Abstract
Obesity is a chronic inflammatory state and adipocytes are capable of contributing to this inflammation by their production of inflammatory mediators. The present study used fibroblast-derived adipocytes and normal spleen cells as a model to determine if adipocytes can also serve as immune regulatory cells by modulating the functions of conventional immune cells. Media conditioned by the adipocytes stimulated release of the Th1-type cytokines IL-2, IFN-γ and GM-CSF from cultures of normal spleen cells. The adipocytes also stimulated spleen cell release of inhibitory cytokines, although to varying degrees. This included IL-10, IL-13 and, to a lesser extent, IL-4. Spleen cell production of the inflammatory cytokines IL-6, TNF-α and IL-9 was stimulated by adipocytes, although production of the Th17-derived cytokine, IL-17, was not stimulated. The adipocyte-conditioned medium did not stimulate production of predominantly monocytes-derived chemokines CXCL9, CCL2, CCL3, CCL4, but stimulated production of the predominantly T-cell-derived chemokine CCL5. In all cases where cytokine/chemokine production from spleen cells was stimulated by adipocytes, it was to a far greater level than was produced by the adipocytes themselves. Studies initiated to determine the identity of the adipocyte-derived mediators showed that the spleen cell modulation could not be attributed to solely adiponectin or leptin. Studies to determine the source of some of the cytokines whose production was stimulated by adipocytes showed that expression of the inflammatory cytokine IL-6 was not increased in either CD4+ or CD8+ T-cell. When the splenic T-cells were examined for IFN-γ, the adipocyte stimulation of IFN-γ was within CD8+ T-cells, not CD4+ T-cells. These studies show that adipocytes may be able to serve as immune regulatory cells to stimulate conventional immune cells to release a spectrum of immune mediators.
Keywords: Adipocytes, Cytokines, Immune regulation, Inflammation, T-cells
1. Introduction
The World Health Organization estimates that 1.5 billion people are overweight and more than 500 million are obese worldwide [1]. Obesity is associated with a multitude of complications including diabetes, increased chronic periodontal disease, diffficulties with wound healing, and increased cancer risk, and takes an immense economic toll in multiple countries throughout the world [1–6].
The obese state has been recognized as being in a chronic inflammatory condition, with macrophages and T-cells accumulating within adipose tissue [7,8]. Adipocytes secrete a wide variety of hormones and proteins that regulate whole body homeostasis involving nearly all organs and cell types. This includes the pro-angiogenic adipokines such as VEGF and leptin [9,10]. The levels of adipokines that are produced can vary with the extent of obesity. Studies with animals have shown that feeding a high-fat diet results in early increases in plasma levels of the adipokines leptin, resistin and adipsin, and a later reduction in levels of adiponectin [11]. Similar results are seen in the obese human population, with levels of leptin and adiponectin returning to normal levels following gastric bypass surgery [12]. Adipocytes are not only potent producers of adipokines such as adiponectin, adipsin, leptin, and visfatin, but respond to and produce various cytokines including interleukin (IL)-6, tumor necrosis factor (TNF)-α and IL-10 [7,13]. In obesity, levels of IL-6, TNF-α and leptin are increased [14]. In terms of the biological importance of adipokines, adiponectin has anti-diabetic, anti-atherosclerotic, and anti-inflammatory properties and, thus, may influence cardiovascular disease and some types of cancer [15–20]. Leptin, which regulates food intake and energy expenditure, also has an immune regulatory functions [21].
Immune function can be impacted at several levels such as through immune regulatory cells that can amplify or diminish immune responses. So far, more than 150 cytokines have been identified and classified into several categories, although the plasticity of immune cells such as T-cell subpopulations makes categorization of these cells and their functions somewhat complex. Clustering T-cells into Th1- and Th2-type categories can simplify discussion of their immunological functions, but it cannot be used rigidly as there are other T-cell subpopulations that can produce overlapping cytokines. Traditionally, Th1-type cytokines have been considered to have a role in cellular reactivity such as against intracellular pathogens, such as viruses, and against cancerous cells. Among the Th1-type cytokines are IFN-γ, IL-12 and GM-CSF [22,23]. Th2-type cytokines tend to control humoral immunity by up-regulating antibody production to protect against extracellular pathogens [24]. These include cytokines such as IL-4, IL-5, IL-10 and IL-13 [23]. Closely related to Th2 cells are Th9 cells, which produce IL-9 and IL-10 [25]. Other categories include Th17 and Treg cells, which share a common lineage with significant plasticity, exhibit opposing immunological effects [26,27]. Th17 cells produce IL-17A, IL-17F, IL-22, IL-21 and IFN-γ [28] In contrast, Tregs produce immune inhibitory mediators such as IL-10 and TGF-β [29]. There are also cytokines with dual functions and which are involved in inflammatory responses. Inflammatory cytokines that have been shown to be over-expressed in obese mice include IL-6, TNF-α and IL-1 [14]. Leptin has been suggested to mediate inflammation, including in inflamed dental pulp tissue, and can stimulate human dendritic cell differentiation and their capacity to stimulate Th1 reactivity [30,31]. Treatment of healthy subjects with leptin increased production of inflammatory mediators [32]. In contrast, adiponectin tends to have anti-inflammatory properties. In a sepsis mouse model, adiponectin reduced mortality through an anti-inflammatory mechanism whereby serum levels of TNF-α and IL-6 were reduced [33].
While the direct inflammatory effects of obesity and adipocytes have been studied, less well known is if adipocytes might serve as immune regulatory cells through modulation of conventional immune regulatory cell types. Furthermore, most of the studies of the inflammatory and anti-inflammatory adipocyte-derived mediators have focused on individual mediators or were conducted in the context of obesity and associated disorders. The present studies used fibroblast-derived adipocytes to examine how the sum of the mediators that they produce function in an immune regulatory capacity to production of Th1-type cytokines, inhibitory cytokines, inflammatory mediators and chemokines by conventional immune spleen cells from healthy mice. The results show adipocyte stimulation of spleen cell production of Th1-type cytokines and, to varying degrees, inhibitory cytokines. Adipocytes also stimulated spleen cell production of inflammatory cytokines, although not Th17. In contrast, adipocytes did not stimulate spleen cell secretion of chemokines, with the exception of CCL5. These results indicate the capacity of adipocytes to serve as immune regulatory cells by triggering cytokine production by conventional immune cells.
