Abstract
The anti-Candida activity of the innate defense protein human lactoferrin was investigated. Lactoferrin displayed a clear fungicidal effect against Candida albicans only under low-strength conditions. This candidacidal activity was inversely correlated with the extracellular concentration of the monovalent cations and was prevented by Na+ and K+ (≥30 mM) and by divalent cations (Ca2+ and Mg2+ at ≥4 mM). A slight cellular release of K+, cytosolic acidification, and a change in the membrane potential were observed in C. albicans cells treated with lactoferrin, suggesting that this protein directly or indirectly interacts with the cytoplasmic membrane. Mitochondrial inhibitors (carbonyl cyanide m-chlorophenylhydrazone, 2,4-dinitrophenol, azide, and antimycin) as well as anaerobic conditions significantly reduced the killing effect of lactoferrin. These results suggest that low-strength conditions and the cellular metabolic state may modulate the candidacidal activity of human lactoferrin.
Candida albicans is a commensal yeast that colonizes the oral mucosa of most healthy individuals (7), but it is also the major fungal pathogen of humans, causing frequent mucosal and systemic opportunistic infections (7, 35, 41). The colonization of the oral mucosa by C. albicans is modulated by specific and nonspecific defenses present in saliva, which contains proteins (i.e., lactoferrin, lactoperoxidase, and lysozyme) and peptides (i.e., histatins and β-defensins) with demonstrated anticandidal activities in vitro (11, 42). These antimicrobial compounds exert a regulatory role on the microbiota, allowing the colonization of mucosal surfaces by microbial species evolutionarily adapted to the host but preventing the overgrowth of the microorganisms and the invasion of internal host tissues (9).
Human lactoferrin (hLf) is a prominent host defense iron-binding glycoprotein (77 kDa) synthesized by polymorphonuclear neutrophils and acinar epithelial cells and is present at relatively high concentrations (0.2 to 2.2 mg/ml) in all mucosal bathing fluids (i.e., saliva), as well as in blood and milk, with the concentrations increasing significantly with various infections (21, 29). The antimicrobial mechanism of action of lactoferrin has not yet been totally elucidated. It is thought that lactoferrin exerts an antibacterial effect by limiting the availability of iron required for microbial growth (for a review, see reference 24) and/or by directly interacting with the bacterial surface (3), with subsequent damage to the outer membranes of gram-negative bacteria (12). It has also been hypothesized that the antimicrobial activity of this protein could be related to the interaction of the hLf amino acid sequences homologous to the antimicrobial hLf-derived peptides termed lactoferricin (residues 1 to 42) or kaliocin-1 (residues 153 to 183) with the bacterial membrane (5, 40). Although considerable attention has been focused on the antibacterial activity of lactoferrin (1, 13, 34), very little is known about its antifungal mechanism of action. Early reports demonstrated that hLf has an anti-Candida effect (2, 23). This effect was related to hLf adsorption to the C. albicans cell surface rather than iron deprivation (39), a suggestion recently supported in several reports demonstrating cell wall damage (31, 32, 42).
In this study we investigated the conditions optimal for the candidacidal activity of hLf in vitro, and some intracellular changes related to its killing effects are reported.
MATERIALS AND METHODS
Materials.
hLf (lot nos. 98H3784 and 18H3789) was obtained from Sigma Chemical Co. (St. Louis, Mo.). The purity of hLf was assessed as described previously (8), and the iron saturation (<0.03%) of the protein was determined by atomic absorption spectrometry. Amphotericin B, antimycin A, carbonyl cyanide m-chlorophenylhydrazone (CCCP), 2,4-dinitrophenol (DNP), nystatin, and proteinase K were from Sigma. Zymolyase 20T was from ICN Biomedicals (Costa Mesa, Calif.), 2′,7′-bis-(2-carboxyethyl)-5-(6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM) and 3,3′-dipentyloxacarbocyanine iodide [DiOC5(3)] were purchased from Molecular Probes, Inc. (Eugene, Oreg.), and Sabouraud dextrose broth (SDB) and Sabouraud dextrose agar (SDA) medium were purchased from Difco (Detroit, Mich.).
Yeast culture conditions.
C. albicans ATCC 10231 was purchased from the American Type Culture Collection. C. albicans cells from glycerol stocks were cultured on SDA plates. For the experiments, yeast cells were grown at stationary phase and subcultured (1:400) in SDB to the mid-logarithmic growth phase in a shaker at 30°C. The cell number was determined by phase-contrast microscopy with a hemocytometer chamber, and in some experiments the number of CFU was determined by standard plate counting procedures. The cells remained in the yeast (blastoconidial) phase throughout the studies.
Antifungal activity assays.
