Abstract
Human aging is associated with decline in cognitive and physical functioning. Although pulmonary function predicts long-term performance (up to 10 years) on measures of cognitive function, recent data suggest the opposite relationship: Cognitive decline predicts self-reported physical limitations. In the study reported here, we utilized dual-change-score models to determine the directional relationship between pulmonary and cognitive function. Our sample consisted of 832 participants (ages 50–85 years at baseline), who were assessed in up to seven waves of testing across 19 years as part of the longitudinal Swedish Adoption/Twin Study of Aging. Changes in pulmonary function led to subsequent changes in fluid cognitive function, specifically, in tasks reflecting psychomotor speed and spatial abilities. There was no evidence that declines in cognitive function led to subsequent declines in pulmonary function. Thus, these data indicate a directional relationship from decreased pulmonary function to decreased cognitive function, a finding that underscores the importance of maintaining pulmonary function to ensure cognitive performance.
Keywords: pulmonary function, cognitive function, dual-change-score model, longitudinal model, cognitive ability, statistical analysis, aging, health
Cognitive decline with increasing age among healthy older adults has been well-documented and extensively studied (Salthouse, 2004; Schaie, 1994). Early studies of aging processes have characterized cognitive decline as a component of global age-related behavioral slowing (Birren, 1974), and recent studies have documented cognitive decline associated with a number of different age-related chronic health conditions, including cardiovascular disease (Verhaeghen, Borchelt, & Smith, 2003), chronic obstructive pulmonary disease (Incalzi et al., 1998), and hypertension (Waldstein, Brown, Maier, & Katzel, 2005). Thus, declines in physical function and cognitive function have been associated with the aging process, but few studies have attempted to evaluate whether there is a causal relationship between these functions or to determine a direction of causality.
Pulmonary function, as measured with standardized spirometric assessment, has been utilized as an objective indicator of physical function in studies evaluating the relationship of physical function and cognitive function. Spirometric-testing outcomes—especially forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC)—are commonly used measures of lung capacity and lung responsivity that may reflect pathophysiological changes in the lungs occurring normally with age. FEV1 is the most commonly utilized indicator of pulmonary function in studies investigating the relationship of pulmonary function and cognitive function. Population-based studies have documented that pulmonary function is a longitudinal predictor of cognitive function among older adults (Albert et al., 1995; Chyou et al., 1996; Emery, Pedersen, Svartengren, & McClearn, 1998), as well as a cross-sectional and longitudinal predictor among middle-aged and young adults (Anstey, Windsor, Jorm, Christensen, & Rodgers, 2004; Emery, Huppert, & Schein, 1997; Richards, Strachan, Hardy, Kuh, & Wadsworth, 2005). Pulmonary function typically has been associated with measures of fluid cognitive performance (e.g., problem-solving ability, psychomotor speed, sequencing of information) rather than measures of accumulated crystallized knowledge (e.g., retrieval of information from long-term memory). Data from these population-based studies, in turn, are consistent with data documenting increased dementia risk associated with lower levels of pulmonary function (Schaub et al., 2000).
Despite documentation of longitudinal associations between cognitive function and pulmonary function, most studies have not evaluated whether changes in pulmonary function precede changes in cognitive function or vice versa. Causal hypotheses were addressed in one relatively recent prospective, population-based study (N = 1,778) in Great Britain (Richards et al., 2005). Results of that study indicated that FEV1 was associated with psychomotor speed among middle-aged adults (43 years old) and that FEV1 predicted change in psychomotor speed over a 10-year span (from age 43 to 53). In addition, cognitive ability at age 15 predicted FEV1 at age 43 but did not predict change in FEV1 during the subsequent decade (age 43 to 53). It was concluded that pulmonary function did not have a causal influence on cognitive function; instead, pulmonary function and cognitive function were thought to covary over the life span. However, the causal question remains to be further examined because this study did not include a measure of pulmonary function at the earliest assessment (age 15) and because analyses did not evaluate cognitive function in midlife as a predictor of change in pulmonary function at midlife.
To address the question of cause and effect, it is necessary to evaluate the extent to which each component (pulmonary function and cognitive function) predicts the other component over time using structural equation models that allow for dynamic interaction between the two components. The development of dual-change-score models (DCSMs) to characterize age changes has facilitated specification and testing of dynamic hypotheses about cognitive aging (McArdle, 2001; McArdle & Hamagami, 2003; McArdle, Hamagami, Meredith, & Bradway, 2000). These models assist with identification of leading indicators of cognitive change by measuring the extent to which changes in one variable influence subsequent changes in a second, related variable.
In a recent population-based study of healthy older adults (mean age = 76.2 years) that utilized DCSMs, it was found that episodic memory (as measured by immediate and free recall of a list of 10 words) predicted self-reported functional limitations (e.g., using the telephone, taking medications, shopping, cooking, walking), but functional limitations did not predict memory (Infurna, Gerstorf, Ryan, & Smith, 2011). This study utilized advanced statistical procedures but relied on a self-report measure of physical functioning and incorporated only one aspect of cognitive function (i.e., memory). The purpose of the present study was to apply DCSMs to data from the longitudinal Swedish Adoption/Twin Study of Aging (SATSA) to evaluate directionality in the relationship of pulmonary function, a widely regarded objective indicator of physical functioning, and four components of cognitive performance.