2. Materials and methods
2.1. Adipocyte differentiation from the mouse embryonic fibroblast cell line, 3T3-L1
The model used to assess the immune regulatory capacity of adipocytes was to co-culture media conditioned by fibroblast-derived adipocytes with conventional immune cells of the spleen, and then to analyze cytokine production by the spleen cells. The mouse fibroblast cell line, 3T3-L1, (ATCC) was grown and differentiated post-confluency to adipocytes in DMEM (Invitrogen, Carlsbad, VA, USA) culture medium containing 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, 0.02 M HEPES buffer, 2 mM L-glutamine, and 5 × 10−5 M 2-mercaptoethanol. The medium to differentiate the fibroblasts into adipocytes was additionally supplemented with 25 mM glucose, 0.5 mM of 3-isobutyl-1-methylxantine (Sigma-Aldrich, St Louis, MO) and 1 μM of dexamethasone (Sigma-Aldrich, St Louis, MO). After 72 h of incubation, medium was replaced with DMEM culture medium containing 25 mM glucose plus 1.74 μM of insulin (Sigma-Aldrich) for 48 h. After differentiation, the insulin was removed from the culture medium and adipocytes were maintained in DMEM culture medium containing 25 mM glucose and 10% FBS for 5 to 9 days. Conditioned medium was collected every other day and stored frozen for analyses [34].
2.2. Preparation and culture of splenocytes with medium or adipocyte conditioned medium
Female C57BL/6 mice (Charles Rivers Laboratory) were humanely euthanized by CO2 asphyxiation followed by cervical dislocation. All procedures were conducted with Institutional Animal Care and Use Committee approval. Spleens from healthy mice were collected and used as a source of immune cells. The spleens were homogenized using a Stomacher™80 homogenizer (Seward) set on medium for 60 s. They were placed into DMEM culture and 1 × 106 cells were plated into 24-well plates coated with 2.5 μg/well anti-CD3 antibody. The spleen cells were not fractionated so as to include both T-cells as well as antigen-presenting cells. To further help sustain the spleen cells, the wells were supplemented with 0, 15 or 150 pg/ml IL-2. Control cultures contained culture medium alone while experimental cultures contained medium conditioned by adipocyte cells. In several pilot studies, adiponectin or leptin were each added to spleen cell cultures in lieu of the media conditioned by adipocytes. The total volume per well was 2 ml. Seventy-two hours later, supernatants from spleen cells were collected for measurement of secreted immune regulatory products or, in several select studies, cells were further processed for intracellular immunostaining [35].
2.3. Flow cytometric analysis of intracellular cytokine expression
All reagents used for intracellular immunostaining were from BD Biosciences unless otherwise specified. After incubation with control medium or medium conditioned by fibroblast-derived adipocytes, spleen cells were incubated for 4 h at 37 °C with 50 ng/ml phorbol 12-myristate 12-acetate (PMA), 1 μg/ml ionomycin, and brefelden A solution. They were then surface immunostained with fluorescent-conjugated antibodies to CD8 and CD4. For intracellular immunostaining, cells were then fixed and permeabilized with Cytofix/Cytoperm, and immunostained with fluorescent-tagged antibodies to cytokines or to granzyme B. The extent and frequency of positively stained cells was visualized using flow cytometry (FACSCanto).
2.4. Cytokine bead array
Following 3 days of spleen cell incubation with control medium or adipocyte-conditioned medium, culture supernatants were collected and used to measure levels of soluble immune mediators. Unless otherwise specific, these analyses were conducted with reagents from BD Biosciences. Using the manufacturer’s instructions, levels of cytokines and chemokines in supernatants were measured with the mouse Th1/Th2/Th17 cytometric bead array kits, while levels of chemokines and IL-13 in cell supernatants were measured with cytometric bead array flex sets for the individual mediators. Supernatants used for measurement of TGF-β1 levels were first acid activated in accordance to the manufacturer’s instructions. Relative amounts of each cytokine were analyzed using FCAP Array software.
2.5. Statistical analysis
Data were reported using the mean as a measure of central tendency ± standard error of the mean. To compare one variable condition between groups, the 2-tailed Student’s t test was used. Significance was reported at the 95% confidence interval.
3. Results
3.1. Adipocyte regulation of T-cell production of Th1-type and inhibitory cytokines
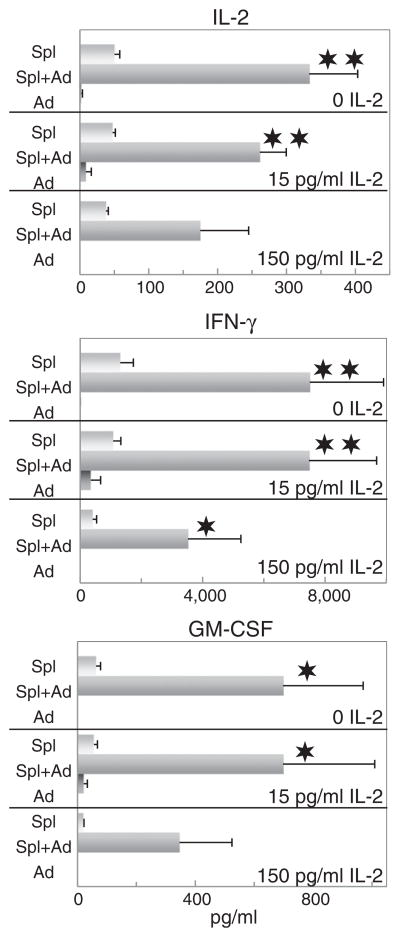
Obesity has been characterized as a state of chronic inflammation and adipocytes have previously been shown to be capable of producing inflammatory cytokines. However, their capacity to function as immune regulatory cells by skewing T-cell production of cytokines has not been previously evaluated. This was first assessed by determining the effect of media conditioned by adipocytes on the production of Th1-type and Th2-type cytokines by spleen cells that were sustained on immobilized CD3 antibody plus either no IL-2 or two relatively low doses of IL-2 so as not to overtly stimulate T-cells and allow visualization of the effect of adipocyte mediators on the T-cells. Under these culture conditioned, T-cell production of IL-2, IFN-γ and GM-CSF was minimal (Fig. 1). As expected, adipocyte-conditioned medium was devoid of these cytokines. However, in the presence of adipocyte-conditioned medium, T-cell production of these Th1-type mediators was significantly increased. Of interest is that this was most prominently visible in the absence of added IL-2 and the stimulation by adipocyte-conditioned medium to produce increased levels of Th1 cytokines became less prominent in the presence of increasing amounts of added IL-2.
