Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE, USA) for 90 min, and incubated with primary antibody for NCAM, sc-10735, or β-actin for 2 h (information on the antibodies can be found in Table 1). The plate was washed 5 times with PBS containing 0.1% Tween-20 and incubated with secondary antibody diluted in Odyssey Blocking Buffer at 1:200, donkey anti-mouse IRDye 800CW (926–32212), and donkey anti-rabbit IRDye 680RD (926–68073) for 1 h at room temperature, and washed 3 times in PBS. Stained cells were imaged and analyzed with an Odyssey Imager (LI-COR).
PEDF treatment
Mouse PEDF (50235-M08H; Sino Biological Inc., Beijing, China) was administered as acute, single-dose (i.v) injections (administered as a 50 μl volume, 10 μg/100 g body weight, dissolved in a saline vehicle) 30 min prior to NE. A similar dose was given the next d 30 min prior to NE. Control animals received the same volume of vehicle. Animals in the PEDF-treated group were euthanized immediately after the cessation of NE. PEDF concentration in the stria vascularis was assessed by ELISA.
Statistics
All experiments were performed multiple times to validate the observations, with the data expressed as means ± sd. Statistical analysis was conducted using the Student's t test for comparison of 2 groups or ANOVA for comparisons of ≥3 groups. A 95% confidence level was considered statistically significant.
RESULTS
Noise affects PVM/M morphology and down-regulates PVM/M-derived PEDF in the stria vascularis
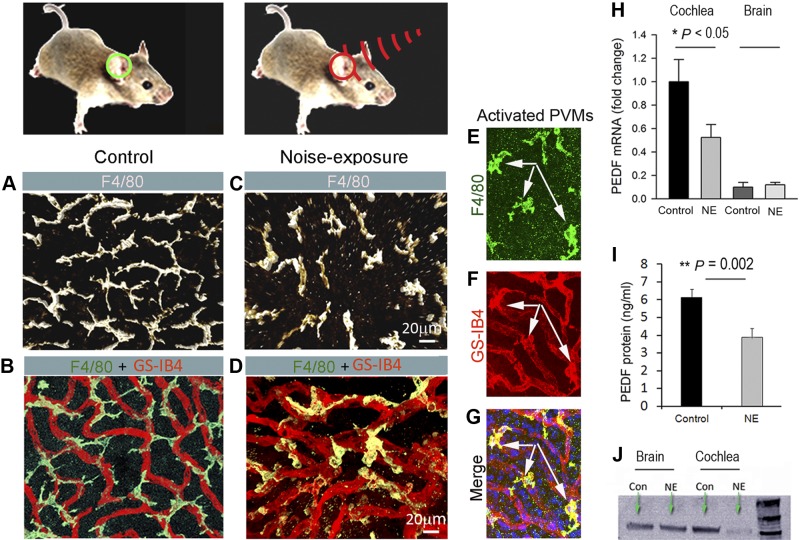
In control tissue, most PVM/Ms exhibit branched (dendrite) morphology. The cells arrange in a self-avoidance pattern (Fig. 1A), which allows for maximum coverage of the capillary wall (Fig. 1B). The morphology of PVM/Ms in animals exposed to wide-band noise at 120 dB SPL for 3 h/d for 2 consecutive days is markedly changed. Some of the PVM/Ms become smaller with shorter processes (Fig. 1C). Approximately 15–20% of the PVM/Ms become flat and amoeboid shaped (Fig. 1C) in less physical contact with the capillary (Fig. 1D). Some PVM/Ms are activated and display a terminal galactopyranosyl group on their membrane surface binding the lectin Griffonia simplicifolia-IB4 (GS-IB4; Fig. 1E–G). The data show that PVM/Ms are highly responsive to noise stimulation.
Figure 1.
NE affects PVM/M morphology and secretion of PEDF. A) Confocal image shows PVM/M morphology. B) PVM/M distribution on strial capillaries labeled with GS-IB4 in a control animal. C) PVM/Ms in noise-exposed animals show reduced branching and withdrawal of ramifications. D) PVM/Ms display less physical contact with capillaries in noise-exposed animals. E–G) Triple-labeled whole-mounted stria vascularis shows PVM/Ms labeled with antibody for F4/80 also positive for GS-IB4. H) qRT-PCR analysis shows decreased mRNA expression for Pedf in noise-exposed mice relative to controls (n=3). *P < 0.05. I) ELISA analysis shows significantly decreased production of PEDF in noise-exposed groups relative to controls (n=6). **P < 0.01; 1-way ANOVA. J) Western blot analysis shows NE to dramatically decrease PEDF protein in the stria vascularis, but not in brain tissue.
PEDF, a 50-kDa glycoprotein secreted by PVM/Ms stabilizing intrastrial fluid–blood barrier integrity (19), is significantly down-regulated by noise at both the transcript mRNA and protein level. Significant down-regulation of Pedf mRNA is shown by quantitative real-time PCR analysis (Fig. 1H). Corresponding to down-regulation of the transcripts, production of PEDF protein in the stria vascularis, measured by ELISA (Fig. 1I) or Western blot (Fig. 1J), is also significantly reduced. In contrast, NE causes no change in expression of PEDF in brain. The data indicate that PVM/M-derived PEDF is significantly down-regulated in the stria vascularis of animals exposed to acoustic trauma.
