Abstract
Background
Until just a few years ago, locally advanced or high-risk prostate cancer was generally considered incurable. Recently, however, evidence has accumulated that, even for these patients, the oncologic outcome after radical prostatectomy is not uniformly poor.
Methods
13 262 evaluable patients with prostate cancer were treated with radical prostatectomy from 1992 to 2012. 4391 had a locally advanced stage, lymphogenous metastases, and/or unfavorable histopathological tumor characteristics. The endpoints of this retrospective, monocentric study were biochemical recurrence-free survival (postoperative PSA value less than 0.2 ng/mL), carcinoma-specific survival, and overall survival.
Results
The rates of biochemical recurrence-free survival, carcinoma-specific survival, and overall survival at 10 years were 53%, 98%, and 89% for patients with extraprostatic tumor growth (tumor stage pT3a, 2675 patients); 19%, 87%, and 79% for patients with demonstrated invasion of the seminal vesicle (pT3b, 1373 patients); and 3%, 77%, and 69% for patients with tumor invasion of neighboring organs (pT4, 53 patients). The corresponding figures were 14%, 81%, and 71% for patients with lymph node metastases (682 patients); 32%, 93%, and 85% for those with a preoperative PSA value above 20 ng/mL (728 patients); and 25%, 70%, and 58% for those with a prostatectomy Gleason score of 8 or more points (559 patients).
Conclusion
Even patients with locally advanced, nodally metastasized, or localized high-risk prostate cancer do not necessarily have a poor outcome. Although most such patients have a biochemical recurrence after radical prostatectomy, their carcinoma-specific mortality within ten years of radical prostatectomy ranges from 2% to 30% depending on the risk constellation, while their overall survival rate over the same period ranges from 58% to 89%.
Prostate cancer (PCa) is the most frequent solid malignancy among men in western countries (1). Following the adoption of routine measurement of prostate-specific antigen (PSA), the majority of PCa detected are now diagnosed at an early stage. One characteristic that sets PCa apart from other solid cancers is the usually protracted natural course of the disease. This is determined principally by the degree of differentiation, which in PCa is described by the Gleason score (2). Nevertheless, a significant proportion of newly diagnosed PCa are first detected at an advanced stage or already display very poor differentiation (high-risk PCa). In the past PCa of this type was thought to be incurable, but a growing number of studies in recent years have shown that radical prostatectomy can achieve cure in a significant proportion of patients and that tumor-specific survival after sequential treatment is not uniformly poor (3– 5). The aim of the present study was to evaluate biochemically recurrence-free, carcinoma-specific, and total survival as well as long-term complications in a large, consecutive, contemporary cohort of patients treated with radical prostatectomy for locally advanced PCa, PCa with nodal metastases, or high-risk PCa. The efficacy of radical prostatectomy compared with other forms of treatment, e.g., radiotherapy, was not directly evaluated because there are no relevant original data.
Patients and methods
Since 1992 all patients treated with radical prostatectomy in the Department of Urology at the University Medical Center Hamburg-Eppendorf (UKE) or at the Martini Hospital of the UKE have been registered prospectively in an electronic database. All patients are contacted by post at yearly intervals and asked for relevant oncological (biochemical recurrence, last recorded PSA level, additional treatment, etc.) and functional information (descriptions of continence, potency, etc.). The data are then entered into the database by specially trained members of staff. The data can therefore be evaluated retrospectively at any time.
In the great majority of patients treated with radical prostatectomy since 1992, access has been via a retropubic route (6). Starting in 2005, however, some patients are treated by means of robot-assisted laparoscopic prostatectomy (DaVinci). The staging of PCa is oriented on the TNM classification of 2002/2009 (7) (Box).
