Abstract
Aerobic training (AT) improves the metabolic syndrome (MS) and its component risk factors; however, to our knowledge, no randomized clinical studies have addressed whether resistance training (RT) improves the MS when performed alone or combined with AT. Sedentary, overweight dyslipidemic men and women, aged 18 to 70 years completed a 4-month inactive run-in period and were randomized to 1 of 3 eight-month exercise programs (n = 196). The exercise programs were (1) RT (3 days/week, 3 sets/day of 8 to 12 repetitions of 8 different exercises targeting all major muscle groups); (2) AT (~120 minutes/week at 75% of the maximum oxygen uptake), and (3) AT and RT combined (AT/RT) (exact combination of AT and RT). Of the 196 randomized patients, 144 completed 1 of the 3 exercise programs. The 86 participants with complete data for all 5 MS criteria were used in the present analysis, and a continuous MS z score was calculated. Eight months of RT did not change the MS score. AT improved the MS score (p <0.07) and showed a trend toward significance compared to RT (p <0.10). AT/RT significantly decreased the MS score and was significantly different from RT alone. In conclusion, RT was not effective at improving the MS score; however, AT was effective. Combined AT and RT was similarly effective but not different from AT alone. When weighing the time commitment versus health benefit, the data suggest that AT alone was the most efficient mode of exercise for improving cardiometabolic health.
Numerous studies have examined the beneficial effects of aerobic training (AT) on the measures of obesity and cardiometabolic risk factors1–3; however, few randomized trials have examined the optimal mode of exercise or combination of modalities for specific cardiometabolic health benefits. The few studies evaluating the effects of resistance training (RT) on the metabolic syndrome (MS) have failed to use a robust RT program and did not directly compare RT to AT.4,5 Therefore, questions remain unaddressed whether RT alone improves cardiometabolic health in overweight and obese adults; whether AT is more effective than RT at improving cardiometabolic health; and whether AT and RT combined (AT/RT) provide additional improvements that outweigh or justify the additional time required for a combined program for improving this cardiometabolic end point. The Studies of a Targeted Risk Reduction Intervention through Defined Exercise (STRRIDE-AT/RT) was designed, in part, to address these questions in a large randomized trial of overweight and obese adults.
Methods
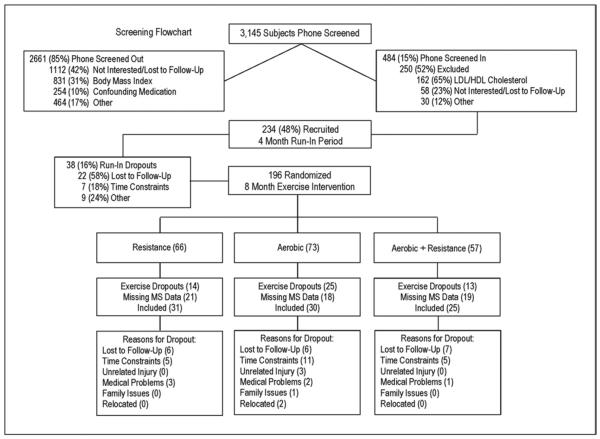
The institutional review boards at Duke University Medical Center (Duke) and East Carolina University (ECU) reviewed and approved the protocol. The subjects recruited for the STRRIDE-AT/RT study were selected from the 3,145 who responded to newspaper, magazine, and Internet advertisements and word of mouth from Durham, Greenville and the surrounding communities in North Carolina. The subjects were initially screened by telephone. Of these, 2,661 were screened out, leaving 484 eligible subjects. Of these, 250 were excluded after a more detailed assessment of interest and clinical inclusion criteria at an initial consent meeting, leaving 234 subjects who were recruited into the study (Figure 1). Of the 234 subjects, 75% were recruited at Duke and the remaining 25% at ECU.
Figure 1.
Flowchart of screening and randomization inclusion and exclusion.
The inclusion criteria included age 18 to 70 years, sedentary (exercising ≤2 times/week), overweight or moderately obese (body mass index 25 to 35 kg/m2), with mild to moderate dyslipidemia (either low-density lipoprotein [LDL] cholesterol 130 to 190 mg/dl or high-density lipoprotein [HDL] cholesterol ≤40 mg/dl for men or ≤45 mg/dl for women). The subjects were excluded if they used tobacco; had a history of diabetes, hypertension (systolic blood pressure >160 mm Hg and/or diastolic blood pressure >90 mm Hg or taking blood pressure medication), musculoskeletal disorders, or coronary artery disease; were currently dieting or intending to diet; were taking confounding medications; or were unwilling to be randomized into 1 of 3 study groups.