Method
Participants
Participant data for the present study were obtained from the SATSA sample, which is a subset of twins from the population-based Swedish Twin Registry (Finkel & Pedersen, 2004). Testing at Wave 1 took place in person at a location convenient to the participants, such as district nurses’ offices, health-care schools, and long-term-care clinics. Testing was completed during a single 4-hr visit. The second and third waves of in-person testing occurred at 3-year intervals. In-person testing did not occur during Wave 4; the next in-person testing occurred at Wave 5, after a 7-year interval (see Finkel & Pedersen, 2004). Regular 3-year testing continued after Wave 5; therefore, the total time span from Wave 1 to Wave 7 was 19 years.
Participant data were included from all available time points. Dementia status at each wave was determined by clinical diagnosis based on current diagnostic criteria (Gatz et al., 1997), and data were retained in analyses for all available assessments prior to any dementia diagnosis. In total, 832 individuals had cognitive and pulmonary data available from at least one testing occasion. Of those participants, 67% had data from three or more time points, and 12% participated in all six waves. Fifty-nine percent of participants were women, and 41% were men. Table 1 presents descriptive statistics at each wave. Because SATSA has a cohort-sequential design, new participants were added at Waves 2 through 5, and some participants were lost to attrition. Statistical modeling accounts for missing data by giving more weight to participants with more time points of data.
Table 1.
Means for the Two Pulmonary Measures and the Four Cognitive Factors at Each Wave
| Wave | N | Age (years) | Cognitive factors
|
Pulmonary measures
|
||||
|---|---|---|---|---|---|---|---|---|
| Verbal ability | Spatial ability | Memory | Processing speed | FEV1 | FVC | |||
| Wave 1 | 598 | 65.3 (8.3) | 50.0 (10.0) | 50.0 (10.0) | 50.0 (10.0) | 50.0 (10.0) | 2.22 (0.71) | 2.67 (0.85) |
| Wave 2 | 500 | 65.9 (8.9) | 51.7 (9.2) | 51.5 (11.0) | 51.3 (10.1) | 50.8 (9.9) | 2.24 (0.72) | 2.73 (0.86) |
| Wave 3 | 482 | 68.8 (9.2) | 52.2 (9.7) | 51.4 (10.8) | 51.9 (10.6) | 51.2 (10.8) | 2.21 (0.75) | 2.45 (0.80) |
| Wave 5 | 470 | 70.6 (10.0) | 53.3 (10.0) | 51.5 (11.0) | 51.0 (10.1) | 49.3 (10.9) | 2.09 (0.74) | 2.26 (0.81) |
| Wave 6 | 380 | 72.2 (9.3) | 54.3 (9.3) | 51.6 (11.0) | 51.5 (9.7) | 50.3 (11.3) | 2.04 (0.72) | 2.14 (0.78) |
| Wave 7 | 302 | 74.3 (9.0) | 55.2 (9.3) | 54.5 (11.2) | 52.6 (9.6) | 51.1 (11.5) | 2.01 (0.74) | 2.28 (0.93) |
| Participants with cognitive and pulmonary data from at least one wave | 832 | 69.0 (9.6) | 52.1 (9.8) | 51.1 (10.5) | 51.1 (10.1) | 50.3 (10.6) | 2.15 (0.73) | 2.44 (0.87) |
Note: For the cognitive factors, the table presents T scores; for forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC), mean uncorrected values (in liters) are expressed in body temperature and pressure saturated (BTPS) with water vapor. Standard deviations are given in parentheses. No data are reported for Wave 4 because in-person testing was not conducted during that wave.
To maintain consistency with the 3-year testing interval and to maximize the age range available for inclusion in the study, we divided data into thirteen 3-year age intervals from age 50 to 86. However, the data for the cognitive factors were too sparse after age 86 to support statistical modeling (i.e., samples contained less than 15 participants); therefore, only data up to age 86 were included in these analyses.
Measures
Cognitive performance
Four cognitive domains are represented in the SATSA cognitive test battery (see Nesselroade, Pedersen, McClearn, Plomin, & Bergeman, 1988; Pedersen, Plomin, Nesselroade, & McClearn, 1992). Verbal abilities, reflecting primarily crystallized intelligence, are tapped by tests of Information, Synonyms, and Analogies. The other three domains reflect components of fluid intelligence. Block Design and Card Rotations tests assess spatial abilities, memory tests include Digit Span and Picture Memory, and Digit Symbol and Figure Identification tests measure processing speed (see Pedersen et al., 1992, for original sources of all cognitive tests). Reliabilities for these tests range from .82 to .96 (Pedersen et al., 1992).
Principal component analysis was used to construct latent factors from the individual tests within each domain: verbal ability, spatial ability, memory, and processing speed. Factor loadings ranged from .79 to .92. Previous comparisons of factor structure between cohorts and across testing occasions indicated that the factor structure did not vary systematically across age or time (Finkel, Reynolds, McArdle, & Pedersen, 2005). To avoid variance in measurement (cf. Wicherts et al., 2004), we created an invariant definition of factors at each testing occasion by standardizing the cognitive measures relative to the respective means and variances at Wave 1. Then, loadings from the factor analyses conducted at Wave 1 were used to construct the verbal, spatial, memory, and speed factors. For visual interpretation, all factor scores were later transformed to T scores using factor means and variances from Wave 1. Mean T scores at each wave are included in Table 1.