Down-regulation of PVM/M-derived PEDF causes intrastrial fluid barrier breakdown
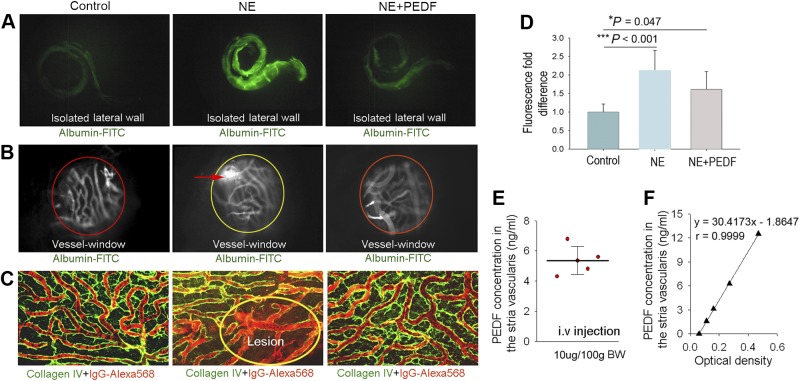
The intrastrial fluid barrier, restricting movement of molecules from blood into the ear, is essential for maintaining cochlear homeostasis under normal conditions. The barrier becomes highly permeable to large substances such as albumin-FITC and IgG Alexa Fluro-568 with exposure to wide-band noise at 120 dB SPL for 3 h/d for 2 consecutive days. Albumin-FITC administered 30 min prior to harvesting accumulates in isolated noise-exposed lateral walls from leakage (Fig. 2A, middle panel). Significant leakage of albumin-FITC across the barrier in noise-exposed animals was also observed in “vessel-windows” in vivo under intravital fluorescence microscopy (Fig. 2B, middle panel). Immunohistochemical staining in combination with confocal microscopy demonstrated extravasation of high-molecular-weight IgG Alexa Fluro-568 tracers in the tissues of noise-exposed animals (Fig. 2C, middle panel). Treatment of noise-exposed animals with PEDF at a dose of 10 μg/100 g bw dramatically reduces local lesioning and significantly decreases vascular permeability to low and high-molecular-weight tracers (Fig. 2A–D, right panel). Using an ELISA technique, we measured on the order of 5 ng/ml of PEDF protein, approximately the normal level, in the stria vascularis of noise-exposed animals assessed at the close of the noise regime after administration of PEDF at 10 μg/100 g bw (Fig. 2E). Figure 2F is a representative ELISA standard calibration curve for PEDF. The data show application of PEDF to significantly protect against noise-induced deterioration in barrier integrity.
Figure 2.
Down-regulation of PEDF causes disruption of the intrastrial fluid-blood barrier. A) Fluorescent tracers (albumin-FITC) accumulate in the cochlear lateral wall of noise-exposed animals (middle panel) but not in control animals (left panel). The accumulation is attenuated in PEDF treated noise-exposed animals (rignt panel). B) Albumin-FITC tracer is shown to extravasate from capillaries in noise-exposed living animals (open “vessel-window” method, middle panel) but not in control animals (left panel). Less extravasation is seen in PEDF-treated noise-exposed animals (right panel). C) High-molecular-weight IgG-Alexa Fluor 568 tracer, visualized by collagen IV immunostaining (green), extravasates from capillaries in noise-exposed mice (middle panel) but not in control (left panel) and PEDF-treated animals (right panel). Fluorescence IgG-Alexa fluor 568 tracer (red) leaks from lesioned capillaries (oval circle region). D) Treatment with PEDF significantly reduces capillary leakage (n=9). Data are expressed as means ± sd. *P < 0.05, ***P < 0.01; 1-way ANOVA. E) Localized PEDF concentration in the stria vascularis was assessed for a range of intravenously administered PEDF doses. F) Representative ELISA standard calibration curve for PEDF.
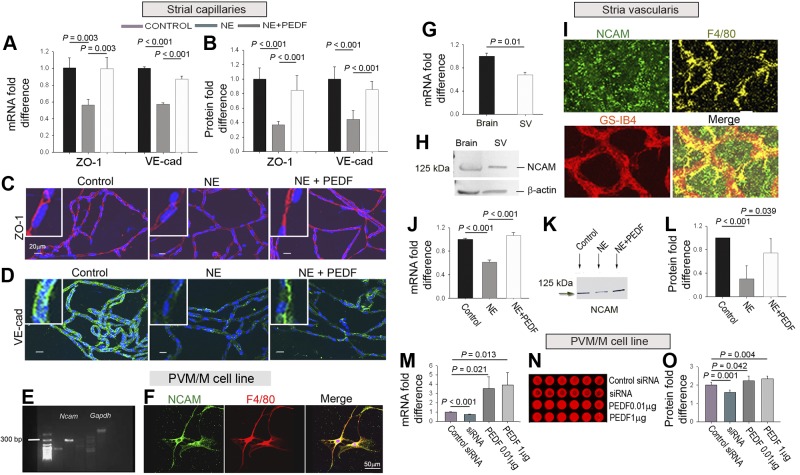
Figure 3.