Fig. 1.
Medium derived from fibroblast-derived adipocytes stimulates spleen cell production of Th1-type cytokines. Spleen cells were incubated on anti-CD3-coated plates with adipocyte-conditioned medium plus 0, 15 or 150 pg/ml IL-2. After 3 days, supernatants were collected and used to measure levels of IL-2, IFN-γ and GM-CSF. Data shown are mean values ± SEM. Significance of differences between cytokine levels in supernatants of spleen cells in the presence versus absence of adipocyte-conditioned medium is shown as: ✶ = p < 0.05; ✶✶ = p < 0.001.
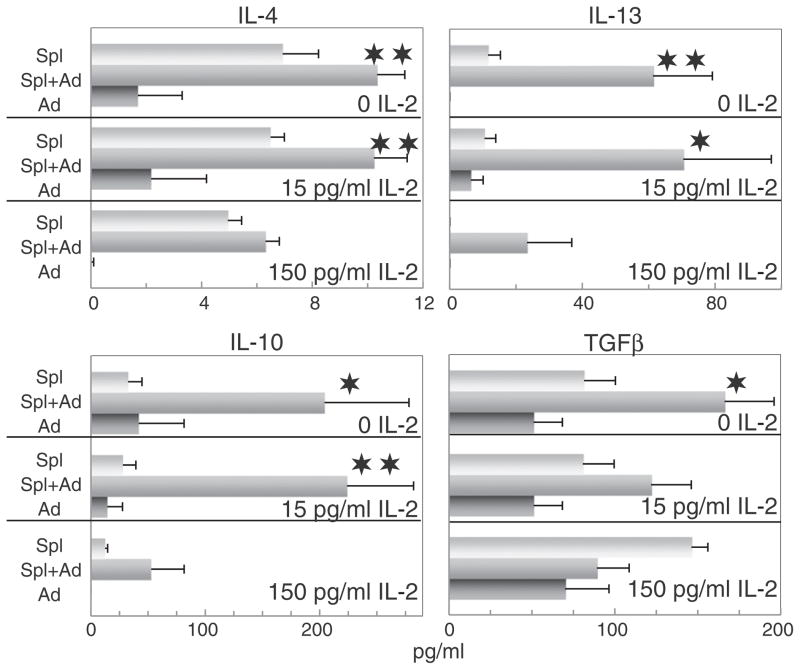
While a similar type of pattern was observed with Th2-type cytokines as was seen with the Th1-type cytokines in the presence of adipocyte-conditioned medium, the effects were more variable (Fig. 2). Levels of IL-10 and IL-13 produced by control spleen cells were relatively low. As was seen with the Th1-type cytokines, addition of adipocyte-conditioned medium increased levels of IL-10 and IL-13. In contract to that seen for IL-10 and IL-13, spleen cells produced low levels of IL-4 and, while levels of IL-4 were increased in the presence of adipocyte-conditioned medium, this appeared to simply be the additive effect of IL-4 levels in the adipocyte-conditioned medium plus the baseline produced by the spleen cells alone. This also appeared to be the case for the effect of adipocyte-conditioned medium on spleen cell production of the immune inhibitory mediator TGF-β. Also noted was that adipocyte-conditioned medium alone contained significant levels of TGF-β. In the absence of IL-2, levels of TGF-β in supernatants of spleen cells cultured with adipocyte-conditioned medium increased, although this increase tended to be more variable than was seen for the other cytokines and was likely to be the result of an additive effect of levels of TGF-β produced by spleen cells as well as the levels in the adipocyte-conditioned medium. With increasing levels of added IL-2, these increases in levels of TGF-β in medium of spleen cells that were cultured with adipocyte-conditioned medium declined. Thus, contrasting with the overall stimulatory effects of adipocyte-conditioned medium on spleen cell production of Th1-type cytokines, the effects on production of inhibitory cytokines were less consistent and more variable.
Fig. 2.
Adipocyte-conditioned medium stimulates spleen cell production of the inhibitory cytokines IL-4, IL-10, IL-13 and, to a lesser extent, TGF-β. The same experimental design was used to measure the effects of adipocyte-conditioned medium on spleen cell production of inhibitory cytokines as was described for results shown in Fig. 1 for Th1-type cytokines.
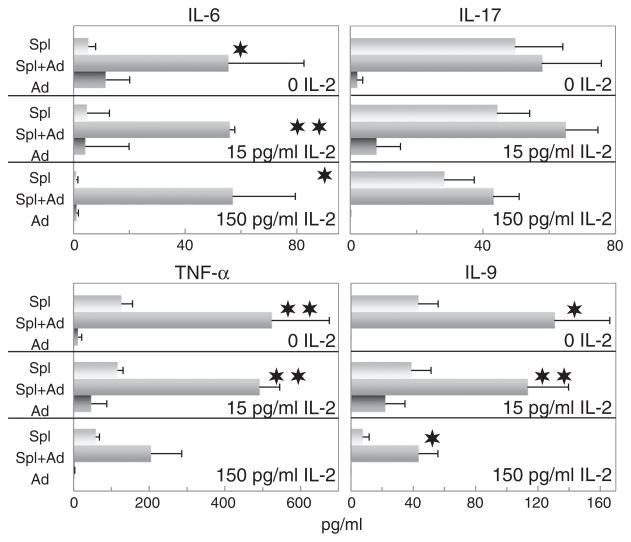
3.2. Adipocytes as stimulators of immune cell production of inflammatory mediators
While adipocytes have been shown to have the capacity to produce inflammatory mediators, the present studies determined whether they can act to exacerbate the inflammatory process by stimulating spleen cell production of inflammatory mediators. The effect of adipocyte-conditioned medium on spleen cell production of the inflammatory cytokines IL-6, IL-9 and TNF-α mirrored the effect on Th1 cytokine production (Fig. 3). Adipocytes produced minimal amounts of these cytokines, but stimulated an increased production of these inflammatory cytokines from spleen cells. Levels of IL-1α, IL-1β, and IL-12 produced by the spleen cells were minimal and addition of adipocyte supernatant did not increase production of these cytokines (not shown).
Fig. 3.