The anti-Candida effect of hLf was monitored by using C. albicans cell suspensions (105 cells/ml) in 5 mM potassium phosphate buffer (PPB; K2HPO4-KH2PO4 [pH 7.4]) incubated at 37°C. Killing assays under anaerobic conditions were performed as described previously (16, 27) in an anaerobic cabinet (model 1024; Forma Scientific Inc., Marietta, Ohio) or in the presence of the redox potential reducer l-cysteine (2.5 mM). The influences of K+ and Na+ at various concentrations on the killing effect of hLf were determined in PPB or sodium phosphate buffer (SPB; Na2HPO4-NaH2PO4 [pH 7.4]) at different molarities. The effects of divalent cations were determined in Tris-HCl (pH 7.4) containing different concentrations of CaCl2 and MgCl2 instead of PPB to avoid the possible precipitation of Ca2+ or Mg2+ by phosphate. The effect of the pH on the activity of hLf was tested in 5 mM PPB adjusted to different pHs (5.5 to 8.0) by varying the concentrations of K2HPO4 and KH2PO4. The effects of several respiratory inhibitors were evaluated by using C. albicans cells (105 cells/ml) that were incubated at 37°C for 2 h in 5 mM PPB (pH 7.4) with or without azide, CCCP, DNP, or antimycin A (16, 18) and then treated with hLf (5 μM) for 2 h. The cells used in the antimycin inhibition assays were cultured in the presence of 10 μM antimycin A (18). Aliquots from the mixtures were serially diluted, spread onto SDA plates, and incubated for 48 h at 30°C. The number of viable cells was determined by counting the colonies on the SDA plates. Cell viability was expressed as a percentage of the viability of the control, and the loss of viability was calculated as [1 − (CFU of hLf-treated cells/CFU of control cells)] × 100.
Spheroplast preparation and killing assays.
Conversion of C. albicans cells to spheroplasts was carried out as described previously (10, 19). Cells growing in logarithmic phase were centrifuged at 1,500 × g for 10 min, and the pellet was washed in TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 7.4]). Cell suspensions (106 cells/ml) were prepared in TE buffer containing 1 M sorbitol and were incubated with 30 mM 2-mercaptoethanol and Zymolyase 20T (150 U/g [wet weight]) at 30°C for 1 h to obtain spheroplasts. The spheroplasts were washed twice with 5 mM PPB (pH 7.4) containing 1 M sorbitol, and suspensions prepared in the same buffer were immediately used for the killing assays. To test for spheroplast formation, samples treated with 5% (wt/vol) sodium dodecyl sulfate were observed under a microscope. When required, spheroplasts were incubated for 30 min on ice with proteinase K (300 μg/ml), and proteolysis was stopped by adding phenylmethylsulfonyl fluoride (final concentration, 10 mM). After 3 min, the samples were washed twice as described above and used immediately.
For the killing assays, the spheroplast suspensions (105 cells/ml) were exposed to selected concentrations of hLf for 2 h at 37°C, and then aliquots were poured into regeneration SDA plates as described previously (20).
Measurement of cation concentrations.
Determination of the extracellular K+ contents was performed as described previously (4), with slight modifications. For intracellular Na+ concentration determinations, the cells were loaded with Na+ after exposure (20 min) in Na+ loading buffer (10 mM morpholineethanesulfonic acid, 2% glucose, 0.1 mM MgCl2, 0.5 mM KCl, 100 mM NaCl [pH 6.0]) (4). The cell suspensions were centrifuged (2,000 × g, 2 min) and then washed twice with ice-cold MgCl2 (20 mM) containing an isosmotic concentration of sorbitol. The cells (109 cells/ml) were rapidly resuspended in 5 mM PPB or 5 mM SPB (pH 7.4) for determination of the Na+ and K+ concentrations, respectively. Lactoferrin (5 μM) or nystatin (100 μg/ml) was added, and the mixture was incubated at 37°C. Samples (0.5 ml) were taken at intervals and centrifuged, and the supernatant was collected to determine the extracellular K+ content. For determination of the intracellular Na+ content, the pellet was treated with 0.5% (vol/vol) perchloric acid and heat (95°C for 1 h) and centrifuged to remove the cell debris. The supernatant was then analyzed for the intracellular Na+ content. The monovalent cation concentration was determined by flame photometry with samples of nontreated cells and hLf solutions without cells. The percentages of Na+ and K+ were calculated on the basis of the total cellular Na+ and K+ contents of untreated cells (which were considered 100%).
Measurement of intracellular pH.
Flow cytometric analysis was performed with the pH indicator BCECF (43). Cell suspensions (5 × 106 cells/ml) in 5 mM PPB (pH 7.4) were incubated with BCECF-AM (final concentration, 15 μM) for 90 min. Subsequently, the cells were washed with the same buffer, and 105 cells/ml were incubated with 5 μM hLf for 2 h at 37°C. Amphotericin B (16 μg/ml) was used as a positive control (33). In parallel, the hLf-treated and untreated cells were analyzed with a fluorescence microscope (Leica DMR-XA; Leica Microsystems, Wetzlar, Germany).
Measurement of Δψ in C. albicans cells.
The cytoplasmic transmembrane electrical potential (Δψ) of C. albicans cells was measured as described previously (25). Cell suspensions (106 cells/ml) in 5 mM PPB (pH 7.4) were incubated with 5 μM hLf for 2 h at 37°C. The samples were reincubated for an additional 10 min with the membrane potential-sensitive fluorescent probe DiOC5(3) (final concentration, 0.5 μM) and immediately analyzed by cytofluorometry.
RESULTS
Killing of intact cells and spheroplasts.
