Abstract
Background
Intracranial hemorrhage occurs in 20% to 25% of neonates born before the 30th week of gestation or weighing less than 1500 grams at birth. These hemorrhages carry a risk of long-term neurocognitive damage. Measures for lowering the incidence of intracranial hemorrhage were evaluated.
Methods
A working group at the University of Ulm, Germany, developed a prospective monitoring program for risk factors and a bundle of measures including altered clinical approaches to delivery, initial care of the neonate in the delivery room immediately after birth, and intensive care in the first few days thereafter. Adherence to these measures was checked once per week. The evaluation was performed prospectively for a period of 23 months (August 2010 to July 2012) with a 31-month period of historical controls (January 2008 to July 2010).
Results
In the reference period before the intervention was introduced, 263 neonates weighing less than 1500 grams and with a median (quartile) gestational age at birth of 27.4 (25.4–29.9) weeks were treated. The incidence of intracranial hemorrhage was 22.1%, and that of high-grade hemorrhage was 9.1%. The mortality was 6.1%, and the rate of survival without brain hemorrhage was 74.5%. After the bundle of preventive measures was introduced, 191 neonates weighing less than 1500 grams and with a median (quartile) gestational age at birth of 28.0 (26.0, 30.3) weeks were treated. The incidence of intracranial hemorrhage dropped to 10.5% (odds ratio [OR] 0.43, 95% confidence interval [CI] 0.25–0.73); the incidence of high-grade hemorrhage dropped to 3.7% (OR 0.36; 95% CI 0.14–0.89). The mortality was no different at 6.3%, and 85.3% of the children survived without a hemorrhage (OR 1.95, 95% CI 1.20–3.15). After statistical adjustment for higher gestational age, the OR for intracranial hemorrhage (IVH) was 0.49 (0.28–0.86) and the probability of survival without IVH improved (OR 1.68, 95% CI 1.01–2.81).
Conclusion
The rate of brain hemorrhage in premature neonates can be considerably lowered by prospective monitoring of risk factors.
The survival chances of very premature infants have risen in recent decades (1, 2). This change has been accompanied by an increase in the significance of morbidities in premature infants, including long-term morbidities (3). The risk of brain damage is particularly significant (4, 5). However, the incidence of brain damage has not fallen like that of other morbidities (2). Hemorrhages into the germinal matrix and into the ventricle and hemorrhagic infarctions of the cerebral parenchyma are commonly denoted by the umbrella term “intraventricular hemorrhage” (IVH) (6). Current opinion (6) is that they are caused by reperfusion following ischemia and a range of other factors. They are a specific problem in very premature infants and have significant, long-term neurocognitive consequences (7).
Perinatal units that treat large numbers of cases seem to have a lower IVH rate (8, 9) and mortality rate (10). However, when extremely premature infants are treated, financial constraints obviously also play a role (11). The perinatal unit in Ulm, Germany treats a large number of preterm infants from an area that covers approximately one-fifth of the territory of the region of Baden–Württemberg, as unusually high numbers of pregnant women at risk of preterm delivery are referred there as part of the regional neonatal network (ARGE Ulm) (12). Although comparison of the unit’s neonatal figures with those of Baden–Württemberg as a whole was very favorable, with a mortality rate of 1.44% versus a median standardized mortality rate of 7.15%, the IVH rate was “only” average (13) and was within the range found in the literature (14, 15). Prompted by the experiences of other authors (16, 17), the decision was made to undertake an initiative to reduce the IVH rate at the Ulm perinatal unit.
The goal of this work was to evaluate whether the introduction and prospective monitoring of a bundle of measures that were in line with local procedures and had been developed on the basis of risk factors could reduce the incidence of IVH in preterm infants with a birth weight below 1500 grams (monitoring of quality of process and outcome).
Methods
We founded an “IVH Working Group” consisting of the heads of the Departments of Neonatology (Helmut Hummler) and Obstetrics (Frank Reister), senior physicians, interns, and nursing staff. The group met weekly and discussed the pathophysiology of IVH in detail. A delegation of the group visited the hospital that had had the lowest rate of IVH in the federal state for a similar patient and care structure for several years (Heidelberg University Hospital). The hospitals’ treatment standards with regard to individual professional groups were then compared and allocated to one of the following three categories:
Measures to be adopted directly
Measures to be examined on the basis of the literature
Measures not to be adopted
On this basis an individual bundle of measures for IVH prevention that was specific and tailor-made to the Ulm perinatal unit was developed and revised several times. Adherence to the bundle of measures, and thus the quality of the treatment process for all preterm infants with IVH, was discussed at weekly IVH sessions that lasted approximately one hour and brought together various professional groups. By way of “devil’s advocate,” all issues that might have contributed to a hemorrhage were examined. In particular, discussion focused on whether the bundle of measures had been adhered to and whether there were any risk factors.
Ultrasound examination of the head was performed regularly three and seven days after birth, and then at least every four weeks, and the highest grade of hemorrhage according to Papile (18) was recorded. No distinction was made between earlier and later IVH. Mortality was defined as death in hospital.
Patients
Statistical evaluation included the data of all infants receiving curative treatment with a birth weight of less than 1500 grams. Data of infants from the cohort born after the bundle of measures was introduced (August 1, 2010 to July 8, 2012) were compared with those of infants born before it had been introduced (January 1, 2008 to July 31, 2010). Infants referred postnatally to Ulm from other pediatric hospitals were excluded, because the approach taken at Ulm could not affect the onset of intracranial hemorrhage in these infants. The study design was that of an interventional cohort study. The dataset before introduction of the bundle of measures was gathered retrospectively; the dataset after its introduction was gathered prospectively.
Statistics
Statistical evaluation was performed using SigmaPlot 12.0 (Systat Software, San Jose, CA, 2011). Differences in qualitative outcomes were reported using the chi-square test; for quantitative outcomes, the t-test was used to report differences in those with normal distribution, and the Mann–Whitney rank sum test for those with non-parametric distributions. Multivariate logistic regression was performed using SAS 9.2 (SAS Institute, Cary, North Carolina) to investigate differences in the intracranial hemorrhage rate after introduction of the bundle of measures (significance level p = 0.05). The study received a favorable ruling from the Ulm Ethics Committee (no. 213/11).
Results
The IVH prevention bundle included measures concerning delivery, immediate care in the delivery room, and infants’ first few days in the ICU (eBox 1). One of its major component was “minimal handling” (eBox 2), i.e. keeping intervention by physicians and nurses to the absolute minimum. It contained only changes to procedure and cannot be considered in isolation from the pre-existing standard procedure on which it was based (eBox 3). The most important differences it introduced were the following:
eBox 1. Working group: bundle of measures for IVH.