Adipocyte-conditioned medium stimulates spleen cell production of the inflammatory cytokines IL-6, TNF-α and IL-9, but not IL-17. The same experimental design was used to measure the effects of adipocyte-conditioned medium on spleen cell production of inflammatory mediators as was described for results shown in Fig. 1 for Th1-type cytokines. Levels of the pro-inflammatory cytokines IL-1α, IL-1β and IL-12 produced by the spleen cells were minimal and addition of adipocyte supernatant did not increase production of these cytokines (not shown).
In contrast to that seen for IL-6, IL-9 and TNF-α, production of IL-17 was unaffected by adipocyte-conditioned medium. IL-17 is predominantly produced by Th17 cells, which suggests the absence of adipocyte regulation of Th17 cells.
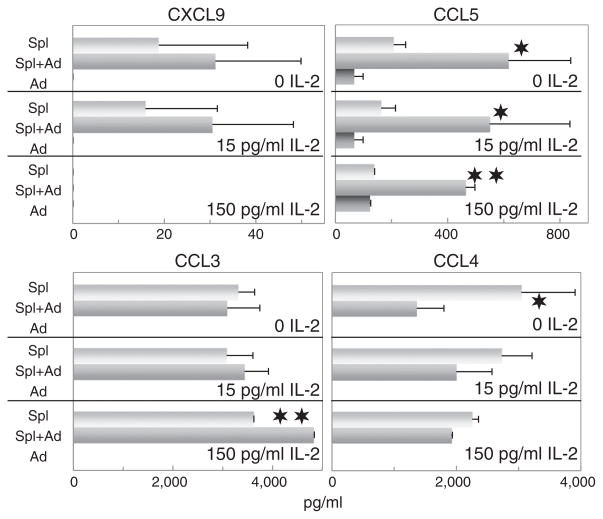
3.3. Regulation of spleen cell production of inflammatory chemokines by adipocytes
The CC chemokine family members CCL3 and CCL4 (MIP-1α and MIP-1β), and the CXC chemokine member CXCL9 (MIG) are important in inflammatory cell recruitment during inflammatory processes and were originally shown to be monocyte products. Although the results above showed that adipocyte-conditioned medium stimulated spleen cell production of inflammatory cytokines, the effect on chemokines/monokines was typically not significant (Fig. 4). Similar results were also obtained for CCL2 (not shown). In fact, in the presence of adipocyte-conditioned medium, levels of CCL4 in the cultures tended to decline. The absence of a stimulatory effect of adipocyte-conditioned medium on spleen cell production of CCL3, CCL4 and CSCL9 contrasts to the stimulatory effect seen on spleen cell production of the inflammatory cytokines IL-6, IL-9 and TNF-α.
Fig. 4.
Adipocyte-conditioned medium stimulates spleen cell production of the T-cell chemokine CCL5, but not the monokine/chemokines CXCL9, CCL2 (not shown), CCL3 or CCL4. The same experimental design was used to measure the effects of adipocyte-conditioned medium on spleen cell production of chemokines as was described for results shown in Fig. 1 for Th1 cytokines.
While the chemokines described immediately above are likely to be derived from the macrophage fraction of the spleen cell population, the chemokine CCL5 is considered to be of T-cell origin. While supernatants of spleen cells contained readily detectable levels of CCL5, these levels were significantly increased by the addition of adipocyte-conditioned medium (Fig. 4). The presence of IL-2 tended to diminish levels of CCL5 in the cultures, but this decline was not significant. Thus, in contrast to the other chemokines that were measured, T-cell production of the chemokine CCL5 was positively regulated by adipocyte-conditioned medium.
3.4. Preliminary analysis of possible adipokines that may be responsible for modulating spleen cells cytokine production
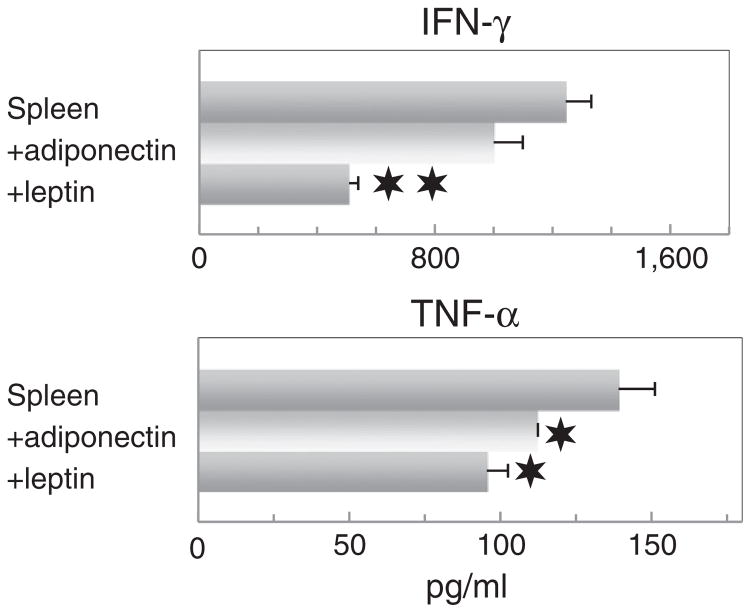
Two adipokines that are prominently produced by adipocytes include adiponectin, which tends to have anti-inflammatory properties, and leptin, which tends to have pro-inflammatory properties [32,33]. Therefore, pilot studies were initiated to determine if these two adipokines might be mediators of the adipocyte stimulation of spleen cell cytokine production. Adiponectin and leptin were added to spleen cells in lieu of adipocyte-conditioned media at levels that are comparable to those produced by the fibroblast-derived adipocytes (approximately 10 μg/ml and 30 ng/ml, respectively). Addition of adiponectin to spleen cells had an insignificant inhibitory effect on spleen cell production of a representative Th1-type cytokine, IFN-γ, and an inhibitory effect on spleen cell production of a representative inflammatory cytokine, TNF-α (Fig. 5). Leptin similarly inhibited spleen cell production of both IFN-γ and TNF-α. These results suggest that neither adiponectin nor leptin are individually responsible for the stimulatory effect of adipocyte-conditioned media on spleen cell production of IFN-γ or TNF-α.
Fig. 5.
Addition of adiponectin or leptin to spleen cells inhibits their production of IFN-γ and TNF-α. Either adiponectin (10 μg/ml) or leptin (30 ng/ml) were added to spleen cells. After 3 days, supernatants were collected and used to measure levels of the Th1 cytokine IFN-γ and the inflammatory cytokine TNF-α. Data shown are mean values ± SEM. Significance of differences between cytokine levels in supernatants of spleen cells in the presence versus absence of adiponectin or leptin is shown as: ✶ = p < 0.05; ✶✶ = p < 0.001.