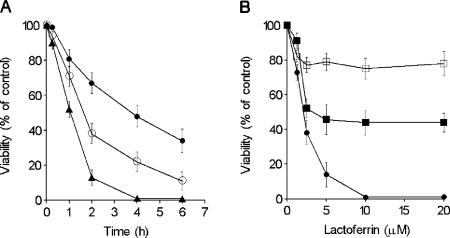
The anti-Candida effect of hLf was time and concentration dependent (Fig. 1). The results showed that 5 μM hLf incubated with yeast cells in 5 mM PPB (pH 7.4) for 2 h caused a loss of cell viability (mean ± standard deviation, 85% ± 4%), and no living cells were detected after 4 h (Fig. 1A). A short lag phase (15 min) followed by a rapid decrease (>10%) in cellular viability was observed in cell suspensions incubated with low concentrations (1.25 and 2.5 μM) of hLf.
FIG. 1.
Killing effect of lactoferrin on C. albicans cells. (A) C. albicans cells were incubated with 1.25 μM (•), 2.5 μM (○), or 5 μM (▴) hLf in 5 mM PPB (pH 7.4). (B) Spheroplasts treated with proteinase K (□), untreated spheroplasts (▪), and intact cells (•) of C. albicans were incubated with lactoferrin for 2 h. The results are the means ± standard deviations from duplicates of at least three independent assays.
Since previous reports have demonstrated that hLf mediates destabilization of the C. albicans cell wall (31, 42), an interaction of hLf with the cytoplasmic membrane could be possible. We tested whether cell wall-free cells (spheroplasts) obtained by enzymatic treatment would more susceptible to hLf activity. Spheroplasts of C. albicans were susceptible to hLf (5 μM hLf caused an approximately 55% loss of viability), but the level of spheroplast killing did not increase at higher concentrations (>5 μM). In contrast, hLf killed intact cells in a dose-dependent manner (Fig. 1B). Remarkably, spheroplasts treated with proteinase K were less susceptible to hLf (5 μM hLf caused an approximately 21% loss of viability) than untreated spheroplasts. The simultaneous monitoring of the cell number with a hemocytometer chamber did not show a significant decrease in the numbers of spheroplasts or whole cells (data not shown), indicating that the cell death caused by hLf was not due to a cytolytic effect.
Influences of external cation concentration, pH, and temperature on hLf killing activity.
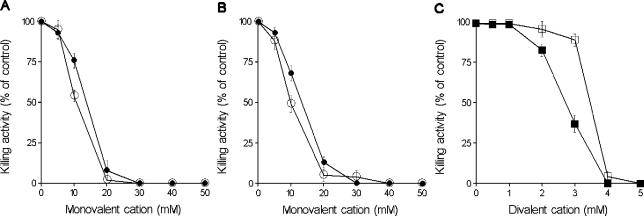
As we had previously reported that monovalent cations (Na+, K+) adversely affect the bactericidal activity of lactoferrin in buffers with low ionic strengths (40), we tested the influence of monovalent cations (Na+, K+) and divalent cations (Ca2+, Mg2+) on the candidacidal activity of hLf. We found that the killing effect progressively decreased with increasing mono- and divalent cation concentrations in the buffer (Fig. 2). The killing activity was prevented at Na+ or K+ concentrations ≥30 mM (Fig. 2A) or Ca2+ or Mg2+ concentrations ≥4 mM (Fig. 2C). The inhibition observed with different molarities of PPB or SPB was independent of the different concentrations of phosphate anions present in each assay, because similar inhibition patterns were obtained in experiments performed with 10 mM Tris buffer (pH 7.4) containing a similar range of NaCl or KCl concentrations (Fig. 2B). The inhibitory effects of Na+ and K+ were not reverted by addition of hLf (up to 30 μM) to cell suspensions prepared in 10 mM Tris buffer (pH 7.4) that contained 50 mM NaCl or KCl (data not shown). The candidacidal activity of hLf (5 μM) was not significantly modified when the killing assays were performed in a solution in which 0.32 M sorbitol replaced 140 mM NaCl to maintain similar osmotic conditions (data not shown).
FIG. 2.
Effects of extracellular cations on candidacidal effects of hLf. (A) C. albicans cells were suspended in SPB (•) or PPB (○) (both at pH 7.4) adjusted to different molarities and then incubated with lactoferrin for 2 h. (B) The candidacidal effect of lactoferrin was also tested in 10 mM Tris buffer (pH 7.4) containing either NaCl (•) or KCl (○). (C) Killing activity of lactoferrin against C. albicans cells suspended in 10 mM Tris buffer (pH 7.4) containing different concentrations of CaCl2 (□) or MgCl2 (▪). The results are the means ± standard deviations from duplicates of three separate experiments.
Additional experiments were performed to evaluate the influences of pH and temperature on the candidacidal effect of lactoferrin. Cell suspensions in 5 mM PPB at different pHs (5.5 to 8.0) were incubated with or without hLf (5 μM). The killing effect of hLf was greater at pH 5.5 than at pH 7.4 (Table 1). A similar effect was observed when Tris buffer was used (data not shown). In several experiments (n = 7), the cells incubated at 37°C were more susceptible (approximately 28%) to the antifungal effect of hLf than cells incubated at 4°C (Table 1).
TABLE 1.
Effects of pH and temperature on the candidacidal activity of lactoferrina
| Agent | nb | Viability (% of control) |
|---|---|---|
| External pH | ||
| 5.4 | 5 | 0 |
| 6.0 | 5 | 3 ± 2 |
| 6.4 | 4 | 8 ± 2 |
| 7.0 | 5 | 15 ± 4 |
| 7.4 | 7 | 15 ± 4 |
| 8.0 | 5 | 14 ± 2 |
| Temp (°C) | ||
| 4 | 7 | 22 ± 2 |
| 37 | 7 | 15 ± 4 |
C. albicans cells were treated with 5 μM hLf for 2 h. Each value represents the mean ± standard deviation from duplicates of at least three separate experiments.