Box. TNM classification of prostate cancer after radical prostatectomy (pathological stage).
pT2
Tumor confined to prostate
pT2a
Tumor involves <50% of one lateral lobe
pT2b
Tumor involves >50% of one lateral lobe
pT2c
Tumor involves both lateral lobes
pT3a
Infiltration of periprostatic fatty tissue
pT3b
Infiltration of one or seminal vesicles
pT4
Infiltration of adjacent organs
pN0
No metastases in regional lymph nodes
pN1
Metastases in regional lymph nodes
The grading of prostate biopsy samples and prostate tissue removed by radical prostatectomy was performed as described by Gleason and classified using the Gleason score (2). The prostatectomy specimens are processed in 3-mm slices according to the Stanford protocol (8). Lymphadenectomy was not carried out in all patients, but only when there was evidence of moderate to high danger of lymph-node metastasis, e.g., a preoperative PSA level of >10 ng/mL and/or a Gleason score of ≥7 at biopsy (9).
A PSA level of 0.2 ng/mL and rising, measured 3 months or more after radical prostatectomy, was defined as biochemical recurrence (PSA recurrence). Radiotherapy within the first 4 months after radical prostatectomy, regardless of PSA level, was classified as adjuvant radiotherapy. Additional irradiation more than 4 months after radical prostatectomy was termed salvage radiotherapy. Adjuvant and salvage androgen deprivation were defined analogously.
The study endpoints were biochemically recurrence-free, carcinoma-specific, and overall survival following radical prostatectomy, determined by Kaplan–Meier analysis. First, patients with locally advanced (pT3a, pT3b, or pT4) prostate cancer or metastasis to lymph nodes were evaluated. Next, the biochemically recurrence-free and carcinoma-specific survival for patients with a high-risk tumor constellation were investigated (preoperative PSA level >20 ng/mL and/or Gleason score of ≥8 in the prostatectomy specimen). With regard to PSA we oriented ourselves on D’Amico et al.’s definition of high-risk PCa. In contrast to D’Amico et al., however, we used the prostatectomy Gleason score rather than the biopsy Gleason score, because correlation of the two scores with one another is limited and the prostatectomy Gleason score is accordingly better suited for definition of high-risk PCa (10).
Furthermore, the rates of postoperative incontinence and erectile dysfunction (ED) 1 year after surgery were recorded. Postoperative incontinence was deemed to be present if the patient used one or more pads daily. We excluded patients who wore a pad just to be on the safe side although they displayed no significant involuntary leakage. ED was evaluated by means of the International Index of Erectile Function (IIEF-5) questionnaire. Here we included only those who had no ED before operation (IIEF-5 score 22–25). The software package SPSS, version 17, was used for statistical analysis.
Results
Descriptive data
Between 1 January 1992 and 22 March 2012, a total of 15 045 patients were treated with radical prostatectomy in the authors’ department. Of these, 732 were excluded because measurements of relevant variables were missing. A further 1051 patients were excluded from the study because they had received neo-adjuvant androgen deprivation, which can considerably hamper histological processing, potentially leading to false conclusions (11). We included patients with adjuvant or salvage androgen deprivation or adjuvant or salvage radiotherapy, however, because leaving them out would have had an inappropriate positive effect on biochemically recurrence-free, carcinoma-specific, and overall survival. The reason for this is that additional treatment tends to be given to patients perceived to have an unfavorable prognosis, or after recurrence. Moreover, we included only those patients who displayed a tumor stage ≥pT3a and/or preoperative PSA >20 ng/mL and/or prostatectomy Gleason score ≥8 and/or lymph-node metastases. A collective of 4391 patients remained for analysis. The characteristics of this cohort are summarized in Table 1.