After informed written consent was obtained and the baseline tests were completed, all subjects were asked to maintain their current lifestyle for 4 months, followed by clinical testing and randomization into 1 of 3 exercise training groups. This period for all subjects constituted the inactive run-in period. Randomization was performed using a standard computer-based random number generator using a randomized design, blocked by gender, race, and study site. Of the subjects recruited, 196 (84%) completed the 4-month run-in period and were randomized. Of those randomized, 144 subjects (74%) completed the study. A subset of that group (86 of the 144) had data for all 5 criteria that constitute the MS at both testing points (after run-in/before training and after 8 months of exercise training), and the data from these subjects were included in the present analysis (Table 1). Of the 5 MS criteria, the most often missed variable was the mean arterial pressure.
Table 1.
Baseline demographics and exercise prescription
| Variable | RT (n = 31) | AT (n = 30) | AT + RT (n = 25) |
|---|---|---|---|
| Age (years) | 51.8 ± 11.0 | 51.1 ± 9.49 | 45.8 ± 11.8 |
| Body mass index (kg/m2) | 30.3 ± 3.10 | 30.8 ± 3.20 | 30.4 ± 3.76 |
| Race (n) | |||
| White | 27 | 25 | 21 |
| Black | 3 | 5 | 4 |
| Other | 1 | 0 | 0 |
| Gender (n) | |||
| Female | 15 | 14 | 12 |
| Male | 16 | 16 | 13 |
| Resistance exercise | |||
| Frequency (sessions/week) | 3 | 3 | |
| Intensity | Progressive | Progressive | |
| Amount (sets/week)* | 72 | 72 | |
| Time (min/week)† | 135–180 | 135–180 | |
| Adherence (%) | 83.8 ± 13.8 | 77.6 ± 15.6 | |
| Actual frequency (sessions/week) | 2.52 ± 0.41 | 2.36 ± 0.45 | |
| Actual amount (sets/week)‡ | 60.4 ± 9.91 | 55.9 ± 11.3 | |
| Aerobic exercise | |||
| Intensity (% peak oxygen consumption) | 65–80 | 65–80 | |
| Amount (kcal × kg−1 · week−1)§ | 14 | 14 | |
| Time (min/week) | 130 ± 22.7 | 128 ± 26.7 | |
| Adherence (%) | 91.0 ± 10.0 | 77.9 ± 18.1 | |
| Actual frequency (sessions/week) | 3.07 ± 0.46 | 2.68 ± 0.64 | |
| Actual time (min/week)¶ | 117 ± 19.6 | 99.7 ± 30.2 |
Data are presented as mean ± SD.
No significant baseline differences present among groups.
Amount (72 sets/week) = 3 days/week, 3 sets of 8 to 12 reps, using 8 different machines.
Actual amount (min/week) = approximate range of number of minutes per week to complete prescribed sets/week.
Actual amount (sets/week) = amount × adherence.
Amount (14 and 23 kcal × kg−1 week−1) approximately calorically equivalent to 12 and 20 miles of jogging per week, respectively.
Actual time (min/week) = time × adherence.
The exercise groups were as follows: (1) RT (3 days/week, 3 sets/day, 8 to 12 repetitions/set); (2) AT (calorically equivalent to ~12 miles/week at 65% to 80% peak oxygen consumption); (3) AT/RT (AT, calorically equivalent to ~12 miles/week at 65% to 80% peak oxygen consumption plus RT, 3 days/week, 3 sets/day, 8 to 12 repetitions/set).
A ramp period of 8 to 10 weeks, designed to gradually increase the amount of aerobic exercise over time, was prescribed to all subjects followed by 5 to 6 additional training months at the appropriate exercise prescription. For subjects randomized to RT, the ramp period began with 1 set during weeks 1 and 2, 2 sets during weeks 3 and 4, building up to the prescribed amount of 3 sets by week 5.