Number of assays performed.
Lactoferrin induces K+ efflux in C. albicans cells.
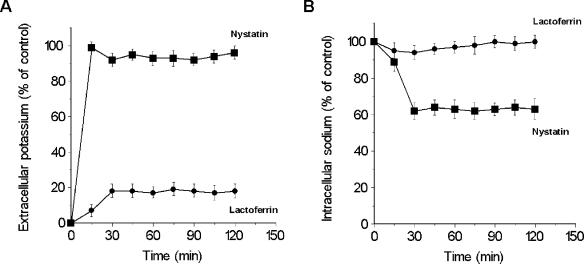
Since membrane pore formation, which causes a nonspecific efflux of ions, is assumed to be a major antimicrobial mechanism of action of many peptides (15), we assessed the ability of hLf to permeabilize the cell membrane to intracellular cations. Measurements of the extracellular K+ and intracellular Na+ concentrations from cell suspensions incubated with hLf revealed that an estimated 19% ± 2% (n = 5) of the total K+ content was released (Fig. 3A). The percentage of K+ released remained constant after 30 min in all other samples tested. Higher hLf concentrations (up to 30 μM) were unable to increase the percentage of K+ released. C. albicans cells loaded with an intracellular Na+ concentration 21% ± 3% greater than that of cells not loaded with Na+ were used to determine whether hLf could cause Na+ efflux. No decrease in the intracellular Na+ concentration was detected in cells treated with 5 μM hLf (Fig. 3B). However, control assays performed with cells treated with nystatin, an ionophore that causes leakage of univalent cations (14), showed a more rapid rate of release of intracellular K+ and an increased level of release of intracellular K+ (at 15 min, 96% ± 2%; n = 5) than those observed with hLf-treated cells (Fig. 3A). A decrease in the level of intracellular Na+ (46% ± 5%; n = 4) from Na+-loaded cells treated with nystatin was also observed (Fig. 4B).
FIG. 3.
Effect of lactoferrin on intracellular cation contents. Extracellular potassium (A) and intracellular sodium (B) concentrations were determined by flame photometry of C. albicans cells treated with hLf (•) or nystatin (▪). Values (means ± standard deviations; n = 3) are given as percentages relative to the total cation contents determined after cell lysis.
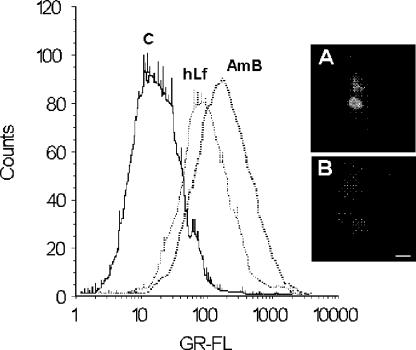
FIG. 4.
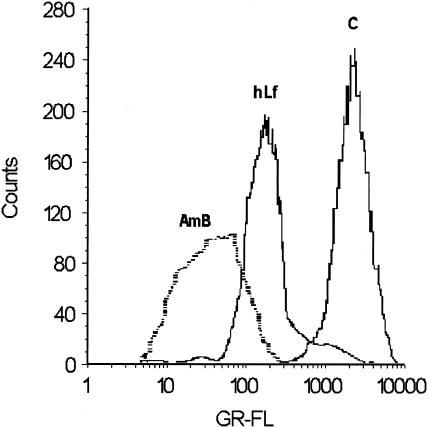
Effect of lactoferrin on intracellular pH of C. albicans cells. C. albicans cells preloaded with the pH-sensitive fluorescent dye BCECF-AM were exposed to hLf or amphotericin B (AmB) for 2 h, and then the intracellular BCECF-AM fluorescence intensities of treated and nontreated (control [C]) cells were analyzed by flow cytometry. GR-FL, green fluorescence. The micrographs show BCECF- loaded cells incubated in the presence (A) or the absence (B) of lactoferrin and examined by fluorescence microscopy. Bar, 5 μm.
Changes in intracellular pH induced by hLf.
The internal pH of C. albicans cells treated with hLf was examined by using cells preloaded with BCECF-AM. This fluorescent probe can diffuse into cells and can be hydrolyzed by intracellular esterases. The fluorescence of the BCECF trapped inside cells is pH dependent, and this compound can serve as an indicator of the intracellular pH (43). Under candidacidal conditions cells treated with 5 μM hLf for 2 h showed a slight increase in the level of BCECF fluorescence intensity, indicating acidification of the cytoplasm (Fig. 4). In control assays, amphotericin B (16 μg/ml) caused intracellular acidification, as described previously (43). Lactoferrin-treated cells showed a higher BCECF fluorescence intensity than nontreated cells (controls), as observed by fluorescence microscopy (Fig. 4).
Effect of hLf on transmembrane Δψ of C. albicans cells.
