Abstract
AIM: To assess the clinical significance and the prognostic value of preoperative serum carbohydrate antigen 19-9 (CA 19-9) level in gastric cancer.
METHODS: Between January 2005 and December 2006, 1960 patients underwent surgery for histologically confirmed gastric cancer. Of these, 163 patients had elevated serum levels of CA 19-9 preoperatively, and 1628 patients had normal serum levels of CA 19-9 preoperatively. For this study, 325 patients were selected from the group of 1628 patients by age, sex, and cancer stage to serve as controls. Statistically significant differences in survival rates were calculated using the log-rank test. A P value less than 0.05 was considered statistically significant and was determined using SAS software.
RESULTS: The baseline characteristics showed some differences between the two groups with regard to histology. Overall survival (OS) in the elevated and non-elevated group was 37.90 and 68.67 mo, respectively (P < 0.001). N stage (P = 0.001) was a significant predictor of disease-free survival by multivariate analysis. Also, N stage (P < 0.001), and the presence of peritoneal metastasis (P < 0.001) remained independent factors in predicting OS by multivariate analysis. Additionally, preoperative serum CA 19-9 levels were significantly associated with OS in univariate (P = 0.009) and multivariate (P = 0.021) analyses.
CONCLUSION: Serum CA 19-9 can be considered an independent prognostic factor in predicting OS in patients anticipating surgery for gastric cancer.
Keywords: Gastric cancer, Carbohydrate antigen 19-9, Disease-free survival, Overall survival
Core tip: The exact functions of preoperative carbohydrate antigen (CA) 19-9 in stomach cancer have yet to be uncovered. We sought to assess the clinical significance of preoperatively high levels of CA 19-9 in patients with gastric cancer and aimed to investigate the relationship between serum levels of CA 19-9 and disease-free survival and overall survival (OS). We conclude that OS in gastric cancer patients with elevated CA 19-9 levels was lower than that in patients with non-elevated levels. Serum CA 19-9 can be considered an independent prognostic factor in predicting OS in patients anticipating surgery for gastric cancer.
INTRODUCTION
Gastric cancer is one of the most common malignancies and the cause of many cancer-related deaths worldwide. Although a single tumor marker is limited for the diagnosis of cancer, it can be used in various clinical aspects, including assessment of clinical status, monitoring of treatment response, prediction of prognoses, and as a surveillance marker for recurrence[1-6]. Carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 are tumor markers that are commonly used for the early diagnosis and prognostic evaluation of gastric cancer[7-9], potentially reflecting tumor biology[10]. Additionally, the relatively new marker cancer antigen, CA 72-4 provides prognostic information in gastric cancer[11,12].
Recent clinical studies have shown that CEA and CA 19-9 are recognized as poor prognostic factors for gastric cancer[13-15] and are related to its recurrence[6,14]. The prognostic relevance of such tumor markers in patients with gastric cancer is not comparable with those markers used in other carcinomas[5,16-18]. Specifically, CA 19-9 has been reported to be elevated in certain forms of gastric cancer[11,19,20]. However, because little research on the prognoses of gastric cancer patients with elevated preoperative CA 19-9 levels has been performed, the clinical significance of preoperative CA 19-9 levels has not been fully verified[5,13].
Therefore, it is important to interpret the prognostic value of CA 19-9 levels in patients with gastric cancer, especially in patients anticipating surgery for postoperative survival. Thus, we sought to assess the clinical significance of preoperatively high levels of CA 19-9 in patients with gastric cancer and aimed to investigate the relationship between serum levels of CA 19-9 and disease-free survival (DFS) and overall survival (OS).
MATERIALS AND METHODS
Patients
Between January 2005 and December 2006, 1960 patients underwent surgery for histologically confirmed gastric cancer at Severance Hospital, Seoul, South Korea. Sixty-nine patients who did not have preoperative serum CA 19-9 were excluded. Of the remaining 1891, 163 patients had elevated serum levels of CA 19-9 preoperatively, and 1628 patients had normal serum levels of CA 19-9 preoperatively. For this study, 325 patients were selected from the group of 1628 patients by age, sex, and cancer stage to serve as controls. A separate group of 488 patients who received surgery as a treatment modality for confirmed gastric cancer was included and analyzed retrospectively in this study.
Classification of gastric cancers
The endoscopic findings of early gastric cancer were classified according to the criteria of the Japanese Research Society for Gastric Cancer (JRSGC) as follows: elevated (types I or IIA), flat (type IIB), depressed (types IIC, IIC + III, or IIA + IIC), and mixed. Advanced gastric cancers were categorized according to Borrmann’s classification. Histological evaluation was performed according to the Japanese General Rules for Gastric Cancer Study in Surgery and Pathology from the JRSGC[21].
In addition, patients were classified into three groups, which were based on the location of the primary lesion: upper third, middle third, and lower third. The upper third-designated cancer developed in the gastric cardia and fundus, the middle third-designated cancer developed in the gastric body, and the lower third-designated cancer was found in the antrum and pylorus[22].