The aerobic exercise prescription for the AT and AT/RT groups was 14 kcal/kg of body mass/week, which is calorically equivalent to approximately 12 miles/week of walking or jogging. The number of minutes of exercise needed per week was dependent on the prevailing fitness level. The calculation of the caloric expenditure was determined by each 1 L of oxygen consumed during exercise being equivalent to 5.0 kcal (this was not corrected for the respiratory exchange ratio). The details of the prescribed and actual exercise training amounts and frequency by group are listed in Table 1. Although the amount of exercise is expressed in terms of walking or jogging, the actual exercise modes included treadmill, elliptical trainers, and cycle ergometers for the aerobic exercise.
All aerobic exercise sessions were verified by direct supervision and/or the use of a heart rate monitor that provided recorded, downloadable data (Polar Electro, Woodbury, New York). Aerobic compliance was calculated each week as a percentage, equal to the number of minutes completed within the prescribed heart rate range divided by the number of total minutes prescribed. All weekly compliance percentages were averaged to yield the overall aerobic adherence for each subject for the study (Table 1).
The RT groups at Duke were prescribed 3 sessions/week of 3 sets of 8 to 12 repetitions on 8 Cybex weight lifting machines, 4 upper body and 4 lower body, designed to target all major muscle groups. The resistance training groups at ECU were prescribed 3 sessions/week of 3 sets of 8 to 12 repetitions on 8 Cybex machines, 2 free weight exercises, plus abdominal crunches, designed to target all major muscle groups. Eight Cybex machines were used at the Duke site throughout the study; however, at ECU, the prescribed resistance training program during the first 13 weeks used 8 Cybex machines until week 14, in which they began using free weights instead of the Cybex machines for all upper body exercises. The Cybex machine exercises involving the lower body remained the same throughout the study at ECU. For all participants, at both sites, study trainers determined the baseline amount of weight lifted for each exercise by selecting a weight that the participant could lift 8 to 12 times with proper form. Throughout the training intervention, the weight amounts were increased by 2.3 kg (5 lb) each time the participant performed 12 repetitions with proper form for all 3 sets during 2 consecutive workout sessions.
All RT sessions at Duke were verified by direct supervision and/or the use of the FitLinxx Strength Training Partner, a state-of-the-art technological computer system designed to monitor and track workouts electronically (FitLinxx, Norwalk, Connecticut). The “training partner” automatically sent data from each workout to the FitLinxx server computer, where it was aggregated once each week for compliance reports by the study staff. RT compliance was calculated each week as a percentage, equal to the number of sets completed divided by the number of sets prescribed. All weekly compliance percentages were averaged to yield the overall RT adherence (Table 1).
No dietary prescriptions or instructions were provided to the participants, except for the prescription to not change their diet during the study period.
Height was measured to the nearest 0.25 cm. Body mass was measured with the subject in light clothing without shoes to the nearest 0.1 kg on a digital scale (Scale 5005, ScaleTronix, Wheaton, Illinois). The body mass index was calculated by dividing the body mass (kg) by the height (m2). The waist circumference was taken around the abdomen at the level of the iliac crest twice and averaged. Two blood pressure readings were taken at rest approximately 20 minutes apart and averaged.
HDL cholesterol and triglycerides (TG) were determined from fasting plasma samples using nuclear magnetic resonance spectroscopy (LipoScience, Raleigh, North Carolina). Glucose was determined from fasting plasma samples at the beginning of an intravenous glucose tolerance test.
As previously described in earlier STRRIDE studies,3 the MS z score used for the present study was a continuous score of the 5 MS variables. A modified z score was calculated for each variable using individual subject data, the Adult Treatment Panel (ATP) III criteria, and standard deviations using data from the entire STRRIDE-AT/RT cohort at baseline (n = 234). Gender-specific MS z score equations were used to account for variations in the ATP III criteria for men and women. Standard deviations for HDL cholesterol and waist circumference were also determined separately for men and women. The equations used to calculate the MS z score were as follows: {z-Score = [(40 − HDL)/6.2] + [(TG − 150)/66.2] + [(fasting blood glucose − 100)/10.4] + [(waist circumference − 102)/9.3] + [(mean arterial pressure − 100)/8.7]} for men and {z score = [(50 − HDL)/11.8] + [(TG − 150)/66.2] + [(fasting blood glucose − 100)/10.4] + [(waist circumference − 88)/9.2] + [(mean arterial pressure − 100)/8.7]} for women. A MS risk factor score using the ATP III guidelines was also determined for each subject as a sum of the number of ATP III criteria met before and after the exercise intervention.
