Abstract
The human leukocyte antigen (HLA)-G is a tolerogenic molecule, whose expression by allografts is associated with better acceptance. An increasing interest in producing HLA-G as a clinical-grade molecule for therapy use is impaired by its complexity and limited stability. Our purpose was to engineer simpler and more stable HLA-G-derived molecules than the full-length HLA-G trimolecular complex that are also tolerogenic, functional as soluble molecules, and compatible with good manufacturing practice (GMP) production conditions. We present two synthetic molecules: (α3-L)x2 and (α1-α3)x2 polypeptides. We show their capability to bind the HLA-G receptor LILRB2 and their functions in vitro and in vivo. The (α1-α3)x2 polypeptide proved to be a potent tolerogenic molecule in vivo: One treatment of skin allograft recipient mice with (α1-α3)x2 was sufficient to significantly prolong graft survival, and four weekly treatments induced complete tolerance. Furthermore, (α1-α3)x2 was active as a soluble molecule and capable of inhibiting the proliferation of tumor cell lines, as does the full length HLA-G trimolecular complex. Thus, the synthetic (α1-α3)x2 polypeptide is a stable and simpler alternative to the full-length HLA-G molecule. It can be produced under GMP conditions, it functions as a soluble molecule, and it is at least as tolerogenic as HLA-G in vivo.—LeMaoult, J., Daouya, M., Wu, J., Loustau, M., Horuzsko, A., Carosella, E. D. Synthetic HLA-G proteins for therapeutic use in transplantation.
Keywords: engineered molecules, immune regulation, therapy, tolerance
Over the past few years, human leukocyte antigen G (HLA-G) has been described as a tolerogenic molecule that allows the fetus to elude the maternal immune response. The expression of the nonclassical HLA class I molecule HLA-G was originally observed and characterized on throphoblasts, and it was demonstrated that it conferred protection to the semiallogeneic fetus from the maternal immune system (1, 2). HLA-G differs from classical MHC class I molecules by its genetic diversity, expression, structure, and functions. HLA-G is characterized by a relatively low allelic polymorphism and a highly restricted tissue distribution. HLA-G constitutive expression is restricted mainly to trophoblast cells (1) and to adult thymic medulla (3), pancreatic islets (4), and stem cells (5, 6). However, HLA-G can be neoexpressed in pathological conditions, such as cancers (7), transplantation (8), inflammatory and autoimmune diseases (9), and viral infections (10).
Seven isoforms of HLA-G have been identified, products of the alternative splicing of a unique mRNA transcript. Four of these are membrane bound (HLA-G1 to -G4), and 3 are soluble isoforms (HLA-G5 to -G7). HLA-G1 and HLA-G5 isoforms present the typical structure of the classical HLA class I molecules formed by a 3 globular domain heavy-chain, noncovalently associated to β-2-microglobulin (B2M) and a nonapeptide. The truncated isoforms lack 1 or 2 domains, although they all contain the α1 domain, and they are all B2M-free isoforms (11).
HLA-G is a potent tolerogenic molecule that has exclusive inhibitory functions. Indeed, neither stimulatory functions nor responses to allogeneic HLA-G have been reported to date. Previous studies have shown that HLA-G proteins can inhibit natural killer (NK) cells and cytotoxic T-lymphocyte cytolytic activity (12–14); allogeneic responses, such as proliferative T-cell response and T-cell and NK-cell ongoing proliferation (15–17); and dendritic maturation (18, 19). Recent studies have also shown that HLA-G can induce the differentiation of regulatory T cells, which can then inhibit allogeneic responses themselves and are known to participate in the tolerance of allografts (15, 18, 20–22).
HLA-G is expressed in various types of primary tumors, metastases, and malignant effusions (23), and can be found in tumor cells and tumor-infiltrating cells (24). Indeed, its expression has been detected in several human cancers, including melanoma, renal cell carcinoma, breast carcinoma, and large-cell carcinoma of the lung (7, 25–29). The expression of HLA-G by tumor cells has been shown to be important for the escape of immunosurveillance mediated by host T lymphocytes and NK cells (7, 25, 26, 30–32). Thus, the expression of HLA-G by malignant cells may prevent tumor immune eradication by inhibiting the activity of tumor-infiltrating NK cells, cytotoxic T lymphocytes (CTLs), and antigen presenting cells (APCs). The clinical relevance of HLA-G expression by tumors as a prominent immune escape mechanism was supported by the observation that HLA-G expression in B-cell chronic leukemia correlated with a strong immunodeficiency and poor clinical evolution (33, 34).
HLA-G's biological function is the induction of immune tolerance through binding to inhibitory receptors. Three receptors have been well reported to bind HLA-G: leukocyte immunoglobulin-like receptor, subfamily B, member 1 (LILRB1)/immunoglobulin-like transcript 2 (ILT2)/CD85j; leukocyte immunoglobulin-like receptor, subfamily B, member 2 (LILRB2)/immunoglobulin-like transcript 4 (ILT4)/CD85d; and killer cell immunoglobulin-like receptor, two domains, long cytoplasmic tail, 4 (KIR2DL4)/CD158d. KIR2DL4 expression is mainly restricted to decidual NK cells (35), and HLA-G is its sole known ligand (36). LILRB1 and LILRB2 receptors have been shown to bind a wide range of classical HLA molecules through the α3 domain and B2M. However, HLA-G is their ligand of highest affinity (37), and its binding is unlikely to be affected by the peptide. LILRB1 is expressed on B cells, some T cells, some NK cells, and all monocytes and dendritic cells, whereas LILRB2 is myeloid-specific and expressed only by monocytes and dendritic cells (38). LILRB1 and LILRB2 receptors have a higher affinity for HLA-G multimers than monomeric structures (37). It is important to highlight the difference between the way LILRB1 and LILRB2 bind to their ligands: LILRB1 recognizes only B2M-associated HLA-G structures, whereas LILRB2 can recognize both B2M-associated and B2M-free HLA-G heavy chains (39, 40). Indeed, LILRB2 shows remarkably distinct HLA class I-binding recognition by binding preferentially the α3 domain rather than B2M, involving the aromatic amino acids Phe-195 and Tyr-197. This explains the B2M-independent HLA-G binding of the latter receptor and its higher affinity for B2M-free isoforms. Furthermore, we recently demonstrated that dimers of the HLA-G2 isoform (α1 and α3 HLA-G domains only) do not bind LILRB1 but bind LILRB2 and, through this interaction, can induce skin allograft tolerance in mice (41).