I. Prenatal and perinatal care:
-
Prenatal steroids:
A complete course of betamethasone, even in a prospective approach in pregnancies <24 wks
In pregnancies <28 wks, a 12 mg betamethasone (booster) dose is administered as soon as cesarean section is indicated or if spontaneous delivery is imminent, if induction of lung maturation was last completed >72 hours ago (i.e. last administration was >96 hours ago).
-
Method of delivery:
No vaginal birth in case of fetal malpresentation; at this time no routine indication for caesarian section in case of vertex presentation
Delayed clamping or repeated milking of the umbilical cord in all preterm infants
When pregnant women with chorioamnionitis or suspected chorioamnionitis are delivered, the surgeon sends a placental/amniotic swab or an amniotic fluid sample, and neonatologists send pharyngeal aspirates for microbiological testing for pathogens/resistance.
II. Immediate care after delivery:
Combined warming bed / incubator for all preterm infants <30 wks (five machines were purchased for this purpose)
Intubation of preterm infants with gestational age <30 wks for one week after birth by neonatal fellows or senior physicians only
Prevention of postnatal hypothermia (<36 °C) and hyperthermia (>38 °C) by close monitoring and documentation or using servo-controlled devices
Prevention of hypocapnia (pCO2 <35 mm Hg)
III. Intensive care unit:
Prevention of hypocapnia (pCO2 <35 mm Hg)
Use of surfactant: If a surfactant is indicated, it should be administered within 30 minutes of being indicated (currently when FiO2 >0.40). Additional medical staff must be recruited if necessary. Prophylactic surfactant administration (currently administered to preterm infants <25 wks) must be performed within 30 minutes of birth.
Pneumothorax: If pneumothorax is diagnosed in an infant receiving mechanical ventilation, it must be drained immediately so as not to affect venous return flow unnecessarily. Additional medical staff must be recruited if necessary.
Arterial hypotension: Mean blood pressure (in mm Hg) should not remain below gestational age (in wks) for more than 1 hour.
Stress reduction: A novel minimal handling protocol will be used in future.
Volume administration: 15 mL/kg body weight over 30 minutes; if evidence of hypovolemia/hemorrhage (suspected or evident blood loss), give fluids faster and monitor hemodynamics closely
NaHCO3: as little as possible (pH >7.20 generally acceptable)
Physiotherapy: not indicated in the first week after birth
IV. Interdisciplinary case discussions:
-
Who:
Neonatology: involved physicians, all senior physicians, nursing staff if possible
Obstetrics: head of division
Other interested physicians and nurses
When: Wednesdays, 2 to 3 p.m.
Form: discussion of case with medical notes and images
What: all preterm infants <28 wks with or without IVH, all preterm infants ≥28 wks with IVH
-
Aims:
Were all measures set out in the bundle (cf. above) adhered to?
Useful measures for improvement?
V. Other:
Noninvasive blood pressure measurement in delivery room: Data should be generated in delivery room (and documented using a standardized monitoring procedure).
New detailed patient history form and medical documentation form for immediate postnatal care
New protocol for sustained inflation
eBox 2. Minimal handling (care procedure).
Definition:
“Minimal handling” means keeping intervention by physicians and nurses, particularly painful and burdensome interventions, to the minimum necessary for safe treatment.
Nursing interventions:
General “gentle care“
Care rounds should be suited to infants’ sleep–wake rhythms. Individual care and nursing interventions should always be queried.
Prevent constant exposure to light: no direct light in infants’ eyes, always cover infants’ heads with small blankets or special blankets to partially cover incubators (visual monitoring must be possible)
Closed aspiration system for all infants
Prevent noise: alarms must be set as loud as necessary but as quiet as possible. Mute alarms swiftly, do not place objects on incubator
Nurses’ and physicians’ cellphones must be set to vibrate mode.
Both nurses and physicians must react swiftly if sound-activated light-alarm indicates elevated noise levels
Speak softly at the bedside, no discussions at the bedside
No radios in the unit
Consistently touch infants when making nursing care rounds, place a hand on the infant’s body
Prevent or alleviate stress and pain: offer one drop glucose 40% and pacifier
Enemas within 3 days of birth only if no bowel movement within 12 hours
Do not measure head circumference and body length before the second day of life
Combine interventions by physicians and nurses to minimize disturbance to the baby
Special care for preterm infants with gestational age <30 wks during the first week after birth
Elevate upper body by 20° during first week after birth
Preterm infants with <30 wks should be supine for the third first 3 days after birth with the head in axial position until 1 week after birth. Do not place in prone position
Preterm infants <30 wks: two members of nursing staff must change bed linen and weigh infant
During the first week after birth preterm infants <30 wks should be cared for by staff who have been working in the ICU for at least 1 year if they have no previous experience of neonatology, or at least 6 months if they do have experience of neonatology.
Preterm infants <30 wks: no body washing during the first 7 days after birth.
Preterm infants <<30 wks: change undersheet daily, sheets on day 4, and incubator on day 7
Preterm infants <30 wks must be weighed on day 4 and 7 after birth only
Interventions by physicians
Preterm infants <30 wks should only be intubated by fellows or senior physicians in the first week after birth.
For preterm infants <30 wks the first ultrasound of the head should not be performed until 3 days after birth
Ultrasound of the head should be performed only by experienced physicians during the first week after birth. No routine Doppler examinations
Perform interventions by physicians and nurses at the same time and respect infants’ rest periods
eBox 3. Treatment of preterm infants weighing <1500 grams.
2008–2010 before introduction of measures to prevent intraventricular hemorrhage (IVH) (meanwhile revised)
Guiding principle: target values act as guidelines and must be adjusted based on clinical cirumstances.
Prenatal
-
Transportation in utero
Pregnant women from the catchment area of regional neonatal network (ARGE Ulm) who go into early labor are referred to the appropriate hospital according to the criteria established by the network. Women who go into labor before 26 to 30 weeks’ gestation (wks) or who have severe additional risk factors are moved to Ulm prenatally. If pregnancy can be maintained, the situation is stable but the persisting risk of preterm delivery prevents from discharge from the hospital, patients are referred back to the hospital near their home if they have reached the agreed cut-off point for gestational age. Because of this procedure, the most premature infants are concentrated in one hospital with extensive experience as a result of its high numbers of cases. If infants meet a set of established referral criteria in Ulm after birth, they are referred to a pediatric hospital near their home until they are discharged home, in consultation with their parents.