Cardiopulmonary exercise tests with 12-lead electrocardiography and expired gas analysis were performed with the subject on a treadmill using a TrueMax 2400 Metabolic Cart (ParvoMedics, Sandy, Utah). The 2 highest, consecutive, 15-second readings from each test were averaged to determine the peak oxygen consumption (L/min). A respiratory exchange ratio ≥1.10 was sought and achieved during 86% of the pre-training cardiopulmonary exercise test and 74% of the post-training cardiopulmonary exercise test (range 0.93 to 1.32).
The upper and lower body total amounts of weight lifted in kilograms from a single training session during week 5 were used as the baseline measure of overall strength and the same totals from a single session at approximately week 32 were used as the end of training measure of overall strength. By calculating the difference in the amount of weight lifted at week 32 from week 5, we determined the overall strength gains expressed in kilograms lifted/session.
All of the tests were completed after the 4-month run-in/pre-training and after the 8-month exercise training intervention.
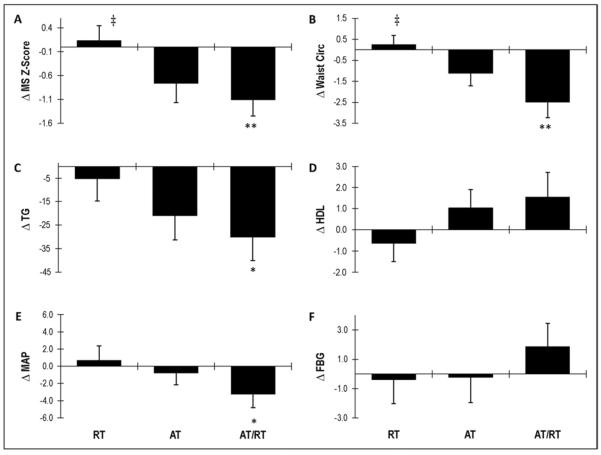
The data were analyzed using analysis of variance (Stat-View or SAS software, SAS Institute, Cary, North Carolina). When the analysis of variance was impressionable (p <0.10), Fisher's protected least significant difference post hoc analysis was performed to determine the differences between the groups (Figure 2). Three pairwise comparisons (AT, RT, and AT/RT exercise groups compared to each other) were of interest. p Values <0.05 were considered significant in post hoc testing. Paired t tests, 2-tailed, were used to determine whether the post- versus pre-intervention score for changes within each group were significant (Table 2).
Figure 2.
Effects of exercise modes on changes in (A) MS z score, (B) waist circumference (cm), (C) TG (mg/dl), (D) HDL (mg/dl), (E) mean arterial pressure (mm Hg), and (F) fasting plasma glucose (mg/dl). Error bars indicate SE. **p <0.05 and *p <0.10 compared to RT; ‡p <0.10 compared to AT.
Table 2.
Baseline values and change scores
| Variable | RT (n = 31) |
AT (n = 30) |
AT + RT (n = 25) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Change | p Value | Baseline | Change | p Value | Baseline | Change | p Value | |
| Body mass (kg) | 89.2 ± 14.5 | 0.70 ± 2.36 | 0.110 | 89.3 ± 10.8 | −1.54 ± 2.59 | 0.003* | 90.1 ± 13.2 | −1.90 ± 3.58 | 0.014* |
| Peak oxygen consumption (ml/kg/min) | 26.5 ± 6.35 | 1.23 ± 3.14 | 0.037* | 28.4 ± 5.96 | 3.33 ± 3.95 | <0.0001* | 28.8 ± 6.48 | 3.67 ± 3.61 | <0.0001* |
| Strength (kg/session) | 9,302 ± 3,688 | 4,212 ± 2,392 | <0.0001* | NA | NA | NA | 8,964 ± 2,771 | 3,425 ± 2,681 | <0.0001* |
| High-density lipoprotein cholesterol (mg/dl) | 46.8 ± 13.9 | −0.63 ± 4.81 | 0.469 | 41.5 ± 14.2 | 1.03 ± 4.81 | 0.250 | 45.0 ± 11.0 | 1.55 ± 5.84 | 0.197 |
| Triglycerides (mg/dl) | 140 ± 81.0 | −5.25 ± 52.6 | 0.583 | 154 ± 81.3 | −21.0 ± 56.0 | 0.049* | 152 ± 93.9 | −30.1 ± 49.8 | 0.006* |
| Waist circumference (cm) | 104 ± 9.68 | 0.25 ± 2.45 | 0.577 | 104 ± 10.1 | −1.12 ± 3.20 | 0.064 | 103 ± 11.2 | −2.48 ± 3.78 | 0.003* |
| Fasting glucose (mg/dl) | 99.8 ± 11.6 | −0.37 ± 9.22 | 0.823 | 96.3 ± 13.4 | −0.22 ± 9.54 | 0.902 | 90.3 ± 9.12 | 1.86 ± 7.95 | 0.253 |
| Systolic blood pressure (mm Hg) | 120 ± 13.2 | 2.32 ± 10.8 | 0.241 | 122 ± 13.2 | −0.57 ± 10.7 | 0.775 | 118 ± 14.6 | −3.08 ± 12.0 | 0.210 |