Treatment modalities
Surgical treatments were considered curative and palliative according to the Union for International Cancer Control criteria[23]. The standard surgical treatment was radical total or subtotal gastrectomy with D2 lymph node dissection in accordance with JRSGC rules[21]. Curative resection (R0) was defined as the absence of tumor either macroscopically or microscopically after surgery. In selected inoperable cases, palliative gastrectomy was performed when necessary.
Initial work-up and follow-up
A follow up period was started on January 1st, 2005 and ended on August 22th, 2008. Initial evaluation included complete medical history and physical examination, paying special attention to symptoms often associated with stomach cancer. Chest radiography and laboratory tests were performed, including complete blood cell count, blood urea nitrogen and creatinine levels, and liver function tests. Serum CA 19-9 concentrations were measured using a commercial chemiluminescent enzyme immunoassay with a normal upper limit of 37 U/mL[24]. Serum CA 19-9 levels were routinely measured immediately before surgery. The entire study population underwent esophagogastroduodenoscopy and computed tomography of the abdomen. After surgery, esophagogastroduodenoscopy, computed tomography of the abdomen and laboratory tests performed during the initial work-up were repeated at each follow-up visit.
Statistical analysis
This study was based on matched pair data considering age, sex and cancer stage. In the mixed model, comparisons between patients with elevated CA 19-9 levels and those with normal levels based on age, sex, cancer stage, and survival were performed. Categorical data were evaluated by the χ2 test or Fisher’s exact test, and all continuous variables were expressed as the median (range) and analyzed using the Mann-Whitney U test. Multivariate analysis of survival was performed using the Cox proportional hazards model. OS was defined from the date of surgery until death or the date of last follow-up. DFS was defined as the interval from the operation date to the date of confirming recurrence, death from any cause other than cancer, or last visiting date. Paired Kaplan-Meier curves and Cox regression analyses using robust standard error for survival analysis were performed. Statistically significant differences in survival rates were calculated using the log-rank test. A P value less than 0.05 was considered statistically significant and was determined using SAS software (version 9.1.3, SAS Institute Inc., Cary, NC, United States).
RESULTS
Patient characteristics
We compared the outcomes and clinicopathologic characteristics of 163 patients with elevated preoperative serum CA 19-9 levels (elevated group) with those of 325 patients with non-elevated preoperative serum CA 19-9 levels (non-elevated group), which are summarized in Table 1. Baseline characteristics did not show a significant statistical relationship between the two groups except for histology and serum CA 19-9 levels, which revealed a significantly higher proportion of less differentiated adenocarcinoma in patients with elevated preoperative serum CA 19-9 levels and mean serum CA 19-9 values of 575.74 ± 518.09 U/mL in the elevated group and 8.45 ± 8.42 U/mL in the non-elevated group. However, there were no significant differences in baseline characteristics with regard to other variables, such as sex, age, endoscopic findings, and other serum tumor markers. In 56 patients, surgery was performed as a palliative treatment. Of these, gastrojejunostomy was performed for bypass in 27 patients. Hepatic and peritoneal metastases were appraised by radiological findings, histological examination, and/or intraoperative observation.
Table 1.
Baseline characteristics with a comparison of patients with elevated serum carbohydrate antigen 19-9 levels and normal serum carbohydrate antigen 19-9 levels
| Characteristic | Elevated group (n = 163) | Non-elevated group (n = 325 ) | P value |
| Sex | 0.963 | ||
| Male | 111 (68.1) | 222 (68.3) | |
| Female | 52 (31.9) | 103 (31.7) | |