-
Obstetric approach
Risk of spontaneous preterm delivery (preterm contractions with or without preterm rupture of the membranes):
After examination for acute need for delivery (e.g. as a result of amniotic infection syndrome), begin induction of lung maturation (no repeat cycles) and oral nifedipine tocolysis
Stop tocolysis after completion of lung maturation
Decreased physical activity but not bed rest
For early rupture of the membranes, antibiotic treatment with amoxicillin/clavulanic acid for 48 hours IV, then orally for 5 days
If amniotic membranes are intact, antibiotics only on evidence of infection
For elevated infection parameters, careful monitoring, including amniocentesis, to rule out amniotic infection
For suspected amniotic infection, delivery is indicated at low-threshold. Method of delivery: normal vaginal delivery for vertex presentation, if labor is progressing well, and there is no fetal distress
-
Placental insufficiency:
Induction of lung maturation, close monitoring using cardiotocography (CTG), ultrasound, and Doppler
Decreased physical activity
Delivery indicated on the strength of CTG and Doppler (e.g. severely abnormal ductus venosus, absent end-diastolic flow at 30 wks or more)
Method of delivery: usually cesarean section
-
Medical staff
Senior physicians are neonatologists who have many years’ experience in caring for very premature infants and who have cared for between 200 and 1000 preterm infants with birth weight <1500 grams
Experienced residents or fellows are pediatricians in training for subspecialty neonatology or already having accomplished it. They are expected to be able to perform endotracheal intubation; insertion of umbilical venous and arterial catheters, central venous access routes, peripheral arterial cannulae, and pleural drains; and resuscitation.
Delivery room
-
Staff
One senior physician and at least one fellow or experienced resident (immediate care) per infant with gestational age <30 wks
Fellow / resident performs immediate care, senior physician supervises and assists
Preparation of equipment: temperature control, noninvasive and invasive respiratory support, peripheral access and umbilical vessel access, emergency equipment
-
Temperature control
Prewarmed open unit in a separate room in labor and delivery ward
No air drafts, no passage of uninvolved persons
Place infant in transparent side-opening plastic wrap without drying first
Seal wrap completely using adhesive tape, leaving face uncovered
The umbilical cord must exit the plastic wrap through a small opening.
-
Monitoring
Place pulse oximeter sensor on right wrist when placing infant in elastic wrap
-
Ventilation
Use ventilation device during immediate postnatal care
Nasopharyngeal tube as patient–machine interface; ID 2.5 mm using Seldinger procedure on 4 CH aspiration catheter, insert to 4 cm
Continuous positive airway pressure (CPAP) 5 cm H2O
Initial FiO2 0.4
-
Perform up to 3 sustained inflations as follows:
Close other nostril and mouth carefully
Inspiratory time for each sustained inflation: 15 seconds
Initial inspiratory pressure 20 cm H2O, second and third sustained inflations 25 or 30 cm H2O
Measure vital signs before each sustained inflation. Perform second and third sustained inflations only if there is apnea and persistent bradycardia or insufficient increase in O2 saturation
Between sustained inflations use nasopharyngeal ventilation, f = 60/min
Avoid bag ventilation due to insufficient control of peak pressure, inspiratory time, and positive end-expiratory pressure (PEEP); use bag only if ventilator fails or intended peak pressure exceeds ventilator capacity
-
Surfactant
Prophylactic intubation and surfactant <25 + 0 wks (exception: excellent lung function: FiO2 0.21, no dyspnea)
-
Other intubation criteria:
FiO2 >0.4 for SpO2 80% to 92% for >1 hour
FiO2 >0.6 for SpO2 80% to 92% and no trend towards improvement
Irregular respiration and significant dyspnea with no trend towards improvement
Usually no intubation due to hypercapnia
If intubated as a result of abnormal oxygenation or hypercapnia, surfactant is always administered (even if FiO2 or respiratory pressure has already improved as a result of intubation and ventilation)
If natural bovine surfactant used, initial dose 100 mg/kg body weight. If natural porcine surfactant used, initial dose 200 mg/kg body weight
Bolus administration of surfactant in at least two fractions
-
Access routes
Peripheral venous access in all preterm infants weighing <1500 grams
Check glucose and blood gas levels
Preprepared mixture of amino acids and glucose as IV fluid
Birth weight <1000 grams or history of amniotic infection: antibiotic treatment after blood culture taken
Umbilical venous and arterial catheter for all preterm infants <26 wks and preterm infants <28 wks if expected to be needed for intubation or cardiovascular therapy
Not to be used until after radiological confirmation of correct location (transition to right atrium for umbilical venous catheter, high [target] or deep position for umbilical arterial catheter).
Blood pressure not usually measured. Volume bolus (restrictive) in case of pale skin and prolonged capillary refill time
-
Transportation
5 minutes’ journey within building: in transportation incubator
Short visit to parents if possible
ICU
-
Ventilation
-
Aims
PaCO2 target area for first 3 days: 45 to 55 mm Hg
pH >7.20; metabolic acidosis caused by high acid levels to be treated causally; for acidosis caused by loss of bicarbonate (base excess < –8mmol/L), substitute with bicarbonate
-
Intubation criteria in the first 72 hours in ICU
FiO2 >0.4 for 1 hour due to respiratory distress syndrome
pCO2 >65 mm Hg or >60 mm Hg with significant dyspnea
Repeat episodes of apnea and bradycardia after methylxanthine therapy, noninvasive ventilation, and supportive measures have been explored
-
Surfactant
Initially: all infants with respiratory distress syndrome (RDS) who are intubated; 100 mg/kg body weight natural bovine surfactant or 200 mg/kg body weight natural porcine surfactant
Repeat: second increase in FiO2 >0.4 or >0.3 with dyspnea; 50 mg/kg body weight natural bovine surfactant or 100 mg/kg body weight natural porcine surfactant
Physician providing surfactant remains at incubator, continuous CO2 monitoring compulsory
-
Monitoring of ventilation
Frequency 60 to 80/min
Maximum I:E ratio 1:2
Positive end-expiratory pressure (PEEP) after oxygenation, diaphragm position on expiration at 8th to 9th rib
Peak inspiratory pressure (PIP) as low as possible for CO2 target region
Demand flow for gas
If peak inspiratory pressure (PIP) >24 cm H2O, consider high frequency oscillatory ventilation
Nursing staff to keep ventilator settings within boundaries established by physician
-
-
Indomethacin as prophylaxis to prevent intracranial hemorrhage
Indicated in case of intubation and ventilation within 12 hours after birth and
Birth weight <1000 grams or gestational age <28 wks
Dose 0.1 mg/kg body weight every 24 hours
Do not administer if donor in twin-to-twin transfusion syndrome
Platelet transfusion if thrombocytopenia <70 000/µL during indomethacin therapy
-
Patent ductus arteriosus (PDA)
-
For hemodynamically relevant PDA (i.e. murmur, bounding pulse, large PDA with signs of strain on ECG, respiratory involvement):
Indomethacin 3 doses of 0.2 mg/kg body weight given every 12 hours (every 8 hours from 8 days after birth onwards)
Repeat indomethacin administration up to 3 cycles (including IVH prophylaxis)
Surgical ligation if extubation unsuccessful due to PDA
Ligation to be performed in ICU
-
-
Arterial hypotension
Minimum mean arterial pressure (MAP): gestational age in wks
-
If lower:
Ringer’s solution 15 mL/kg body weight administered at a rate of 1 mL/kg/hr, prepare dopamine infusion
Dopamine up to maximum 15 µg/kg body weight/min, begin via peripheral access route if necessary
Epinephrine
Hydrocortisone (individual desicion; avoid if indomethacin has been administered)
Higher threshold for indicating delivery
More preference for indicating cesarean section as method of delivery
Milking the umbilical cord
An additional single dose of betamethasone
Timing for the treatment of respiratory distress syndrome and arterial hypotension.