Analysis of the Δψ of the cytoplasmic membrane was performed with the fluorescent probe DiOC5(3). This probe is a charged lipophilic dye which partitions into the cytoplasm and is dependent on an intact Δψ for intracellular retention. The fluorescence intensity of DiOC5(3) decreased in yeast cells incubated with 5 μM hLf, indicating a depolarization of the cytoplasmic membranes of the C. albicans cells. Figure 5 shows the results of a representative experiment performed under candidacidal conditions in which cell suspensions in 5 mM PPB (pH 7.4) were exposed to hLf (5 μM) for 2 h. A similar result was obtained in control assays with cells treated with amphotericin B (Fig. 5), an antifungal agent that causes depolarization of C. albicans cell membranes (22).
FIG. 5.
Effect of lactoferrin on C. albicans cytoplasmic membrane potential. C. albicans cells suspended in 5 mM PPB (pH 7.4) were treated with hLf or amphotericin B (AmB) or were not treated (control [C]) for 2 h. The fluorescent probe DiOC5(3) was then added to the incubation mixture, and the fluorescence distribution was analyzed by flow cytometry. GR-FL, green fluorescence.
Effects of respiratory inhibitors on candidacidal activity of lactoferrin.
The importance of the metabolic state on susceptibility to hLf was tested in the presence of several agents and conditions that inhibit respiration of C. albicans cell mitochondria (16, 18). The killing activity of hLf was assayed with cell suspensions (105 cells/ml) in 5 mM PPB (pH 7.4) that were preincubated with the respiration uncouplers CCCP (500 μM) and DNP (10 μM), which stop mitochondrial ATP synthesis without blocking the respiratory chain or oxygen consumption (36). The candidacidal effect of hLf (5 μM) was significantly decreased by CCCP (mean ± standard deviation cell survival, 94% ± 3%; n = 5) and DNP (cell survival, 47% ± 4%; n = 3) (Table 2). Sodium azide (5 mM) was also able to inhibit the killing effect of hLf (cell survival, 96% ± 4%; n = 5) (Table 2). In control assays, sodium azide was replaced by equimolar concentrations of NaCl in order to discount the inhibitory effect caused by Na+. The concentrations and the exposure times of the inhibitors (CCCP, DNP, and azide) assayed here were not candidacidal, as described previously (16, 36). The fungicidal activity of hLf was also clearly inhibited (cell survival, >81%) by anaerobic conditions (Table 2).
TABLE 2.
Effects of cellular respiratory inhibitors on the candidacidal activity of lactoferrina
| Incubation | Inhibitor (concn) | Cell viability (% of control)b |
|---|---|---|
| Aerobic | 15 ± 2 | |
| Aerobic | Azide (5 mM) | 96 ± 4 |
| Aerobic | CCCP (500 μM) | 94 ± 3 |
| Aerobic | DNP (10 μM) | 47 ± 4 |
| Aerobic | Antimycin (10 μM) | 92 ± 5 |
| Anaerobicc | 81 ± 4 | |
| Anaerobicd | 92 ± 3 |
C. albicans cells were incubated with or without the respiratory inhibitors and were then treated with lactoferrin for 2 h.
Values are the means ± standard deviations from duplicates of at least three independent experiments.
Assays were performed under anaerobic conditions (80% N2, 10% CO2, 10% H2).
Assays were performed in the presence of 2.5 mM l-cysteine.
In addition to the conventional respiratory pathway, C. albicans can also express an alternative terminal oxidase involved in the alternative (cyanide-resistant) respiratory pathway (18). The hLf susceptibilities of C. albicans cells respiring via the conventional or alternative respiratory pathways were tested with cells cultured and assayed with or without antimycin A (10 μM). Antimycin-treated cells showed a lower level of susceptibility (cell survival, 92% ± 5%; n = 3) to hLf than cells not exposed to this inhibitor (Table 2).
DISCUSSION
Concentration and time course assays demonstrated the ability of hLf to kill C. albicans cells in a dose- and time-dependent manner. Several factors (extracellular cation concentration, pH, and temperature) were investigated for their abilities to modify the killing activity of hLf. Since previous reports have described the influence of the extracellular monovalent cation concentration on the antibacterial activity of hLf (38, 40), we examined whether a similar effect could modify the antifungal effect of this protein. Interestingly, the candidacidal effect of hLf progressively diminished as the Na+ or K+ concentration in the buffer increased and was abolished at concentrations ≥30 mM. Our data also show that CaCl2 or MgCl2 concentrations ≥4 mM inhibited the candidacidal effect of hLf. Previous work indicated that Ca2+ and Mg2+ inhibit the bactericidal effect of hLf due to the inability of hLf to bind to the cell surface (13). Even though the cause of the inhibitory effect on candidacidal activity was not investigated, it could be a consequence of different factors, such as the increase in the ionic strength or the formation of hLf tetramers in the presence of divalent cations (6). Furthermore, the inhibition did not appear to be related to an osmotic effect, because when the salt was replaced by an equimolar concentration of sorbitol, the killing effect of lactoferrin was not blocked. The candidacidal effect of lactoferrin was enhanced at acidic pH, probably due to the increase in the amount of negatively charged groups on the cell envelope, which facilitates electrostatic interactions between the cell surface and lactoferrin (pI = 8.7). Furthermore, the susceptibility of C. albicans cells was slightly modified by the temperature, with cell susceptibility to hLf decreasing at 4°C, in agreement with previous reports (37).
The results presented above suggest that the candidacidal effect of hLf is concomitant with low ionic strength. We have also reported previously that the bactericidal effect of lactoferrin is dependent on the extracellular cation concentration (40). The differences in the ionic strengths of the test buffers could explain in part the discrepancies in the different candidacidal effects of hLf reported previously (26, 30, 42).
