Summary
Objective
To investigate the effectiveness of an exercise intervention for decreasing fatigue severity and increasing physical activity in individuals with pulmonary arterial hypertension (PAH). A small, phase 2 randomized clinical trial of the effect of aerobic exercise training on fatigue severity and physical activity in patients with idiopathic or PAH associated with other conditions was conducted.
Methods
Twenty-four patients with PAH (24 female; age: 54.4 ± 10.4 years; BMI: 30.8 ± 7.2 kg/m2) participated in the study. A convenience sample was recruited in which 9% (28 of 303) of screened patients were enrolled. The project was carried out in a clinical pulmonary rehabilitation clinic during existing pulmonary rehabilitation program sessions.
Patients with PH were randomized into a 10-week program that consisted of patient education only or patient education plus an aerobic exercise-training regimen. Both groups received 20 lectures, two per week over the 10-weeks, on topics related to PAH and its management. The aerobic exercise training consisted of 24–30 sessions of treadmill walking for 30–45 min per session at an intensity of 70–80% of heart rate reserve, three days per week over the 10 weeks.
Results
After 10-weeks of intervention, patients receiving aerobic exercise training plus education reported routinely engaging in higher levels of physical activity (p < 0.05) and a decrease in fatigue severity (p = 0.03). Patients in the education only group did not report changes in fatigue severity or participation in physical activity.
Conclusions
The 10-week aerobic exercise training intervention resulted in increased physical activity and decreased fatigue in individuals with PAH.
Keywords: Hypertension, Pulmonary, Fatigue, Motor activity, Exercise
Pulmonary Arterial Hypertension (PAH) is a rare disease which affects between 3 and 5 of every 10,000 people in the United States.1 Overall survival in patients with PAH is poor and ranges between 60 and 70% three years after initial diagnosis.2 The disease has multiple etiologies and can be either idiopathic or associated with other conditions such as autoimmune diseases, connective tissue disease, or sickle cell disease.3 Clinical diagnosis of PAH is made when mean pulmonary artery pressure equals or exceeds 25 mmHg as identified by right heart catheterization.4 The elevated pulmonary pressures result in decreased pulmonary blood flow with impaired cardiorespiratory function that ultimately progresses to right heart failure.5 Symptoms of PAH include shortness of breath, syncope and excessive fatigue, all of which limit patients’ ability to engage in physical activity.6 There are currently seven FDA-approved compounds4,7,8 available for the treatment of PAH. Although 3-year survival remains poor, these medications have improved the lives of patients with PAH.9
Current medications have been somewhat effective in reducing the severity of symptoms and improving physical functioning in patients who have PAH. However, shortness of breath, dizziness and perceptions of excessive fatigue typically persist despite medical treatment.6 Severe fatigue is common and debilitating among nearly all patients with PAH.6 The pathophysiological basis of fatigue in PAH is complex and its precise mechanisms remain uncharacterized. In patients who have PAH, fatigue may be associated with primary factors, which are related to the disease process itself, or secondary factors such as sleep disturbance and depression.10 The interactions among these contributors to fatigue severity are confluent and difficult to isolate. There has been some evidence that patients with PAH do not routinely engage in physical activity11 and it seems reasonable to suspect that fatigue may deter them from participating.
Regular participation in aerobic exercise has been reported to improve cardiorespiratory fitness, increase physical activity levels, and diminish the severity of fatigue in patients with severe illnesses. Indeed patients with heart failure, cancer and unexplained physical symptoms12 have benefited from participation in aerobic exercise. In patients with PAH, significant improvements in cardiorespiratory fitness and the vitality and physical function domains of HRQoL have been reported after aerobic exercise training.13–15 Only one of these studies13 used an aerobic exercise training regimen that conformed to accepted recommendations for individuals in need of rehabilitative exercise which means that it could be carried out in existing, outpatient pulmonary rehabilitation settings.
The purpose of this study was to determine whether 10 weeks of medically supervised, outpatient, aerobic exercise training would alter severity of fatigue and participation in physical activity. It was hypothesized that this aerobic exercise training regimen could improve patient-reported fatigue severity and participation in physical activity in patients with PAH.