Although the relevance of HLA-G in the down-regulation of the immune system and in tumor evasion of immunosurveillance has been largely studied and demonstrated, several hurdles remain that impair the use of HLA-G as a therapeutic molecule. First of all, the best-characterized HLA-G isoform, B2M-associated HLA-G1/G5, is a trimolecular complex of noncovalently associated heavy-chain, B2M, and a peptide. Besides being challenging to produce, this structure is of limited stability. Second, most of the in vitro data obtained so far on the immunoinhibitory function for HLA-G were obtained using membrane-bound HLA-G1 molecules or B2M-associated HLA-G molecules aggregated on beads. Studies do report the function of nonaggregated HLA-G molecules, but these were usually purified from transfected cell lines or body fluids, and their actual structure is unknown, particularly their monomeric/multimeric status, or their association with other molecules. Because of this, the development of a therapeutically compatible HLA-G molecule is still slow. Engineering an HLA-G molecule, simpler than the full-length HLA-G1, which can be produced in good manufacturing practice (GMP) conditions (compatible) and would mimic HLA-G functions, is a strategy to overcome these limitations. In the present study, we addressed 3 points: whether we can engineer a simpler HLA-G protein that exerts some of the full-length's functions; whether we can engineer a molecule that is simpler to produce and that can be produced under GPM conditions; and whether we can engineer an HLA-G protein that would function as a soluble molecule and not only as a bead-aggregated structure. We present here one such molecule.
For this study, we took advantage of our knowledge that the HLA-G2 molecule (α1-α3 domains) binds and may function through the LILRB2 HLA-G receptor (41). Furthermore, based on crystallographic data (42, 43), we reasoned that a dimer of the HLA-G α3 domain may be sufficient to induce a function through LILRB2. Thus, we synthesized and evaluated the functions of 2 dimeric molecules: a dimer of the α3 domain of HLA-G called (α3-L)x2 and a dimeric (α1-α3)x2 protein. (α1-α3)x2 is a synthetic dimer of 2 HLA-G α1-α3monomers. These synthetic monomers were based on the extracellular domains of the HLA-G2/G6 isoforms, and dimerization was achieved through disulfide bonds between 2 free cysteins in position 42, as reported for the natural HLA-G molecule (41, 44, 45). Despite their capability to bind LILRB2, (α3-L)x2 dimers proved to be noninhibitory in our assays. However, we report here that the (α1-α3)x2 synthetic molecule, which is simpler than B2M-associated HLA-G, mimics it functionally. Indeed, this molecule is highly active in vitro and in vivo: (α1-α3)x2 polypeptide can induce complete tolerance to allogeneic skin grafts in mice and can inhibit the cellular multiplication of tumor cell lines of leukocytic and myelocytic origins as a soluble protein. Also, HLA-G (α1-α3)x2 molecule inhibits the proliferation of tumor lines that did not express LILRB2, thus demonstrating the existence of yet-unknown receptors for HLA-G.
MATERIALS AND METHODS
HLA-G molecules
Three polypeptides have been synthesized, named (α1)x2, (α1-α3)x2, and (α3-L)x2. Dimerization was sought because it is demonstrated that HLA-G binds with higher affinity to its inhibitory receptors as dimeric forms than as monomers, and it is under this conformation that HLA-G exerts its functions (37, 42, 44, 45). The synthesis and biochemical validation were done by Synoprosis (Fuveau, France). The HLA-G synthetic polypeptides that we used in this study contained >70% of homodimers. The (α1)x2 polypeptide has already been described (46) and is constituted of the HLA-G α1 domain. The (α1-α3)x2 polypeptide is the synthetic equivalent of the extracellular domains of the HLA-G2 and HLA-G6 isoforms, which are constituted of the α1 followed by the α3 domain of HLA-G. Folding of the α1-α3 monomers was directed during protein generation prior to dimerization. The α1 and α1-α3 polypeptides may dimerize through the generation of intermolecular disulfide bonds involving the free cysteine in position 42 (C42). The α3-L monomer consists of a GC(GGGGS)x2 linker sequence (named L), followed by the α3 domain of HLA-G. The linker sequence contains a cysteine for dimerization through disulfide bond generation. All HLA-G polypeptides contain a free cysteine for homodimerization. The complete sequences of the HLA-G polypeptides and a schematic representation of the putative structures of HLA-G polypeptide dimers are shown in Fig. 1. Validation experiments demonstrated that these polypeptides, at the doses used in this study, were not toxic for PBMCs and did not induce their proliferation (data not shown). The B2M-HLA-G5 fusion protein was obtained from culture medium supernatant of HeLa human cell line [American Type Culture Collection (ATCC), Manassas, VA, USA] transfected with a plasmid containing the B2M-HLA-G5 complementary DNA (cDNA) sequence, as described previously (46).
Figure 1.
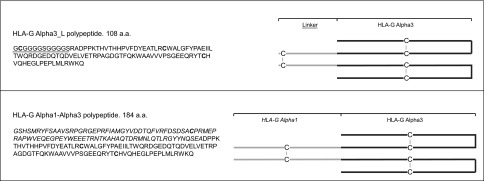
(α1-α3)x2 and (α3-L)x2 HLA-G synthetic polypeptides. Left: amino acid sequences of the HLA-G polypeptides used in this study. HLA-G α3 polypeptides: linker sequences are underlined, and cysteines appear in bold type. HLA-G α1-α3polypeptide: sequence for HLA-G α1 domain appears in italic type. Cysteines appear in bold type, including that in position 42 of the α1 domain, which mediates dimerization. Right: putative structures of the HLA-G polypeptide dimeric forms.