Pulmonary functioning
Spirometric testing was performed during in-person testing on one of two intercalibrated portable 10-1 dry bellows Vicatest spirometers (Mijnhardt, Bunnik, The Netherlands) with subjects in a seated position and their nasal passages blocked with nose clips. Two measures of pulmonary function were collected: FEV1 and FVC. At Wave 1 and Wave 7, only one trial was collected. During Waves 2 through 6, two spirometric trials were completed, and data from the best trial were used in the present analyses. During the course of the study, it became necessary to change spirometric equipment because of the increasing difficulty of transporting the Vicatest spirometers and the availability of new equipment that was lighter and easier for the nurses to use. Thus, at Wave 3, pulmonary function for 30% of the subjects was measured using the Vicatest, and the remaining subjects were assessed with a portable ML 330 spirometer (Micor Medical, Kent, England). The two spirometers were intercalibrated to ensure consistent measurement. FEV1 and FVC values for both spirometers were expressed in body temperature and pressure saturated (BTPS) with water vapor. Mean uncorrected FEV1 and FVC at each testing wave is reported in Table 1. For the purposes of analysis, FEV1 and FVC were corrected for height and gender, and the standardized score was then transformed to the T score metric for ease of interpretation.
Statistical method
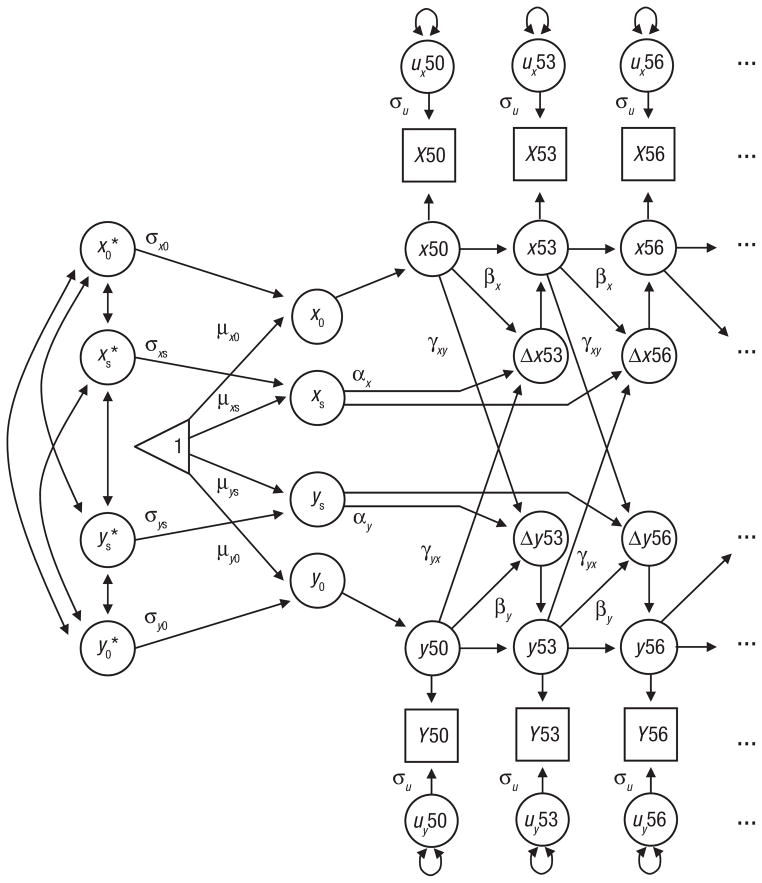
DCSMs were utilized to examine age-related changes in cognitive performance and pulmonary functioning both independently and as part of bivariate relationships. Extensive discussions of the model are available (McArdle, 2001; McArdle & Hamagami, 2003; McArdle et al., 2004), as well as comparisons of DCSMs with latent-growth-curve models (Ghisletta & de Ribaupierre, 2005; Lövdén, Ghisletta, & Lindenberger, 2005). As can be seen in Figure 1, the model is based on latent difference scores that create a growth curve based not on performance at a single age but on change from one age to another age (Δy), which is modeled as a function of both constant change (α) that accumulates over time in an additive fashion and proportional change (β) based on the previous score. In the full DCSM, α is set to 1, and the parameter β differs from 0 to the extent that the longitudinal change is nonlinear. The bivariate DCSM allows for a coupling mechanism (γ), where change in trait x depends on the previous value of y, and vice versa. Because previous analyses of data from SATSA have revealed minimal practice effects, retest was not included in the statistical models.
Fig. 1.
Bivariate dual-change-score model used in this study. Alpha α) indicates the linear change (with age, and β captures proportional (nonlinear) change. Cross-trait dynamics are indicated by β. Intercept (x0, y0) and slope (xs, ys) parameters are estimated, as are their mean (μ) and standard deviation (σ). Asterisks indicate the standardized scores of intercepts and slopes. The unique variance at each age bracket not captured by the model is represented by σu. Observed scores are represented by boxes with capital letters. Latent scores are represented by circles with lowercase letters. The numbers 50, 53, and 56 refer to participants’ age at each measurement occasion.