Methods
Patients
Patients with a diagnosis of PAH were recruited from local outpatient pulmonary hypertension and advanced lung disease clinics between September 2009 and April 2012. Patients with PAH were included if they were between 21 and 82 years of age, not pregnant, and tobacco free. Patients were excluded if they participated in structured aerobic exercise three days a week for 30 min or more, if they were classified as World Health Organization (WHO) functional class I and could walk more than 400 m during a 6-min walk test (6 MWT), or WHO functional class IV and could not walk more than 50 m during a 6 MWT. Additional exclusion criteria included an FEV1/FVC ratio ≤65%; a history of ischemic heart disease; an ejection fraction <40% or a documented pulmonary capillary wedge pressure ≥18 mmHg; significant hepatic, renal, metabolic or mitochondrial dysfunctions; severe psychiatric disease; use of beta-adrenergic blockers or antiretroviral therapies; and any musculoskeletal or neurological condition that would limit walking or exercise performance. PAH was diagnosed by a resting mean pulmonary arterial pressure ≥25 mmHg determined by right heart catheterization. Patients were on stable PAH medical regimens for at least 3 months prior to their enrollment. Signed informed consent was obtained from all participants prior to any procedures or data collection. This protocol was approved by the respective Institutional Review Boards of the collaborating institutions.
Study design
Patients who enrolled in the protocol were sequentially assigned subject numbers that randomly corresponded to a group receiving concurrent patient education plus aerobic exercise training (EXE) or to a group that received only the patient education portion of the regimen (EDU). A 2 × 2 sequential block randomization schema of the subject numbers was used in an attempt to achieve and maintain near-equal group symmetry throughout the study duration. Study personnel were blind to the randomization of patients during all baseline evaluations.
Following inclusion, patients with PAH filled out questionnaires that included the Fatigue Severity Scale and the Human Activity Profile. These questionnaires were measures of the primary variables of interest for this study, fatigue severity and physical activity. In addition to these self-report measures, cardiorespiratory fitness was determined by measures of symptom-limited treadmill test endurance and peak power output and 6 MWT distance. Following the baseline evaluations, patients were informed of the group to which they were randomly assigned. Patients in both the EXE and EDU groups received 20, 1-h education sessions over 10 weeks, which were identical in content for both groups and included lectures on anatomy and physiology, lung disease processes, medication use, oxygen therapy, community resources, advance directives, panic control and social well-being.
In addition to the lectures, the EXE group concurrently participated in 24–30 sessions of medically supervised treadmill walking for 30–45 min per session over 10-weeks. A target exercise intensity range of 70–80% of each subject’s heart rate reserve obtained from the respective baseline treadmill test was used to guide each exercise training session. The target heart rate range was calculated as: [0.7 and 0.8 × (peak HR – resting HR)] + (resting HR), in accordance with the method of Karvonen.16 Subject’s perceived dyspnea and exertion, oxygen saturation, and heart rate were continuously monitored throughout the sessions. Treadmill speed and/or inclination were adjusted by the designated research staff to keep each subject within or as close as possible to target heart rate range. The cardiorespiratory fitness tests and questionnaires were repeated immediately following the 10-week EXE and EDU regimens.
Assessments
The Fatigue Severity Scale (FSS) measures the patient’s perception of the influence of fatigue on physical and social functioning through patient responses to nine different physical and social functioning situations. Scoring is based on a Likert scale where 1 indicates a strong disagreement and 7 a strong agreement with the statement to quantify the perceived fatigue severity over the preceding week. Scoring is completed by calculating the mean response to the questions, with a score of 4 or greater indicative of severe fatigue.17 The FSS has been shown to be a valid tool for individuals with varying health conditions.18
The Human Activity Profile (HAP) was developed in 1982 to assess the activity level of patients in rehabilitation. The HAP is a self-administered test validated against a maximum oxygen consumption (VO2 max) test.19 The questionnaire consists of 94 questions presented in ascending order based on metabolic demand. The list encompasses activities from a variety of life situations, from social and physical functioning to activities of daily living such as self-care and occupational-related tasks.20 For each activity that is listed, participants were asked if they are (1) still doing this activity, (2) have stopped doing this activity, or (3) never did this activity. The HAP generates two scores, the Maximum Activity Score (MAS) and the Adjusted Activity Score (AAS). The MAS represents the activity still being performed that requires the highest oxygen consumption or metabolic equivalents (METs) and is considered a marker of an individual’s capacity for physical activity. The AAS is calculated by subtracting the activities no longer performed from MAS, and is a measure of an individual’s daily physical activities and tasks. Higher scores are indicative of higher levels of physical activity.