| Mean age, yr (range) | 60.70 ± 12.00 (29-84) | 59.71 ± 12.40 (27-85) | 0.295 |
| Number of lesions | 0.436 | ||
| Single | 145 (89.0) | 281 (86.5) | |
| Multiple | 18 (11.0) | 44 (13.5) | |
| Size (cm) | |||
| Horizontal | 6.38 ± 3.51 | 5.78 ± 3.16 | 0.060 |
| Vertical | 5.15 ± 2.79 | 4.66 ± 2.60 | 0.053 |
| Endoscopic findings | 0.135 | ||
| EGC | |||
| Elevated | 10 (6.1) | 23 (7.1) | |
| Flat | 3 (1.8) | 15 (1.6) | |
| Depressed | 10 (6.1) | 18 (5.5) | |
| AGC | |||
| Borrmann I | 9 (5.5) | 8 (2.5) | |
| Borrmann II | 25 (15.3) | 38 (11.7) | |
| Borrmann III | 89 (54.6) | 203 (62.5) | |
| Borrmann IV | 17 (10.4) | 20 (6.2) | |
| Histology | 0.033 | ||
| Well differentiated | 19 (11.7) | 23 (7.1) | |
| Moderately differentiated | 57 (35.0) | 86 (26.5) | |
| Poorly differentiated | 54 (33.1) | 145 (44.6) | |
| Signet ring cell cancer | 33 (20.3) | 71 (21.9) | |
| Location | 0.404 | ||
| Lower | 108 (66.3) | 210 (61.6) | |
| Middle | 16 (9.8) | 49 (15.1) | |
| Upper | 31 (19.0) | 52 (16.0) | |
| Diffuse | 8 (1.9) | 14 (4.3) | |
| T stage | 0.843 | ||
| T0-2 | 55 (36.7) | 117 (37.6) | |
| T3, 4 | 95 (63.3) | 194 (62.4) | |
| N stage | 0.908 | ||
| N0, 1 | 79 (52.7) | 162 (52.1) | |
| N2, 3 | 71 (47.3) | 149 (47.9) | |
| Mean metastatic | 0.893 | ||
| Lymph nodes (N) | 9.88 ± 13.03 | 10.09 ± 11.94 | |
| TNM stage | > 0.999 | ||
| 0 | 1 (0.6) | 2 (0.6) | |
| I | 26 (16.0) | 52 (16.0) | |
| II | 21 (12.9) | 42 (12.9) | |
| III | 57 (35.0) | 114 (35.1) | |
| IV | 58 (35.6) | 115 (35.4) | |
| Operation | 0.114 | ||
| For radical | 139 (85.3) | 293 (90.2) | |
| For palliative | 24 (14.7) | 32 (0.8) | |
| Lymphovascular invasion | 0.225 | ||
| Positive | 98 (76.0) | 190 (70.1) | |
| Negative | 31 (24.0) | 81 (29.9) | |
| Peritoneal metastasis | 0.899 | ||
| Positive | 10 (6.1) | 19 (5.8) | |
| Negative | 153 (93.9) | 306 (94.2) | |
| Hepatic metastasis | 0.853 | ||
| Positive | 5 (3.1) | 11 (3.4) | |
| Negative | 158 (96.9) | 314 (96.6) | |
| Neoadjuvant chemotherapy | 0.217 | ||
| Positive | 6 (3.7) | 6 (1.8) | |
| Negative | 157 (96.3) | 319 (98.2) | |
| Mean serum CA 19-9 (range) | 575.74 ± 518.09 (37.4-12800) | 8.45 ± 8.42 (0-36.8) | < 0.001 |
| Mean serum CEA (range) | 6.00 ± 21.86 (0.01-260.27) | 5.49 ± 17.30 (0.01-189.21) | 0.777 |
| Mean serum CA 72-4 (range) | 9.31 ± 20.52 (0.33-164) | 14.42 ± 68.61 (0.2-600) | 0.376 |
Data are expressed as mean ± SD or n (%). AGC: Advanced gastric cancer; CA: Carbohydrate antigen; CEA: Carcinoembryonic antigen; EGC: Early gastric cancer; TNM: Tumor-node-metastasis.
Survival outcome according to preoperative CA 19-9 levels
The median OS was 58.433 mo (95%CI: 43.07-70.90). A significantly longer median OS was observed in the non-elevated group than in the elevated group (68.67 mo vs 37.90 mo, 95%CI: 25.07-56.13; P < 0.001; Figure 1A). Because the majority of patients died near the end of the study, the upper limit of the confidence interval in the non-elevated group was not calculated.
Figure 1.
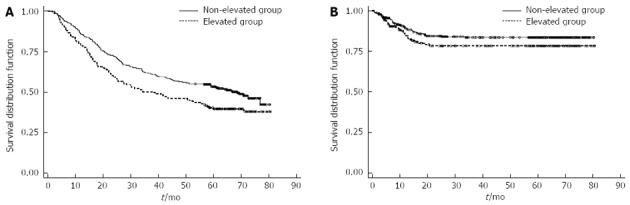
Kaplan-Meier curves. A: Overall survival, P-value by log-rank test < 0.001; B: Disease free survival in patients with elevated serum carbohydrate antigen 19-9 (CA 19-9) levels (n = 163) and those with normal serum CA 19-9 levels (n = 325), P-value by log-rank test = 0.099.
As survival curves for DFS did not reach 50% after Kaplan-Meier analysis, a median DFS was not defined. A longer DFS was seen in the non-elevated group than in the elevated group, but the difference was not statistically significant (P = 0.099; Figure 1B).
Prognostic factors
Potential prognostic variables for DFS are presented in Table 2. In univariate analysis, DFS was significantly associated with T stage (P = 0.001), N stage (P < 0.001), and the presence of lymphovascular invasion (P = 0.002). Other factors, including age, sex, number of lesions, differentiation, histology, peritoneal metastasis, and hepatic metastasis, were not significantly associated with DFS. Only N stage (P = 0.001) remained significantly linked with DFS in multivariate analysis. Neither univariate nor multivariate analyses revealed that preoperative serum CA 19-9 levels affected DFS.
Table 2.