| Diastolic blood pressure (mm Hg) | 78.8 ± 9.28 | −0.16 ± 9.85 | 0.928 | 80.6 ± 9.14 | −0.87 ± 8.20 | 0.567 | 77.8 ± 8.36 | −3.32 ± 7.80 | 0.044* |
| Mean arterial blood pressure (mm Hg) | 92.7 ± 10.2 | 0.67 ± 9.46 | 0.697 | 94.3 ± 9.81 | −0.77 ± 7.59 | 0.584 | 91.2 ± 9.68 | −3.24 ± 7.76 | 0.048* |
| Adult Treatment Panel III score | 2.19 ± 1.28 | 0.36 ± 0.99 | 0.054* | 2.63 ± 1.10 | −0.03 ± 1.19 | 0.879 | 2.28 ± 0.98 | −0.64 ± 1.04 | 0.005* |
| Metabolic syndrome z score | −0.22 ± 3.47 | 0.13 ± 1.76 | 0.677 | 0.45 ± 3.59 | −0.76 ± 2.20 | 0.067 | −1.07 ± 3.06 | −1.10 ± 1.70 | 0.004* |
Data are presented as mean ± SD.
No significant baseline differences found among groups.
Significant (p <0.05) change (postintervention compared to preintervention) score using paired t test.
Results
The baseline demographics are presented for each group in Table 1. No significant between-group differences were noted for any baseline measures. A similar number of men and women were in each group, and minorities constituted 15% of the study population. The exercise prescription and adherence are also described in detail in Table 1. Adherence was slightly lower for each portion of the AT/RT group than for either AT or RT; however, the total time accumulated for AT/RT remained almost double that of the other exercise groups. Figure 1 describes the flow of participants from recruitment to post-intervention testing. There was a 26.5% drop-out rate from the exercise intervention across all groups. The most commonly missing values were for the mean arterial pressure (64% of missing values). No baseline differences were found in the other characteristics listed in Table 1 for those 58 participants without a calculated MS z score and the 86 included in the present analysis, implying that the data were missing at random (data not shown).
The pre-training and post-training change scores for body mass, peak oxygen consumption, strength, all 5 MS risk factors, and the MS z score are presented in Table 2, with the t test results for significant pre- to post-intervention changes highlighted. The significant change in the peak oxygen consumption in each AT group demonstrated the effectiveness of the training stimulus, as did the results for strength in the RT groups. The body mass significantly decreased with AT and AT/RT but did not change with RT. The waist circumference significantly decreased in the AT/RT group and showed a trend toward a significant decrease with AT. However, the waist circumference did not change with RT. TG decreased significantly with AT and AT/RT but showed no significant change with RT. No significant changes were found in the HDL cholesterol, fasting plasma glucose, or systolic blood pressure in any exercise group. The diastolic blood pressure and mean arterial pressure decreased significantly with AT/RT but failed to change with AT or RT.
AT/RT induced a significant improvement in the MS z score (p = 0.004) and AT alone exhibited a trend toward improvement (p <0.07). However, RT alone failed to significantly alter the MS z score. When calculating an ATP III score using the current ATP III guidelines, AT/RT elicited a significant decrease in the ATP III score but AT did not; interestingly, RT elicited a trend toward a significant improvement in the ATP III score (p = 0.054; Figure 3).
Figure 3.