We assumed that the highly cationic protein hLf interacted with the C. albicans cytoplasmic membrane to exert its candidacidal effect, as has been described for many antimicrobial peptides. For instance, the antimicrobial mechanism of action of cationic peptides is believed to arise from the binding to the negatively charged plasma membrane, which results in the formation of transmembrane pore-like structures or structures resembling ion channels. In this case, the subsequent permeabilization of the membrane causes leakage of intracellular constituents, leading to cell death (for a review, see reference 15). Although previous studies have demonstrated that hLf causes alteration of C. albicans cell walls (31, 42), the interaction of this protein with the cytoplasmic membrane or intracellular organelles has not been reported. The cell wall destabilization caused by hLf could facilitate the translocation of the protein to the cytoplasmic membrane and then cause a small disruptive effect on the membrane. In this case, we could expect that the direct exposure of the membrane of cell wall-free spheroplasts would result in an increase in the antifungal effect of hLf. Nevertheless, the susceptibilities of the spheroplasts did not increase in the presence hLf concentrations ≥5 μM, thus indicating that the integrity of the C. albicans cytoplasmic membrane was not modified by lactoferrin. The increased resistance of spheroplasts to lactoferrin could be due to the absence of a cell wall compound required for hLf activity. It could also be due to modification of a membrane element (i.e., a protein) by other enzymes (i.e., proteases) present in the Zymolyase preparations (10), which would avoid the hypothetical interaction of hLf with a surface membrane element (i.e., protein), as previously suggested to explain the decreased activity of histatin 5 against spheroplasts (10). The last suggestion is supported by the significantly lower levels of susceptibility to hLf of spheroplasts treated with proteinase K than untreated spheroplasts.
The release of intracellular potassium was detected, even though lactoferrin was unable to disrupt the C. albicans cytoplasmic membrane (data not shown). However, the difference between the K+ release induced by lactoferrin (19% in 30 min) compared with that caused by nystatin (96% in 15 min) was significant. In contrast, Na+ efflux from cells previously loaded with sodium was not detected. This selective efflux differed from that reported for the majority of the antimicrobial cationic peptides (15). Interestingly, the low level of K+ efflux appears to be an early event related to the killing effect but is not the primary cause of cell death. This suggestion is based on the absence of a correlation between K+ efflux and the loss of viability of hLf-treated cells. For instance, the K+ release (approximately 19% of total intracellular K+) did not increase after 30 min of exposure to hLf; meanwhile, cell viability progressively decreased (approximately 85% until 2 h). How the selective K+ release is exactly generated is unknown at present, but the K+ release suggests that membrane components other than membrane phospholipids are involved. This could be a consequence of cell homeostatic mechanisms that compensate for intracellular ionic changes, as seems to be indicated by the decrease in the membrane Δψ and the intracellular acidification.
Cell susceptibility to certain candidacidal peptides is dependent on the metabolic state. For instance, histatin 5 targets mitochondria and induces the dissipation of the membrane potential, whereas human defensins HNP-1, HNP-2, and HNP-3 block the metabolic processes of C. albicans cells (16, 17, 28). The killing effects of these different human peptides are not observed under anaerobic conditions (16, 25, 27). In a similar way, the candidacidal effect of hLf was clearly higher against cells maintained under aerobic conditions than that against cells incubated under anaerobic conditions (85 and 19% loss of cell viability, respectively). Moreover, it was striking that the candidacidal activity of hLf against C. albicans cells preincubated with different respiration inhibitors or uncouplers (sodium azide, DNP, CCCP, and antimycin) was significantly decreased. The results presented above suggest that the in vitro candidacidal activity of hLf depends on mitochondrial energy as well.
Further studies exploring the intracellular events implicated in the candidacidal effect of lactoferrin are required; here we present evidence indicating that extracellular cation concentrations, as well as the metabolic state of C. albicans cells, modulate the in vitro candidacidal activity of hLf.
Acknowledgments
We thank M. E. Díaz and A. Salas for assistance with atomic emission spectrometry and flow cytometric analysis, respectively.
This work was supported by the University of Oviedo (CN-96-133-B1/Laboratory of Oral Microbiology).