The 6 MWT was conducted by having subjects walk as far as possible for 6 min on a marked course. The 6 MWT is scored as the total distance covered in the 6-min duration of the test. The 6 MWT is the method perhaps most frequently used to evaluate cardiorespiratory fitness in both the research and clinical environments in patients with PAH. In fact, all seven of the medications currently available for the treatment of PAH have been approved at least in part as a result of an improvement in 6 MWT distance following their administration and sustained use.9 At specific intervals during the 6MWT, patients reported their ratings of perceived dyspnea.
The symptom limited treadmill exercise test was conducted by having subjects walk on a treadmill with regular increments in work rate to a target endpoint of volitional exhaustion. Volitional exhaustion was defined as the participants’ expressed inability to continue exercising, despite strong encouragement to continue by the testing staff. Treadmill tests were performed on a Trackmaster® (Full Vision, Inc., Newton, KS) motor-driven treadmill with computer regulated adjustments in speed and inclination. A modified Naughton protocol was followed.13 Heart rate was measured by electrocardiogram. The resting and peak heart rates attained during the baseline treadmill test were used to calculate the target heart rate range during the aerobic exercise training. Peak power output and the time the treadmill test could be sustained were recorded as outcome measures of cardiorespiratory fitness.
Statistical analysis
Data were analyzed using the statistical software package SPSS version 18.0 (IBM Corporation, Somers, NY). Chi-square and independent t-tests were used to determine differences between the individuals randomly assigned to the EXE versus the EDU group. To investigate the effect of the exercise intervention on fatigue, physical activity, exercise test duration, 6 MWT distance, and peak power output, repeated measures analyzes of variance were used to examine the interaction between group assignment (EXE vs EDU) and time of assessment (baseline vs. post-intervention). If a statistically significant interaction existed, pairwise comparisons were then performed. Statistical significance was set to p ≤ 0.05.
Results
Demographic and clinical characteristics
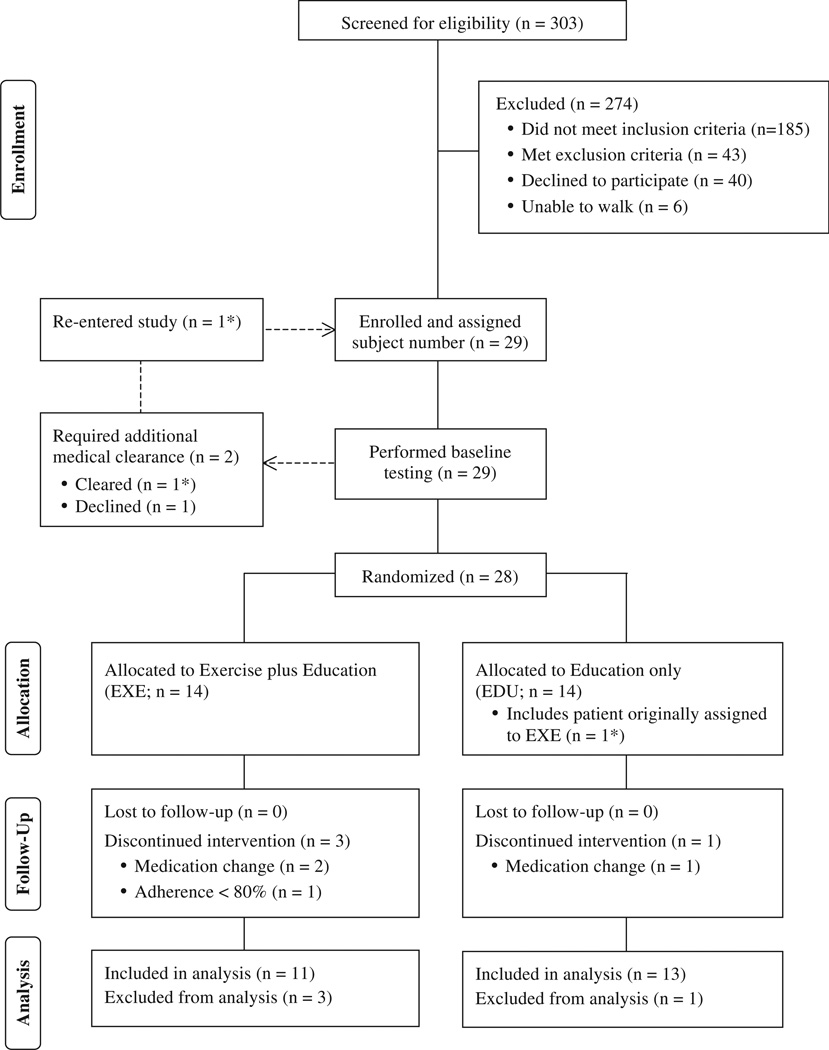
Twenty-nine (9.6%) of the 303 total patients screened met the inclusion criteria without identification of an exclusion criterion (Fig. 1). All 29 of these patients performed baseline testing. Based on their test responses, two of these patients were required to obtain additional medical clearance prior to beginning the intervention. One patient declined further participation while the other patient was cleared for participation and subsequently assigned a new subject number upon re-entry into the protocol. This patient was originally assigned a subject number corresponding to EXE, but at re-entry the randomization procedure resulted re-assignment to EDU. As such, 28 patients in total participated in either the EXE or EDU groups (Fig. 1). Of the 14 patients allocated to the EXE group, two patients withdrew due to changes in medication and one withdrew due to low attendance at the exercise sessions. One patient in the EDU group was withdrawn from the study due to medication changes. Data for the 24 patients (EXE, n = 11; EDU, n = 13) that completed the interventions was included in the analyzes.