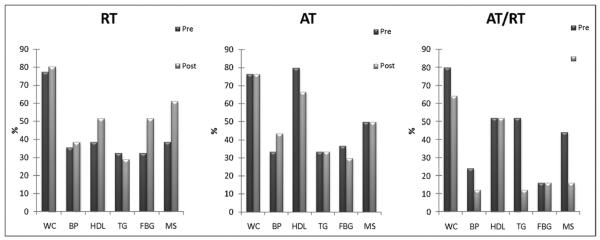
ATP III risk factor and MS prevalence before and after exercise intervention. MS defined as having 3 of 5 criteria: waist circumference (WC) ≥102 cm in men and ≥88 cm in women, blood pressure (BP) ≥130 mm Hg systolic or ≥85 diastolic, HDL cholesterol <40 mg/dl in men and <50 mg/dl in women, TG ≥150 mg/dl, and fasting plasma blood glucose (FBG) ≥100 mg/dl.
Figure 2 depicts the effects of exercise mode on the changes in the MS z score and its risk factors. Several between-group differences were observed; however, several only showed a trend toward significance (Figure 2). A difference in the effects on the MS z score and waist circumference were observed in the AT/RT group compared to the RT group (p <0.05) and in the AT group compared to the RT group (p <0.10). A trend toward significance was observed (p <0.10) when comparing the effects of AT/RT and RT on the changes in TG and mean arterial pressure.
Discussion
The effects of AT on the cardiometabolic variables associated with an increased risk of cardiovascular disease, diabetes, obesity, and premature mortality are well established.1,2,6–10 However, little research has been done on the effects of RT on the MS and its risk factors. Therefore, the optimal mode of exercise or the combination thereof with respect to its effects on cardiometabolic risk is unclear. The present report represents the largest randomized trial to directly compare the changes in MS induced by RT and AT or the combination of both in non-diabetic, sedentary, and overweight adults.
We believe the most important findings of the present study were the following: RT did not significantly improve any of the MS parameters or the comprehensive MS z score; AT improved the MS z score; AT/RT showed significant, robust improvements in the MS z score; and the direct comparison of AT and RT showed that AT improved MS more than RT, just at the border of statistical significance. In addition, the subjects in the AT/RT group exercised significantly longer (almost twice as much) than those in the AT or RT group; therefore, the study did not directly address whether the significant benefits accruing to those in the AT/RT group resulted from the longer exercise duration or the additive or synergistic nature of the combined training stimuli.
Although the MS is a relatively new clinical diagnosis, it is clear that AT beneficially affects the MS. Specifically, large randomized controlled trials, reviews, and meta-analyses have shown that AT decreases the fasting TG levels and increases the HDL concentrations,6,8,9 reduces the waist circumference,10–12 lowers blood pressure,7,13,14 and either lowers the fasting glucose and/or prevents the increases associated with a continued sedentary lifestyle.15,16 In addition to these well-characterized individual effects of AT, some studies have shown that AT improves the overall MS z score.1,3 Other comprehensive reviews have also been reported.2,16
In the present study, although AT did not significantly improve all 5 of the ATP III-defined MS variables, the combination of these changes into a MS z score was responsive to AT and highlights the power of this construct (MS z score) to reflect more accurately and more sensitively the totality of these cardiometabolic health benefits of AT than does the ATP III score as a linear combination of the presence or absence of any single dichotomous variable using the clinical diagnostic criteria.
Fewer studies have been published on the cardiometabolic health effects of RT. In the published data, RT has been shown to be consistently superior to AT for improving muscular strength and lean body mass.17–20 However, specifically with respect to cardiometabolic health, the beneficial effects of RT are less clear. The most consistently reported metabolic benefit of RT is an improvement in insulin resistance and glycemic control.21–23 RT also improves glycosylated hemoglobin in individuals with type 2 diabetes.21,22 In a recent study of patients with type 2 diabetes, a combination of AT and RT showed an improvement in glycosylated hemoglobin levels compared to a control group.24 RT also improves glucoses tolerance in glucose-intolerant subjects and improves insulin sensitivity in insulin-resistant subjects.18 However, it is important to note that there is a dearth of comparative studies, especially large, randomized trials of AT versus RT on cardiometabolic health and the MS. It is not known whether RT reduces fasting glucose values or even prevents the increase in fasting glucose observed with continued sedentary life-style. However, from the effects of RT on glycosylated hemoglobin, one would hypothesize that it lowers and/or prevents increases in fasting glucose concentrations in those without diabetes. With regard to serum lipid concentrations, previous data have suggested that RT has little effect in improving TG or HDL.2,4 Studies of the effects of RT on blood pressure14,25 have reported that there is a small, but significant, reduction in systolic blood pressure but no significant effect on diastolic blood pressure. Some studies have reported that RT reduces the waist circumference,5 but others have reported that it does not.21 RT expends fewer calories in each duration of exercise session than does AT,4 probably owing to the rest periods in between the bouts of exercise; therefore, RT inconsistently reduces the total body fat.18 As a result, one might expect that the effects of RT on the waist circumference would decrease between inactive controls and AT. In summary, our observations of the effect of RT are in general agreement with the published effects of RT on the individual components of MS and with the American Heart Association statement on the role of RT in those with and without cardiovascular disease,18 concluding that AT induces “greater improvements in aerobic capacity and associated cardiopulmonary and metabolic variables and more effectively modifies CVD [cardiovascular disease] risk factors” than does RT.