Cell lines and transfectants
Several cell lines were used for a proliferation assay: U937, Raji, KG-1, NKL-LILRB1+, and NKL-LILRB1+-LILRB2+. All cells were phenotyped by flow cytometry for known HLA-G receptor expression. The KG-1 cell line (myelogenous leukemia) is LILRB1−, LILRB2−, KIR2DL4−; the U937 (histiocytic lymphoma) and Raji (Burkitt's lymphoma) cell lines are LILRB1+, LILRB2−, KIR2DL4−. All cell lines were obtained from ATCC. The NKL line (50) is an NK-cell line that endogenously expresses both KIR2DL4 and LILRB1. This line is henceforth referred to as NKL-LILRB1+. NKL-LILRB1+-LILRB2+ cells are NKL-LILRB1+ cells transduced with a lentivirus containing the LILRB2 cDNA, as described previously (43).
Monoclonal antibodies (mAbs) and flow cytometry analysis
The following mAbs were used: from Exbio Praha (Vestec, Czech Republic), anti-HLA-G G233 and phycoerythrin (PE)-conjugated anti-HLA-G MEM-G/09; from Sigma (St. Louis, MO, USA), goat-anti-mouse HRP- and PE-conjugated goat-anti-human Fc (hFc); from Novus Biologicals (Littleton, CO, USA), fluorescein isothiocyanate (FITC)-conjugated anti-glutathione S-transferase (GST) antibody; and from Beckman Coulter (Fullerton, CA, USA), PE-conjugated anti-LILRB1 and PE-conjugated anti-LILRB2.
Cytometry-based (synthetic protein:LILRB) interaction analysis
In order to document (α3-L)x2, (α1-α3)x2, and B2M-HLA-G5 protein interaction with LILRB1 and LILRB2 receptors, either G233 capture antibody or (α3-L)x2 and (α1-α3)x2 proteins were coated on Bio-Plex COOH beads (Bio-Rad, Hercules, CA, USA) using the Bio-Plex Amine Coupling kit (Bio-Rad) following manufacturer's recommendations. G233-coated beads were used to capture B2M-HLA-G5 protein from culture cell supernatant. As negative control, Bio-Plex beads were identically prepared using phosphate-buffered saline (PBS) or HeLa mock supernatant. Of these coupled beads, 2000 were then incubated with sample for 90 min at room temperature and then again for 60 min at room temperature in the presence of 20 μg/ml of human IgG, LILRB1-Fc, or LILRB2-Fc. Finally, all samples were incubated with anti-LILRB1-PE or anti-LILRB2-PE, respectively, used as secondary antibodies, and analyzed by flow cytometry.
In vivo analysis
C57BL/6 and B6.C-H-2bm12 (bm12) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). The use of animals for this work was approved by the animal care committee of the Medical College of Georgia. The in vivo experimental procedures were approved by the animal care committee of the Medical College of Georgia (approval ID: BR08-06–070), and the experiments were conducted in accordance with institutional guidelines for animal care and use. Specific pathogen-free C57BL/6 mice (8–10 wk of age) were used as skin graft recipients throughout the study. Recipient mice received (α3-L)x2 and (α1-α3)x2 polypeptide-coated beads, which were injected 4 times, at d −1, 7, 14, and 21 of skin transplantation for both peptides, and in a single dose by injecting (α1-α3)x2-coated beads at d −1, as described previously (46). Donor skin was from MHC class II-disparate B6.CH-2bm12 (bm12, H-2b) mice. Allogeneic skin grafts were performed by standard methods. Briefly, skin (1.0 cm2) from the tail of donor mice (12–14 wk old) was grafted onto the flank of recipient, anesthetized mice. The graft was covered with gauze and plaster, which were removed on d 10. Grafts were scored daily until rejection (defined as 80% of grafted tissue becoming necrotic and reduced in size). All skin grafting survival data were tested by Kaplan-Meier survival analysis.
Tumor cell line proliferation assay
U937, Raji, KG-1, NKL-LILRB1+, and NKL-LILRB1+-LILRB2+ cells (105) were set in wells of a 96-well plate, in 150 μl of culture medium. Cells were stimulated with 50 μg/ml (α1-α3)x2, (α3-L)x2, or (α1-L)x2 proteins. A similar volume of PBS was used for control. After 24 h of incubation at 37°C with 5% CO2, 1 μCi of 3H-thymidine was added. After 36 h of incubation, content of the wells was transferred to a membrane (Printed Filtermat A 1450-421, Perkin Elmer, Wellesley, MA, USA) and soaked in a scintillation liquid (Ultima Gold MV; Perkin Elmer) in order to read the radioactive labeling in a 1450 Microbeta Trilux Wallac equipment (Perkin Elmer).
RESULTS
Proper polypeptide conformation evidenced by binding to known HLA-G receptors
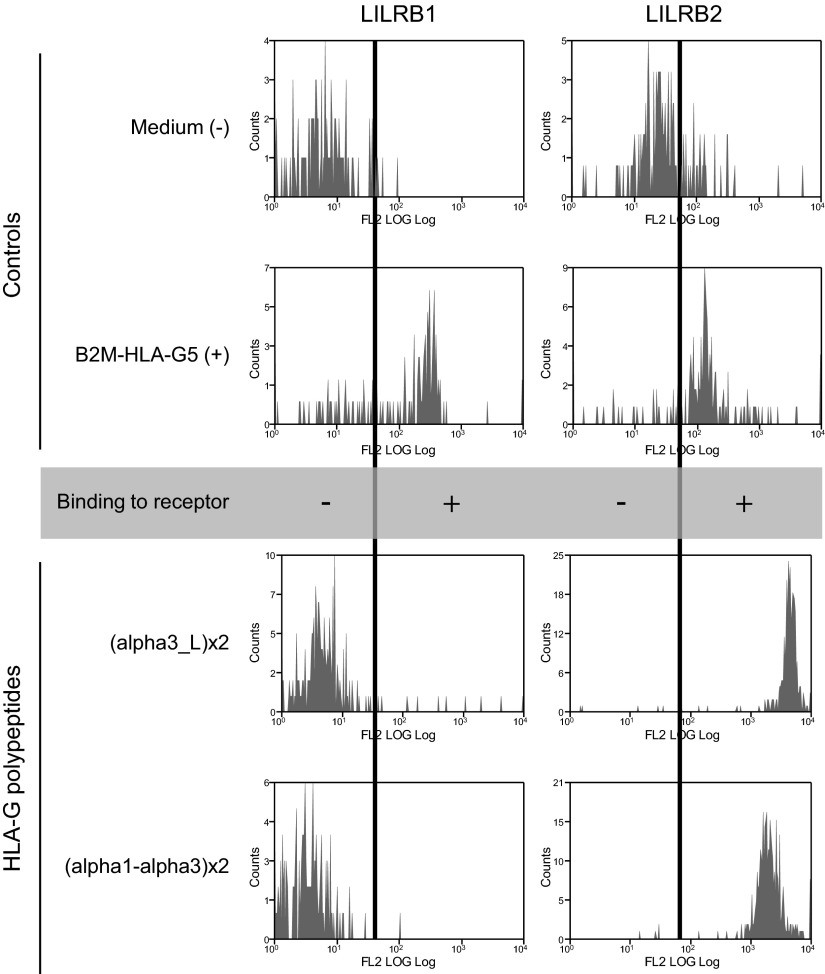
To verify the correct folding and conformation of the synthetic proteins, we investigated whether the HLA-G synthetic polypeptides could be recognized by the two main HLA-G receptors, LILRB1 and LILRB2. For this purpose, we generated (α1-α3)x2 and (α3-L)x2-coated Bio-Plex COOH beads, by directly coupling the polypeptides to the activated beads and G233-coated beads to capture the B2M-HLA-G5 protein. The binding of LILRB1-Fc and LILRB2-Fc molecules to the HLA-G-coated beads was then evaluated. The results obtained are shown in Fig. 2. They demonstrate that, whereas the B2M-associated recombinant protein B2M-HLA-G5 (46) was recognized by both LILRB1 and LILRB2, (α1-α3)x2 and (α3-L)x2 were recognized by LILRB2 but not by LILRB1.