PEDF ameliorates noise-induced suppression of TJ proteins. A) qRT-PCR shows PEDF to attenuate noise-induced down-regulation of ZO-1 (n=3) and VE-cadherin mRNA (n=3). B) PEDF attenuates down-regulation of ZO-1 (n=9) and VE-cadherin protein (n=9). C, D) PEDF-treated noise-exposed groups show reduced suppression of ZO-1 (C, red) and VE-cadherin (D, green). E) Agarose gel of Ncam mRNA in the PVM/M cell line. F) PVM/M cell line labeled for NCAM (green), F4/80 (red), and nuclei (Hoechst, blue). G) qRT-PCR Ncam mRNA in strial tissue and brain tissue control. H) Western blot of NCAM protein in strial tissue and brain tissue control. I) NCAM (green) labeled whole-mounted stria vascularis, capillaries labeled by GS-IB4 (red) and PVM/Ms for F4/80 (yellow). J) qRT-PCR Ncam mRNA in the stria vascularis, transcript mRNA for Ncam expression in the 3 groups (n=3). K) Western blot of NCAM in the 3 groups. L) PEDF restores NCAM in noise-exposed groups (n=4). M–O) Concurrent expression of PEDF and NCAM transcript mRNA (M, N) and protein (O) (n=6).
Intrastrial fluid barrier breakdown is associated with down-regulation of endothelial-endothelial TJ proteins and NCAM-120
Intrastrial fluid-blood barrier integrity is reflected in the state of endothelial TJs (33). PEDF plays a central role in functionally regulating endothelial cell-cell junction proteins, including ZO-1 and VE-cadherin (19). Consistent with this regulation, we find that PEDF treatment attenuates noise-induced down-regulation of ZO-1 and VE-cadherin at both the transcript (Fig. 3A) and protein level (Fig. 3B). Immunohistochemical examination by confocal microscopy clearly shows attenuation of the noise-induced reduction in ZO-1 and VE-cadherin expression between endothelial cells in PEDF treated noise-exposed animals (Fig. 3C, D).
Intrastrial fluid-blood barrier integrity not only correlates with the state of endothelial cell TJs but also with the interaction between endothelial cells and PVM/Ms (33). NCAM, a membrane-bound glycoprotein cell adhesion molecule with an essential role in cell-cell and cell-extracellular matrix binding, was recently detected in our PVM/M primary cell line by next-generation sequencing (https://dnanexus.com). We report that, of the 3 identified isoforms of NCAM (NCAM-120, NCAM-140, and NCAM-180) (34), NCAM-120 expressed in PVMs and in the surrounding extracellular matrix. Figure 3E shows expression of the Ncam gene in the PVM/M cell line detected with reverse transcriptase PCR, and Fig. 3F shows NCAM protein expression detected by immunohistochemical staining. Ncam mRNA in the strial tissue was assessed by quantitative real-time PCR (qRT-PCR), with brain tissue serving as the control (Fig. 3G), while NCAM protein expression was assessed by Western blot (Fig. 3H), with its distribution immunohistochemically verified by confocal fluorescent microscopy (Fig. 3I). Our results show NCAM primarily expressed in PVM/Ms and in the extracellular matrix around PVM/Ms. NCAM expression in the stria vascularis is significantly decreased at the transcript mRNA (Fig. 3J) and protein level when animals are exposed to noise (Fig. 3K). Administration of PEDF attenuates the down-regulation of NCAM (Fig. 3L). Parallel expression of PEDF and NCAM at the transcript mRNA and protein level was further confirmed in an in vitro model using primary cell lines (Fig. 3M–O). The data suggest that PEDF not only up-regulates endothelial cell-endothelial TJ protein expression but also promotes PVM/M-endothelial contacts.
PEDF restores the intrastrial barrier
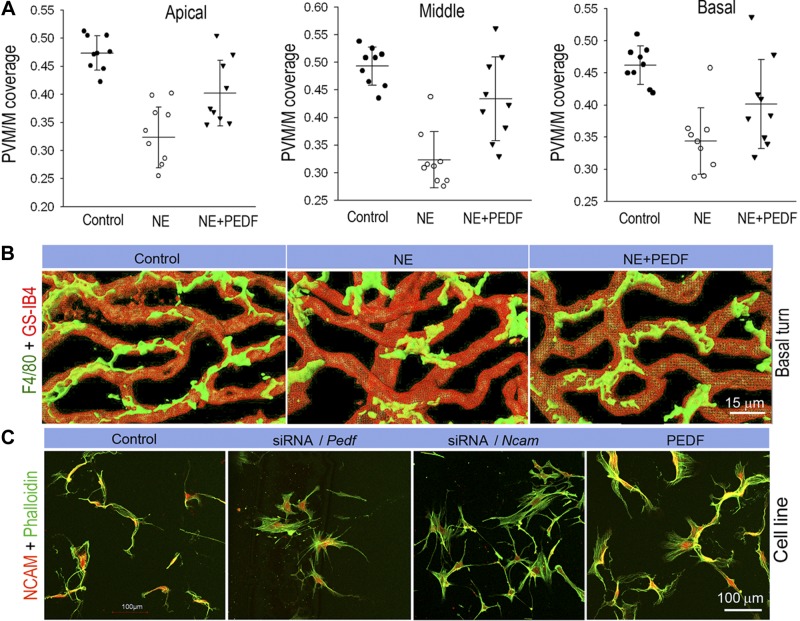
Noise-exposed groups display significantly reduced PVM/M coverage on blood vessels in all apical, middle, and basal regions in noise-exposed groups (Fig. 4A). Confocal z stacks show PVM/Ms from the control group to have ramified processes interfacing with blood vessels (Fig. 4B, left panel) in comparison to the noise-exposed group which shows less branching (Fig. 4B, middle panel). PVM/M coverage is improved with PEDF treatment of the noise-exposed group (Fig. 4B, right panel).