Our study had several major strengths: (1) the randomized, run-in design; (2) the direct verification of the amount and intensity of nearly all exercise sessions using heart rate monitors, Fitlinxx, and training supervision; (3) a substantial RT program that reduced the likelihood that negative findings resulted from an inadequate RT stimulus; (4) a large number of subjects leading to excellent statistical power to detect exercise exposure effects across groups; and (5) the additive nature of the AT/RT exercise program, permitting the assessment of interacting (interfering, additive, or synergistic) effects of AT and RT. One limitation of the present study was that the participants were motivated men and women who were asked to exercise in a semi-supervised setting; therefore, the results might not generalize to a non-supervised group in the general population. Second, the combined AT/RT group required approximately double the time effort of either group alone, resulting in slightly lower protocol adherence. In conclusion, RT did not result in significant improvements in the MS risk factors or the MS z score; however, AT was an effective and efficient method to improve MS. Therefore, when weighing the time commitment versus cardiometabolic health benefit, our data suggest that aerobic exercise is the most efficient mode of exercise for addressing the health issues associated with MS.
Acknowledgment
A special acknowledgment to all study staff at Duke and East Carolina University who worked extremely hard to make this study successful.
This study was conducted in Durham and Greenville, North Carolina, with grant HL-57354 provided by the National Health, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland (Clinical Trial Registration No. NCT00275145).
References
- 1.Katzmarzyk PT, Leon AS, Wilmore JH, Skinner JS, Rao DC, Rankinen T, Bouchard C. Targeting the metabolic syndrome with exercise: Evidence from the heritage family study. Med Sci Sports Exerc. 2003;35:1703–1709. doi: 10.1249/01.MSS.0000089337.73244.9B. [DOI] [PubMed] [Google Scholar]
- 2.Lakka TA, Laaksonen DE. Physical activity in prevention and treatment of metabolic syndrome. Appl Physiol Nutr Metab. 2007;32:76–88. doi: 10.1139/h06-113. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JL, Slentz CA, Houmard JA, Samsa GP, Duscha BD, Aiken LB, McCartney JS, Tanner CJ, Kraus WE. Exercise training amount and intensity effects on metabolic syndrome (from studies of a targeted risk reduction intervention through defined exercise) Am J Cardiol. 2007;100:1759–1766. doi: 10.1016/j.amjcard.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strasser B, Siebert U, Schobersberger W. Resistance training in the treatment of the metabolic syndrome. Sports Med. 2010;40:397–415. doi: 10.2165/11531380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Stensvold D, Tjonna AE, Skaug E-A, Aspenes S, Stolen T, Wisloff U, Slordahl SA. Strength training versus aerobic interval training to modify risk factors of metabolic syndrome. J Appl Physiol. 2010;108:804–810. doi: 10.1152/japplphysiol.00996.2009. [DOI] [PubMed] [Google Scholar]
- 6.Durstine JL, Haskell WL. Effects of exercise training on plasma lipids and lipoproteins. Exer Sports Sci Rev. 1994;22:477–522. [PubMed] [Google Scholar]
- 7.Fagard RH. Exercise is good for your blood pressure: effects of endurance training and resistance training. Clin Expt Pharmacol Physiol. 2006;33:853–856. doi: 10.1111/j.1440-1681.2006.04453.x. [DOI] [PubMed] [Google Scholar]
- 8.Kraus W, Houmard J, Duscha B, Knetgzer K, Wharton M, McCartney J, Bales C, Henes S, Samsa G, Otvos J, Kulkarni K, Slentz C. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 9.Leon A, Sanchez O. Response of blood lipids to exercise training alone or combined with dietary intervention. Med Sci Sports Exerc. 2001;33:S502–S515. doi: 10.1097/00005768-200106001-00021. [DOI] [PubMed] [Google Scholar]