REFERENCES
- 1.Arnold, R. R., M. F. Cole, and J. R. McGhee. 1977. A bactericidal effect for human lactoferrin. Science 197:263-265. [DOI] [PubMed] [Google Scholar]
- 2.Arnold, R. R., M. Brewer, and J. J. Gauthier. 1980. Bactericidal activity of human lactoferrin: sensitivity of a variety of microorganisms. Infect. Immun. 28:893-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold, R. R., J. E. Russell, W. J. Champion, M. Brewer, and J. J. Gauthier. 1982. Bactericidal activity of human lactoferrin: differentiation from the stasis of iron deprivation. Infect. Immun. 35:792-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bañuelos, M. A., and A. Rodríguez-Navarro. 1998. P-type ATPases mediate sodium and potassium effluxes in Schwanniomyces occidentalis. J. Biol. Chem. 273:1640-1646. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy, W., M. Takase, K. Yamauchi, H. Wakabayashi, K. Kawase, and M. Tomita. 1992. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1121:130-136. [DOI] [PubMed] [Google Scholar]
- 6.Bennett, R. M., G. C. Bagby, and J. Davis. 1981. Calcium dependent polymerization of lactoferrin. Biochem. Biophys. Res. Commun. 101:88-95. [DOI] [PubMed] [Google Scholar]
- 7.Cannon, R. D., and W. L. Chaffin. 1999. Oral colonization by Candida albicans. Crit. Rev. Oral Biol. Med. 10:359-383. [DOI] [PubMed] [Google Scholar]
- 8.de Lillo, A., L. M. Quirós, and J. F. Fierro. 1997. Relationship between antibacterial activity and cell surface binding of lactoferrin in species of genus Micrococcus. FEMS Microbiol. Lett. 150:89-94. [DOI] [PubMed] [Google Scholar]
- 9.Devine, D. A. 2003. Antimicrobial peptides in defence of the oral and respiratory tracts. Mol. Immunol. 40:431-443. [DOI] [PubMed] [Google Scholar]
- 10.Edgerton, M., S. E. Koshlukova, T. E. Lo, B. G. Chrzan, R. M. Straubinger, and P. A. Raj. 1998. Candidacidal activity of salivary histatins. Identification of a histatin 5-binding protein on Candida albicans. J. Biol. Chem. 273:20438-20447. [DOI] [PubMed] [Google Scholar]
- 11.Edgerton, M., and S. E. Koshlukova. 2000. Salivary histatin 5 and its similarities to the other antimicrobial proteins in human saliva. Adv. Dent. Res. 14:16-21. [DOI] [PubMed] [Google Scholar]
- 12.Ellison, R. T., III, T. J. Giehl, and F. M. LaForce. 1988. Damage of the outer membrane of enteric gram-negative by lactoferrin and transferrin. Infect. Immun. 56:2774-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellison, R. T., III, F. M. LaForce, T. J. Giehl, D. S. Boose, and B. E. Dunn. 1990. Lactoferrin and transferrin damage of the gram-negative outer membrane is modulated by Ca2+ and Mg2+. J. Gen. Microbiol. 136:1437-1446. [DOI] [PubMed] [Google Scholar]
- 14.Hammond, S. M., P. A. Lambert, and B. N. Kliger. 1974. The mode of action of polyene antibiotics; induced potassium leakage in Candida albicans. J. Gen. Microbiol. 81:325-330. [DOI] [PubMed] [Google Scholar]
- 15.Hancock, R. E. W., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helmerhorst, E. J., P. Breeuwer, W. van't Hof, E. Walgreen-Weterings, L. C. J. M. Oomen, E. C. I. Veerman, A. V. N. Amerongen, and T. Abee. 1999. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J. Biol. Chem. 274:7286-7291. [DOI] [PubMed] [Google Scholar]
- 17.Helmerhorst, E. J., W. van't Hof, P. Breeuwer, E. C. I. Veerman, T. Abee, R. F. Troxler, A. V. N. Amerongen, and F. G. Oppenheim. 2001. Characterization of histatin 5 with respect to amphipathicity, hydrophobicity, and effects on cell and mitochondrial membrane integrity excludes a candidacidal mechanism of pore formation. J. Biol. Chem. 276:5643-5649. [DOI] [PubMed] [Google Scholar]
- 18.Helmerhorst, E. J., M. P. Murphy, R. F. Troxler, and F. G. Oppenheim. 2002. Characterization of the mitochondrial respiratory pathways in Candida albicans. Biochim. Biophys. Acta 1556:73-80. [DOI] [PubMed] [Google Scholar]
- 19.Jazwinsky, S. M. 1990. Preparation of extracts from yeast. Methods Enzymol. 182:154-174. [DOI] [PubMed] [Google Scholar]
- 20.Kakar, S. N., R. M. Partridge, and P. T. Magee. 1983. A genetic analysis of Candida albicans: isolation of a wide variety of auxotrophs and demonstration of linkage and complementation. Genetics 104:241-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanyshkova, T. G., V. N. Buneva, and G. A. Nevinsky. 2001. Lactoferrin and its biological functions. Biochemistry (Moscow) 66:1-7. [DOI] [PubMed] [Google Scholar]
- 22.Kim, D. H., D. G. Lee, K. L. Kim, and Y. Lee. 2001. Internalization of tenecin 3 by a fungal cellular process is essential for its fungicidal effect on Candida albicans. Eur. J. Biochem. 268:4449-4458. [DOI] [PubMed] [Google Scholar]
- 23.Kirkpatrick, C. H., I. Green, R. R. Rich, and A. L. Schade. 1971. Inhibition of growth of Candida albicans by iron-unsaturated lactoferrin: relation to host-defense mechanisms in chronic mucocutaneous candidiasis. J. Infect. Dis. 124:539-544. [DOI] [PubMed] [Google Scholar]