Table 1. Characteristics of the 4391 patients*.
| Variable | Number of patients |
|---|---|
|
Age (years) Mean (median) Range |
64 (65) 41–80 |
|
PSA (ng/mL)
Mean (median) Range |
13.7 (8.8) 0.6–290 |
|
Type of radical prostatectomy Retropubic DaVinci |
4142 (94.3%) 249 (5.7%) |
|
Biopsy Gleason score ≤6 3+4 4+3 ≥8 |
1291 (29.4%) 1411 (32.1%) 805(18.3%) 884 (20.1%) |
|
pT stage pT2 pT3a pT3b pT4 |
290 (6.6%) 2675 (60.9%) 1373 (31.3%) 53 (1.2%) |
|
pN stage pN0 pN1 pNx |
2915 (66.4%) 680 (15.5%) 795 (18.1%) |
|
Prostatectomy Gleason score ≤6 3+4 4+3 ≥8 |
231 (5.3%) 2287 (52.1%) 1314 (29.9%) 559 (12.7%) |
|
Androgen deprivation Adjuvant Salvage |
118 (2.7%) 479 (10.9%) |
|
Radiotherapy Adjuvant Salvage |
303 (6.9%) 618 (14.1%) |
| Number of biochemical recurrences | 1279 (29.1%) |
|
Follow-up of censored patients BCR (months)
Mean (median) Range |
43.1 (25) 1–219 |
|
Number of deaths Number of carcinoma-specific deaths |
209 (4.8%) 85 (1.9%) |
|
Follow-up of censored patients prostate carcinoma-specific survival (months) Mean (median) Range |
51.6 (36.4) 1–230 |
|
Follow-up of censored patients overall survival (months) Mean (median) Range |
50.5 (36.3) 1–230 |
*These patients were all treated with radical prostatectomy in the authors’ department between 1992 and 2012; clinical parameters and prognostically unfavorable histopathological parameters were recorded. PSA, prostate-specific antigen; BCR, biochemical recurrence
Altogether, 921 patients (21%) received additional radiotherapy after radical prostatectomy; 303 (6.9%) as adjuvant and 618 (14.1%) as salvage treatment. Adjuvant androgen deprivation was carried out in 118 patients (2.7%), salvage androgen deprivation in 479 patients (10.9%).
Results of oncological treatment
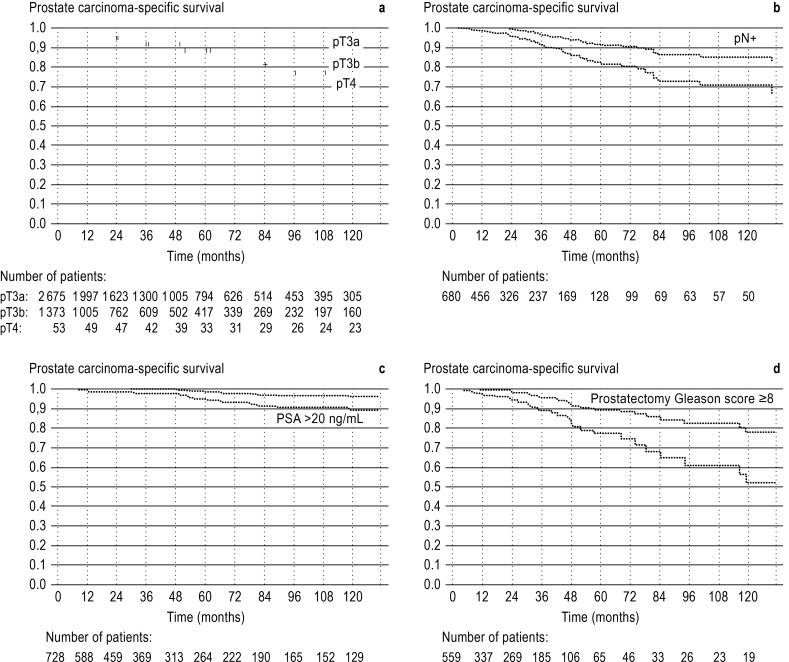
Figure 1 shows the Kaplan–Meier curves for carcinoma-specific survival in patients with tumor stage pT3a, pT3b, pT4, pN1 and/or preoperative PSA >20 ng/mL and/or prostatectomy Gleason score ≥8.
Figure.