- 10.Slentz C, Duscha B, Johnson J, Ketchum K, Aiken L, Samsa G, Houmard J, Bales C, Kraus W. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE—a randomized controlled study. Arch Intern Med. 2004;164:31–39. doi: 10.1001/archinte.164.1.31. [DOI] [PubMed] [Google Scholar]
- 11.Ross R, Janssen I. Physical activity total and regional obesity: dose–response considerations. Med Sci Sports Exerc. 2001;33:S521–S527. doi: 10.1097/00005768-200106001-00023. [DOI] [PubMed] [Google Scholar]
- 12.Ross R, Janssen I, Dawson J, Kungl A, Kuk J, Wong S, Nguyen-Duy T, Lee S, Kilpatrick K, Hudson R. Exercise-induced reduction in obesity and insulin resistance in women: a randomized, controlled trial. Obesity. 2004;12:789–798. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 13.Cornelissen VA, Fagard RH. Effect of resistance training on resting blood pressure: a meta-analysis of randomized controlled trials. J Hypertens. 2005;23:251–259. doi: 10.1097/00004872-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kelley GA, Kelley KS. Progressive resistance exercise and resting blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2000;35:838–843. doi: 10.1161/01.hyp.35.3.838. [DOI] [PubMed] [Google Scholar]
- 15.Slentz C, Houmard J, Kraus W. Modest exercise prevents the progressive disease associated with physical inactivity. Exer Sports Sci Rev. 2007;35:18–23. doi: 10.1249/01.jes.0000240019.07502.01. [DOI] [PubMed] [Google Scholar]
- 16.Kraus W, Slentz C. Metabolic syndrome: recognition, etiology and physical fitness as a component. In: Opara E, editor. Nutrition and Diabetes: Pathophysiology and Management. Taylor & Francis; New York: 2006. pp. 57–78. [Google Scholar]
- 17.Pollock ML, Franklin BA, Balady GJ, Chaitman BL, Fleg JL, Fletcher B, Limacher M, Pina IL, Stein RA, Williams M, Bazzarre T. Resistance exercise in individuals with and without cardiovascular disease. Circulation. 2000;101:828–833. doi: 10.1161/01.cir.101.7.828. [DOI] [PubMed] [Google Scholar]
- 18.Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, Gulanick M, Laing ST, Stewart KJ. Resistance exercise in individuals with and without cardiovascular disease: 2007 update. Circulation. 2007;116:572–584. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 19.Braith RW, Stewart KJ. Resistance exercise training: its role in the prevention of cardiovascular disease. Circulation. 2006;113:2642–2650. doi: 10.1161/CIRCULATIONAHA.105.584060. [DOI] [PubMed] [Google Scholar]
- 20.Davidson LE, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM, Lee S, Lam M, Ross R. Effects of exercise modality on insulin resistance and functional limitation in older adults. Arch Intern Med. 2009;169:122–131. doi: 10.1001/archinternmed.2008.558. [DOI] [PubMed] [Google Scholar]
- 21.Sigal R, Kenny G, Boule N, Wells G, Prud'Homme D, Fortiere M, Reid R, Tulloch H, Coyle D, Phillips P, Jennings A, Jaffey J. Effects of aerobic training, resistance training or both on glycemic control in type 2 diabetes. Ann Intern Med. 2007;147:357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 22.Castaneda C, Layne J, Munoz-Orians L, Gordon P, Walsmith J, Foldvari M, Roubenoff R, Tucker K, Nelson M. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25:2335–2341. doi: 10.2337/diacare.25.12.2335. [DOI] [PubMed] [Google Scholar]
- 23.Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients. Diabetes Care. 2006;29:2518–2527. doi: 10.2337/dc06-1317. [DOI] [PubMed] [Google Scholar]
- 24.Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, Mikus CR, Myers V, Nauta M, Rodarte RQ, Sparks L, Thompson A, Earnest CP. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304:2253–2262. doi: 10.1001/jama.2010.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fagard RH, Cornelissen VA. Effect of exercise on blood pressure control in hypertensive patients. Eur J Cardiovasc Prev Rehabil. 2007;14:7–12. doi: 10.1097/HJR.0b013e3280128bbb. [DOI] [PubMed] [Google Scholar]