Univariate and multivariate analysis of factors associated with disease-free survival
| Variable |
Univariate analysis |
Multivariate analysis |
||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| Disease-free survival | ||||
| Age (< 60 vs ≥ 60 yr) | 0.737 (0.475-1.143) | 0.737 | 0.620 (0.379-1.015) | 0.053 |
| Sex (male vs female ) | 1.140 (0.720-1.806) | 0.575 | 0.777 (0.454-1.331) | 0.359 |
| Lesions (single vs multiple) | 1.350 (0.746-2.446) | 0.322 | 0.930 (0.458-1.892) | 0.842 |
| T staging (T 0-2 vs T 3, 4) | 2.469 (1.442-4.228) | 0.001 | 1.841 (0.961-3.527) | 0.066 |
| N staging (N 0, 1 vs N 2, 3) | 4.069 (2.445-6.772) | < 0.001 | 2.993 (1.587-5.646) | 0.001 |
| Differentiation (well vs poorly) | 1.378 (0.863-2.201) | 0.179 | 1.419 (0.786-2.563) | 0.246 |
| Histology (adenocarcinoma vs signet ring cell cancer) | 0.979 (0.573-1.674) | 0.940 | 0.599 (0.315-1.142) | 0.119 |
| Lymphovascular invasion (negative vs positive ) | 3.054 (1.514-6.161) | 0.002 | 1.15 (0.495-2.674) | 0.745 |
| Peritoneal metastasis (negative vs positive ) | 0.956 (0.301-3.034) | 0.939 | 0.507 (0.069-3.704) | 0.503 |
| Hepatic metastasis (negative vs positive ) | 0.048 (0.000-29.505) | 0.354 | 0.000 (0.000-1.254) | 0.962 |
| CA19-9 (< 37.0 U/mL vs ≥ 37.0 U/mL) | 1.385 (0.883-2.172) | 0.156 | 1.179 (0.710-1.958) | 0.525 |
| Overall survival | ||||
| Age (< 60 vs ≥ 60 yr) | 1.047 (0.816-1.342) | 0.719 | 1.218 (0.905-1.639) | 0.193 |
| Sex (male vs female ) | 0.979 (0.755-1.269) | 0.874 | 0.995 (0.732-1.355) | 0.977 |
| Lesions (single vs multiple) | 1.144 (0.804-1.626) | 0.455 | 1.163 (0.769-1.761) | 0.474 |
| T staging (T 0-2 vs T 3, 4) | 2.498 (1.846-3.382) | < 0.001 | 1.437 (0.986-2.094) | 0.059 |
| N staging (N 0, 1 vs N 2, 3) | 3.577 (2.713-4.715) | < 0.001 | 2.817 (1.984-4.001) | < 0.001 |
| Differentiation (well vs poorly) | 1.595 (1.227-2.073) | < 0.001 | 1.408 (0.990-2.002) | 0.057 |
| Histology (adenocarcinoma vs signet ring cell cancer) | 1.171 (0.880-1.560) | 0.279 | 1.000 (0.702-1.424) | 0.999 |
| Lymphovascular invasion (negative vs positive ) | 2.527 (1.739-3.673) | < 0.001 | 1.004 (0.643-1.569) | 0.985 |
| Peritoneal metastasis (negative vs positive ) | 4.620 (3.098-6.6887) | < 0.001 | 3.213 (1.792-5.762) | < 0.001 |
| Hepatic metastasis (negative vs positive ) | 2.294 (1.285-4.097) | 0.005 | 2.114 (0.916-4.880) | 0.079 |
| CA19-9 (< 37.0 U/mL vs ≥ 37.0 U/mL) | 1.395 (1.087-1.791) | 0.009 | 1.414 (1.053-1.898) | 0.021 |
CA: Carbohydrate antigen.
Potential prognostic variables are shown in Table 2. Focusing on OS, univariate analysis demonstrated an association with T stage (P < 0.001), N stage (P < 0.001), differentiation (P < 0.001), the presence of lymphovascular invasion (P < 0.001), the presence of peritoneal metastasis (P < 0.001), and hepatic metastasis (P = 0.005). Multivariate analysis showed that N stage (P < 0.001), and the presence of peritoneal metastasis (P < 0.001) remained independent factors in predicting OS. Additionally, preoperative serum CA 19-9 levels were significantly associated with OS in univariate (P = 0.009) and multivariate (P = 0.021) analyses. Cox proportional hazards regression analysis indicated that patients with elevated levels of CA 19-9 had a 1.4-fold higher risk of worse OS than patients with low levels of this marker.
DISCUSSION
Gastric cancer is one of the most common cancers worldwide with approximately 989600 new cases and 738000 deaths per year, accounting for approximately 8% of new cancers[25]. Thus, gastric cancer continues to be a global health problem. However, gastric cancer-specific tumor markers have not yet been identified. The tumor markers currently in use have limited clinical utility due to insufficient specificity and poor sensitivity[14,26,27].