In this structural-equation-model formulation, it is possible to evaluate dynamic hypotheses about the temporal order of changes in variables through restrictions on model parameters. For that reason, we created five models. In Model 1, the relationship between the two variables was bidirectional, such that x affected changes in y, and y affected changes in x (i.e., both γyx and γxy were not equal to 0). Models 2 and 3 tested the dynamic relationship functions in one direction only, either with x as a leading indicator of change in y (i.e., γyx = 0) or with changes in y preceding changes in x (i.e., γxy = 0). In contrast, Model 4 was used to determine whether the crossvariable dynamic effects were equivalent (i.e., γxy = γyx). Finally, in the most reduced model (Model 5), no dynamic coupling between the variables was included (i.e., γxy = γyx = 0).
It is important to note that one of the fundamental assumptions underlying DCSMs is that data are missing at random. Previous investigations of SATSA data have suggested that, on such factors as personality ratings (Pedersen & Reynolds, 1998) and cognitive ability (Dominicus, Palmgren, & Pedersen, 2006), participants who continue in the study are significantly different from those who drop out. Of most importance in age-based DCSMs is demonstrating that the pattern of missing data does not differ for older and younger participants. Cohort comparisons of missing data (Finkel, Reynolds, McArdle, & Pedersen, 2007) indicate that the patterns of participation are fairly similar, although older participants (71%) are somewhat more likely than younger participants (64%) to participate in at least three time points simply as a result of a greater number of opportunities for participation.
Univariate and bivariate DCSMs were fit to the data using Mplus (Muthén & Muthén, 2005). Model fit was indicated by the −2 log likelihood (−2LL) and the root mean square error of approximation (RMSEA; Browne & Cudeck, 1993). Adequate fit of the full model to the data is indicated when the RMSEA is less than or equal to .1, and an RMSEA of .05 or less indicates close fit. Hypotheses were tested by comparing model fit indices; nested models were compared using the difference chi-square test obtained by taking the difference between the obtained model fits (−2LL) and testing the significance of the chi-square test with the degrees of freedom equal to the difference in the number of parameters of the two models. Given the number of model comparisons conducted, the significance level was set at .01 to reduce the likelihood of Type I error. The current analyses focused on individual performance by including a correction for relatedness of twin pairs in the modeling (i.e., twin pairness was coded and included in models). Furthermore, all models were run in two subsamples, composed of one member of each pair, to confirm consistency with full sample results.
Results
Univariate analyses
In the first step of the analysis, the univariate DCSM was fit separately to the four cognitive factors and the two measures of lung function to verify the shape of the change trajectory across ages and to provide starting values for the bivariate DCSM. Two models were fit to the data for each measure: a full model and a reduced model, in which β was set to 0. Parameter estimates resulting from fitting the full model are presented in Table 2, along with fit statistics and the results of testing the reduced model. The fit statistics demonstrate that the univariate DCSM provides at least an adequate fit to the data for all six measures. Fit statistics for the reduced model testing nonlinear change are provided in the last row of Table 2. For all four cognitive factors, removing β from the model resulted in a significant reduction in model fit, which indicates that the change trajectory for each factor was nonlinear. Removing β from the model did not result in a significant change in fit for either measure of lung function, which indicates that in this age range, decline in both FEV1 and FVC is primarily linear.
Table 2.
Parameter Estimates and Fit Statistics From the Univariate Dual-Change-Score Model
| Parameter | Cognitive factors
|
Pulmonary measures
|
||||
|---|---|---|---|---|---|---|
| Verbal ability | Spatial ability | Memory | Processing speed | FEV1 | FVC | |
| Constant change (α) | 1 | 1 | 1 | 1 | 1 | 1 |
| Proportional change (β) | 0.41 (0.06) | 0.16 (0.02) | 0.23 (0.04) | 0.12 (0.02) | −0.03 (0.02) | −0.06 (0.03) |
| Mean intercept (μ0) | 52.44 (0.36) | 55.10 (0.47) | 53.34 (0.45) | 57.17 (0.45) | 55.58 (0.56) | 55.50 (0.67) |
| Mean slope (μs) | −21.56 (3.28) | −9.72 (1.16) | −12.51 (2.07) | −7.86 (0.79) | 0.32 (1.11) | 2.01 (1.55) |
| Intercept deviation (σ0) | 87.46 (4.90) | 88.25 (6.00) | 75.81 (5.62) | 67.52 (5.16) | 72.57 (7.38) | 60.12 (9.03) |
| Slope deviation (σs) | 14.80 (4.55) | 2.56 (0.67) | 4.34 (1.38) | 1.27 (0.28) | −0.06 (0.21) | 0.51 (0.28) |
| Intercept-slope correlation (ρs0) | −.99 (.18) | −.99 (.20) | −.98 (.24) | −.95 (.20) | −.06 (.21) | .30 (.23) |
| Error deviation (σu) | 9.21 (0.34) | 17.41 (0.66) | 26.49 (0.93) | 17.69 (0.66) | 23.36 (0.84) | 36.75 (1.50) |
| Misfit index: −2LL (7 parameters) | −8,343 | −8,569 | −9,560 | −9,166 | −9,395 | −8,344 |
| Root mean square error of approximation | .03 [.02, .04] | .03 [.02, .04] | .03 [.02, .04] | .03 [.02, .04] | .03 [.02, .04] | .03 [.02, .04] |
| Misfit index with β = 0: −2LL (6 parameters)a | −8,385* | −8,598* | −9,582* | −9,199* | −9,396 | −8,345 |
Note: Standard errors are given in parentheses; 90% confidence intervals are given in brackets. FEV1 = forced expiratory volume in 1 s; FVC = forced vital capacity; LL = log likelihood.