Figure 1.
Participant flow chart. (n = 1*) represents the same patient through the enrollment and randomization process.
The demographic and clinical characteristics of patients are presented in Table 1. The PAH cohort consisted of all female patients, ranging in age from 32 to 68 years (54.4 ± 10.4 years), with BMI from 17.3 to 43.6 (30.8 ± 7.2 kg/m2). Age and BMI were not significantly different at baseline for EXE and EDU groups (Table 1). Groups were heterogenous and included patients with idiopathic PAH and PAH associated with other conditions such as scleroderma, Sjogren’s syndrome and systemic lupus erythematosus (Table 1). Patient’s medication regimens were stable for 3 months prior to beginning participation in the study and remained unchanged throughout the study’s duration.
Table 1.
Baseline participant characteristics.
| Characteristics | All patients (n = 24) |
EXE Group (n = 11) |
EDU Group (n = 13) |
Comparison between groups (P value) |
|---|---|---|---|---|
| Age, mean (SD), y | 54.4 (10.4) | 53.4 (12.4) | 55.3 (8.7) | 0.658 |
| Female, no. (%) | 24 (100) | 11 (100) | 13 (100) | 0.999 |
| Race/ethnicity, no. (%) | ||||
| Black | 11 (45.8) | 3 (27.3) | 8 (61.5) | 0.273 |
| White | 9 (37.5) | 6 (54.5) | 3 (23.1) | |
| Other | 4 (16.7) | 2 (18.2) | 2 (15.4) | |
| BMI, mean (SD), kg/m2 | 30.8 (7.2) | 29.4 (7.0) | 31.9 (7.4) | 0.455 |
| WHO/NYHA functional class, no. (%) | ||||
| Class I | 1 (4.2) | 1 (9.1) | 0 | 0.363 |
| Class II | 12 (50) | 4 (36.4) | 8 (61.5) | |
| Class III | 10 (41.6) | 5 (45.4) | 5 (38.5) | |
| Class IV | 1 (4.2) | 1 (9.1) | 0 | |
| PAH etiology, no. (%) | ||||
| Idiopathic pulmonary arterial hypertension | 6 (25) | 2 (18.2) | 4 (30.8) | 0.723 |