Patient characteristics are summarized in Table 1. Infants included in the cohorts before and after the introduction of the bundle of measures had a median gestational age of 27.4 and 28.0 weeks’ gestation (wks) respectively, and a median birth weight of 910 and 962 grams respectively. The difference in gestational age is statistically significant. There were no significant differences in the following parameters:
Table 1. Patient characteristics: gestational age, birth weight, and other basic parameters of neonatal morbidity.
| Before intervention | After intervention | Difference (95% CI) | p-value | |
|---|---|---|---|---|
| n (number of premature infants) | 263 (31 months) | 191 (23 months) | ||
| Mean gestational age (SD) | 27.6 wks (±3.0) |
28.3 wks (±3.0) |
+0.7 wks (0.14 to 1.26) |
0.014 |
| Median gestational age (IQR) | 27.4 wks (25.4 to 29.9) |
28.0 wks (26.0 to 30.3) |
0.015 | |
| Mean birth weight (SD) | 936g (±315) | 962g (±307) | +26g (–32 to 84) |
0.381 |
| Median birth weight (IQR) | 910g (680 to 1230) | 955g (720 to 1228) | 0.365 | |
| Antenatal steroid treatment begun | 242 (92.0%) | 179 (93.7%) | +1.7% (–3.1 to 6.5) |
0.612 |
| Antenatal steroid treatment completed | 196 (74.5%) | 143 (74.9%) | +0.4% (–7.8 to 8.5) |
0.979 |
| Male sex | 143 (54.4%) | 95 (49.7%) | -4.6% (–13.9 to 4.7) |
0.378 |
| Multiple birth | 78 (29.7%) | 64 (33.5%) | +3.8% (–4.8 to 12.5) |
0.441 |
| Hypotrophic preterm infant (birth weight <10th percentile) | 34 (12.9%) | 24 (12.6%) | -0.4% (–6.6 to 5.9) |
0.977 |
| Cesarean section | 219 (83.3%) | 174 (91.1%) | +7.8% (1.5 to 14.2) |
0.023 |
| Median time between premature rupture of membranes and birth (IQR) | 87h (7 to 156) | 108h (8 to 454) | 0.210 | |
| Median 1-minute Apgar score (IQR) | 6 (4 to 8) | 5 (4 to 7) | 0.218 | |
| Median 5-minute Apgar score (IQR) | 9 (8 to 10) | 8 (7 to 9) | <0.001 | |
| Median 10-minute Apgar score (IQR) | 10 (9 to 10) | 9 (9 to 10) | <0.001 | |
| Median umbilical artery pH (IQR) | 7.32 (7.27 to 7.36) |
7.34 (7.28 to 7.39) |
0.024 | |
| Cardiac massage immediately after birth | 10 (3.8%) | 3 (1.6%) | -2.2% (–5.3 to 0.9) |
0.262 |
| Surfactant administration immediately after birth | 54 (20.5%) | 50 (26.2%) | +5.6% (–2.2 to 13.5) |
0.194 |
| Median surfactant dose* (IQR) | 2 (1 to 3) | 1 (1 to 2) | <0.001 | |
| Perinatal infection | 129 (49.0%) | 103 (53.9%) | +4.8% (–0.4 to 14.2) |
0.352 |
| Catecholamines | 133 (50.6%) | 75 (39.3%) | -11.3% (–2.0 to 20.6) |
0.022 |
| Surgical PDA ligation | 27 (10.3%) | 5 (2.6%) | -7.7% (–12.4 to 2.9) |
0.003 |
| BPD (28 days O2) | 107 (40.7%) | 67 (35.1%) | -5.6% (–14.7 to 3.5) |
0.265 |
| BPD (36 wks) | 28 (10.6%) | 17 (8.9%) | -1.7% (–7.3 to 3.8) |
0.649 |
| Postnatal administration of dexamethasone | 1 (0.4%) | 1 (0.5%) | +0.1% (–10.9 to 13.8) |
0.624 |
| Discharged home with additional O2 | 10 (3.8%) | 5 (2.6%) | -1.2% (–4.51 to 2.2) |
0.666 |
| ROP with laser treatment | 14 (5.3%) | 10 (5.2%) | -0.1% (–0.10 to 0.08) |
0.908 |
| NEC stage II onwards | 9 (3.4%) | 9 (4.7%) | 1.3% (–2.3 to 4.9) |
0.651 |
| Extraalveolar air (e.g. pneumothorax. pulmonary ‧interstitial emphysema. pneumopericardium) | 34 (12.9%) | 18 (9.4%) | -3.5% (–9.4 to 2.4) |
0.313 |
| PVL | 5 (1.9%) | 1 (0.5%) | -1.4% (–3.5 to 0.07) |
0.392 |
CI: confidence interval, SD: standard deviation, IQR: interquartile range (25th to 75th percentile), PDA: patent ductus arteriosus, p-value: significance level, BPD: bronchopulmonary dysplasia, ROP: retinopathy of prematurity, NEC: necrotizing enterocolitis, PVL: periventricular leukomalacia, wks: weeks’ gestation.
*Only infants who received surfactant
Rate of completed antenatal steroid cycles
Sex
Percentage of infants with dystrophy
Frequency of bronchopulmonary dysplasia (BPD)
Discharge home with oxygen
Necrotizing enterocolitis (NEC)
Retinopathy of prematurity (ROP).