Figure 4.
PEDF has a restorative effect on blood tissue barrier architecture after noise damage. A) Noise-exposed groups display significantly reduced PVM/M density on blood vessels in apical, middle, and basal regions in noise exposed groups. PEDF treatment promotes PVM/M coverage in apical (n=9; P < 0.001 for NE vs. control, P = 0.03 for NE+PEDF vs. NE; 1-way ANOVA test), middle (n=9; P < 0.01 for NE vs. control, P < 0.001 for NE+PEDF vs. NE; 1-way ANOVA test), and basal (n=9; P < 0.001 for NE vs. control, P = 0.008 for NE+PEDF vs. NE; 1-way ANOVA test) regions. Data are expressed as means ± sd. B) Three-dimensional rendering of confocal z stacks highlights the degree of ramification of PVM/M processes in noise-exposed, and PEDF-treated noise-exposed groups. PVM/Ms are labeled with antibody for F4/80 and GS-IB4. C) Control in vitro PVM/Ms are double labeled with phalloidin for F-actin and an antibody for NCAM-120 to show the branched morphology. PVM/Ms in vitro show withdrawal of ramified branches when Pedf and Ncam expressions are down-regulated with siRNA. PEDF treatment promotes ramified branching in PVM/M.
How does PEDF affect PVM/M coverage on ECs? Does PEDF regulate PVM/M dendrite morphology? To explore these effects on PVM/M morphology, we used an siRNA-targeting vector to suppress Pedf expression in an in vitro model and observed for changes in PVM/M morphology. PVM/Ms were transfected with siRNA for 48 h and double-labeled with phalloidin for F-actin and an antibody for NCAM. A number of obvious morphological changes were observed in PVM/Ms with down-regulation of PEDF. Down-regulation of PEDF caused PVM/Ms to withdraw dendrite branching relative to controls. Conversely, elevation of PEDF promoted ramified branching (Fig. 4C). The data suggest PEDF is required to maintain dendrite arbors in PVM/Ms.
PEDF improves EP and hearing function
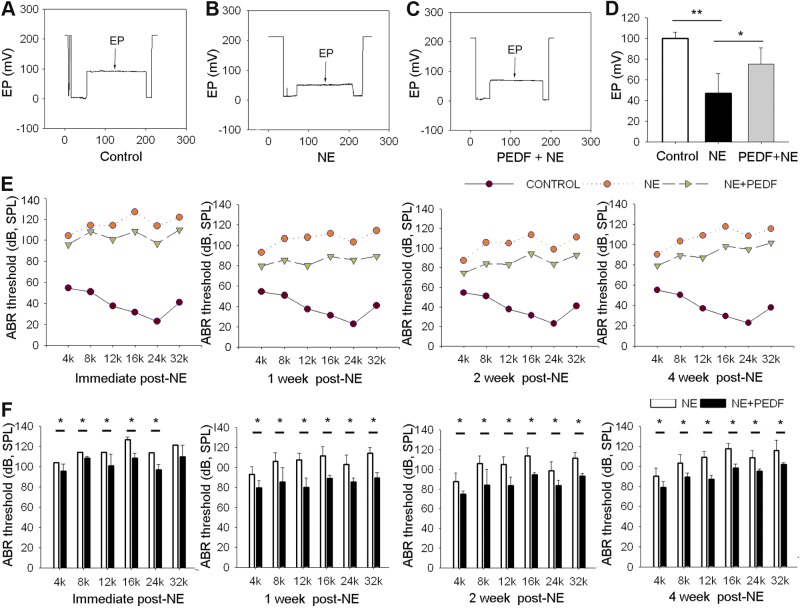
EP and hearing function are significantly improved with PEDF treatment. In mice, the EP is usually about +100 mV (35, 36). In this study, normal EP in our control group is ∼80–100 mV (Fig. 5A). EP in animals exposed to wide-band noise at 120 dB SPL for 3 h/d for 2 consecutive days is reduced ∼50 mV (Fig. 5B). However, PEDF treatment attenuates the noise caused drop in EP (Fig. 5C). Figure 5D shows the average EP in control-, NE-, and PEDF+NE-treated groups. Consistent with our previous report (11), wide-band noise causes profound hearing loss at most frequencies as assessed by auditory brain-stem response (Fig. 5E). PEDF treatment, however, reduces hearing loss and significantly improves hearing function at 1, 2, and 4 wk after NE (Fig. 5F).