| Mixed connective tissue disease | 1 (4.2) | 0 | 1 (7.7) | |
| Rheumatoid arthritis | 1 (4.2) | 0 | 1 (7.7) | |
| Scleroderma | 12 (50) | 7 (63.6) | 5 (38.4) | |
| Sjogren’s syndrome | 2 (8.3) | 1 (9.1) | 1 (7.7) | |
| Systemic lupus erythematosus | 2 (8.3) | 1 (9.1) | 1 (7.7) | |
| Drug combination therapy, no. (%) | ||||
| None | 1 (4.2) | 0 | 1 (7.7) | 0.186 |
| Mono therapy | 7 (29.3) | 5 (45.4) | 2 (15.4) | |
| Dual therapy | 6 (25) | 1 (9.1) | 5 (38.4) | |
| Triple therapy | 9 (37.5) | 5 (45.4) | 5 (38.4) | |
| Human activity profile, mean (SD) | ||||
| Maximal activity score | 63 (11) | 58 (11) | 67 (10) | 0.061 |
| Adjusted activity score | 50 (15) | 43 (13) | 57 (14) | 0.021 |
| Fatigue severity score (FSS), mean (SD) | 4.2 (1.8) | 4.9 (1.6) | 3.5 (1.8) | 0.059 |
| FSS score > 4, no. (%) | 14 (58.3) | 9 (81.8) | 5 (38.4) | 0.032 |
| Cardiorespiratory fitness measures | ||||
| 6 MWT distance, m | 396 (84) | 412 (69) | 383 (96) | 0.418 |
| Treadmill time to exercise intolerance, min | 6.2 (1.9) | 6.6 (1.8) | 5.8 (2.0) | 0.320 |
| Peak power output, W | 91.8 (45) | 97.5 (41) | 86.9 (50) | 0.579 |
The exercise sessions were well attended (only one participant was withdrawn from the study due to poor adherence), with an attendance ranging between 24 and 30 (80–100%) sessions, and averaging 26.8 ± 2.2 sessions. The EXE regimen resulted in a 53 ± 44 m increase in 6 MWT distance (p = 0.003), a 2.1 ± 0.75 min increase in symptom limited treadmill exercise test duration (p = 0.001), an increase in peak power output of 25 ± 22 W (p = 0.003) and no change in perceived dyspnea at the completion of the 6 MWT (−0.3 ± 2.11; p > 0.50). No significant differences in 6 MWT distance, treadmill test duration, peak power output, or perceived dyspnea were observed following the EDU regimen.