- 24.Kontoghiorghes, G. J., and E. D. Weinberg. 1995. Iron: mammalian defense systems, mechanisms of disease, and chelation therapy approaches. Blood Rev. 9:33-45. [DOI] [PubMed] [Google Scholar]
- 25.Koshlukova, S. E., T. L. Lloyd, M. W. B. Araujo, and M. Edgerton. 1999. Salivary histatin 5 induces non-lytic release of ATP from Candida albicans leading to cell death. J. Biol. Chem. 274:18872-18879. [DOI] [PubMed] [Google Scholar]
- 26.Kuipers, M. E., H. G. de Vries, M. C. Eikelboom, D. K. Meijer, and P. J. Swart. 1999. Synergistic fungistatic effects of lactoferrin in combination with antifungal drugs against clinical Candida isolates. Antimicrob. Agents Chemother. 43:2635-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehrer, R. I., T. Ganz, D. Szklarek, and M. E. Selsted. 1988. Modulation of the in vitro candidacidal activity of human neutrophil defensins by target cell metabolism and divalent cations. J. Clin. Investig. 81:1829-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lichtenstein, A. K., T. Ganz, T. M. Nguyen, M. E. Selsted, and R. I. Lehrer. 1988. Mechanism of target cytolysis by peptide defensins. Target cell metabolic activities, possibly involving endocytosis, are crucial for expression of cytotoxicity. J. Immunol. 140:2686-2694. [PubMed] [Google Scholar]
- 29.Lonnerdal, B., and S. Iyer. 1995. Lactoferrin: molecular structure and biological function. Annu. Rev. Nutr. 15:93-110. [DOI] [PubMed] [Google Scholar]
- 30.Lupetti, A., A. Paulusma-Annema, M. M. Welling, S. Senesi, J. T. van Dissel, and P. H. Nibbering. 2000. Candidacidal activities of human lactoferrin peptides derived from the N terminus. Antimicrob. Agents Chemother. 44:3257-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikawa, H., L. P. Samarayanake, J. Tenovuo, K. M. Pang, and T. Hamada. 1993. The fungicidal effect of human lactoferrin on Candida albicans and Candida krusei. Arch. Oral Biol. 38:1057-1063. [DOI] [PubMed] [Google Scholar]
- 32.Nikawa, H., L. P. Samarayanake, and T. Hamada. 1995. Modulation of the anti-Candida activity of apo-lactoferrin by dietary sucrose and tunicamycin in vitro. Arch. Oral Biol. 40:581-584. [DOI] [PubMed] [Google Scholar]
- 33.Peyron, F., A. Favel, H. Guiraud-Dauriac, M. El Mzibri, C. Chastin, G. Dumenil, and P. Regli. 1997. Evaluation of a flow cytofluorometric method for rapid determination of amphotericin B susceptibility of yeast isolates. Antimicrob. Agents Chemother. 41:1537-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sallmann, F. R., S. Baveye-Descamps, F. Pattus, V. Salmon, N. Branza, G. Spik, and D. Legrand. 1999. Porins OmpC and PhoE of Escherichia coli as specific cell-surface targets of human lactoferrin. Binding characteristics and biological effects. J. Biol. Chem. 274:16107-16114. [DOI] [PubMed] [Google Scholar]
- 35.Sangeorzan, J. A., S. F. Bradley, X. He, L. T. Zarins, G. L. Ridenour, R. N. Tiballi, and C. A. Kauffman. 1994. Epidemiology of oral candidiasis in HIV-infected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am. J. Med. 97:339-346. [DOI] [PubMed] [Google Scholar]
- 36.Shepherd, M. G., C. M. Chin, and P. A. Sullivan. 1978. The alternate respiratory pathway of Candida albicans. Arch. Microbiol. 116:61-67. [DOI] [PubMed] [Google Scholar]
- 37.Soukka, T., J. Tenovuo, and M. Lenander-Lumikari. 1992. Fungicidal effect of human lactoferrin against Candida albicans. FEMS Microbiol. Lett. 90:223-228. [DOI] [PubMed] [Google Scholar]
- 38.Travis, S. M., B. D. Conway, J. Zabner, J. J. Smith, N. N. Anderson, P. K. Singh, E. P. Greenberg, and M. J. Welsh. 1999. Activity of abundant antimicrobials of the human airway. Am. J. Respir. Cell Mol. Biol. 20:872-879. [DOI] [PubMed] [Google Scholar]
- 39.Valenti, P., P. Visca, G. Antonini, and N. Orsi. 1986. Interaction between lactoferrin and ovotransferrin and Candida cells. FEMS Microbiol. Lett. 33:271-275. [Google Scholar]
- 40.Viejo-Díaz, M., M. T. Andrés, J. Pérez-Gil, M. Sánchez, and J. F. Fierro. 2003. Potassium-efflux induced by a new lactoferrin-derived peptide mimicking the effect of native human lactoferrin on the bacterial cytoplasmic membrane. Biochemistry (Moscow) 68:217-227. [DOI] [PubMed] [Google Scholar]
- 41.Wozniak, K. L., J. E. Leigh, S. Hager, R. K. Swoboda, and P. L. Jr. Fidel. 2002. A comprehensive study of Candida-specific antibodies in the saliva of human immunodeficiency virus-positive individuals with oropharyngeal candidiasis. J. Infect. Dis. 185:1269-1276. [DOI] [PubMed] [Google Scholar]
- 42.Xu, Y. Y., Y. H. Samaranayake, L. P. Samaranayake, and H. Nikawa. 1999. In vitro susceptibility of Candida species to lactoferrin. Med. Mycol. 37:35-41. [DOI] [PubMed] [Google Scholar]
- 43.Ziegelbauer, K., G. Grusdat, A. Schade, and W. Paffhausen. 1999. High throughput assay to detect compounds that enhance the proton permeability of Candida albicans membranes. Biosci. Biotechnol. Biochem. 63:1246-1252. [DOI] [PubMed] [Google Scholar]