3.5. Preliminary analysis of spleen cells whose cytokine production is regulated by adipocyte-conditioned medium
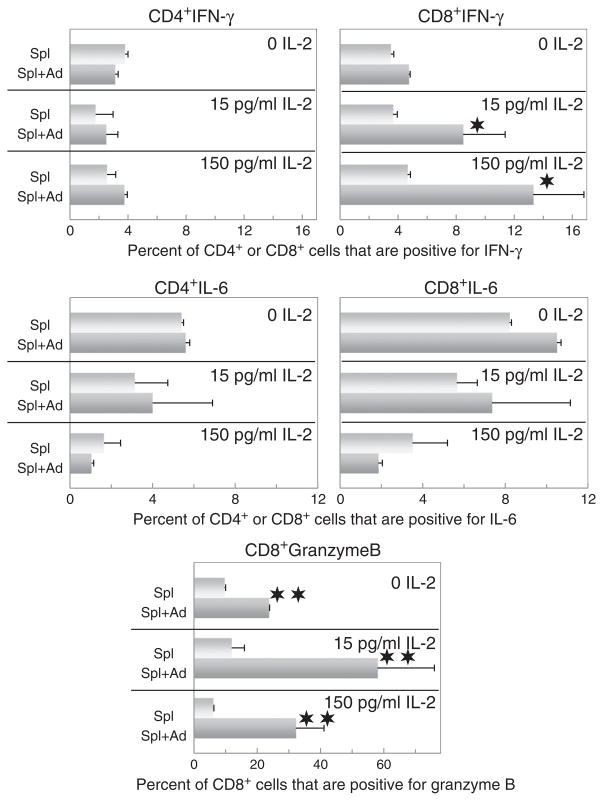
The above studies showing adipocyte-conditioned medium regulating production of a multitude of cytokines and chemokines by spleen cells prompted initial analysis to identify the source among the spleen cells for the mediators that were being upregulated. Toward that goal, spleen cells from the cultures from which the supernatants were collected for analysis were immunostained extracellularly for CD4 and CD8, and then intracellularly for select cytokines that were prominently stimulated by adipocyte-conditioned medium. This included IFN-γ and IL-6. In contrast to expectation, intracellular levels of IFN-γ were not increased in CD4+ cells that were cultured with adipocyte-conditioned medium (Fig. 6). Instead the increased levels of IFN-γ were seen in the CD8+ cells, suggesting them to be responsible for the increases in IFN-γ seen in the spleen cell supernatants. These CD4+ and CD8+ cells were also stained intracellularly for IL-6 since secretion of this inflammatory cytokine was prominently stimulated by adipocyte-conditioned medium. This immunostaining showed that intracellular staining for IL-6 was not increased in either CD4+ or CD8+ T-cells, suggesting a non-T-cell origin for this cytokine.
Fig. 6.
Adipocyte-conditioned medium stimulates CD8+ T-cell expression of IFN-γ and granzyme B, but not expression of IL-6 by either CD4+ or CD8+ T-cells, or IFN-γ by CD4+ T-cells. After 72 h culture in the presence or absence of adipocyte-conditioned medium and/or IL-2, spleen cells were treated for 4 h with PMA/ionomycin/brefeldin A solutions and then surface immunostained for CD4 and CD8. After permeabilization, cells were immunostained for intracellular expression of IFN-γ, IL-6 or granzyme B. Shown are the percent of CD4+ or CD8+-staining cells that also express either IFN-γ, IL-6 or granzyme B.
The unexpected adipocyte-stimulated increase in IFN-γ expression within CD8+ cells but not in CD4+ cells prompted inquiry into what other non-cytokine mediators might also be triggered within CD8+ cells. Therefore, the spleen cells were also immunostained intracellularly for granzyme B to determine if this also was upregulated by adipocyte-conditioned medium. As was seen for the increased expression of IFN-γ in CD8+ cells, expression of granzyme B was also prominently stimulated by the adipocyte-conditioned medium (Fig. 6). Thus, regulation of CD8+ cells by adipocytes appears not to be limited to stimulation of cytokines, but also mediators involved in the cytolytic activity of the CD8+ T-cells.
4. Discussion
Obesity and its associated health complications are significant hazards in the U.S. and an increasingly greater proportion of the population is becoming obese. Adipose tissue is considered to be in a chronic state of inflammation [7,8]. While adipose tissue and adipocytes have previously been shown to have the capacity to produce various mediators that can impact on inflammation [7], the possibility of adipocytes serving as immune regulatory cells has not been explored. Using fibroblast-derived adipocytes and normal spleen cells as a model for assessing the impact of adipocytes on conventional immune cells, the present studies indicated that adipocytes can have the capacity to skew immune cell production of Th1-type cytokines and inhibitory cytokines, as well as to stimulate production of the T-cell chemokine CCL5. The results also indicated that adipocytes could stimulate spleen cell production of select inflammatory cytokines, although not production of IL-17, which is predominantly produced by Th17 cells. In contrast, production of monokine chemokines was generally unaffected.
Studies initiated to identify the subpopulation of spleen cells whose production of cytokines was being stimulated by adipocytes revealed some expected and unexpected results. Adipocyte stimulation of spleen cell production of inflammatory cytokines such as IL-6 was expected and consistent with their own capacity to produce inflammatory mediators such as IL-1, TNF-α and IL-6 [13]. However, the increased production of IL-6 did not come from either CD4+ or CD8+ T-cells. While adipocyte-conditioned medium stimulated a strong increase in spleen cell secretion of IFN-γ, it was surprising that the CD4+ T-cell population was not whose IFN-γ production was being stimulated but, instead, the CD8+ population of T-cells was being stimulated to produce IFN-γ. This led to additional probing into the characteristics of CD8+ cells that were being stimulated, which showed a significant stimulation of CD8+ cell levels of granzyme B following exposure to adipocyte-conditioned medium. Granzyme B is involved in CD8+ cell cytolytic activity [36], but the significance of an increase in CD8+ cell granzyme B levels as it relates to obesity is unclear and yet to be explored.