Figure 2.
HLA-G synthetic polypeptides binding to specific inhibitory receptors. Synthetic polypeptides were directly coated onto Bioplex beads, and recombinant B2M-HLA-G5 was captured by a specific antibody-coated beads. Positive signal was detected after specific binding of the inhibitory receptors.
(α1-α3)x2 synthetic polypeptide induces skin graft tolerance in vivo
To prove that the polypeptides had retained some HLA-G functions, we chose to evaluate their capability to induce skin graft tolerance in in vivo experiments. The capability of HLA-G recombinant proteins, including B2M-HLA-G5 and α1-α3-Fc (41, 46), to inhibit allogeneic skin graft rejection in mice has been repeatedly published by us and others (18, 19, 46). The standard protocol to evaluate the tolerogenic function of HLA-G molecules is one intraperitoneal injection of HLA-G-coated beads. Thus, based on the precedent experience, we injected (α1-α3)x2-coated beads at d −1 of skin graft. In addition, we performed a 4-injection protocol by inoculating the animals 7 d apart with (α1-α3)x2- or (α3-L)x2-coated beads to determine whether the tolerogenic effects could be cumulative and capable of prolonging skin allograft survival. Figure 3 shows that even 4 injections of (α3-L)x2-coated beads had a very limited, albeit significant, effect on skin allograft survival. Indeed, these injections increased the median graft survival time by 3 d, from 18 to 21 d (n=9, P=0.0486), and the maximum graft survival time from 27 to 28 d. However, the (α1-α3)x2 synthetic polypeptide proved to be an extremely potent tolerogenic structure: one injection of (α1-α3)x2-coated beads was sufficient to increase the median graft survival time by 11 d, from 18 d in controls, to 29 d (n=10, P<0.0001). In this experiment, the maximum graft survival time was increased from 29 d in controls, to 48 d in mice treated with one injection of (α1-α3)x2-coated beads. By comparison, B2M-HLA-G5-coated beads increased the median graft survival time from 18 to 29 d (n=9, P=0.0001), and the maximal graft survival time from 24 to 29 d (46). These previously published data are shown in Supplemental Fig. S1 (see also refs. 18, 49). Thus, the tolerogenic effect of (α1-α3)x2 was equal to that of HLA-G when only one injection was performed.
Figure 3.
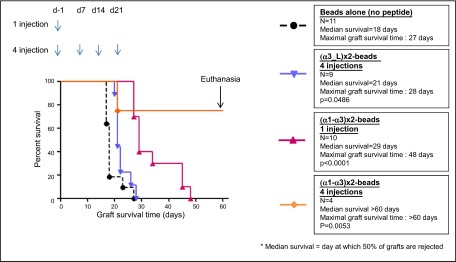
Tolerogenic function of HLA-G synthetic polypeptides in vivo. (α1-α3)x2 increases skin allograft median graft survival time by 11 d with 1 injection and induces complete tolerance with a 4-injection treatment.
We next evaluated the cumulative effect of 4 injections of (α1-α3)x2-coated microspheres. The results show that repeated (α1-α3)x2-coated bead injections had a major effect on skin allograft survival: 3 of 4 transplanted mice that received this treatment still tolerated their transplant at d 60; i.e., 39 d after the last treatment. At this time, complete tolerance was declared, and the experiment was stopped. These results are shown in Fig. 3. Thus, the (α1-α3)x2 synthetic polypeptide proved to have potent tolerogenic functions and was at least as tolerogenic as the complete HLA-G protein.
(α1-α3)x2 synthetic polypeptide is active as a soluble molecule and inhibits the proliferation of tumor cell lines
Once proven that soluble synthetic polypeptides bind to the LILRB2 receptor and that they are biologically functional in vivo by inducing tolerance, we sought to determine whether they could conserve their biological effect as soluble molecules in vitro. For this, we evaluated the capability of (α1-α3)x2 to inhibit proliferation of different cell lines that express HLA-G receptors, as described previously (17) for HLA-G1 and NKL cell lines. Because NKL cells express only LILRB1, which does not bind α1-α3, we used LILRB2-transfected NKL cells (NKL-LILRB1+-LILRB2+). These cells were incubated in the presence of (α1)x2, (α3-L)x2, or (α1-α3)x2. For NKL-LILRB1+-LILRB2+ cells, we observed that (α1)x2 and (α3-L)x2 did not have any inhibitory effect on cell proliferation. However, (α1-α3)x2 polypeptide induced a strong inhibitory effect, as shown in Fig. 4A. The same experiment was carried out for NKL-LILRB1+, Raji, KG-1 and U937 cell lines. Similar results were obtained in these cell lines regardless of their expression of known HLA-G receptors, which demonstrated that the (α1-α3)x2 polypeptide is a potent inhibitory molecule and can function as a soluble molecule by strongly decreasing the tumor cell proliferation, as shown in Fig. 4B. Of note: no cell death and no apoptosis were detected after coincubation of the tumor cell lines with (α1-α3)x2 polypeptide (data not shown).