Asterisks indicate that the change in model fit compared with the fit of the full model was significant (p < .01).
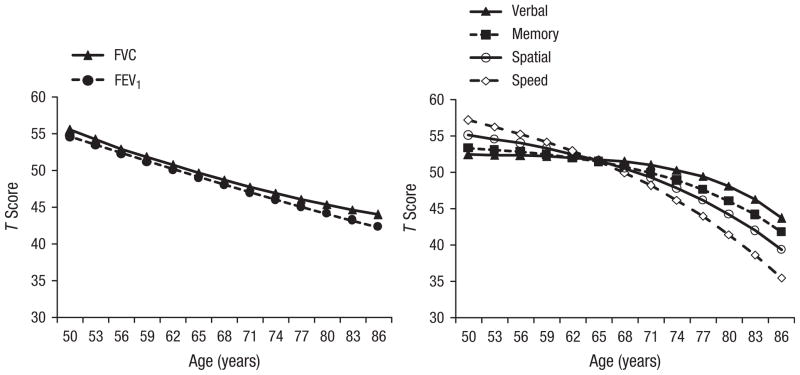
Change trajectories indicated by the results of the univariate DCSM are presented in Figure 2. Larger values of β are associated with more moderate acceleration of decline, as cognitive performance at the previous age contributed more stability to the estimation of change over time. The results presented in Table 2 and Figure 2 indicate that, as expected from previous investigations (e.g., Finkel et al., 2007), accelerating decline is steepest for the spatial and speed factors, moderate for the memory factor, and modest for the verbal factor. Data in Figure 2 and negative β values for measures of lung function suggest decelerating decline; however, nonlinear change did not achieve significance.
Fig. 2.
T score derived from the univariate dual-change-score model as a function of participants’ age and measures of lung function (left panel) and cognitive ability (right panel). FEV1 = forced expiratory volume in 1 s; FVC = forced vital capacity.
Bivariate analyses
Model fit statistics for the five comparison models are presented in Table 3; all reduced models were compared with the full model. As expected, in most cases, the results for FEV1 and FVC were parallel, thus providing a measure of replication of the model-fitting conclusions. For the verbal factor, for example, setting the coupling between lung function and verbal ability to 0 resulted in a significant loss of fit for both FEV1 (Δfit = 12, Δdf = 1, p < .01) and FVC (Δfit = 11, Δdf = 1, p < .01), whereas setting the coupling between verbal ability and lung function to 0 had no effect on model fit for either measure of lung function. However, testing Model 4 indicated that the two coupling parameters could be equated, for models including either FEV1 or FVC, without significant loss of fit (Δfit = 5 or 6, Δdf = 1, n.s.), although the comparison with the full model was significant (p < .05). Taken together, these results indicate that although lung function is a leading cause of subsequent changes in verbal ability, the effect is at most modest.
Table 3.
Model Fit Statistics (−2LL) for the Five Comparison Models Testing the Bivariate Relationships Between Measures of Cognitive Ability and Lung Function
| Model | Verbal ability
|
Spatial ability
|
Memory
|
Processing speed
|
||||
|---|---|---|---|---|---|---|---|---|
| FEV1 | FVC | FEV1 | FVC | FEV1 | FVC | FEV1 | FVC | |
| Model 1: full model (21 parameters) | −17,782 | −16,664 | −18,011 | −16,892 | −19,011 | −17,896 | −18,607 | −17,486 |
| Model 2: lung function → cognitive ability set to 0 (20 parameters) | −17,794* | −16,675* | −18,017 | −16,901* | −19,011 | −17,896 | −18,621* | −17,502* |
| Model 3: cognitive ability → lung function set to 0 (20 parameters) | −17,785 | −16,666 | −18,016 | −16,898 | −19,011 | −17,896 | −18,607 | −17,486 |
| Model 4: all couplings equal (20 parameters) | −17,788 | −16,669 | −18,018* | −16,901* | −19,011 | −17,896 | −18,616* | −17,493* |
| Model 5: no couplings (19 parameters) | −17,795* | −16,676* | −18,018 | −16,902* | −19,011 | −17,896 | −18,621* | −17,502* |
Note: Asterisks indicate that the change in model fit compared with the fit of the full model was significant (*p < .01). FEV1 = forced expiratory volume in 1 s; FVC = forced vital capacity; LL = log likelihood.