Prostate carcinoma-specific survival after radical prostatectomy for patients with confirmed locally advanced tumors (pT3a, pT3b, or pT4) and/or confirmed lymph-node metastases (pN+) and/or preoperative PSA >20 ng/mL and or Gleason score ≥8 in the prostatectomy specimen. The dotted lines show the 95% confidence interval. a) Prostate carcinoma-specific survival, stratified according to pT stage. b) Prostate carcinoma-specific survival in patients with lymph-node metastases. c) Prostate carcinoma-specific survival in patients with preoperative PSA >20 ng/mL. d) Prostate carcinoma-specific survival in patients with prostatectomy Gleason score ≥8.
The 10-year carcinoma-specific survival rates were:
98% for patients with stage pT3a (N = 2675; 10-year biochemically recurrence-free survival 53%; 10-year overall survival 89%)
87% for patients with stage pT3b (N = 1373; 10-year biochemically recurrence-free survival 18%; 10-year overall survival 79%)
77% for patients with stage pT4 (N = 53; 10-year biochemically recurrence-free survival 3%; 10-year overall survival 69%)
81% for patients with confirmed lymph-node metastases (N = 682; 10-year biochemically recurrence-free survival 14%; 10-year overall survival 71%)
Among patients with a high-risk tumor constellation, the 10-year carcinoma-specific and 10-year overall survival rates were 93% and 85% respectively for those with a preoperative PSA level >20 ng/mL (N = 728) and 70% and 58% for those with a prostatectomy Gleason score ≥8 (N = 559). The corresponding 10-year biochemically recurrence-free survival rates were 32% and 25%.
Functional outcome
Table 2 shows the rates of postoperative stress incontinence and erectile dysfunction. One year after operation 87.7% of patients were completely continent. The findings with regard to potency in preoperatively potent men were as follows:
Table 2. Rates of postoperative stress incontinence and erectile dysfunction*.
| Variable | Number of patients (%) |
|---|---|
|
Continence Continent Incontinent |
3850 (87.7%) 541 (12.3%) |
|
Erectile dysfunction (ED) No ED (IIEF-5 score 22–25) Mild ED (IIEF-5 score 17–21) Mild to moderate ED (IIEF-5 score 12–16) Moderate ED (IIEF-5 score 8–11) Severe ED (IIEF-5 score 5–7) |
472 (25.2%) 452 (24.2%) 224 (12%) 179 (9.6%) 543 (29%) |
*Postoperative stress incontinence defined as use of ≥ 1 pads/day, postoperative erectile dysfunction defined as International Index of Erectile Function (IIEF-5) score of <22, both 1 year after radical prostatectomy. For analysis of erectile dysfunction, only patients who had a preoperative IIEF score of ≥ 22 and complete postoperative data were included (N=1870).
No ED in 25% of cases
Slight ED in 24%
Slight to moderate ED in 12%
Moderate ED in 10%
Severe ED in 29%
Discussion
The frequency and pronounced heterogeneity of prostate cancer present considerable challenges. On one hand, there are indolent variants with a very protracted natural course that sometimes never need active treatment (12). On the other hand, one finds locally advanced tumors or tumors whose properties correspond to high-risk PCa, some of which are not amenable to cure even by radical surgery and/or irradiation. The latter are the subject of this study.
Up to only a few years ago, the recommended initial treatment in patients with locally advanced or high-risk PCa was usually palliative, predominantly radiotherapy, and only rarely radical prostatectomy. In 2006 Denberg et al. (13) published a study in which they had investigated the preferred treatment option in clinically advanced prostate cancer (clinical stage T3) in the USA between 1995 and 2001. It emerged that the proportion of patients treated primarily with radical prostatectomy sank from 18% to 9% during the study period, while the rate of radiotherapy as primary treatment rose from 58% to 69%.