Figure 4.
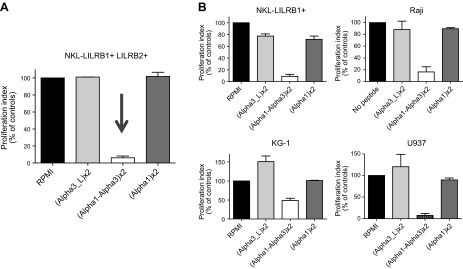
Function of soluble HLA-G synthetic polypeptides in vitro. A) Different tumor cell lines were incubated in presence of (α1-L)x2, (α3-L)x2, and (α1-α3)x2. B) A 36-h proliferation assay demonstrates that (α1-α3)x2 strongly inhibits the proliferation of tumor cell lines.
DISCUSSION
The relevance of the HLA-G molecule for the induction of immune tolerance and its implication in the escape mechanisms of tumor cells has been proven, and many attempts at using this molecule as a therapeutic agent have been made. However, its mechanisms of expression regulation, which still remain to be elucidated, and the lack of a stable purified molecule render the clinical use of HLA-G far removed. To overcome such hurdles, and based on previous studies that demonstrated that truncated isoforms of HLA-G bind its specific receptors and carry its biological function, we designed two synthetic molecules, derived from HLA-G, that are simpler to produce than B2M-associated HLA-G under GMP-compatible conditions: (α1-α3)x2 and (α3-L)x2. Their validation as HLA-G equivalents was the goal of this study.
We synthesized dimeric polypeptides because most HLA-G functions are due to dimers, not monomers. We first synthesized an (α1-α3)x2 structure because studies from our laboratory had demonstrated that α1-α3-Fc recombinant proteins bind the LILRB2 receptor and are tolerogenic in vivo (41). (α3-L)x2 was then synthesized as an even simpler alternative to full-length HLA-G, based on the notion that the α3 domain of HLA-G is crucial to the binding with the LILRB receptors. The capability of both (α1-α3)x2 and (α3-L)x2 to specifically bind LILRB2 but not LILRB1, in conformance with previous studies (41), proved that this hypothesis was sound and that both molecules had the potential to be functional.
The biological function of the synthetic molecules was assessed using two systems, one in vitro and one in vivo. To prove that the synthetic polypeptides functionally behaved as HLA-G, we chose to follow a well-described protocol of skin allograft transplantation in which recipient animals are treated with HLA-G-coated beads. With this method, we evaluated whether the synthetic polypeptides were tolerogenic in vivo. Because synthetic proteins aggregated on beads are unlikely to constitute a viable clinical tool, and given that in vivo experiments (allogenic skin transplant) with native HLA-G injections of soluble protein have been performed, and no effects on graft survival time were observed, we evaluated whether the synthetic polypeptides were still active as soluble molecules in an in vitro experiment. For this, a tumor cell line proliferation inhibition assay was performed based on that which had been reported for membrane-bound HLA-G1 (17). In the latter report, membrane-bound HLA-G1 strongly inhibited the proliferation of the NKL-LILRB1+ cell line through the LILRB1 HLA-G receptor. Because the synthetic polypeptides do not bind LILRB1, the NKL-LILRB1+cell line was transfected with LILRB2. In both in vitro and in vivo functional evaluations, (α3-L)x2 proved to be noninhibitory. (α1-α3)x2, however, was highly tolerogenic in vivo and could inhibit the proliferation of NKL-LILRB1+-LILRB2+ cells, as well as other tumor lines of immune origin. Of note, the latter results imply that HLA-G may function through other receptors than the ones currently known. Indeed, out of all the cell lines used here, only NKL-LILRB1+-LILRB2+ express cell surface LILRB1 and LILRB2. The other cell lines expressed only cell surface LILRB1 (U937, Raji, NKL-LILRB1+) or no detectable cell surface HLA-G receptors (KG-1). Bead-based assays showed that (α1-α3)x2 does not bind LILRB1 but only LILRB2. Hence, if tumor cell proliferation inhibition was mediated through the only known HLA-G receptor (α1-α3)x2 can bind, i.e., LILRB2+, only NKL-LILRB1+-LILRB2+ should have been inhibited. The fact that cell lines expressing only LILRB1 and cells expressing no detectable levels of either of HLA-G receptors were also inhibited points to the notion that other HLA-G receptors exist. This point is strengthened by the observation that (α3-L)x2 systematically increased KG-1 proliferation, possibly through a yet-unknown activating receptor.
HLA-G specific (α1-α3)x2 was functional, but (α3-L)x2 was not. This finding implies that the α1 HLA-G domain is a structurally important component for the function of (α1-α3)x2 and not only the α3 domain. Because of the folding procedure, it is known that the cysteins of the α3 domain are properly bonded and that the free cysteine of the α1 domain is involved in homodimerization. Because (α3-L)x2 and (α1-α3)x2 both efficiently bind ILT4, it is also known that the α3 domain is at least partially structurally similar to that of the previously crystallized B2M-associated HLA-G1. However, beyond this, the actual structure of (α1-α3)x2 remains unknown, as are those of the natural HLA-G2/6 molecules. This finding raises the question of whether the α1 and α3 dimers must be specific for HLA-G, or whether (α1-α3)x2 dimers of other HLA molecules, such as HLA-A2, should have the same function. Several lines of evidence indicate that they should not; only HLA-G possesses the free C42 that allows it to dimerize through its α1 domain. Hence, in order to obtain classical HLA dimers, dimerization should be induced artificially, either through the addition of a linker, which is what was done in this work for (α3-L)x2 molecules, or through modification of the classical HLA class I α1 domain sequence to introduce a free C42 resembling that of HLA-G. Either way, the α1 domain of a classical HLA class I molecule cannot substitute for that of HLA-G. Even if (α1-α3)x2 dimers of other HLA molecules were made, we think it unlikely that they should be as tolerogenic as those of HLA-G. Indeed, in vivo tolerance, such as we describe here, is mediated through the LILRB2 homologue PIR-B (49), and it is known that HLA-G is LILRB2's ligand of highest affinity because of its unique FDY sequence in the α3 domain. Thus, any α1-α3 dimer of a classical HLA class I molecule should be functionally weaker than that of HLA-G.