Nevertheless, current serum tumor markers are primarily used for the preoperative staging of neoplasms, postoperative monitoring of treatment effectiveness, and early diagnosis of recurrence, as they can be easily and cost-effectively identified[28]. Specifically, tumor markers, including alpha-fetoprotein (AFP), CEA, CA 19-9, CA 50, and CA 72-4, have been reported to be elevated in certain gastric cancer patients[11,19,20]. AFP, a marker commonly used for germ cell and hepatocellular carcinoma, is elevated in AFP-producing gastric cancer, often presenting as liver metastasis and leading to a poor prognosis. However, the value of these tumor markers in gastric cancer is still controversial[29-31]. In the case of CEA, preoperative serum CEA levels have been reported to be useful for determining or predicting gastric cancer prognosis[11,32,33]. However, some authors have indicated that CEA positivity is not a prognostic factor in gastric cancer[15]. In addition, earlier studies have reported that CA 72-4 is more relevant than other tumor markers for gastric cancer, but this has not been verified[13,33-36]. Of currently used markers, CA 19-9 is known to have a positive correlation with depth of invasion, nodal involvement, and peritoneal metastasis in gastric cancer[15,17,37]. However, CA 19-9 can be elevated in endometrial, lung, breast, and pancreatic cancers as well as benign conditions, including cholecystitis, cholangitis, acute pancreatitis, and liver cirrhosis[38,39]. An association between CA 19-9 levels and prognosis has not yet been established and remains controversial[5,13]. Thus, none of these markers are used alone to diagnose, monitor disease, or predict prognosis. Although prognosis is mainly determined by tumor stage at the time of gastric cancer surgery, recent studies have assessed the usefulness of preoperative tumor marker levels to predict invasiveness and prognosis[5,6,18,32,33,40].
Therefore, we focused on the prognostic significance of high preoperative levels of CA 19-9 in patients with gastric cancer. The results of our study indicate that measurements of preoperative serum CA 19-9 levels may be useful in the prediction of survival and prognoses in patients with gastric cancer, confirming its association with OS and DFS. OS is thought to be influenced by preoperative CA 19-9 levels.
Previous studies identified the usefulness of postoperative CA 19-9 levels to predict prognosis and recurrence of gastric cancer after gastrectomy[6,37]. In many studies, however, preoperative CA 19-9 levels were neither prognostic nor were associated with survival. According to studies by Dilege et al[17] and Ishigami et al[41], preoperative CA 19-9 levels were only significantly correlated with lymph node metastasis; patient survival did not correlate with preoperative CA 19-9 levels. In addition, Ucar et al[15] showed that CA 19-9 was only significantly related to lymph node metastasis and peritoneal carcinomatosis, but prognosis and survival were not relevant. These authors suggested that CA 72-4 was an independent prognostic factor for risk of death in this study.
These studies did not provide any predictive information for preoperative CA 19-9 levels on prognosis or survival in gastric cancer patients. One study showed by univariate analysis that preoperative CA 19-9 levels could predict specific clinical outcomes such as DFS. Another study showed poor OS in CA 19-9-positive patients by the log-rank test[5]. However, the independent prognostic value of CA 19-9 on OS by Cox regression multivariate analysis was not shown[5]. The association between CA 19-9 levels and stage of disease, lymph node metastasis, and depth of invasion has been reported, but none have been assigned an independent prognostic value by multivariate analysis[5,16,41]. In contrast, our study showed survival outcome according to preoperative CA 19-9 levels and prognostic factors for OS and DFS. There were significant differences regarding OS between the non-elevated group and the elevated group. Preoperative CA 19-9 levels were a reliable prognostic factor for OS in our study. With respect to DFS, despite an insignificant prognostic value for CA 19-9 levels, we can see possibilities for future research, as our findings are confined to the limited data presented here.
A recent study by Jo et al[42] showed that an elevated CA 19-9 concentration before chemotherapy was significantly associated with shorter survival especially in metastatic or recurrent gastric cancer. The patients in this study had metastatic or recurrent gastric cancer. However, our study was designed to analyze treatment-naïve patients who were planning to undergo gastrectomy. Therefore, we have superiority and originality compared to previous studies due to the differences in the subject and focus of study.
We aimed to determine whether the preoperative tumor marker CA 19-9 could provide useful information on clinical outcome and postoperative prognosis similar to other common prognostic factors. Unlike the study by Marrelli et al[14], we analyzed not only 432 R0 resection cases, but also 56 palliative gastrectomy cases; of these, 27 cases of bypass surgery were also included. We cannot exclude the possibility that survival rates will appear low due to inclusion of the latter cases and influence tumor progression. These factors might affect the tumor burden and predominance of advanced cancer among marker-positive patients[18].
Our study has limitations associated with its retrospective nature, single-center design and relatively small sample numbers. In addition, preoperative CA 19-9 sampling was not performed at the same time before surgery due to the retrospective design.
Also, analyses regarding the presence of chemotherapy or radiation therapy after surgery were not performed in this study. Twelve patients who had neoadjuvant chemotherapy were included in this study. However, for patients who had adjuvant chemotherapy or other therapies after surgery, analyses were not performed. Although neither adjuvant nor neoadjuvant chemotherapy showed any clear significant survival benefit in gastric cancer[18], these factors might be crucial in influencing survival, therefore further study is necessary. In the future, it would be interesting to measure CA 19-9 consistently during the preoperative examination before surgery and analyze the effects of additional therapies such as adjuvant chemotherapy or radiation therapy. Therefore, multi-center and prospective studies should be designed to certify the prognostic significance of CA 19-9.
We conclude that OS in gastric cancer patients with elevated CA 19-9 levels was lower than that in patients with non-elevated levels. Serum CA 19-9 can be considered an independent prognostic factor in predicting OS in patients anticipating surgery for gastric cancer.