Figure 5.
PEDF restores endocochlear potential and hearing function. A–C) Representative EP values in control (A), noise-exposed (B), and PEDF-treated noise-exposed animals. D) Mean value of EP in the 3 groups. PEDF significantly attenuates the drop in EP (n=6). *P < 0.05, ***P < 0.001; 1-way ANOVA test. E) Hearing function is assessed by ABR at 1, 2, and 4 after NE. F) Mean ABR threshold in PEDF-treated mice is significantly lower than in untreated noise-exposed mice (n=6). *P < 0.05; Student's t test.
DISCUSSION
In this study, we identify a mechanism by which reactive PVM/Ms mediate disruption of the blood barrier in the ear. The mechanism may provide the means for inhibiting the breakdown of the blood barrier, opening a novel therapeutic approach for remediating noise-induced hearing loss. Our findings show that activation of PVM/Ms and concurrent down-regulation of tissue resident macrophage-derived PEDF expression are key factors in the hyperpermeability of the intrastrial fluid-blood barrier caused by acoustic trauma. Application of PEDF to the noise-damaged ear remediates vascular hyperpermeability in animal models, reduces noise-induced EP drop, and significantly improves hearing outcomes. These studies point to the critical role PVM/Ms and secreted PEDF play in regulating intrastrial fluid-blood barrier integrity in healthy and noise-damaged ears.
The intrastrial fluid–blood barrier in the inner ear is critical for maintaining ionic and metabolic homeostasis essential for hearing function (2, 37). Dysfunction of the barrier is associated with a number of hearing disorders (3–8, 10, 11). In a previous article, we demonstrated that PVM/Ms, requisite for establishing the EP, control the integrity of the intrastrial fluid–blood barrier by affecting the expression of TJ and adherens-junction proteins. PEDF secreted by PVM/Ms was identified as an essential signaling molecule. In the present study, we further show that acoustic trauma causes PVM/Ms to lose the capacity to produce PEDF. The reduction in PEDF causes substantial down-regulation of tight- and adherens-junction proteins. PEDF delivery to the acoustic damaged ear reduces noise-induced barrier leakage and attenuates the down-regulation of junction proteins such as ZO-1 and VE-cadherin. Although the mechanism by which PEDF regulates TJs in the intrastrial fluid–blood barrier is not known, studies in other tissues have shown PEDF to regulate TJs by targeting the PI3K/Akt signaling pathway (19), phosphorylating adherens-junction proteins through a γ-secretase–mediated pathway (33).
In addition to the PEDF effect on TJs, PEDF is also shown to promote PVM/M dendrite growth and arborization, restoring PVM/Ms to a normal morphology. PEDF has also been demonstrated to regulate dendrite arborization and growth in a neuronal system. An NCAM-based mechanism is plausibly involved, as in vitro-cultured PVM/Ms transfected with Ncam siRNA for 48 h results in obvious morphological changes including withdrawal of dendrite branching. Our findings are in accord with NCAM regulation of dendrite arborization and growth in a neuronal system (38, 39). NCAM, a membrane-bound glycoprotein playing an essential role in cell-cell and cell-extracellular matrix binding (40), is shown to be expressed in PVM/Ms. In in vitro models using primary PVM/M cell lines we find parallel expression of PEDF and NCAM at the transcript mRNA and protein level. Likewise, in an in vivo animal model we find noise causes NCAM expression to significantly decrease at the mRNA and protein level. Elevation of PEDF in the cochlea attenuates the down-regulation of NCAM. PEDF promotes NCAM expression and intercellular contacts between PVM/Ms and endothelial cells, and thereby reinforces intrastrial fluid-blood barrier integrity.
Normal functioning of PVM/Ms is critical for establishing the EP (28, 41, 42) and normal strial function (28, 29, 43, 44). Hearing loss results when the EP, normally sustained by the fluid-blood barrier, is not adequately maintained, resulting in an intrastrial electric shunt (6, 19). PVM/Ms, and its secreted PEDF, have a salutary effect on EP by restoring the integrity of the intrastrial fluid–blood barrier, providing immediate or delayed improvement to hearing function after noise damage (A synopsis of PEDF's restorative effects on the intrastrial fluid–blood barrier is illustrated in Fig. 6). Improved hearing function from PEDF treatment could be related to the salutary effect it has on functional recovery of PVM/Ms.
Figure 6.