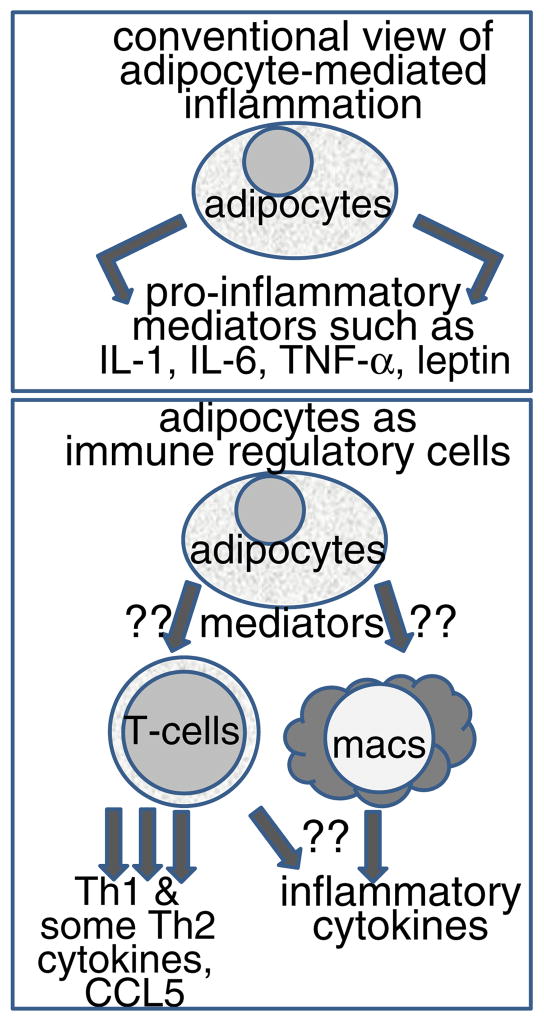
It has been previously shown that adipocytes can produce immune mediators such as IL-6 and TNF-α [13]. However, our studies suggest that their immune capacity might not be limited to directly producing immune mediators, but may include modulation of other immune cells (diagrammed in Fig. 7). Studies still need to be conducted with freshly isolated adipocytes from obese and non-obese environments so as to determine whether they too can regulate conventional immune cells and how obesity impacts on this immune regulatory capacity. While the design of the present study included stimulation of T-cells by anti-CD3, the spleen cells were unfractionated so as to include the presence of antigen-presenting cells. However, future studies will need to be conducted in a model that involves antigen presentation and antigen-specific responses to better understand how adipocytes in their immunoregulatory role can influence host defenses.
Fig. 7.
Summary showing the difference between the current views of adipocytes stimulating inflammatory responses versus their potential to serve as immune regulatory cells.
Not yet identified are the mediators through which adipocytes regulate cytokine production by conventional immune cells, although such studies were initiated. Among the prominent mediators that adipocytes produce are adiponectin and leptin, with the former having anti-inflammatory properties and the latter having inflammatory properties [32,33]. However, preliminary studies to determine the identity of the adipocyte-derived mediators that regulate conventional immune cell production of cytokines suggested that neither adiponectin nor leptin alone stimulated spleen cell cytokine production as was seen with adipocyte-conditioned media. Other possible adipokines that might be candidates are resistin and adipsin, which increase in concentration in obesity [11,12]. In reality, the balance in the levels of adipocyte-derived mediators may be more important than the absolute quantity of individual mediators.
The biological impact of the immune modulatory potential of adipocytes is expected to be diverse based on the multitude of disorders that are co-associated with obesity and cytokines. Chronic persistent inflammation has an important role in the disease pathology of cancer, diabetes, cardiovascular disease, and metabolic syndrome. The capacity of adipocytes to stimulate conventional immune cell production of inflammatory mediators could be a contributor to the association between obesity and an increased risk of cancer. Cancer-related inflammation is an important step in the process toward malignant disease [37,38]. Obese patients have a higher probability of developing cancers such as hepatic cancer [39]. Women who are obese have a higher risk of developing premalignant or malignant endometrial polyps [40]. Carcinogen-induced development of premalignant colonic lesions in mice is increased in obese mice [41]. Following irradiation or chemotherapy, there is an increase in adipocytes within bone marrow, which affects the frequency of hematopoietic stem cells and the capacity of immune repopulation of the bone marrow [42]. Immune capability can be impaired in other instances where adipocyte levels are increased in the bone marrow such as a result of vitamin C deficiency and aging [43,44]. Diabetes is another disorder that is associated with obesity. Increased levels of cytokines such as IL-6 and IL-1β have been shown to increase the risk for type 2 diabetes [45]. Inflammatory cytokine levels increase with obesity and have been associated with increased insulin resistance [14,46]. Overweight individuals with metabolic syndrome, which is frequently associated with obesity, have increased circulating levels of cytokines [47]. Because of the multitude of health conditions that are precipitated by obesity and the concurrent increases in cytokine levels, efforts have been initiated to temper, not just obesity, but also the cytokine-mediated inflammatory and immune responses. This includes dietary weight loss approaches, gastric bypass surgery, treatment of patients having insulin resistance ω-3 polyunsaturated fatty acids, and even immunological approaches such as adoptive transfer of regulatory invariant NKT cells [12,48,49]. Additional approaches include use of pharmacological compounds, such as metformin or the seaweed extract fucoidan, to reduce inflammation and cytokine levels so as to, in turn, reduce the complications of obesity [50,51]. These results underscore the immune regulatory importance of adipocytes in their contribution to the complications that are associated with obesity.
5. Conclusion
Using a model for adipocytes and conventional immune cells, the present study indicates that, in addition to being able to produce inflammatory mediators, adipocytes may also have a regulatory capacity to stimulate conventional immune cell production of cytokines and inflammatory mediators. The mechanisms by which adipocytes regulate immune cell cytokine production have not yet been defined, but are not mediated by adiponectin or leptin individually. These studies suggest an additional means by which adipocytes interface with the conventional immune system.
Acknowledgments
This work has been supported by awards from the Biomedical Laboratory and Clinical Sciences Programs of the Department of Veterans Affairs and by grants from the National Institute of Health (MRIY, I01-CX000100, R01-CA128837 and R01-DE018268).
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
Contributor Information
Silvana A. Vielma, Email: vielmasa@musc.edu.
Richard L. Klein, Email: kleinrl@musc.edu.
Corinne A. Levingston, Email: cos25@musc.edu.