COMMENTS
Background
Gastric cancer is one of the most common cancers worldwide with approximately 989600 new cases and 738000 deaths per year, accounting for approximately 8% of new cancers. Thus, gastric cancer continues to be a global health problem. However, gastric cancer-specific tumor markers have not yet been identified. The tumor markers currently in use have limited clinical utility due to insufficient specificity and poor sensitivity.
Research frontiers
Recent clinical studies have shown that carcinoembryonic antigen and carbohydrate antigen 19-9 (CA 19-9) are recognized as poor prognostic factors for gastric cancer and are related to its recurrence. The prognostic relevance of such tumor markers in patients with gastric cancer is not comparable with those markers used in other carcinomas. Specifically, CA 19-9 has been reported to be elevated in certain forms of gastric cancer. However, because little research on the prognoses of gastric cancer patients with elevated preoperative CA 19-9 levels has been performed, the clinical significance of preoperative CA 19-9 levels has not been fully verified.
Innovations and breakthroughs
In most current research related to preoperative CA 19-9 levels in gastric cancer, discussions on survival have only focused on overall survival (OS). The association between CA 19-9 levels and stage of disease, lymph node metastasis, and depth of invasion has been reported, but none have been assigned an independent prognostic value by multivariate analysis. This study showed survival outcome according to preoperative CA 19-9 levels and prognostic factors for OS and disease-free survival (DFS). There were significant differences regarding OS between the non-elevated group and the elevated group. Preoperative CA 19-9 levels were a reliable prognostic factor for OS in our study. With respect to DFS, despite an insignificant prognostic value for CA 19-9 levels, we can see possibilities for future research, as the findings are confined to the limited data presented here.
Applications
Prior to surgery, CA 19-9 levels could be used to predict poor prognosis and to suggest adjuvant therapies in early stages of the disease when more aggressive surgical approaches are warranted.
Terminology
Serum CA 19-9 concentrations were measured using a commercial chemiluminescent enzyme immunoassay with a normal upper limit of 37 U/mL. Serum CA 19-9 levels were routinely measured immediately before surgery.
Peer review
The authors have focused on the prognostic significance of high preoperative levels of CA 19-9 in patients with gastric cancer. The results of the study indicate that measurements of preoperative serum CA 19-9 levels may be useful in the prediction of survival and prognoses in patients with gastric cancer, confirming its association with OS and DFS. OS is thought to be influenced by preoperative CA 19-9 levels.
Footnotes
P- Reviewers Sun LM, Sun QM S- Editor Gou SX L- Editor Webster JR E- Editor Zhang DN
References
- 1.Mihmanli M, Dilege E, Demir U, Coskun H, Eroglu T, Uysalol MD. The use of tumor markers as predictors of prognosis in gastric cancer. Hepatogastroenterology. 2004;51:1544–1547. [PubMed] [Google Scholar]
- 2.Dittrich C, Jakesz R, Havelec L, Lenzhofer R, Breyer S, Moser K. Carcinoembryonic antigen (CEA) plasma level determination in the management of gastric cancer patients. Cancer Detect Prev. 1985;8:181–187. [PubMed] [Google Scholar]
- 3.Shimizu N, Wakatsuki T, Murakami A, Yoshioka H, Hamazoe R, Kanayama H, Maeta M, Koga S. Carcinoembryonic antigen in gastric cancer patients. Oncology. 1987;44:240–244. doi: 10.1159/000226486. [DOI] [PubMed] [Google Scholar]
- 4.Wobbes T, Thomas CM, Segers MF, Nagengast FM. Evaluation of seven tumor markers (CA 50, CA 19-9, CA 19-9 TruQuant, CA 72-4, CA 195, carcinoembryonic antigen, and tissue polypeptide antigen) in the pretreatment sera of patients with gastric carcinoma. Cancer. 1992;69:2036–2041. doi: 10.1002/1097-0142(19920415)69:8<2036::aid-cncr2820690805>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Duraker N, Celik AN. The prognostic significance of preoperative serum CA 19-9 in patients with resectable gastric carcinoma: comparison with CEA. J Surg Oncol. 2001;76:266–271. doi: 10.1002/jso.1044. [DOI] [PubMed] [Google Scholar]