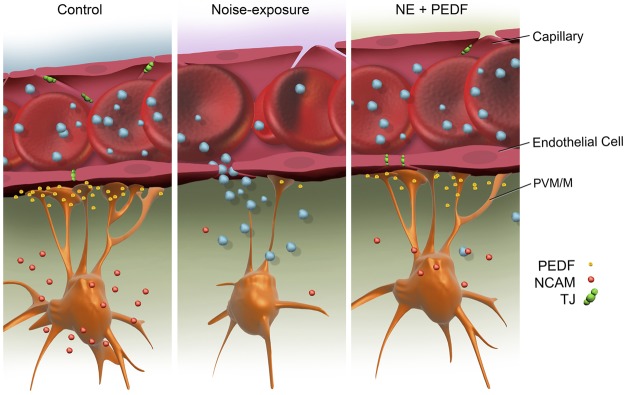
PEDF remodeling of the intrastrial fluid–blood barrier. Under normal conditions, tight-adherens junctions between capillary endothelial cells link adjacent cells and limit paracellular transport. PEDF secreted by PVM/Ms regulates vascular permeability by affecting expression of tight- and adherens-junction proteins. The branched (dendrite) morphology of PVM/Ms maximizes coverage of the capillary wall. Acoustic trauma causes down-regulation of PEDF, detachment of PVM/M end-feet from endothelial cells, and decreased expression of TJ and adherens-junction proteins, disrupting the intrastrial fluid-blood barrier. Leakage of serum proteins from loosened TJs results in cochlear edema. Delivery of PEDF to the damaged ear ameliorates hearing loss by restoring intrastrial fluid-blood barrier integrity. PEDF up-regulates the expression of TJ and adherens-junction proteins, including ZO-1 and VE-cadherin, and adhesive proteins such as NCAM, and restores intrastrial fluid-blood barrier integrity.
Normal PVM/Ms, as melanocytes, are biosensors responsive to biological and physicochemical signals in the local environment, producing melanin pigment in response to noxious factors (45, 46). The melanin has an essential role in buffering calcium, scavenging heavy metals, and promoting antioxidant activity (44, 45, 47–51), although the contributions of this melanogenic activity to sustaining the integrity of the intrastrial fluid-blood barrier are yet to be investigated.
In summary, our report provides new insights into the role played by PVM/Ms and down-regulated secretion of the signaling molecule PEDF in the pathological deterioration of ear health after noise damage. The effectiveness of PEDF in ameliorating noise-induced effects on vascular permeability and PVM/M morphology suggests new strategies for treating hearing disorders in which barrier integrity is compromised.
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) grants NIH NIDCD DC008888-02A1 (X.R.S.), NIH NIDCD DC008888-02S1 (X.R.S.), NIH NIDCD R21 DC012398 (X.R.S.), NIH NIDCD R01-DC010844 (X.R.S.), NIHP30-DC005983.
The authors declare no conflicts of interest.
Footnotes
- ABR
- auditory brain-stem response
- EP
- endocochlear potential
- GS-IB4
- Griffonia simplicifolia-IB4
- NCAM
- neural cell adhesion molecule
- NE
- noise exposure
- PEDF
- pigment epithelium growth factor
- PVM/M
- perivascular resident macrophage-like melanocyte
- TJ
- tight junction;
REFERENCES
- 1. Juhn S. K., Hunter B. A., Odland R. M. (2001) Blood-labyrinth barrier and fluid dynamics of the inner ear. Int. Tinnitus J. 7, 72–83 [PubMed] [Google Scholar]
- 2. Juhn S. K., Rybak L. P. (1981) Labyrinthine barriers and cochlear homeostasis. Acta Otolaryngol. 91, 529–534 [DOI] [PubMed] [Google Scholar]
- 3. Lin D. W., Trune D. R. (1997) Breakdown of stria vascularis blood-labyrinth barrier in C3H/lpr autoimmune disease mice. Otolaryngol. Head Neck Surg. 117, 530–534 [DOI] [PubMed] [Google Scholar]
- 4. Doi K., Mori N., Matsunaga T. (1992) Adenylate cyclase modulation of ion permeability in the guinea pig cochlea: a possible mechanism for the formation of endolymphatic hydrops. Acta Otolaryngol. 112, 667–673 [DOI] [PubMed] [Google Scholar]
- 5. McMenomey S. O., Russell N. J., Morton J. I., Trune D. R. (1992) Stria vascularis ultrastructural pathology in the C3H/lpr autoimmune strain mouse: a potential mechanism for immune-related hearing loss. Otolaryngol. Head Neck Surg. 106, 288–295 [DOI] [PubMed] [Google Scholar]
- 6. Cohen-Salmon M., Regnault B., Cayet N., Caille D., Demuth K., Hardelin J. P., Janel N., Meda P., Petit C. (2007) Connexin30 deficiency causes instrastrial fluid-blood barrier disruption within the cochlear stria vascularis. Proc. Natl. Acad. Sci. U. S. A. 104, 6229–6234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klein M., Koedel U., Kastenbauer S., Pfister H. W. (2008) Nitrogen and oxygen molecules in meningitis-associated labyrinthitis and hearing impairment. Infection 36, 2–14 [DOI] [PubMed] [Google Scholar]
- 8. Kellerhals B. (1972) Acoustic trauma and cochlear microcirculation. An experimental and clinical study on pathogenesis and treatment of inner ear lesions after acute noise exposure. Adv. Otorhinolaryngol. 18, 91–168 [PubMed] [Google Scholar]
- 9. Hukee M. J., Duvall A. J., 3rd (1985) Cochlear vessel permeability to horseradish peroxidase in the normal and acoustically traumatized chinchilla: a reevaluation. Ann. Otol. Rhinol. Laryngol. 94, 297–303 [PubMed] [Google Scholar]