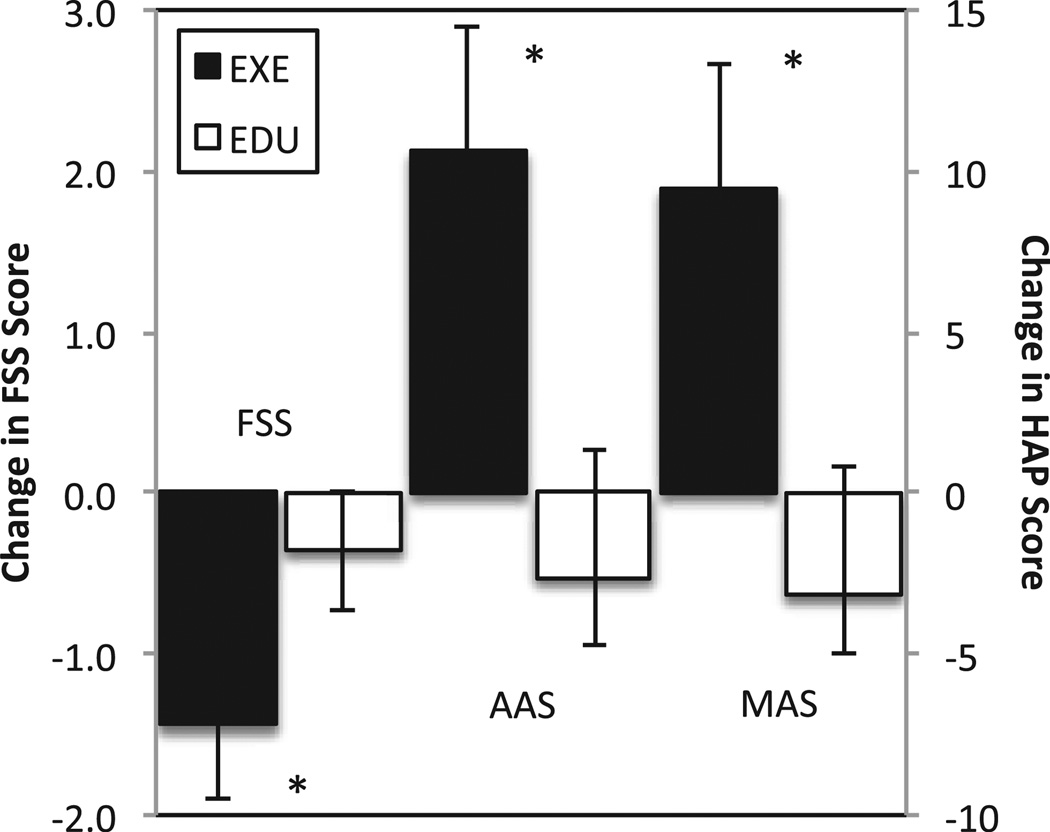
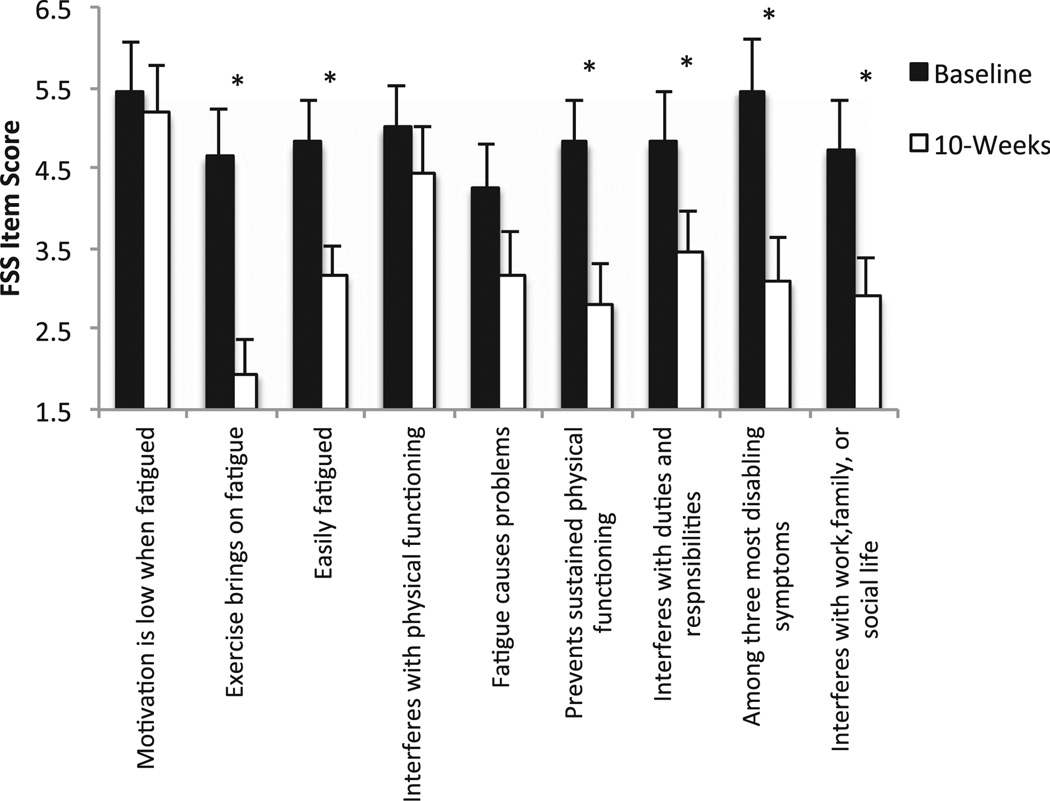
Concomitant with observed increases in cardiorespiratory fitness, patients in the EXE group reported improvements in our main variables of interest, physical activity and fatigue severity (Fig. 2). Significant increases in both AAS (p = 0.036) and MAS (p= 0.022) were observed for the EXE group following aerobic exercise training. A significant (p = 0.003) decrease in FSS composite score (post-training = 3.45 ± 1.1 versus pre-training = 4.9 ± 1.6) was observed after aerobic exercise training in EXE group (Fig. 2). Patients in the EXE group reported decreases on 6 of the 9 individual FSS items (Fig. 3). A change in fatigue severity was not observed in EDU. Nine patients in EXE had severe fatigue at baseline, only 2 of the 9 patients originally reporting severe fatigue continued to report it after the aerobic exercise training regimen (p = 0.03).
Figure 2.
Change in fatigue severity scale (FSS) and human activity profile (HAP) scores in response to 10-weeks of aerobic exercise training plus education (EXE) or education alone (EDU). *p < 0.05 for difference in change score between EXE group versus EDU group. AAS is the adjusted activity score on the HAP and MAS is the maximal activity score on the HAP.
Figure 3.
Item analysis of the fatigue severity scale (FSS) for the aerobic exercise training plus education group. *p < 0.05 for baseline versus post-intervention score (10-weeks score) on the individual items of the FSS.