Because cross-trait relationships are the focus of model comparisons, coupling parameters are presented in Table 4, in addition to fit statistics. (Complete modeling results are available from the authors.) As indicated by the RMSEA, the full model provided adequate fit to the data. The estimate of the coupling between FEV1 and verbal ability in the full model was 0.12, indicating that higher scores on the FEV1 measure precede shallower decline in the verbal factor. In contrast, the estimate of the coupling between verbal ability and FEV1 did not differ significantly from 0, thus indicating no effect of the verbal factor on the rate of decline in FEV1.
The pattern of model-fitting results presented in Table 3 for the spatial ability and processing speed factors are generally similar. Results of testing the five DCSMs indicate one clear best-fitting model for the speed factor: the model in which the coupling between cognitive ability and lung function was set to 0. Results for the spatial factor were not consistent across models including either FEV1 or FVC; coupling parameters could either not be dropped from the model (FVC) or could not be set equal to each other (FEV1) without significantly reducing model fit. Thus the model-fitting results are consistent with the hypothesis that changes in lung function drive subsequent changes in spatial abilities and processing speed, but the results provide no support for changes in cognitive performance driving subsequent changes in lung function. Strong positive values for the couplings between FEV1 and each of the factors (shown in Table 4) indicate that higher scores on FEV1 precede shallower decline in spatial ability and processing speed.
Table 4.
Coupling Parameters (γ) and Fit Statistics From the Full Bivariate Dual-Change-Score Model (Model 1) for Each of the Cognitive Factors
| Coupling | Verbal ability | Spatial ability | Processing speed | Memory |
|---|---|---|---|---|
| FEV1 → cognitive ability | 0.12 (0.02) | 0.29 (0.09) | 0.34 (0.07) | 0.03 (0.06) |
| Cognitive ability → FEV1 | −0.18 (0.07) | −0.23 (0.08) | 0.02 (0.04) | −0.02 (0.07) |
| Misfit index: −2LL (21 parameters) | −17,782 | −18,011 | −18,607 | −19,011 |
| Root mean square error of approximation | .031 [.027, .035] | .031 [.027, .035] | .033 [.029, .037] | .031 [.026, .035] |
Note: Standard errors are given in parentheses, and 90% confidence intervals are given in brackets. FEV1 = forced expiratory volume in 1 s; LL = log likelihood.
Results for the memory factor are markedly different. Model-fitting indicates that the most reduced model, in which both coupling parameters were set to 0, provided an adequate fit to the data for both FEV1 and FVC (Δfit < 1, Δdf = 1, n.s.). In fact, the parameter estimate for the coupling between FEV1 and processing speed was 0.03 (SE = 0.06). The DCSM estimates correlations among slope parameters, and these correlation estimates also support the conclusion that the relationship between changes in memory and lung function is minimal. Correlations between the slope parameters of FEV1 and the verbal, spatial, and speed factors were .70, .88, and .68, respectively. In contrast, the correlation between the slope estimates for FEV1 and the memory factor was .17. Similarly, correlations between the slopes of FVC and the verbal, spatial, speed, and memory factors were .80, .93, .92, and .26, respectively. Thus, we can conclude that lung function has no impact on subsequent changes in memory, and memory has no impact on subsequent changes in lung function.
Discussion
Declines in components of cognitive function are associated with declines in standard indicators of pulmonary function. This relationship appears to be directional, with decline in pulmonary function leading to subsequent decline on cognitive tasks reflecting spatial performance and processing speed, common indicators of fluid cognitive abilities. Changes in pulmonary function also lead to changes in performance on verbal tasks, but the effect is very modest. It is interesting that there was no influence of pulmonary function on memory performance. In addition, there was no evidence that decline on any of the four cognitive factors led to decline in pulmonary function. Thus, the directional hypothesis was supported by these data, most strongly for speeded tasks and spatial functioning. These results extend findings of prior research, in which the directional hypothesis has been addressed (Richards et al., 2005), by utilizing the DCSM approach to identify causal relationships. These results contrast with recent data from Infurna et al. (2011) suggesting that decreased memory function led to decreased self-reported functional abilities among older adults. Despite being conducted with advanced statistical procedures, the latter study included no objective measure of physical performance, such as pulmonary function. This study is the first to utilize advanced statistical-modeling procedures to identify temporal causation between pulmonary function and cognitive function. Thus, these results provide a unique perspective on the interrelationship of physical function and cognitive function with age.
Consistent with prior research, our results showed that the decline in pulmonary function over the longitudinal interval was linear, whereas the decline in cognitive function was curvilinear and accelerating across all four of the cognitive factors studied. As expected, the decline was most steep for the spatial and speed factors, moderate for the memory factor, and modest for the verbal factor. The modest slope of decline in verbal performance may help to explain the limited relationship between pulmonary function and verbal function. Also, these results are consistent with prior studies documenting stronger associations of pulmonary function with fluid measures of cognitive performance than with crystallized measures (Anstey et al., 2004; Emery et al., 1998; Richards et al., 2005).