- 6.Choi SR, Jang JS, Lee JH, Roh MH, Kim MC, Lee WS, Qureshi W. Role of serum tumor markers in monitoring for recurrence of gastric cancer following radical gastrectomy. Dig Dis Sci. 2006;51:2081–2086. doi: 10.1007/s10620-006-9166-5. [DOI] [PubMed] [Google Scholar]
- 7.Heptner G, Domschke S, Domschke W. Comparison of CA 72-4 with CA 19-9 and carcinoembryonic antigen in the serodiagnostics of gastrointestinal malignancies. Scand J Gastroenterol. 1989;24:745–750. doi: 10.3109/00365528909093116. [DOI] [PubMed] [Google Scholar]
- 8.Harrison JD, Stanley J, Morris DL. CEA and CA 19.9 in gastric juice and serum: an aid in the diagnosis of gastric carcinoma? Eur J Surg Oncol. 1989;15:253–257. [PubMed] [Google Scholar]
- 9.Cidón EU, Bustamante R. Gastric cancer: tumor markers as predictive factors for preoperative staging. J Gastrointest Cancer. 2011;42:127–130. doi: 10.1007/s12029-010-9161-0. [DOI] [PubMed] [Google Scholar]
- 10.Webb A, Scott-Mackie P, Cunningham D, Norman A, Andreyev J, O’Brien M, Bensted J. The prognostic value of serum and immunohistochemical tumour markers in advanced gastric cancer. Eur J Cancer. 1996;32A:63–68. doi: 10.1016/0959-8049(95)00504-8. [DOI] [PubMed] [Google Scholar]
- 11.Ychou M, Duffour J, Kramar A, Gourgou S, Grenier J. Clinical significance and prognostic value of CA72-4 compared with CEA and CA19-9 in patients with gastric cancer. Dis Markers. 2000;16:105–110. doi: 10.1155/2000/595492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaspar MJ, Arribas I, Coca MC, Díez-Alonso M. Prognostic value of carcinoembryonic antigen, CA 19-9 and CA 72-4 in gastric carcinoma. Tumour Biol. 2001;22:318–322. doi: 10.1159/000050633. [DOI] [PubMed] [Google Scholar]
- 13.Reiter W, Stieber P, Reuter C, Nagel D, Cramer C, Pahl H, Fateh-Moghadam A. Prognostic value of preoperative serum levels of CEA, CA 19-9 and CA 72-4 in gastric carcinoma. Anticancer Res. 1997;17:2903–2906. [PubMed] [Google Scholar]
- 14.Marrelli D, Roviello F, De Stefano A, Farnetani M, Garosi L, Messano A, Pinto E. Prognostic significance of CEA, CA 19-9 and CA 72-4 preoperative serum levels in gastric carcinoma. Oncology. 1999;57:55–62. doi: 10.1159/000012001. [DOI] [PubMed] [Google Scholar]
- 15.Ucar E, Semerci E, Ustun H, Yetim T, Huzmeli C, Gullu M. Prognostic value of preoperative CEA, CA 19-9, CA 72-4, and AFP levels in gastric cancer. Adv Ther. 2008;25:1075–1084. doi: 10.1007/s12325-008-0100-4. [DOI] [PubMed] [Google Scholar]
- 16.Duraker N, Naci Celik A, Gençler N. The prognostic significance of gastric juice CA 19-9 and CEA levels in gastric carcinoma patients. Eur J Surg Oncol. 2002;28:844–849. doi: 10.1053/ejso.2002.1295. [DOI] [PubMed] [Google Scholar]
- 17.Dilege E, Mihmanli M, Demir U, Ozer K, Bostanci O, Kaya C, Aksakal O, Sakiz D. Prognostic value of preoperative CEA and CA 19-9 levels in resectable gastric cancer. Hepatogastroenterology. 2010;57:674–677. [PubMed] [Google Scholar]
- 18.Tocchi A, Costa G, Lepre L, Liotta G, Mazzoni G, Cianetti A, Vannini P. The role of serum and gastric juice levels of carcinoembryonic antigen, CA19.9 and CA72.4 in patients with gastric cancer. J Cancer Res Clin Oncol. 1998;124:450–455. doi: 10.1007/s004320050198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao ZL, Zhang C, Du GY, Lu ZJ. Clinical significance of changes in tumor markers, extracellular matrix, MMP-9 and VEGF in patients with gastric carcinoma. Hepatogastroenterology. 2007;54:1591–1595. [PubMed] [Google Scholar]
- 20.Adachi Y, Tsuchihashi J, Shiraishi N, Yasuda K, Etoh T, Kitano S. AFP-producing gastric carcinoma: multivariate analysis of prognostic factors in 270 patients. Oncology. 2003;65:95–101. doi: 10.1159/000072332. [DOI] [PubMed] [Google Scholar]
- 21.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition - Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 22.Han KB, Jang YJ, Kim JH, Park SS, Park SH, Kim SJ, Mok YJ, Kim CS. Clinical significance of the pattern of lymph node metastasis depending on the location of gastric cancer. J Gastric Cancer. 2011;11:86–93. doi: 10.5230/jgc.2011.11.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wittekind C, Compton C, Quirke P, Nagtegaal I, Merkel S, Hermanek P, Sobin LH. A uniform residual tumor (R) classification: integration of the R classification and the circumferential margin status. Cancer. 2009;115:3483–3488. doi: 10.1002/cncr.24320. [DOI] [PubMed] [Google Scholar]