Discussion
Patients in the EXE group had improved cardiorespiratory fitness13 concomitant with increased participation in physical activity and decreased fatigue severity, following the aerobic exercise-training regimen compared to baseline. Not only was overall fatigue severity diminished, fewer of these patients reported experiencing fatigue severe enough to interfere with physical or social functioning following aerobic exercise training. This finding may help to explain improvements in HRQoL reported in previous exercise interventions.13,15 The aerobic exercise regimen used in this study was similar in intensity, session duration, and frequency of participation to programs known to improve cardiorespiratory fitness in the general population. Increases in cardiorespiratory fitness observed here were in agreement with previous reports.13–15 Results of this study suggest that this type of exercise training is likely to be sufficient for improving cardiorespiratory fitness, decreasing the severity of fatigue, and increasing participation in physical activity in patients with PAH of various etiologies.
Previous information regarding the effect of an aerobic exercise intervention on fatigue severity and participation in physical activity is lacking in patients with PAH. Some earlier studies have suggested that aerobic exercise training may be safe and efficacious for patients with PAH, but measures of fatigue or physical activity were not included among their outcome variables.14,15,21 The long-term effects of aerobic exercise training on PAH survival have not been determined and this may be a next logical step in examining the efficacy of rehabilitative aerobic exercise training in patients with PAH. Moreover, durability of the effect of aerobic exercise training on cardiorespiratory fitness, fatigue severity and physical activity, and the feasibility of long-term compliance with continued participation in an aerobic exercise training regimen have yet to be determined.
In this study, the PAH cohort was heterogenous with respect to etiology. The cohort included patients with idiopathic PAH as well as those with PAH associated with autoimmune diseases such as scleroderma, systemic lupus erythematosus, and mixed connective tissue disease. Decreased cardiorespiratory fitness and physical activity, and increased fatigue severity are associated with each of these conditions independently, as well as with idiopathic PAH. In some patients with these autoimmune conditions who do not have PAH, aerobic exercise training has been reported to improve cardiorespiratory fitness, decrease fatigue, and increase physical activity.22–25 The current study demonstrates the effectiveness of aerobic exercise training for reducing fatigue severity and increasing physical activity even when PAH occurs secondarily to an underlying connective tissue disease.
Limitations
The current investigation included a small sample, which increases the likelihood of Type II error. Statistical significance was found in the main variables of interest and thus Type II error was minimized in this investigation. Although heterogenous, the small, convenience sample precludes widespread generalization of the results of this study. Physical activity was self-reported. Individuals in the EXE group may have been sensitized to physical activity following their participation in an exercise trial and therefore, may have been more likely to report their physical activity than the EDU group. Finally, all the participants were females and classified as having WHO Group 1 PAH. Further work is needed to determine if these results would be applicable to men or to patients with PH who are in WHO Groups 2 through 5.
Clinical implications
The results of this study indicate that exercise training may decrease the severity of fatigue and increase physical activity in patients who have idiopathic PAH or PAH associated with other conditions. These findings expand the available information regarding the benefits of medically supervised aerobic exercise training in female patients with Group 1 PAH, underscoring its potential importance as an adjunct therapy. The aerobic exercise training regimen used in this study is similar to one often recommended to the general population or prescribed for patients participating in cardiac and pulmonary rehabilitation exercise programs. The results demonstrate that the positive effects of aerobic exercise training may extend beyond measures of cardiorespiratory fitness.
Conclusion
Ten weeks of thrice weekly, medically supervised, treadmill walking, at 70–80% of HRR, for 30–45 min per week, was found to reduce patient-reported fatigue, increase participation in physical activity and improve cardiorespiratory fitness and 6 MWT distance in Group 1 PAH. The regimen that was used was easily incorporated into an existing clinical pulmonary rehabilitation program. Overall, the results support the use of aerobic exercise training as an adjunct to existing medical regimens in patients with PAH.
Acknowledgment
This work was supported by the National Institutes of Health [Intramural Funds 1 Z01 CL060068-05].
Footnotes
Conflict of interest statement
No conflicts of interest exist.