Despite lower umbilical artery pH values, the cohort before the bundle of measures was introduced had higher Apgar scores. However, subsequently these infants received catecholamines and surfactant more frequently.
Seven infants were born in other maternity units and transferred to Ulm after initial care. Two of these infants suffered intracranial hemorrhages, one before and one after the bundle of measures was introduced. These infants’ data were not evaluated separately, due to the small number of cases.
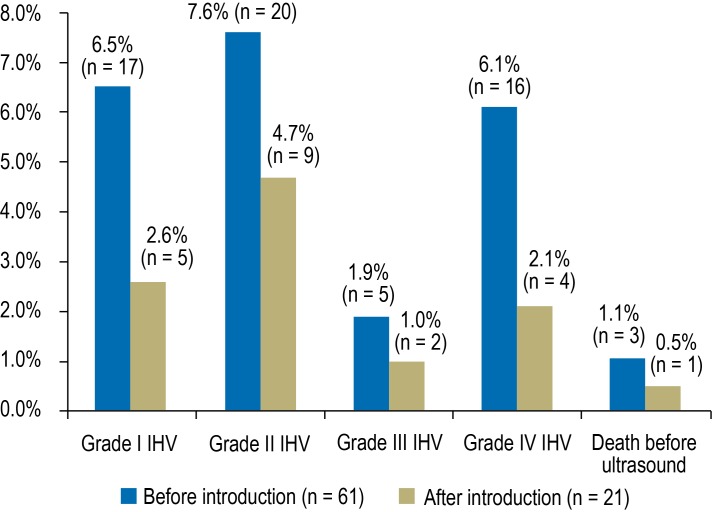
The incidence of IVH fell from 22.1% to 10.5% (p = 0.002). It decreased in every grade of IVH (Figure), all birth weight categories (Table 2a), and almost all gestational age categories (Table 2b). The fall in IVH incidence was not caused by an increase in mortality: The survival rate remained stable at 94%. The combined target outcome of IVH-free survival was achieved significantly more frequently. Twenty-six infants died, 13 of them less than one week after birth and 10 within one day of birth. Four of the 13 infants had not undergone ultrasound examination of the head, and three were diagnosed with grade III or IV IVH. Of the six remaining infants, one died five days after birth and five died within one day of birth. Five of these deaths occurred before the bundle of measures was introduced, and one afterwards.
Figure.
Incidence of IVH by severity before and after introduction of the bundle of measures
Table 2a. Rates of IVH, severe IVH, survival, and IVH-free survival by birth weight.
| n | IVH | Grade III/IV IVH or death with no ultrasound performed | Survival | IVH-free survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | p-value | Before | After | p-value | Before | After | p-value | Before | After | p-value | |
| <500g | 21 | 15 | 28.6% | 20.0% | 0.845 | 9.5% | 6.7% | 0.760 | 81.0% | 86.7% | 1.0 | 57.1% | 80.0% | 0.282 |
| 500 to 749g | 67 | 39 | 37.3% | 17.9% | 0.061 | 14.9% | 10.3% | 0.669 | 89.6% | 89.7% | 0.765 | 58.2% | 76.9% | 0.082 |
| 750 to 999g | 68 | 48 | 20.6% | 10.4% | 0.229 | 11.8% | 2.1% | 0.117 | 95.6% | 93.8% | 0.988 | 76.5% | 83.3% | 0.505 |
| 1000 to 1249g | 46 | 48 | 13.0% | 6.3% | 0.442 | 8.7% | 2.1% | 0.333 | 95.7% | 95.8% | 0.640 | 84.8% | 89.6% | 0.698 |
| 1250 to 1499g | 61 | 41 | 11.5% | 4.9% | 0.426 | 0.0% | 0.0% | 100.0% | 97.6% | 0.841 | 88.5% | 92.7% | 0.724 | |
| <1000g | 156 | 102 | 28.8% | 14.7% | 0.013 | 12.8% | 5.9% | 0.11 | 91.0% | 91.2% | 0.856 | 66.0% | 80.4% | 0.018 |
| <1500g | 263 | 191 | 22.1% | 10.5% | 0.002 | 9.1% | 3.7% | 0.037 | 93.9% | 93.7% | 0.912 | 74.5% | 85.3% | 0.007 |
IVH: intraventricular hemorrhage; p-value: significance level
Table 2b. Rates of IVH, severe IVH, survival, and IVH-free survival by gestational age.
| Gestational age category | n | IVH | Grade III/IV IVH or death with no ultrasound performed | Survival | IVH-free survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | p-value | Before | After | p-value | Before | After | p-value | Before | After | p-value | |
| <24 wks | 26 | 13 | 61.5% | 30.8% | 0.141 | 26.9% | 15.4% | 0.687 | 76.9% | 92.3% | 0.461 | 30.8% | 69.2% | 0.052 |
| 24 to 25 wks | 71 | 32 | 31.0% | 25.0% | 0.701 | 18.3% | 6.3% | 0.192 | 90.1% | 87.5% | 0.955 | 62.0% | 65.6% | 0.893 |
| 26 to 27 wks | 48 | 48 | 20.8% | 6.3% | 0.074 | 8.3% | 4.2% | 0.673 | 95.8% | 91.7% | 0.673 | 77.1% | 87.5% | 0.285 |
| 28 to 29 wks | 56 | 45 | 8.9% | 8.9% | 0.731 | 0.0% | 0.0% | 98.2% | 100.0% | 0.912 | 89.3% | 91.1% | 0.976 | |
| ≥30 wks | 62 | 53 | 8.1% | 1.9% | 0.287 | 0.0% | 1.9% | 0.937 | 100.0% | 94.3% | 0.190 | 91.9% | 94.3% | 0.891 |
| <28 wks | 145 | 93 | 33.1% | 16.1% | 0.006 | 16.6% | 6.5% | 0.037 | 89.7% | 90.3% | 0.944 | 61.4% | 77.4% | 0.015 |
| Total | 263 | 191 | 22.1% | 10.5% | 0.002 | 9.1% | 3.7% | 0.037 | 93.9% | 93.7% | 0.912 | 74.5% | 85.3% | 0.007 |
IVH: intraventricular hemorrhage; p-value: significance level
The risk profile of the infants changed during the monitored period. The mean gestational age after introduction of the bundle of measures was significantly higher, and the number of infants receiving palliative care in the delivery room after 22 + 0 wks or more rose. Before the bundle of measures was introduced, two infants (0.8%) born after 22 wks received palliative care and died without being admitted to the Division of Neonatology. The corresponding figure after introduction of the bundle of measures was 11 (5.4%) infants, and this difference is significant (+4.7% [95% CI: 1.7 to 7.7%], p = 0.006). If preterm infants with a gestational age of less than 24 wks are excluded, the size of the cohorts is reduced to 237 and 178 infants respectively; the incidence of IVH falls from 18.6% to 10.1% (–8.5% [95% CI: –15.4 to –1.5%], p = 0.024); and the incidence of severe IVH (grade III or IV IVH or death with no ultrasound examination) falls from 7.2% to 3.4% (–3.6% [95% CI: –8.3 to +0.6%], p = 0.145). The combined target outcome of IVH-free survival was achieved in 79.7% of cases before the intervention and 86.5% afterwards (+6.8% [95% CI: –0.6 to +14.1%], p = 0.095).