These experiments prove that molecules simpler and easier to produce than HLA-G may mimic its functions. In the experiments that we present, one injection of beads coated with (α1-α3)x2 increased allogeneic skin graft survival time by 11 d, which is comparable to the effect obtained using B2M-associated HLA-G5-coated beads (46). What is more, complete skin allograft tolerance was achieved after only 4 injections of the polypeptide-coated beads. In these experiments, allografts were not rejected, and after 60 d, the experiment had to be stopped, which corresponded to 39 d without treatment after the last (α1-α3)x2-coated bead injection. To the best of our knowledge, even though HLA-G is well known for its tolerogenic functions, this is the first time that its capability to induce complete tolerance has been demonstrated. It also opens the possibility that the (α1-α3)x2 polypeptide is more tolerogenic than the full-length HLA-G.
Using HLA-G or HLA-G-derived molecules such as (α1-α3)x2 presents clear advantages over using immunosuppressive drugs, because HLA-G is a natural tolerogenic molecule and not an immunosuppressant. We did not compare the efficacy of (α1-α3)x2 with that of immunosuppressive molecules in our system. However, it is reported that to achieve tolerance of an allogeneic skin graft in mice, daily injections of Tacrolimus (FK506) at doses of 2–5 mg/kg are required, whereas we used only 4 weekly injections of 106 nanobeads loaded altogether with at most 5 μg of (α1-α3)x2 polypeptide (∼0.02 mg/kg/wk, 4 times). In addition to these very basic quantitative comparisons, it must be pointed out that, unlike immunosuppressive drugs with which rejection of the graft is observed immediately after the end of the treatment (49), (α1-α3)x2-induced tolerance allowed the allograft to survive after the treatment was stopped. Further, and even though the toxicology of (α1-α3)x2 has not been assessed, it is obvious that HLA-G, which is expressed primarily at the maternal-fetal interface, should have much lower side effects than immunosuppressive drugs such as FK506.
Of course, the pharmacodynamics and pharmacokinetics of (α1-α3)x2 polypeptides should be carefully investigated. Obviously, HLA-G or HLA-G-derived synthetic molecules do not aim to replace reliable immunosuppressants, the efficacy of which has been proven countless times. However, molecules such as the (α1-α3)x2 synthetic polypeptide may be excellent candidates for combination therapy with classical immunosuppressive drugs. Notwithstanding the economics of the operation, this would benefit the patient by lowering the required levels of immunosuppressive drugs and also by inducing a better tolerance.
Supplementary Material
Acknowledgments
This work was supported by Commissariat a l'Energie Atomique et aux Energies Alternatives, and by U.S. National Institutes of Health grant R56 AI055923 (to A.H.).
The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- APC
- antigen-presenting cell
- B2M
- β2-microglobulin
- HLA-G
- human leukocyte antigen G
- cDNA
- complementary DNA
- FITC
- fluorescein isothiocyanate
- GMP
- good manufacturing practice
- GST
- glutathione S-transferase
- hFc
- human Fc
- ILT2
- immunoglobulin-like transcript 2
- ILT4
- immunoglobulin-like transcript 4
- KIR2DL4
- killer cell immunoglobulin-like receptor, two domains, long cytoplasmic tail, 4
- LILRB1
- leukocyte immunoglobulin-like receptor, subfamily B, member 1
- LILRB2
- leukocyte immunoglobulin-like receptor, subfamily B, member 2
- NK
- natural killer
- PBS
- phosphate-buffered saline
- PE
- phycoerythrin
REFERENCES
- 1. Kovats S., Main E. K., Librach C., Stubblebine M., Fisher S. J., DeMars R. (1990) A class I antigen, HLA-G, expressed in human trophoblasts. Science 248, 220–223 [DOI] [PubMed] [Google Scholar]
- 2. Rouas-Freiss N., Goncalves R. M., Menier C., Dausset J., Carosella E. D. (1997) Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc. Natl. Acad. Sci. U. S. A. 94, 11520–11525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mallet V., Blaschitz A., Crisa L., Schmitt C., Fournel S., King A., Loke Y. W., Dohr G., Le Bouteiller P. (1999) HLA-G in the human thymus: a subpopulation of medullary epithelial but not CD83(+) dendritic cells expresses HLA-G as a membrane-bound and soluble protein. Int. Immunol. 11, 889–898 [DOI] [PubMed] [Google Scholar]
- 4. Cirulli V., Zalatan J., McMaster M., Prinsen R., Salomon D. R., Ricordi C., Torbett B. E., Meda P., Crisa L. (2006) The class I HLA repertoire of pancreatic islets comprises the nonclassical class Ib antigen HLA-G. Diabetes 55, 1214–1222 [DOI] [PubMed] [Google Scholar]
- 5. Selmani Z., Naji A., Zidi I., Favier B., Gaiffe E., Obert L., Borg C., Saas P., Tiberghien P., Rouas-Freiss N., Carosella E. D., Deschaseaux F. (2008) Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 26, 212–222 [DOI] [PubMed] [Google Scholar]
- 6. Verloes A., Van de Velde H., LeMaoult J., Mateizel I., Cauffman G., Horn P. A., Carosella E. D., Devroey P., De Waele M., Rebmann V., Vercammen M. (2011) HLA-G expression in human embryonic stem cells and preimplantation embryos. J. Immunol. 186, 2663–2671 [DOI] [PubMed] [Google Scholar]