References
- 1.Hyduk A, Croft JB, Ayala C, Zheng K, Zheng ZJ, Mensah GA. Pulmonary hypertension surveillance—United States, 1980–2002. MMWR Surveill Summ. 2005;54:1–28. [PubMed] [Google Scholar]
- 2.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from REVEAL. Chest. 2012;142:448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 3.Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2001;38:2101–2113. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 4.Badesch DB, Champion HC, Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–S66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin VV, Presberg KW, Doyle RL, et al. Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:78S–92S. doi: 10.1378/chest.126.1_suppl.78S. [DOI] [PubMed] [Google Scholar]
- 6.Matura LA, McDonough A, Carroll DL. Cluster analysis of symptoms in pulmonary arterial hypertension: a pilot study. Eur J Cardiovasc Nurs. 2012;11:51–61. doi: 10.1177/1474515111429649. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Taichman DB, Doyle RL. Health-related quality of life and patient-reported outcomes in pulmonary arterial hypertension. Proc Am Thorac Soc. 2008;5:623–630. doi: 10.1513/pats.200802-020SK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taichman DB, Shin J, Hud L, et al. Health-related quality of life in patients with pulmonary arterial hypertension. Respir Res. 2005;6:92. doi: 10.1186/1465-9921-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naeije R, Huez S. Expert opinion on available options treating pulmonary arterial hypertension. Expert Opin Pharmacother. 2007;8:2247–2265. doi: 10.1517/14656566.8.14.2247. [DOI] [PubMed] [Google Scholar]
- 10.McDonough A, Matura LA, Carroll DL. Symptom experience of pulmonary arterial hypertension patients. Clin Nurs Res. 2011;20:120–134. doi: 10.1177/1054773810391249. [DOI] [PubMed] [Google Scholar]
- 11.Mainguy V, Provencher S, Maltais F, Malenfant S, Saey D. Assessment of daily life physical activities in pulmonary arterial hypertension. PLoS One. 2011;6:e27993. doi: 10.1371/journal.pone.0027993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connor PJ, Puetz TW. Chronic physical activity and feelings of energy and fatigue. Med Sci Sports Exerc. 2005;37:299–305. doi: 10.1249/01.mss.0000152802.89770.cf. [DOI] [PubMed] [Google Scholar]
- 13.Chan L, Chin LMK, Kennedy M, et al. Intensive treadmill exercise training, cardiorespiratory function and quality of life in patients with pulmonary hypertension. Chest. 2013;143:333–343. doi: 10.1378/chest.12-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grunig E, Lichtblau M, Ehlken N, et al. Safety and efficacy of exercise training in various forms of pulmonary hypertension. Eur Respir J. 2012;40:84–92. doi: 10.1183/09031936.00123711. [DOI] [PubMed] [Google Scholar]
- 15.Mereles D, Ehlken N, Kreuscher S, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation. 2006;114:1482–1489. doi: 10.1161/CIRCULATIONAHA.106.618397. [DOI] [PubMed] [Google Scholar]
- 16.Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate: a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307–315. [PubMed] [Google Scholar]
- 17.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 18.Whitehead L. The measurement of fatigue in chronic illness: a systematic review of unidimensional and multidimensional fatigue measures. J Pain Symptom Manage. 2009;37:107–128. doi: 10.1016/j.jpainsymman.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Daughton DM, Fix AJ, Kass I, Bell CW, Patil KD. Maximum oxygen consumption and the ADAPT quality-of-life scale. Arch Phys Med Rehabil. 1982;63:620–622. [PubMed] [Google Scholar]
- 20.Fix AJ, Daughton DM. Human activity profile professional manual. Odessa, FL: Psychological Assessment Resources, Inc; 1988. [Google Scholar]
- 21.Grunig E, Ehlken N, Ghofrani A, et al. Effect of exercise and respiratory training on clinical progression and survival in patients with severe chronic pulmonary hypertension. Respiration. 2011;81:394–401. doi: 10.1159/000322475. [DOI] [PubMed] [Google Scholar]
- 22.Brady TJ, Kruger J, Helmick CG, Callahan LF, Boutaugh ML. Intervention programs for arthritis and other rheumatic diseases. Health Educ Behav. 2003;30:44–63. doi: 10.1177/1090198102239258. [DOI] [PubMed] [Google Scholar]
- 23.Habers GE, Takken T. Safety and efficacy of exercise training in patients with an idiopathic inflammatory myopathyea systematic review. Rheumatology (Oxford) 2011;50:2113–2124. doi: 10.1093/rheumatology/ker292. [DOI] [PubMed] [Google Scholar]
- 24.O’Grady M, Fletcher J, Ortiz S. Therapeutic and physical fitness exercise prescription for older adults with joint disease: an evidence-based approach. Rheum Dis Clin North Am. 2000;26:617–646. doi: 10.1016/s0889-857x(05)70159-9. [DOI] [PubMed] [Google Scholar]
- 25.Poole JL. Musculoskeletal rehabilitation in the person with scleroderma. Curr Opin Rheumatol. 2010;22:205–212. doi: 10.1097/BOR.0b013e328335a7d2. [DOI] [PubMed] [Google Scholar]