M. Rita I. Young, Email: Rita.Young@va.gov.
References
- 1.Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med. 2013;64:45–57. doi: 10.1146/annurev-med-121211-091527. [DOI] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention. Diabetes Data & Trends. http://appsnccdcdcgov/DDTSTRS/defaultaspx2012.
- 3.LeRoith D, Novosyadlyy R, Gallagher EJ, Lann D, Vijayakumar A, Yakar S. Obesity and type 2 diabetes are associated with an increased risk of developing cancer and a worse prognosis; epidemiological and mechanistic evidence. Exp Clin Endocrinol Diabetes. 2008;116:S4–6. doi: 10.1055/s-2008-1081488. [DOI] [PubMed] [Google Scholar]
- 4.Wagner IJ, Szpalski C, Allen RJ, Jr, Davidson EH, Canizares O, Saadeh PB, et al. Obesity impairs wound closure through a vasculogenic mechanism. Wound Repair Regen. 2012;20:512–22. doi: 10.1111/j.1524-475X.2012.00803.x. [DOI] [PubMed] [Google Scholar]
- 5.Levine R. Obesity and oral disease - a challenge for dentistry. Br Dent J. 2012;213:453–6. doi: 10.1038/sj.bdj.2012.1009. [DOI] [PubMed] [Google Scholar]
- 6.Thompson D, Wolf AM. The medical-care cost burden of obesity. Obes Rev. 2001;2:189–97. doi: 10.1046/j.1467-789x.2001.00037.x. [DOI] [PubMed] [Google Scholar]
- 7.Meijer K, de Vries M, Al-Lahham S, Bruinenberg M, Weening D, Dijkstra M, et al. Human primary adipocytes exhibit immune cell function: adipocytes prime in-flammation independent of macrophages. PLoS One. 2011;6:e17154. doi: 10.1371/journal.pone.0017154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–38. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 9.Vona-Davis L, Rose DP. Angiogenesis, adipokines and breast cancer. Cytokine Growth Factor Rev. 2009;20:193–201. doi: 10.1016/j.cytogfr.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 11.Kwon EY, Shin SK, Cho YY, Jung UJ, Kim E, Park T, et al. Time-course microarrays reveal early activation of the immune transcriptome and adipokine dysregulation leads to fibrosis in visceral adipose depots during diet-induced obesity. BMC Genomics. 2012;13:450. doi: 10.1186/1471-2164-13-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Pamuklar Z, Spagnoli A, Torquati A. Serum leptin levels are inversely correlated with omental gene expression of adiponectin and markedly decreased after gastric bypass surgery. Surg Endosc. 2012;26:1476–80. doi: 10.1007/s00464-011-2059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faber DR, Kalkhoven E, Westerink J, Bouwman JJ, Monajemi HM, Visseren FL. Conditioned media from (pre)adipocytes stimulate fibrinogen and PAI-1 production by HepG2 hepatoma cells. Nutr Diabetes. 2012;2:e52. doi: 10.1038/nutd.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladefoged M, Buschard K, Hansen AM. Increased expression of toll-like receptor 4 and inflammatory cytokines, interleukin-6 in particular, in islets from a mouse model of obesity and type 2 diabetes. APMIS. doi: 10.1111/apm.12018. Epub 09/11/2012. [DOI] [PubMed] [Google Scholar]
- 15.Wang SN, Wang ST, Lee KT. The potential interplay of adipokines with toll-like receptors in the development of hepatocellular carcinoma. Gastroenterol Res Pract. 2011;2011:215986. doi: 10.1155/2011/215986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer. 2007;14:189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- 17.Paz-Filho G, Lim E, Wong M, Licinio J. Associations between adipokines and obesity-related cancer. Front Biosci. 2011;16:1634–50. doi: 10.2741/3810. [DOI] [PubMed] [Google Scholar]
- 18.Moon HS, Chamberland JP, Aronis K, Tseleni-Balafouta S, Mantzoros CS. Direct role of adiponectin and adiponectin receptors in endometrial cancer: in vitro and ex vivo studies in humans. Mol Cancer Ther. 2011;10:2234–43. doi: 10.1158/1535-7163.MCT-11-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kharroubi I, Rasschaert J, Eizirik DL, Cnop M. Expression of adiponectin receptors in pancreatic beta cells. Biochem Biophys Res Commun. 2003;312:1118–22. doi: 10.1016/j.bbrc.2003.11.042. [DOI] [PubMed] [Google Scholar]
- 20.Cheng SP, Liu CL, Hsu YC, Chang YC, Huang SY, Lee JJ. Expression and biologic significance of adiponectin receptors in papillary thyroid carcinoma. Cell Biochem Biophys. 2012 doi: 10.1007/s12013-012-9419-1. [Epub 08/22/2012] [DOI] [PubMed] [Google Scholar]
- 21.Vaira S, Yang C, McCoy A, Keys K, Xue S, Weinstein EJ, et al. Creation and preliminary characterization of a leptin knockout rat. Endocrinology. 2012;153:5622–8. doi: 10.1210/en.2012-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dredge K, Marriott JB, Todryk SM, Dalgleish AG. Adjuvants and the promotion of Th1-type cytokines in tumour immunotherapy. Cancer Immunol Immunother. 2002;51:521–31. doi: 10.1007/s00262-002-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cousins DJ, Lee TH, Staynov DZ. Cytokine coexpression during human Th1/Th2 cell differentiation: direct evidence for coordinated expression of Th2 cytokines. J Immunol. 2002;169:2498–506. doi: 10.4049/jimmunol.169.5.2498. [DOI] [PubMed] [Google Scholar]
- 24.Sato M, Goto S, Kaneko R, Ito M, Sato S, Takeuchi S. Impaired production of Th1 cytokines and increased frequency of Th2 subsets in PBMC from advanced cancer patients. Anticancer Res. 1998;18:3951–5. [PubMed] [Google Scholar]