Multivariate statistical regression was performed to adjust the figures for the higher mean gestational age after the intervention. This reduced the difference in IVH incidence between the two cohorts (Table 3), but the difference in both the IVH rate and the IVH-free survival rate (less than 1500 grams) remained significant.
Table 3. Risk reduction following introduction of prevention program. with and without logistic regression to adjust for gestational age (odds ratio <1 means reduced risk).
| Unadjusted | Adjusted for gestational age | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| IVH (<1500g) | 0.43 (0.25 to 0.73) |
0.0018 | 0.49 (0.28 to 0.86) |
0.013 |
| IVH-free survival (<1500g) | 1.95 (1.20 to 3.15) |
0.0067 | 1.68 (1.01 to 2.81) |
0.047 |
| Grade III/IV IVH (<1500g) | 0.36 (0.14 to 0.89) |
0.028 | 0.45 (0.17 to 1.17) |
0.102 |
| IVH (<1000g) | 0.39 (0.20 to 0.74) |
0.004 | 0.47 (0.23 to 0.93) |
0.030 |
| IVH-free survival (<1000g) | 2.17 (1.20 to 3.92) |
0.01 | 1.78 (0.94 to 3.37) |
0.077 |
| Grade III/IV IVH (<1000g) | 0.48 (0.18 to 1.25) |
0.1331 | 0.61 (0.23 to 1.64) |
0.329 |
IVH: intraventricular hemorrhage; CI: confidence interval; p-value: significance level
Discussion
We conducted an observational study to compare the incidence of IVH in preterm infants with a birth weight below 1500 grams in one cohort established prospectively after the introduction of a bundle of measures to prevent IVH, and one cohort established retrospectively. We observed a 50% drop in the IVH rate in all birth weight and gestational age categories after the bundle of measures was introduced, for all grades of IVH. There were no unclear categorization issues. This success was not achieved at the cost of increased mortality, for example by limiting the care provided to the most seriously ill infants.
Nevertheless, after the bundle of measures was introduced the gestational age of the infants in the study was 0.7 weeks higher, which may have acted as a confounding variable by affecting the incidence of IVH. We therefore performed logistical regression to adjust for the difference in gestational age. As expected, this showed the differences to be smaller (Table 3). It is currently unclear why the mean gestational age was higher after introduction of the bundle of measures. After the bundle was introduced, obstetric care focused more strongly on prolonging pregnancy, whereas previously priority had been given to the prevention of perinatal infections caused by infection of the amnion. However, no increase in perinatal infections was observed. One interpretation of these data is that risk to the fetus and prolongation of pregnancy were weighed against each other more successfully, and pregnant women in whom prolongation of pregnancy would not increase risk were identified.
The reasons for the increase in infants receiving palliative care before 24 wks are unclear. Prenatal counseling of pregnant women at risk of preterm delivery before 24 wks has not changed and complies with Guideline 024–019 of the Association of Scientific Medical Societies in Germany (AWMF, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften) (19). Parents decide whether treatment should be palliative or curative following non-instructional discussion using facility-specific treatment outcomes. Differences in the aim of treatment for preterm infants born before 24 wks and in the selected reference number may lead to very different results in terms of preterm infants’ survival rate (20). In this cohort, as in most information reported in the literature, the number of live fetuses in utero at 22 wks was not systematically used as the “highest common denominator.” We therefore proceeded as recommended elsewhere (20) and performed a separate evaluation including only preterm infants with a birth weight of less than 1500 grams at 24 + 0 wks or more. This analysis showed lower mortality and IVH incidence than in the total patient population. The effect of the intervention was smaller, but the reduction in the incidence of IVH remained statistically significant.
During the intervention period, the cesarean section rate was significantly higher and surfactant and catecholamine treatment were needed less frequently. There is evidence of an increased risk of hemorrhage for spontaneous delivery (21), particularly in the case of fetal malpresentation (22). Although not all working groups found this relationship (23), we have chosen cesarean section as the method of delivery when the fetus is not in vertex presentation. The decreased need for surfactant was due to higher gestational age rather than to a repeat single dose of betamethasone (24); the route of surfactant administration and indication for ventilation remained unchanged during the period examined. Both lower cardiac output (25) and treatment for arterial hypotension (26, 27) are associated with an increased rate of IVH. We suspect that the reduced need for catecholamines is related to umbilical cord milking (28).
Limitations
This work does have methodological weaknesses. It included preterm infants who died too early to be able to develop IVH. Therefore, the combined target outcome of IVH-free survival was evaluated. In addition, five of the six deaths without IVH in the first week after birth occurred before the bundle of measures was introduced. If it were assumed that all infants who died would have developed IVH, the incidence of hemorrhage would increase more before the introduction of the bundle of measures than after it, increasing the effect of the intervention. We set the time of initial publication of the bundle of measures as the boundary between the control period and the intervention period even though individual component measures were already known beforehand as a result of the visit to Heidelberg and discussions of the working group. This was also seen in the trend towards decreasing IVH incidence. This means that the decrease in IVH incidence is more likely to have been underestimated than overestimated.
Because of the partially retrospective design of the study, the reason for the drop in IVH incidence cannot be established definitively. Currently ongoing secondary analyses aim to identify the changes that were decisive to success. In other studies, too (16, 17, 29), it proved impossible to identify a single cause for a decrease in IVH incidence. We are inclined to attach more importance to the newly-introduced measures, particularly umbilical cord milking (30) and the additional single dose of betamethasone (31), than to pre-existing treatment aims. However, this is currently speculative and must be tested in detailed analysis.