- 24.Kodera Y, Yamamura Y, Torii A, Uesaka K, Hirai T, Yasui K, Morimoto T, Kato T, Kito T. The prognostic value of preoperative serum levels of CEA and CA19-9 in patients with gastric cancer. Am J Gastroenterol. 1996;91:49–53. [PubMed] [Google Scholar]
- 25.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 26.Kim DH, Oh SJ, Oh CA, Choi MG, Noh JH, Sohn TS, Bae JM, Kim S. The relationships between perioperative CEA, CA 19-9, and CA 72-4 and recurrence in gastric cancer patients after curative radical gastrectomy. J Surg Oncol. 2011;104:585–591. doi: 10.1002/jso.21919. [DOI] [PubMed] [Google Scholar]
- 27.Lam KW, Lo SC. Discovery of diagnostic serum biomarkers of gastric cancer using proteomics. Proteomics Clin Appl. 2008;2:219–228. doi: 10.1002/prca.200780015. [DOI] [PubMed] [Google Scholar]
- 28.Torre GC, Lucchese V, Rembado R, Barbetti V. Tumour markers: from laboratory to clinical use. Anticancer Res. 1996;16:2215–2219. [PubMed] [Google Scholar]
- 29.Libman E, Feher I, Lemberger J, Mutibarić A. [Clinical significance of alpha-fetoprotein and carcinoembryonic antigen in the serum of patients with liver cirrhosis] Med Pregl. 1979;32:229–232. [PubMed] [Google Scholar]
- 30.Nakajima K, Ochiai T, Suzuki T, Shimada H, Hayashi H, Yasumoto A, Takeda A, Hishikawa E, Isono K. Impact of preoperative serum carcinoembryonic antigen, CA 19-9 and alpha fetoprotein levels in gastric cancer patients. Tumour Biol. 1998;19:464–469. doi: 10.1159/000030038. [DOI] [PubMed] [Google Scholar]
- 31.Chang YC, Nagasue N, Kohno H, Taniura H, Uchida M, Yamanoi A, Kimoto T, Nakamura T. Clinicopathologic features and long-term results of alpha-fetoprotein-producing gastric cancer. Am J Gastroenterol. 1990;85:1480–1485. [PubMed] [Google Scholar]
- 32.Yamashita K, Sakuramoto S, Kikuchi S, Katada N, Kobayashi N, Watanabe M. Surgical resection of stage IV gastric cancer and prognosis. Anticancer Res. 2007;27:4381–4386. [PubMed] [Google Scholar]
- 33.González A, Vizoso F, Allende MT, Sánchez MT, Balibrea JL, Ruibal A. Preoperative CEA and TAG-72 serum levels as prognostic indicators in resectable gastric carcinoma. Int J Biol Markers. 1996;11:165–171. doi: 10.1177/172460089601100305. [DOI] [PubMed] [Google Scholar]
- 34.Ikeguchi M, Katano K, Saitou H, Tsujitani S, Maeta M, Kaibara N. Pre-operative serum levels of CA72-4 in patients with gastric adenocarcinoma. Hepatogastroenterology. 1997;44:866–871. [PubMed] [Google Scholar]
- 35.Spila A, Roselli M, Cosimelli M, Ferroni P, Cavaliere F, Arcuri R, Tedesco M, Carlini S, D’Alessandro R, Perri P, et al. Clinical utility of CA 72-4 serum marker in the staging and immediate post-surgical management of gastric cancer patients. Anticancer Res. 1996;16:2241–2247. [PubMed] [Google Scholar]
- 36.Byrne DJ, Browning MC, Cuschieri A. CA72-4: a new tumour marker for gastric cancer. Br J Surg. 1990;77:1010–1013. doi: 10.1002/bjs.1800770918. [DOI] [PubMed] [Google Scholar]
- 37.Kwon OK, Yu W, Chung H. Prognostic value of postoperative CA19-9 normalization in patients with advanced gastric cancer. Hepatogastroenterology. 2013;60:240–243. doi: 10.5754/hge12585. [DOI] [PubMed] [Google Scholar]
- 38.Lamerz R. Role of tumour markers, cytogenetics. Ann Oncol. 1999;10 Suppl 4:145–149. [PubMed] [Google Scholar]
- 39.Muraro R, Kuroki M, Wunderlich D, Poole DJ, Colcher D, Thor A, Greiner JW, Simpson JF, Molinolo A, Noguchi P. Generation and characterization of B72.3 second generation monoclonal antibodies reactive with the tumor-associated glycoprotein 72 antigen. Cancer Res. 1988;48:4588–4596. [PubMed] [Google Scholar]
- 40.Shiraishi N, Sato K, Yasuda K, Inomata M, Kitano S. Multivariate prognostic study on large gastric cancer. J Surg Oncol. 2007;96:14–18. doi: 10.1002/jso.20631. [DOI] [PubMed] [Google Scholar]
- 41.Ishigami S, Natsugoe S, Hokita S, Che X, Tokuda K, Nakajo A, Iwashige H, Tokushige M, Watanabe T, Takao S, et al. Clinical importance of preoperative carcinoembryonic antigen and carbohydrate antigen 19-9 levels in gastric cancer. J Clin Gastroenterol. 2001;32:41–44. doi: 10.1097/00004836-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Jo JC, Ryu MH, Koo DH, Ryoo BY, Kim HJ, Kim TW, Choi KD, Lee GH, Jung HY, Yook JH, et al. Serum CA 19-9 as a prognostic factor in patients with metastatic gastric cancer. Asia Pac J Clin Oncol. 2012:Epub ahead of print. doi: 10.1111/ajco.12019. [DOI] [PubMed] [Google Scholar]