The absence of an association between pulmonary function and memory function in this study was somewhat surprising and contrasts with recent data from Infurna et al. (2011). However, these results are consistent with data from Richards et al. (2005) and not entirely inconsistent with data from Anstey et al. (2004), who found a weaker relationship between pulmonary function and memory in their oldest cohort (ages 60–64) than in two younger cohorts. The equivocal results across studies are likely due, in part, to variability in the methods of assessing memory (e.g., short-term recall of digits vs. recall of visual information vs. prompted recall of lists). In this study, the relatively lower internal consistency of the memory factor may reflect heterogeneity of the component measures, which may have contributed to the absence of an effect. In addition, the inclusion of data from participants who later received a diagnosis of dementia likely contributed to variability in memory performance. Nevertheless, the present data are consistent with normative aging data, reflecting substantial variability in cognitive performance in all four domains as well as predictable decline in performance with age.
Although dual-change-score modeling appears to have important advantages over other methods for addressing hypotheses about dynamic relationships among variables (see Ghisletta & Lindenberger, 2003), it is also limited by many of the statistical assumptions common to structural equation models. The data are assumed to be missing at random, the sample is assumed to be relatively homogeneous, and structural relations based on interindividual variance and on intra-individual variance are assumed to be equivalent (Lövdén et al., 2005). One benefit of using latent factors instead of individual cognitive tasks is the resulting increase in reliable variance. In the current study, internal consistency was strong for all four cognitive factors, although it was somewhat weaker for the memory factor than for the other three. Thus, it is unlikely that the bivariate DCSM results are a by-product of the psychometric properties of the variables.
Results of this study help elucidate mechanisms by which pulmonary function may influence cognitive function. First, effects were present for vulnerable cognitive domains involving spatial performance and psychomotor speed (“fluid abilities”), and effects were limited or absent in areas of cognitive performance dependent on accumulated knowledge. To the extent that exercise may help maintain pulmonary function, these data are consistent with results of numerous exercise-intervention studies documenting a positive influence of exercise on fluid cognitive abilities but not crystallized cognitive function (Colcombe & Kramer, 2003; Smiley-Oyen, Lowry, Francois, Kohut, & Ekkekakis, 2008). Pulmonary function decline also may contribute to decreases in cognitive performance via hypoxia, reduced neurotransmitter function, increased systemic inflammatory processes, or a combination of factors. In contrast with the data from Infurna et al. (2011), our results showed no support for cognitive changes leading to changes in physical (pulmonary) function. These data also diminish support for a third, common variable because the presence of a third variable influencing both cognitive and pulmonary function would likely result in an absence of causal direction. Thus, the present data suggest that strategies for maintaining or improving pulmonary function may be important for maintaining fluid cognitive function with age.
Acknowledgments
Funding
The Swedish Adoption/Twin Study of Aging (SATSA) is supported by the National Institute on Aging (AG04563, AG10175), the MacArthur Foundation Research Network on Successful Aging, the Swedish Council for Social Research (97:0147:1B), and the Swedish Research Council.
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Reprints and permission: sagepub.com/journalsPermissions.nav
References
- Albert MS, Jones K, Savage CR, Berkman L, Seeman T, Blazer D, Rowe JW. Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychology and Aging. 1995;10:578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Windsor TD, Jorm AF, Christensen H, Rodgers B. Association of pulmonary function with cognitive performance in early, middle and late adulthood. Gerontology. 2004;50:230–234. doi: 10.1159/000078352. [DOI] [PubMed] [Google Scholar]
- Birren JE. Translations in gerontology—From lab to life: Psychophysiology and the speed of response. American Psychologist. 1974;29:808–815. doi: 10.1037/h0037433. [DOI] [PubMed] [Google Scholar]
- Browne M, Cudeck R. Alternative ways of assessing model fit. In: Bollen K, Long S, editors. Testing structural equation models. Beverly Hills, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Chyou PH, White LR, Yano K, Sharp DS, Burchfiel CM, Chen R, Curb JD. Pulmonary function measures as predictors and correlates of cognitive functioning in later life. American Journal of Epidemiology. 1996;143:750–756. doi: 10.1093/oxfordjournals.aje.a008812. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Dominicus A, Palmgren J, Pedersen NL. Bias in variance components due to nonresponse in twin studies. Twin Research and Human Genetics. 2006;9:185–193. doi: 10.1375/183242706776382419. [DOI] [PubMed] [Google Scholar]
- Emery CF, Huppert FA, Schein RL. Do smoking and pulmonary function predict cognitive function? Findings from a British sample. Psychology & Health. 1997;12:265–275. [Google Scholar]