However, it is also possible that none of the factors mentioned above play a dominant role, but rather that each individual measure has only a small effect and that they only result in success when combined. It is possible, of course, that the activities intended to prevent IVH (study of the literature, visits to another hospital, discussions and introduction of the bundle of measures, weekly sessions) sharpened all team members’ awareness of the pathophysiology of brain damage. IVH is no longer seen as an unavoidable disaster (16) but instead requires critical analysis addressing the question “What should we do differently next time?” It is possible that increased awareness of the problem played the decisive role in reducing the incidence of IVH.
Other quality improvement programs (32), prevention programs (33), and programs for the chronically ill (34) have shown that achieving success and maintaining it are two separate aims. If several measures are combined, they are harder to sustain, particularly if the activities to achieve the effect lapse or competing activities begin (35). Ongoing improvements to the bundle of measures in order to improve quality further are made for this reason alone, but also because of changes in patients’ risk profile.
In order for the success observed here to be transferable, general, transferable elements must be separated from facility-specific elements whose transferability is limited. Facility-specific elements with only limited transferability are the specific points listed in the bundle of measures mentioned here. They are like directions which we have provided for getting from location A (medium IVH incidence) to location B (low IVH incidence). These directions might be completely useless if location A is different. The abstract steps (Key Messages) are transferable.
Key Messages.
Intraventricular hemorrhages in extremely premature infants are not necessarily unavoidable accidents of fate.
-
The incidence of intracranial hemorrhages was successfully reduced using a prevention program consisting of the following:
Teamwork between disciplines and between professions
Examination of the literature for specific IVH risk factors
Visits to a hospital with lower IVH incidence, asking, “What do they do differently there?”
Development and implementation of a facility-specific bundle of measures
Verification of adherence to measures at weekly, structured case discussions
Brain damage has a substantial impact on the life of affected infants and their families. We believe that this impact justifies major efforts to prevent IVH.
The regional care structure in the context of the regional neonatal network (ARGE Ulm) (12) results in an unusually high number of treated preterm infants with a gestational age of less than 28 wks for the German federal state of Baden–Württemberg. The resulting concentration of specialized expertise, together with interdisciplinary and interprofessional communication with Heidelberg University Gynecology and Pediatric Hospital, may have played a decisive role in this success. In terms of figures, the quality improvement described here prevented 12 intracranial hemorrhages or 5.6 severe intracranial hemorrhages per year when compared to the previous incidence in Ulm Hospital (which was average for the federal region). 9.2 more preterm infants per year survive without intracranial hemorrhage. Success on this scale throughout Germany would save very large numbers of preterm infants and their parents a great deal of suffering. The lifelong costs resulting from mental (36) or physical (37) disability are high and run into the millions; cost-savings would be tremendous.
A prospective intervention in several large perinatal units, for example as part of a cluster randomized trial conducted by a network, would be useful in testing the efficacy of the described measures in a larger population and in reporting the significance of individual factors. At the same time, this might improve the quality of treatment for many preterm infants.
Box. Key elements for successful intervention to reduce the rate of intracranial hemorrhage.
Openness to make changes
Involvement of all affected disciplines and professions
Examination of currently available knowledge on the origin of IVH
Identification of the hospital with the lowest incidence of IVH in the network and critical evaluation / comparison of standard treatment as part of visits to one another’s facilities
Compilation of a facility-specific bundle of measures
Regular case discussions
Monitoring program to verify that the measures discussed have been implemented
Acknowledgments
Translated from the original German by Caroline Devitt, M.A.
Of the many people who have contributed to this considerable success for the wellbeing of preterm infants, we would like to give particular thanks to Prof. Michael Obladen, who inspired us to do this project; Prof. Johannes Pöschl and the team of the Heidelberg Perinatal Unit for intensive, open discussion; and especially to the nursing staff of Ulm University Hospital, led by Jens Albrecht.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Horbar JD, Badger GJ, Carpenter JH, Carpenter F, et al. Trends in mortality and morbidity for very low birth weight infants, 1991-1999. Pediatrics. 2002;110:143–151. doi: 10.1542/peds.110.1.143. [DOI] [PubMed] [Google Scholar]
- 2.Horbar JD, Carpenter JH, Badger GJ, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129:1019–1026. doi: 10.1542/peds.2011-3028. [DOI] [PubMed] [Google Scholar]
- 3.Allen MC, Cristofalo EA, Kim C. Outcomes of preterm infants: morbidity replaces mortality. Clin Perinatol. 2011;38:441–454. doi: 10.1016/j.clp.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Sherlock RL, Anderson PJ, Doyle LW Victorian Infant Collaborative Study Group. Neurodevelopmental sequelae of intraventricular haemorrhage at 8 years of age in a regional cohort of ELBW/very preterm infants. Early Hum Dev. 2005;81:909–916. doi: 10.1016/j.earlhumdev.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volpe JJ. Neurology of the Newborn. 5th ed. Philadelphia, PA: Saunders; 2008. Intracranial Hemorrhage: Germinal Matrix-Intraventricular Hemorrhage of the Premature Infant; pp. 517–588. [Google Scholar]
- 7.Mercier CE, Dunn MS, Ferrelli KR, Howard DB, Soll RF Vermont Oxford Network ELBW Infant Follow-Up Study Group. Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998-2003. Neonatology. 2010;97:329–338. doi: 10.1159/000260136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Synnes AR, Chien LY, Peliowski A, Baboolal R, Lee SK Canadian NICU Network. Variations in intraventricular hemorrhage incidence rates among Canadian neonatal intensive care units. J Pediatr. 2001;138:525–531. doi: 10.1067/mpd.2001.111822. [DOI] [PubMed] [Google Scholar]
- 9.Synnes AR, Macnab YC, Qiu Z, et al. Neonatal intensive care unit characteristics affect the incidence of severe intraventricular hemorrhage. Med Care. 2006;44:754–759. doi: 10.1097/01.mlr.0000218780.16064.df. [DOI] [PubMed] [Google Scholar]
- 10.Kutschmann M, Bungard S, Kotting J, Trumner A, Fusch C, Veit C. The care of preterm infants with birth weight below 1250 g: risk-adjusted quality benchmarking as part of validating a caseload-based management system. Dtsch Arztebl Int. 2012;109(31-32):519–526. doi: 10.3238/arztebl.2012.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmer K-P. Neonatology departments under economic pressure. Dtsch Arztebl Int. 2012;109(31-32):517–518. doi: 10.3238/arztebl.2012.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pohlandt F, Artlich A, Freihorst A, et al. Regionalisation of preterm births in county districts? Yes we can! Z Geburtshilfe Neonatol. 2009;213:135–137. doi: 10.1055/s-0029-1233451. [DOI] [PubMed] [Google Scholar]
- 13.Arbeitskreis der Neonatalerhebungen der Bundesländer. Neonatalerhebung Baden-Württemberg 2009. Zentrum für Qualität und Management im Gesundheitswesen. 2010. pp. 1–11.