Recently, however, several published studies have shown that a significant proportion of high-risk patients can be cured by radical prostatectomy or that carcinoma-specific survival is not always unfavorable after removal of the prostate despite recurrence. As early as 1994, Partin et al. noted 5-year biochemically recurrence-free survival rates of 43% following radical prostatectomy in patients with poorly differentiated PCa (Gleason score ≥8) (14). Other studies had similar findings (15). Even better results were reported in a recently published study by Stephenson et al., who found 10-year and 15-year carcinoma-specific survival rates of 92% and 81% respectively after radical prostatectomy in patients with high-risk PCa as defined by D’Amico et al. (17).
These oncological data are accompanied by recent studies evaluating the functional outcome after radical prostatectomy in patients with high-risk PCa. In a study published in 2009, our own research group investigated the rates of postoperative incontinence and erectile dysfunction following prostatectomy sparing the nerves involved in erection (6). However, no special attention was paid to the patients’ risk profile. Depending on patient age, the rate of complete continence after surgery varied between 97.4% (age <60 years) and 84.1% (age >70 years). With regard to nerve sparing, the postoperative potency rates (in preoperatively potent men) were 84 to 92% with bilateral and 58 to 70% with unilateral preservation of the pelvic splanchnic nerves. These rates of incontinence broadly correspond to those in the present study. The rate of severe ED is much higher in the present study, but it must be taken into account that nerve preservation was possible in only 48% of the patients with a prostatectomy Gleason score ≥8 on oncological grounds. The rate of nerve-sparing surgery was also only 49% in patients with a preoperative PSA level of >20 ng/mL.
On the basis of the published data, radical prostatectomy is now being increasingly employed in patients with high-risk prostate cancer. This is reflected in the current European and US guidelines, which include radical prostatectomy as a reasonable treatment option even in suitable high-risk patients (11, 18). The current S3 guideline on early detection, diagnosis, and treatment of the various stages of prostate cancer lists radical prostatectomy together with external radiotherapy and high dose-rate brachytherapy (HDR brachytherapy) as possible treatments for high-risk prostate cancer and/or locally advanced prostate cancer, without clearly favoring any one option over the others (19). A recent prospective randomized trial in which patients with localized prostate cancer were randomly allocated to a treatment arm (radical prostatectomy, N = 364) or an observation arm (watchful waiting, N = 367) showed that radical prostatectomy is indicated in patients <75 years of age whose general health permits (12). While there was no difference in overall survival between the two groups for low-risk prostate cancer, radical prostatectomy achieved a 13% reduction in overall mortality in patients with intermediate or high-risk constellations. A risk-stratified analysis in the framework of the Scandinavian Prostate Cancer Group (SPCG-4) Study concluded that patients with aggressive prostate cancer (Gleason score ≥8) or a Gleason score of 7 and clinical tumor stage T2 indubitably benefit from radical prostatectomy, while patients with low-risk prostate cancer and/or those clearly over 70 years of age benefit only slightly or not at all (20).
Next to radical prostatectomy, radiotherapy is the best-established treatment for prostate cancer. To date, these two procedures have not been compared with regard to oncological efficacy. Nevertheless, retrospectively acquired data in high-risk patients permit the conclusion of an oncological advantage for radical prostatectomy. Zelefsky et al. retrospectively compared radical prostatectomy and external radiotherapy (21). While patients in the low-risk group showed no statistically significant difference in the rate of metastatic progression 8 years after treatment, patients in the intermediate risk group and particularly those in the high-risk group showed lower rates of metastatic progression (difference 3.3% and 7.8% respectively) for radical prostectomy compared to radiotherapy. A study by Cooperberg et al. also documented a statistically significantly higher rate of carcinoma-specific survival in patients with high-risk PCa treated by radical prostatectomy (22). Relative to radical prostatectomy, external radiotherapy was associated with a 1.55 to 1.59 times higher risk of carcinoma-specific mortality.