- 7. Paul P., Rouas-Freiss N., Khalil-Daher I., Moreau P., Riteau B., Le Gal F. A., Avril M. F., Dausset J., Guillet J. G., Carosella E. D. (1998) HLA-G expression in melanoma: a way for tumor cells to escape from immunosurveillance. Proc. Natl. Acad. Sci. U. S. A. 95, 4510–4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lila N., Carpentier A., Amrein C., Khalil-Daher I., Dausset J., Carosella E. D. (2000) Implication of HLA-G molecule in heart-graft acceptance. Lancet 355, 2138. [DOI] [PubMed] [Google Scholar]
- 9. Aractingi S., Briand N., Le Danff C., Viguier M., Bachelez H., Michel L., Dubertret L., Carosella E. D. (2001) HLA-G and NK receptor are expressed in psoriatic skin: a possible pathway for regulating infiltrating T cells? Am. J. Pathol. 159, 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lozano J. M., Gonzalez R., Kindelan J. M., Rouas-Freiss N., Caballos R., Dausset J., Carosella E. D., Pena J. (2002) Monocytes and T lymphocytes in HIV-1-positive patients express HLA-G molecule. AIDS 16, 347–351 [DOI] [PubMed] [Google Scholar]
- 11. Carosella E. D., Favier B., Rouas-Freiss N., Moreau P., Lemaoult J. (2008) Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood 111, 4862–4870 [DOI] [PubMed] [Google Scholar]
- 12. Rouas-Freiss N., Marchal R. E., Kirszenbaum M., Dausset J., Carosella E. D. (1997) The alpha1 domain of HLA-G1 and HLA-G2 inhibits cytotoxicity induced by natural killer cells: is HLA-G the public ligand for natural killer cell inhibitory receptors? Proc. Natl. Acad. Sci. U. S. A. 94, 5249–5254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Riteau B., Rouas-Freiss N., Menier C., Paul P., Dausset J., Carosella E. D. (2001) HLA-G2, -G3, and -G4 isoforms expressed as nonmature cell surface glycoproteins inhibit NK and antigen-specific CTL cytolysis. J. Immunol. 166, 5018–5026 [DOI] [PubMed] [Google Scholar]
- 14. Rouas-Freiss N., Khalil-Daher I., Riteau B., Menier C., Paul P., Dausset J., Carosella E. D. (1999) The immunotolerance role of HLA-G. Seminars Cancer Biol. 9, 3–12 [DOI] [PubMed] [Google Scholar]
- 15. LeMaoult J., Krawice-Radanne I., Dausset J., Carosella E. D. (2004) HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc. Natl. Acad. Sci. U. S. A. 101, 7064–7069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bahri R., Hirsch F., Josse A., Rouas-Freiss N., Bidere N., Vasquez A., Carosella E. D., Charpentier B., Durrbach A. (2006) Soluble HLA-G inhibits cell cycle progression in human alloreactive T lymphocytes. J. Immunol. 176, 1331–1339 [DOI] [PubMed] [Google Scholar]
- 17. Caumartin J., Favier B., Daouya M., Guillard C., Moreau P., Carosella E. D., LeMaoult J. (2007) Trogocytosis-based generation of suppressive NK cells. EMBO J. 26, 1423–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ristich V., Liang S., Zhang W., Wu J., Horuzsko A. (2005) Tolerization of dendritic cells by HLA-G. Eur. J. Immunol. 35, 1133–1142 [DOI] [PubMed] [Google Scholar]
- 19. Liang S., Ristich V., Arase H., Dausset J., Carosella E. D., Horuzsko A. (2008) Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6–STAT3 signaling pathway. Proc. Natl. Acad. Sci. U. S. A. 105, 8357–8362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naji A., Le Rond S., Durrbach A., Krawice-Radanne I., Creput C., Daouya M., Caumartin J., LeMaoult J., Carosella E. D., Rouas-Freiss N. (2007) CD3+CD4low and CD3+CD8low are induced by HLA-G: novel human peripheral blood suppressor T-cell subsets involved in transplant acceptance. Blood 110, 3936–3948 [DOI] [PubMed] [Google Scholar]
- 21. Gregori S., Tomasoni D., Pacciani V., Scirpoli M., Battaglia M., Magnani C. F., Hauben E., Roncarolo M. G. (2010) Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood 116, 935–944 [DOI] [PubMed] [Google Scholar]
- 22. Agaugue S., Carosella E. D., Rouas-Freiss N. (2011) Role of HLA-G in tumor escape through expansion of myeloid-derived suppressor cells and cytokinic balance in favor of Th2 versus Th1/Th17. Blood 117, 7021–7031 [DOI] [PubMed] [Google Scholar]
- 23. Carosella E. D., Moreau P., Lemaoult J., Rouas-Freiss N. (2008) HLA-G: from biology to clinical benefits. Trends Immunol. 29, 125–132 [DOI] [PubMed] [Google Scholar]
- 24. Ibrahim E. C., Aractingi S., Allory Y., Borrini F., Dupuy A., Duvillard P., Carosella E. D., Avril M. F., Paul P. (2004) Analysis of HLA antigen expression in benign and malignant melanocytic lesions reveals that upregulation of HLA-G expression correlates with malignant transformation, high inflammatory infiltration and HLA-A1 genotype. Int. J. Cancer 108, 243–250 [DOI] [PubMed] [Google Scholar]
- 25. Ibrahim E. C., Guerra N., Lacombe M. J., Angevin E., Chouaib S., Carosella E. D., Caignard A., Paul P. (2001) Tumor-specific up-regulation of the nonclassical class I HLA-G antigen expression in renal carcinoma. Cancer Res. 61, 6838–6845 [PubMed] [Google Scholar]
- 26. Urosevic M., Kurrer M. O., Kamarashev J., Mueller B., Weder W., Burg G., Stahel R. A., Dummer R., Trojan A. (2001) Human leukocyte antigen G up-regulation in lung cancer associates with high-grade histology, human leukocyte antigen class I loss and interleukin-10 production. Am. J. Pathol. 159, 817–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Polakova K., Russ G. (2000) Expression of the non-classical HLA-G antigen in tumor cell lines is extremely restricted. Neoplasma 47, 342–348 [PubMed] [Google Scholar]
- 28. Davies B., Hiby S., Gardner L., Loke Y. W., King A. (2001) HLA-G expression by tumors. Am. J. Reprod. Immunol. 45, 103–107 [DOI] [PubMed] [Google Scholar]
- 29. Real L. M., Cabrera T., Collado A., Jimenez P., Garcia A., Ruiz-Cabello F., Garrido F. (1999) Expression of HLA G in human tumors is not a frequent event. Int. J. Cancer 81, 512–518 [DOI] [PubMed] [Google Scholar]