- 14.Vogtmann C, Koch R, Gmyrek D, Kaiser A, Friedrich A. Risk-adjusted intraventricular hemorrhage rates in very premature infants: towards quality assurance between neonatal units. Dtsch Arztebl Int. 2012 109;(31-32):527–533. doi: 10.3238/arztebl.2012.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheth RD. Trends in incidence and severity of intraventricular hemorrhage. J Child Neurol. 1998;13:261–264. doi: 10.1177/088307389801300604. [DOI] [PubMed] [Google Scholar]
- 16.Obladen M, Metze B, Henrich W, Aktas A, Czernik C, Schulz-Baldes A. Interdisciplinary surveillance of intraventricular haemorrhage associated conditions in infants. Acta Paediatr. 2008;97:731–737. doi: 10.1111/j.1651-2227.2008.00812.x. [DOI] [PubMed] [Google Scholar]
- 17.Carteaux P, Cohen H, Check J, et al. Evaluation and development of potentially better practices for the prevention of brain hemorrhage and ischemic brain injury in very low birth weight infants. Pediatrics. 2003;111:e489–e496. [PubMed] [Google Scholar]
- 18.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 19.Roll C. Zur Neuauflage der Leitlinie Nr 024-019 „Frühgeburt an der Grenze der Lebensfähigkeit des Kindes”. Z Geburtshilfe Neonatol. 2008 M:114–115. doi: 10.1055/s-2008-1076835. [DOI] [PubMed] [Google Scholar]
- 20.Smith L, Draper ES, Manktelow BN, Pritchard C, Field DJ. Comparing regional infant death rates: the influence of preterm births. Arch Dis Child Fetal Neonatal Ed. 2013;98:103–107. doi: 10.1136/fetalneonatal-2011-301359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leviton A, Fenton T, Kuban KCK, Pagano M. Labor and deliver characteristics and the risk of germinal matrix hemorrhage in low birth weight infants. J Child Neurol. 1991;6:35–40. doi: 10.1177/088307389100600107. [DOI] [PubMed] [Google Scholar]
- 22.Shankaran S, Bauer CR, Bain R, Wright LL, Zachary J. Prenatal and perinatal risk and protective factors for neonatal intracranial hemorrhage. National Institute of Child Health and Human Development Neonatal Research Network. Arch Pediatr Adolesc Med. 1996;150:491–497. doi: 10.1001/archpedi.1996.02170300045009. [DOI] [PubMed] [Google Scholar]
- 23.Malloy MH, Onstad L, Wright E. The effect of cesarean delivery on birth outcome in very low birth weight infants. National Institute of Child Health and Human Development Neonatal Research Network. Obstet Gynecol. 1991;77:498–503. [PubMed] [Google Scholar]
- 24.Peltoniemi OM, Kari MA, Tammela O, et al. Randomized trial of a single repeat dose of prenatal betamethasone treatment in imminent preterm birth. Pediatrics. 2007;119:290–298. doi: 10.1542/peds.2006-1549. [DOI] [PubMed] [Google Scholar]
- 25.Kluckow M, Evans N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2000;82:188–194. doi: 10.1136/fn.82.3.F188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dempsey EM, Hazzani Al F, Barrington KJ. Permissive hypotension in the extremely low birthweight infant with signs of good perfusion. Arch Dis Child Fetal Neonatal Ed. 2009;94:241–244. doi: 10.1136/adc.2007.124263. [DOI] [PubMed] [Google Scholar]
- 27.Dempsey EM, Barrington KJ. Treating hypotension in the preterm infant: when and with what: a critical and systematic review. J Perinatol. 2007;27:469–478. doi: 10.1038/sj.jp.7211774. [DOI] [PubMed] [Google Scholar]
- 28.Hosono S, Mugishima H, Fujita H, et al. Blood pressure and urine output during the first 120 h of life in infants born at less than 29 weeks’ gestation related to umbilical cord milking. Arch Dis Child Fetal Neonatal Ed. 2009;94:328–331. doi: 10.1136/adc.2008.142935. [DOI] [PubMed] [Google Scholar]
- 29.McLendon D, Check J, Carteaux P, et al. Implementation of potentially better practices for the prevention of brain hemorrhage and ischemic brain injury in very low birth weight infants. Pediatrics. 2003;111:e497–e503. [PubMed] [Google Scholar]
- 30.Mercer JS, Vohr BR, McGrath MM, Padbury JF, Wallach M, Oh W. Delayed cord clamping in very preterm infants reduces the incidence of intraventricular hemorrhage and late-onset sepsis: a randomized, controlled trial. Pediatrics. 2006;117:1235–1242. doi: 10.1542/peds.2005-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vermillion ST, Bland ML, Soper DE. Effectiveness of a rescue dose of antenatal betamethasone after an initial single course. Am J Obstet Gynecol. 2001;185:1086–1089. doi: 10.1067/mob.2001.117633. [DOI] [PubMed] [Google Scholar]
- 32.Oretveit J, Staines A. Sustained improvement? Findings from an independent case study of the Jönköping quality program. Quality management in health care. 2007;16:68–83. doi: 10.1097/00019514-200701000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Scheirer MA. The life cycle of an innovation: adoption versus discontinuation of the fluoride mouth rinse program in schools. Journal of health and social behavior. 1990;31:203–215. [PubMed] [Google Scholar]
- 34.Reinehr T, Widhalm K, L’Allemand D, et al. Two-year follow-up in 21,784 overweight children and adolescents with lifestyle intervention. Obesity (Silver Spring) 2009;17:1196–1199. doi: 10.1038/oby.2009.17. [DOI] [PubMed] [Google Scholar]
- 35.Wiltsey Stirman S, Kimberly J, Cook N, Calloway A, Castro F, Charns M. The sustainability of new programs and innovations: a review of the empirical literature and recommendations for future research. Implementation science: IS BioMed Central Ltd. 2012;7 doi: 10.1186/1748-5908-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention (CDC) Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment - United States, 2003. MMWR - Morbidity & Mortality Weekly Report. 2004;53:57–59. [PubMed] [Google Scholar]
- 37.Kruse M, Michelsen SI, Flachs EM, Brønnum-Hansen H, Madsen M, Uldall P. Lifetime costs of cerebral palsy. Dev Med Child Neurol. 2009;51:622–628. doi: 10.1111/j.1469-8749.2008.03190.x. [DOI] [PubMed] [Google Scholar]