- 10. Suzuki M., Yamasoba T., Ishibashi T., Miller J. M., Kaga K. (2002) Effect of noise exposure on blood-labyrinth barrier in guinea pigs. Hear. Res. 164, 12–18 [DOI] [PubMed] [Google Scholar]
- 11. Shi X. (2009) Cochlear pericyte responses to acoustic trauma and the involvement of hypoxia-inducible factor-1alpha and vascular endothelial growth factor. Am. J. Pathol. 174, 1692–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirose K., Liberman M. C. (2003) Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J. Assoc. Res. Otolaryngol. 4, 339–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salt A. N. (2004) Acute endolymphatic hydrops generated by exposure of the ear to nontraumatic low-frequency tones. J. Assoc. Res. Otolaryngol. 5, 203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dai M., Yang Y., Omelchenko I., Nuttall A. L., Kachelmeier A., Xiu R., Shi X. (2010) Bone marrow cell recruitment mediated by inducible nitric oxide synthase/stromal cell-derived factor-1alpha signaling repairs the acoustically damaged cochlear blood-labyrinth barrier. Am. J. Pathol. 177, 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang Y., Dai M., Wilson T. M., Omelchenko I., Klimek J. E., Wilmarth P. A., David L. L., Nuttall A. L., Gillespie P. G., Shi X. (2011) Na+/K+-ATPase alpha1 identified as an abundant protein in the blood-labyrinth barrier that plays an essential role in the barrier integrity. PLoS One 6, e16547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng G., Hu B. H. (2012) Cell-cell junctions: a target of acoustic overstimulation in the sensory epithelium of the cochlea. BMC Neurosci. 13, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prat A., Biernacki K., Wosik K., Antel J. P. (2001) Glial cell influence on the human blood-brain barrier. Glia 36, 145–155 [DOI] [PubMed] [Google Scholar]
- 18. Abbott N. J., Ronnback L., Hansson E. (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53 [DOI] [PubMed] [Google Scholar]
- 19. Zhang W., Dai M., Fridberger A., Hassan A., Degagne J., Neng L., Zhang F., He W., Ren T., Trune D., Auer M., Shi X. (2012) Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc. Natl. Acad. Sci. U. S. A. 109, 10388–10393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu J. T., Chen Y. L., Chen W. C., Chen H. Y., Lin Y. W., Wang S. H., Man K. M., Wan H. M., Yin W. H., Liu P. L., Chen Y. H. (2012) Role of pigment epithelium-derived factor in stem/progenitor cell-associated neovascularization. J. Biomed. Biotechnol. 2012, 871272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ueda S., Yamagishi S. I., Okuda S. (2010) Anti-vasopermeability effects of PEDF in retinal-renal disorders. Curr. Mol. Med. 10, 279–283 [DOI] [PubMed] [Google Scholar]
- 22. Bouck N. (2002) PEDF: anti-angiogenic guardian of ocular function. Trends Mol. Med. 8, 330–334 [DOI] [PubMed] [Google Scholar]
- 23. Park K., Lee K., Zhang B., Zhou T., He X., Gao G., Murray A. R., Ma J. X. (2011) Identification of a novel inhibitor of the canonical Wnt pathway. Mol. Cell. Biol. 31, 3038–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park K., Jin J., Hu Y., Zhou K., Ma J. X. (2011) Overexpression of pigment epithelium-derived factor inhibits retinal inflammation and neovascularization. Am. J. Pathol. 178, 688–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu H., Ren J. G., Cooper W. L., Hawkins C. E., Cowan M. R., Tong P. Y. (2004) Identification of the antivasopermeability effect of pigment epithelium-derived factor and its active site. Proc. Natl. Acad. Sci. U. S. A. 101, 6605–6610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sheikpranbabu S., Haribalaganesh R., Lee K. J., Gurunathan S. (2010) Pigment epithelium-derived factor inhibits advanced glycation end products-induced retinal vascular permeability. Biochimie (Paris) 92, 1040–1051 [DOI] [PubMed] [Google Scholar]
- 27. Argaw A. T., Gurfein B. T., Zhang Y., Zameer A., John G. R. (2009) VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. U. S. A. 106, 1977–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steel K. P., Barkway C. (1989) Another role for melanocytes: their importance for normal stria vascularis development in the mammalian inner ear. Development 107, 453–463 [DOI] [PubMed] [Google Scholar]
- 29. Slominski A., Paus R., Schadendorf D. (1993) Melanocytes as “sensory” and regulatory cells in the epidermis. J. Theor. Biol. 164, 103–120 [DOI] [PubMed] [Google Scholar]