- 30. McMaster M., Zhou Y., Shorter S., Kapasi K., Geraghty D., Lim K. H., Fisher S. (1998) HLA-G isoforms produced by placental cytotrophoblasts and found in amniotic fluid are due to unusual glycosylation. J. Immunol. 160, 5922–5928 [PubMed] [Google Scholar]
- 31. Lefebvre S., Antoine M., Uzan S., McMaster M., Dausset J., Carosella E. D., Paul P. (2002) Specific activation of the non-classical class I histocompatibility HLA-G antigen and expression of the ILT2 inhibitory receptor in human breast cancer. J. Pathol. 196, 266–274 [DOI] [PubMed] [Google Scholar]
- 32. Urosevic M., Willers J., Mueller B., Kempf W., Burg G., Dummer R. (2002) HLA-G protein up-regulation in primary cutaneous lymphomas is associated with interleukin-10 expression in large cell T-cell lymphomas and indolent B-cell lymphomas. Blood 99, 609–617 [DOI] [PubMed] [Google Scholar]
- 33. Nuckel H., Rebmann V., Durig J., Duhrsen U., Grosse-Wilde H. (2005) HLA-G expression is associated with an unfavorable outcome and immunodeficiency in chronic lymphocytic leukemia. Blood 105, 1694–1698 [DOI] [PubMed] [Google Scholar]
- 34. Rebmann V., Wagner S., Grosse-Wilde H. (2007) HLA-G expression in malignant melanoma. Semin. Cancer Biol. 17, 422–429 [DOI] [PubMed] [Google Scholar]
- 35. Cooper M. A., Fehniger T. A., Caligiuri M. A. (2001) The biology of human natural killer-cell subsets. Trends Immunol. 22, 633–640 [DOI] [PubMed] [Google Scholar]
- 36. Rajagopalan S., Long E. O. (1999) A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J. Exp. Med. 189, 1093–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shiroishi M., Kuroki K., Ose T., Rasubala L., Shiratori I., Arase H., Tsumoto K., Kumagai I., Kohda D., Maenaka K. (2006) Efficient leukocyte Ig-like receptor signaling and crystal structure of disulfide-linked HLA-G dimer. J. Biol. Chem. 281, 10439–10447 [DOI] [PubMed] [Google Scholar]
- 38. Colonna M., Samaridis J., Cella M., Angman L., Allen R. L., O'Callaghan C. A., Dunbar R., Ogg G. S., Cerundolo V., Rolink A. (1998) Human myelomonocytic cells express an inhibitory receptor for classical and nonclassical MHC class I molecules. J. Immunol. 160, 3096–3100 [PubMed] [Google Scholar]
- 39. Gonen-Gross T., Achdout H., Arnon T. I., Gazit R., Stern N., Horejsi V., Goldman-Wohl D., Yagel S., Mandelboim O. (2005) The CD85J/leukocyte inhibitory receptor-1 distinguishes between conformed and β2-microglobulin-free HLA-G molecules. J. Immunol. 175, 4866–4874 [DOI] [PubMed] [Google Scholar]
- 40. Shiroishi M., Kuroki K., Rasubala L., Tsumoto K., Kumagai I., Kurimoto E., Kato K., Kohda D., Maenaka K. (2006) Structural basis for recognition of the nonclassical MHC molecule HLA-G by the leukocyte Ig-like receptor B2 (LILRB2/LIR2/ILT4/CD85d). Proc. Natl. Acad. Sci. U. S. A. 103, 16412–16417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. HoWangYin K. Y., Loustau M., Wu J., Alegre E., Daouya M., Caumartin J., Sousa S., Horuzsko A., Carosella E. D., LeMaoult J. (2012) Multimeric structures of HLA-G isoforms function through differential binding to LILRB receptors. Cell. Mol. Life Sci. 69, 4041–4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shiroishi M., Maenaka K. (2009) Crystallization and preliminary X-ray analysis of the low-affinity complex between human leukocyte antigen-G (HLA-G) and leukocyte Ig-like receptor B2 (LILRB2). Protein Peptide Lett. 16, 447–449 [DOI] [PubMed] [Google Scholar]
- 43. Clements C. S., Kjer-Nielsen L., Kostenko L., Hoare H. L., Dunstone M. A., Moses E., Freed K., Brooks A. G., Rossjohn J., McCluskey J. (2005) Crystal structure of HLA-G: a nonclassical MHC class I molecule expressed at the fetal-maternal interface. Proc. Natl. Acad. Sci. U. S. A. 102, 3360–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boyson J. E., Erskine R., Whitman M. C., Chiu M., Lau J. M., Koopman L. A., Valter M. M., Angelisova P., Horejsi V., Strominger J. L. (2002) Disulfide bond-mediated dimerization of HLA-G on the cell surface. Proc. Nat. Acad. Sci. U. S. A. 99, 16180–16185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gonen-Gross T., Achdout H., Gazit R., Hanna J., Mizrahi S., Markel G., Goldman-Wohl D., Yagel S., Horejsi V., Levy O., Baniyash M., Mandelboim O. (2003) Complexes of HLA-G protein on the cell surface are important for leukocyte Ig-like receptor-1 function. J. Immunol. 171, 1343–1351 [DOI] [PubMed] [Google Scholar]
- 46. Favier B., HoWangYin K. Y., Wu J., Caumartin J., Daouya M., Horuzsko A., Carosella E. D., LeMaoult J. (2011) Tolerogenic function of dimeric forms of HLA-G recombinant proteins: a comparative study in vivo. PloS One 6, e21011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Robertson M. J., Cochran K. J., Cameron C., Le J. M., Tantravahi R., Ritz J. (1996) Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp. Hematol. 24, 406–415 [PubMed] [Google Scholar]
- 48. Liang S., Baibakov B., Horuzsko A. (2002) HLA-G inhibits the functions of murine dendritic cells via the PIR-B immune inhibitory receptor. Eur. J. Immunol. 32, 2418–2426 [DOI] [PubMed] [Google Scholar]
- 49. Lopes C. T., Gallo A. P., Palma P. V., Cury P. M., Bueno V. (2008) Skin allograft survival and analysis of renal parameters after FTY720 + tacrolimus treatment in mice. Transpl. Proc. 40, 856–860 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.