A potential further advantage of radical prostatectomy over radiotherapy is that a substantial proportion of patients who suffer a recurrence after radical prostatectomy can be cured with acceptable morbidity by additional irradiation (so-called salvage radiotherapy), or show no sign of cancer activity for a variable period of time. A study published in 2009 found no grade 4 toxicity after salvage radiotherapy. The rates of grade 3 acute and late gastrointestinal toxicity were 1.2 and 0.6 respcectively, and 0.0 and 0.6% for urogenital toxicity, respectively (23). The 4-year biochemically recurrence-free survival rate was 82%.
A recurrence after primary radiotherapy can, in principle, be treated and cured by salvage radical prostatectomy; however, salvage prostatectomy is associated with a far higher rate of serious complications. Van der Poel et al. compared the rates of incontinence and erectile dysfunction after the two kinds of salvage treatment (24). Both incontinence (13% versus 56%) and erectile dysfunction (61% versus 81%) were statistically significantly more frequent after salvage prostatectomy.
It must be emphasized that the survival rates mentioned in the present study were often not achieved by radical prostatectomy alone. Altogether, 21% of patients received additional (adjuvant or salvage) radiotherapy. Moreover, 13.6% of the patients underwent adjuvant or salvage androgen deprivation. This should be considered when interpreting the findings.
Our study displays limitations. First, it was a retrospective investigation. Furthermore, the study was monocentric and the patients were treated at a specialized prostate cancer center. Accordingly, the results do not necessarily justify general conclusions.
In summary, although the majority of patients with locally advanced PCa, metastasis to lymph nodes, or high-risk PCa suffer a recurrence, only a small proportion of them die of their cancer within 10 years. Moreover, up to 53% of patients with a tumor not confined to the prostate remain free of postoperative recurrence in the long term, and with careful selection of patients the 10-year overall survival rate is between 58% and 89%, depending on the risk constellation. Nevertheless, prospective studies are necessary before we can formulate the optimal treatment strategy for such prostate cancers with a high level of evidence.
Key Messages.
This study analyzed 4391 patients with locally advanced or high-risk PCa who were treated surgically by means of radical prostatectomy.
The majority of these patients suffered postoperative recurrence of their disease. The 10-year carcinoma-specific survival rates were 98%, 87%, and 77%, respectively, in patients with stage pT3a, pT3b, and pT4 tumors.
The 10-year overall survival by tumor stage was 89%, 79%, and 69%.
A year after surgery, 87.7% of the patients were continent and 49% of the preoperatively potent men had slight or no erectile dysfunction.
Randomized studies are necessary to further refine the treatment strategy.
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481–488. doi: 10.1093/annonc/mdi098. [DOI] [PubMed] [Google Scholar]
- 2.Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging 1974. J Urol. 2002;167:953–958. discussion 959. [PubMed] [Google Scholar]
- 3.Yossepowitch O, Eggener SE, Bianco FJ, Jr., et al. Radical prostatectomy for clinically localized, high risk prostate cancer: critical analysis of risk assessment methods. J Urol. 2007;178:493–499. doi: 10.1016/j.juro.2007.03.105. discussion 499. [DOI] [PubMed] [Google Scholar]
- 4.Spahn M, Joniau S, Gontero P, et al. Outcome predictors of radical prostatectomy in patients with prostate-specific antigen greater than 20 ng/ml: a European multi-institutional study of 712 patients. Eur Urol. 2010;58:1–7. doi: 10.1016/j.eururo.2010.03.001. discussion 10-11. [DOI] [PubMed] [Google Scholar]