- 30. Ni C., Zhang D., Beyer L. A., Halsey K. E., Fukui H., Raphael Y., Dolan D. F., Hornyak T. J. (2013) Hearing dysfunction in heterozygous Mitf(Mi-wh) /+ mice, a model for Waardenburg syndrome type 2 and Tietz syndrome. Pigment Cell Melanoma Res. 26, 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neng L., Zhang W., Hassan A., Zemla M., Kachelmeier A., Fridberger A., Auer M., Shi X. (2013) Isolation and culture of endothelial cells, pericytes and perivascular resident macrophage-like melanocytes from the young mouse ear. Nat. Protoc. 8, 709–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shi X., Nuttall A. L. (2002) The demonstration of nitric oxide in cochlear blood vessels in vivo and in vitro: the role of endothelial nitric oxide in venular permeability. Hear. Res. 172, 73–80 [DOI] [PubMed] [Google Scholar]
- 33. Neng L., Zhang F., Kachelmeier A., Shi X. (2012) Endothelial cell, pericyte, and perivascular resident macrophage-type melanocyte interactions regulate cochlear intrastrial fluid-blood barrier permeability. J. Assoc. Res. Otolaryngol. 14, 175–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. He H. T., Finne J., Goridis C. (1987) Biosynthesis, membrane association, and release of N-CAM-120, a phosphatidylinositol-linked form of the neural cell adhesion molecule. J. Cell Biol. 105, 2489–2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Steel K. P., Bock G. R. (1980) The nature of inherited deafness in deafness mice. Nature 288, 159–161 [DOI] [PubMed] [Google Scholar]
- 36. Steel K. P., Bock G. R. (1983) Cochlear dysfunction in the jerker mouse. Behav. Neurosci. 97, 381–391 [DOI] [PubMed] [Google Scholar]
- 37. Shi X. (2011) Physiopathology of the cochlear microcirculation. Hear. Res. 282, 10–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tan Z. J., Peng Y., Song H. L., Zheng J. J., Yu X. (2010) N-cadherin-dependent neuron-neuron interaction is required for the maintenance of activity-induced dendrite growth. Proc. Natl. Acad. Sci. U. S. A. 107, 9873–9878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Francavilla C., Loeffler S., Piccini D., Kren A., Christofori G., Cavallaro U. (2007) Neural cell adhesion molecule regulates the cellular response to fibroblast growth factor. J. Cell Sci. 120, 4388–4394 [DOI] [PubMed] [Google Scholar]
- 40. Hinsby A. M., Berezin V., Bock E. (2004) Molecular mechanisms of NCAM function. Front. Biosci. 9, 2227–2244 [DOI] [PubMed] [Google Scholar]
- 41. Trowe M. O., Maier H., Petry M., Schweizer M., Schuster-Gossler K., Kispert A. (2011) Impaired stria vascularis integrity upon loss of E-cadherin in basal cells. Dev. Biol. 359, 95–107 [DOI] [PubMed] [Google Scholar]
- 42. Cable J., Barkway C., Steel K. P. (1992) Characteristics of stria vascularis melanocytes of viable dominant spotting (Wv/Wv) mouse mutants. Hear. Res. 64, 6–20 [DOI] [PubMed] [Google Scholar]
- 43. Tachibana M. (1999) Sound needs sound melanocytes to be heard. Pigment Cell Res. 12, 344–354 [DOI] [PubMed] [Google Scholar]
- 44. Ohlemiller K. K., Rice M. E., Lett J. M., Gagnon P. M. (2009) Absence of strial melanin coincides with age-associated marginal cell loss and endocochlear potential decline. Hear. Res. 249, 1–14 [DOI] [PubMed] [Google Scholar]
- 45. Slominski A. (2009) Neuroendocrine activity of the melanocyte. Exp. Dermatol. 18, 760–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sulaimon S. S., Kitchell B. E. (2003) The biology of melanocytes. Vet. Dermatol. 14, 57–65 [DOI] [PubMed] [Google Scholar]
- 47. Murillo-Cuesta S., Contreras J., Zurita E., Cediel R., Cantero M., Varela-Nieto I., Montoliu L. (2010) Melanin precursors prevent premature age-related and noise-induced hearing loss in albino mice. Pigment Cell Melanoma Res. 23, 72–83 [DOI] [PubMed] [Google Scholar]
- 48. Bush W. D., Simon J. D. (2007) Quantification of Ca(2+) binding to melanin supports the hypothesis that melanosomes serve a functional role in regulating calcium homeostasis. Pigment Cell Res. 20, 134–139 [DOI] [PubMed] [Google Scholar]
- 49. Drager U. C. (1985) Calcium binding in pigmented and albino eyes. Proc. Natl. Acad. Sci. U. S. A. 82, 6716–6720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Slominski A., Zmijewski M. A., Pawelek J. (2012) L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 25, 14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Plonka P. M., Passeron T., Brenner M., Tobin D. J., Shibahara S., Thomas A., Slominski A., Kadekaro A. L., Hershkovitz D., Peters E., Nordlund J. J., Abdel-Malek Z., Takeda K., Paus R., Ortonne J. P., Hearing V. J., Schallreuter K. U. (2009) What are melanocytes really doing all day long.? Exp. Dermatol. 18, 799–819 [DOI] [PMC free article] [PubMed] [Google Scholar]