- 25.Li H, Edin ML, Bradbury JA, Graves JP, Degraff LM, Gruzdev A, et al. COX-2 inhibits Th9 differentiation during allergic lung inflammation via downregulation of IL-17RB. Am J Respir Crit Care Med. doi: 10.1164/rccm.201211-2073OC. Epub 02/28/2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gnerlich JL, Mitchem JB, Weir JS, Sankpal NV, Kashiwagi H, Belt BA, et al. Induction of Th17 cells in the tumor microenvironment improves survival in a murine model of pancreatic cancer. J Immunol. 2010;185:4063–71. doi: 10.4049/jimmunol.0902609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulos CM, Carpenito C, Plesa G, Suhoski MM, Varela-Rohena A, Golovina TN, et al. The inducible costimulator (ICOS) is critical for the development of human TH17 cells. Sci Transl Med. 2010;2:55ra78. doi: 10.1126/scitranslmed.3000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedke T, Pretsch L, Karakhanova S, Enk AH, Mahnke K. Endothelial cells augment the suppressive function of CD4+ CD25+ Foxp3+ regulatory T cells: involvement of programmed death-1 and IL-10. J Immunol. 2010;184:5562–70. doi: 10.4049/jimmunol.0902458. [DOI] [PubMed] [Google Scholar]
- 30.Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174:6820–8. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- 31.Martin-Gonzalez J, Sanchez-Jimenez F, Perez-Perez A, Carmona-Fernandez A, Sanchez-Margalet V, Segura-Egea JJ. Leptin expression in healthy and inflamed human dental pulp. Int Endod J. doi: 10.1111/iej.12009. Epub 09/13/2012. [DOI] [PubMed] [Google Scholar]
- 32.Canavan B, Salem RO, Schurgin S, Koutkia P, Lipinska I, Laposata M, et al. Effects of physiological leptin administration on markers of inflammation, platelet activation, and platelet aggregation during caloric deprivation. J Clin Endocrinol Metab. 2005;90:5779–85. doi: 10.1210/jc.2005-0780. [DOI] [PubMed] [Google Scholar]
- 33.Li S, Bao HG, Han L, Liu L, Wang X. Effects of adiponectin on mortality and its mechanism in a sepsis mouse model. J Invest Surg. 2012;25:214–9. doi: 10.3109/08941939.2011.624257. [DOI] [PubMed] [Google Scholar]
- 34.Lee MH, Hammad SM, Semler AJ, Luttrell LM, Lopes-Virella MF, Klein RL. HDL3, but not HDL2, stimulates plasminogen activator inhibitor-1 release from adipocytes: the role of sphingosine-1-phosphate. J Lipid Res. 2010;51:2619–28. doi: 10.1194/jlr.M003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Costa AM, Schuyler CA, Walker DD, Young MR. Characterization of the evolution of immune phenotype during the development and progression of squamous cell carcinoma of the head and neck. Cancer Immunol Immunother. 2012;61:927–39. doi: 10.1007/s00262-011-1154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curtsinger JM, Lins DC, Johnson CM, Mescher MF. Signal 3 tolerant CD8 T cells degranulate in response to antigen but lack granzyme B to mediate cytolysis. J Immunol. 2005;175:4392–9. doi: 10.4049/jimmunol.175.7.4392. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu M, Shirakami Y, Iwasa J, Shiraki M, Yasuda Y, Hata K, et al. Supplementation with branched-chain amino acids inhibits azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Clin Cancer Res. 2009;15:3068–75. doi: 10.1158/1078-0432.CCR-08-2093. [DOI] [PubMed] [Google Scholar]
- 38.Erdman SE, Poutahidis T. Roles for inflammation and regulatory T-cells in colon cancer. Toxicol Pathol. 2010;38:76–87. doi: 10.1177/0192623309354110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka K, Tsuji I, Tamakoshi A, Matsuo K, Ito H, Wakai K, et al. Obesity and liver cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2012;42:212–21. doi: 10.1093/jjco/hyr198. [DOI] [PubMed] [Google Scholar]
- 40.Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. 2013;33:S79–84. doi: 10.1007/s10875-012-9847-0. [DOI] [PubMed] [Google Scholar]
- 41.Guazzone VA, Jacobo P, Theas MS, Lustig L. Cytokines and chemokines in testicular inflammation: a brief review. Microsc Res Tech. 2009;72:620–8. doi: 10.1002/jemt.20704. [DOI] [PubMed] [Google Scholar]
- 42.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–63. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park JK, Lee EM, Kim AY, Lee EJ, Min CW, Kang KK, et al. Vitamin C deficiency accelerates bone loss inducing an increase in PPAR-g expression in SMP30 knockout mice. Int J Exp Pathol. 2012;93:332–40. doi: 10.1111/j.1365-2613.2012.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saidak Z, Hay E, Marty C, Barbara A, Marie PJ. Strontium ranelate rebalances bone marrow adipogenesis and osteoblastogenesis in senescent osteopenic mice through NFATc/Maf and Wnt signaling. Aging Cell. 2012;11:467–74. doi: 10.1111/j.1474-9726.2012.00804.x. [DOI] [PubMed] [Google Scholar]
- 45.Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European prospective investigation into cancer and nutrition (EPIC)-potsdam study. Diabetes. 2003;52:812–7. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 46.Stoppa-Vaucher S, Dirlewanger MA, Meier CA, de Moerloose P, Reber G, Roux-Lombard P, et al. Inflammatory and prothrombotic states in obese children of European descent. Obesity. 2012;20:1662–8. doi: 10.1038/oby.2012.85. [DOI] [PubMed] [Google Scholar]
- 47.Hardy OT, Kim A, Ciccarelli C, Hayman LL, Wiecha J. Increased Toll-like receptor (TLR) mRNA expression in monocytes is a feature of metabolic syndrome in adolescents. Pediatr Obes. 2013;8:e19–23. doi: 10.1111/j.2047-6310.2012.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spencer M, Finlin BS, Unal R, Zhu B, Morris AJ, Shipp LR, et al. Omega-3 fatty acids reduce adipose tissue macrophages in human subjects with insulin resistance. Diabetes. doi: 10.2337/db12-1042. Epub 01/17/2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–87. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim KJ, Lee BY. Fucoidan from the sporophyll of Undaria pinnatifida suppresses adipocyte differentiation by inhibition of inflammation-related cytokines in 3T3-L1 cells. Nutr Res. 2012;32:439–47. doi: 10.1016/j.nutres.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Evia-Viscarra ML, Rodea-Montero ER, Apolinar-Jimenez E, Munoz-Noriega N, Garcia-Morales LM, Leanos-Perez C, et al. The effects of metformin on inflammatory mediators in obese adolescents with insulin resistance: controlled randomized clinical trial. J Pediatr Endocrinol Metab. 2012;25:41–9. doi: 10.1515/jpem-2011-0469. [DOI] [PubMed] [Google Scholar]