- 5.Isbarn H, Wanner M, Salomon G, et al. Long-term data on the survival of patients with prostate cancer treated with radical prostatectomy in the prostate-specific antigen era. BJU Int. 2010;106:37–43. doi: 10.1111/j.1464-410X.2009.09134.x. [DOI] [PubMed] [Google Scholar]
- 6.Budaus L, Isbarn H, Schlomm T, et al. Current technique of open intrafascial nerve-sparing retropubic prostatectomy. Eur Urol. 2009;56:317–324. doi: 10.1016/j.eururo.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 7.Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg. 2002;87:13–15. [PubMed] [Google Scholar]
- 8.McNeal JE, Redwine EA, Freiha FS, Stamey TA. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. Am J Surg Pathol. 1988;12:897–906. doi: 10.1097/00000478-198812000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Briganti A, Chun FK, Salonia A, et al. Validation of a nomogram predicting the probability of lymph node invasion among patients undergoing radical prostatectomy and an extended pelvic lymphadenectomy. Eur Urol. 2006;49:1019–1026. doi: 10.1016/j.eururo.2006.01.043. discussion 1026-1017. [DOI] [PubMed] [Google Scholar]
- 10.Chun FK, Briganti A, Shariat SF, et al. Significant upgrading affects a third of men diagnosed with prostate cancer: predictive nomogram and internal validation. BJU Int. 2006;98:329–334. doi: 10.1111/j.1464-410X.2006.06262.x. [DOI] [PubMed] [Google Scholar]
- 11.Heidenreich A, Aus G, Bolla M, Joniau S, et al. EAU guidelines on prostate cancer. Actas Urol Esp. 2009;33:113–126. doi: 10.1016/s0210-4806(09)74110-5. [DOI] [PubMed] [Google Scholar]
- 12.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denberg TD, Glode LM, Steiner JF, Crawford ED, Hoffman RM. Trends and predictors of aggressive therapy for clinical locally advanced prostate carcinoma. BJU Int. 2006;98:335–340. doi: 10.1111/j.1464-410X.2006.06260.x. [DOI] [PubMed] [Google Scholar]
- 14.Partin AW, Lee BR, Carmichael M, Walsh PC, Epstein JI. Radical prostatectomy for high grade disease: a reevaluation 1994. J Urol. 1994;151:1583–1586. doi: 10.1016/s0022-5347(17)35308-9. [DOI] [PubMed] [Google Scholar]
- 15.Zwergel U, Suttmann H, Schroeder T, et al. Outcome of prostate cancer patients with initial PSA > or =20 ng/ml undergoing radical prostatectomy. Eur Urol. 2007;52:1058–1065. doi: 10.1016/j.eururo.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 16.Stephenson AJ, Kattan MW, Eastham JA, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. J Clin Oncol. 2009;27:4300–4305. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 18.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 19.S3 Leitlinie. Prostatakarzinom: Früherkennung, Diagnose und Therapie der verschiedenen Stadien. Guideline status: 30 September 2011 currently under revision, valid until 30 September 2013. www.awmf.org/leitlinien/detail/ll/043-022OL.html. Last accessed on 26 April 2013.
- 20.Vickers A, Bennette C, Steineck G, et al. Individualized estimation of the benefit of radical prostatectomy from the Scandinavian Prostate Cancer Group randomized trial. Eur Urol. 2012;62:204–209. doi: 10.1016/j.eururo.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zelefsky MJ, Eastham JA, Cronin AM, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol. 2010;28:1508–1513. doi: 10.1200/JCO.2009.22.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooperberg MR, Vickers AJ, Broering JM, Carroll PR. Comparative risk-adjusted mortality outcomes after primary surgery, radiotherapy, or androgen-deprivation therapy for localized prostate cancer. Cancer. 2010;116:5226–5234. doi: 10.1002/cncr.25456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jereczek-Fossa BA, Zerini D, Vavassori A, et al. Sooner or later? Outcome analysis of 431 prostate cancer patients treated with postoperative or salvage radiotherapy. Int J Radiat Oncol Biol Phys. 2009;74:115–125. doi: 10.1016/j.ijrobp.2008.07.057. [DOI] [PubMed] [Google Scholar]
- 24.van der Poel HG, Moonen L, Horenblas S. Sequential treatment for recurrent localized prostate cancer. J Surg Oncol. 2008;97:377–382. doi: 10.1002/jso.20967. [DOI] [PubMed] [Google Scholar]