- Emery CF, Pedersen NL, Svartengren M, McClearn GE. Longitudinal and genetic effects in the relationship between pulmonary function and cognitive performance. The Journals of Gerontology B: Psychological Sciences and Social Sciences. 1998;53:P311–P317. doi: 10.1093/geronb/53b.5.p311. [DOI] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL. Processing speed and longitudinal trajectories of change for cognitive abilities: The Swedish Adoption/Twin Study of Aging. Aging, Neuropsychology and Cognition. 2004;11:325–345. [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. The longitudinal relationship between processing speed and cognitive ability: Genetic and environmental influences. Behavior Genetics. 2005;35:535–549. doi: 10.1007/s10519-005-3281-5. [DOI] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Age changes in processing speed as a leading indicator of cognitive aging. Psychology and Aging. 2007;22:558–568. doi: 10.1037/0882-7974.22.3.558. [DOI] [PubMed] [Google Scholar]
- Gatz M, Pedersen NL, Berg S, Johansson B, Johansson K, Mortimer JA, Ahlbom A. Heritability for Alzheimer’s disease: The study of dementia in Swedish twins. The Journals of Gerontology A: Biological Sciences and Medical Sciences. 1997;52:M117–M125. doi: 10.1093/gerona/52a.2.m117. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, de Ribaupierre A. A dynamic investigation of cognitive dedifferentiation with control for retest: Evidence from the Swiss Interdisciplinary Longitudinal Study of the Oldest Old. Psychology and Aging. 2005;20:671–682. doi: 10.1037/0882-7974.20.4.671. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, Lindenberger U. Age-based structural dynamics between perceptual speed and knowledge in the Berlin Aging Study: Direct evidence for ability dedifferentiation in old age. Psychology and Aging. 2003;18:696–713. doi: 10.1037/0882-7974.18.4.696. [DOI] [PubMed] [Google Scholar]
- Incalzi RA, Chiappini F, Fuso L, Torrice MP, Gemma A, Pistelli R. Predicting cognitive decline in patients with hypoxaemic COPD. Respiratory Medicine. 1998;92:527–533. doi: 10.1016/s0954-6111(98)90303-1. [DOI] [PubMed] [Google Scholar]
- Infurna FJ, Gerstorf D, Ryan LH, Smith J. Dynamic links between memory and functional limitations in old age: Longitudinal evidence for age-based structural dynamics from the AHEAD Study. Psychology and Aging. 2011;26:546–558. doi: 10.1037/a0023023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lövdén M, Ghisletta P, Lindenberger U. Social participation attenuates decline in perceptual speed in old and very old age. Psychology and Aging. 2005;20:423–434. doi: 10.1037/0882-7974.20.3.423. [DOI] [PubMed] [Google Scholar]
- McArdle JJ. A latent difference score approach to longitudinal dynamic structural analyses. In: Cudeck R, duToit S, Sorbom D, editors. Structural equation modeling: Present and future. Lincolnwood, IL: Scientific Software International; 2001. pp. 342–380. [Google Scholar]
- McArdle JJ, Hamagami F. Structural equation models for evaluating dynamic concepts within longitudinal twin analyses. Behavior Genetics. 2003;33:137–159. doi: 10.1023/a:1022553901851. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F, Jones K, Jolesz F, Kikinis R, Spiro A, III, Albert MS. Structural modeling of dynamic changes in memory and brain structure using longitudinal data from the Normative Aging Study. The Journals of Gerontology B: Psychological Sciences and Social Sciences. 2004;59:P294–P304. doi: 10.1093/geronb/59.6.p294. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F, Meredith W, Bradway KP. Modeling the dynamic hypotheses of Gf-Gc theory using longitudinal life-span data. Learning and Individual Differences. 2000;12:53–79. [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 3. Los Angeles, CA: Author; 2005. [Google Scholar]
- Nesselroade JR, Pedersen NL, McClearn GE, Plomin R, Bergeman CS. Factorial and criterion validities of telephone-assessed cognitive ability measures: Age and gender comparisons in adult twins. Research on Aging. 1988;10:220–234. doi: 10.1177/0164027588102004. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Plomin R, Nesselroade JR, McClearn GE. Quantitative genetic analysis of cognitive abilities during the second half of the lifespan. Psychological Science. 1992;3:346–353. [Google Scholar]
- Pedersen NL, Reynolds CA. Stability and change in adult personality: Genetic and environmental components. European Journal of Personality. 1998;12:365–386. [Google Scholar]
- Richards M, Strachan D, Hardy R, Kuh D, Wadsworth M. Lung function and cognitive ability in a longitudinal birth cohort study. Psychosomatic Medicine. 2005;67:602–608. doi: 10.1097/01.psy.0000170337.51848.68. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. What and when of cognitive aging. Current Directions in Psychological Science. 2004;13:140–144. doi: 10.1177/0963721414535212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW. The course of adult intellectual development. American Psychologist. 1994;49:304–313. doi: 10.1037//0003-066x.49.4.304. [DOI] [PubMed] [Google Scholar]
- Schaub RT, Münzberg H, Borchelt M, Nieczaj R, Hillen T, Reischies FM, Steinhagen-Thiessen E. Ventilatory capacity and risk for dementia. The Journals of Gerontology A: Biological Sciences and Medical Sciences. 2000;55:M677–M683. doi: 10.1093/gerona/55.11.m677. [DOI] [PubMed] [Google Scholar]
- Smiley-Oyen A, Lowry K, Francois S, Kohut M, Ekkekakis P. Exercise, fitness, and neurocognitive function in older adults: The “selective improvement” and “cardiovascular fitness” hypotheses. Annals of Behavioral Medicine. 2008;36:280–291. doi: 10.1007/s12160-008-9064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, Borchelt M, Smith J. Relation between cardiovascular and metabolic disease and cognition in very old age: Cross-sectional and longitudinal findings from the Berlin Aging Study. Health Psychology. 2003;22:559–569. doi: 10.1037/0278-6133.22.6.559. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Brown JRP, Maier KJ, Katzel LI. Diagnosis of hypertension and high blood pressure levels negatively affect cognitive function in older adults. Annals of Behavioral Medicine. 2005;29:174–180. doi: 10.1207/s15324796abm2903_3. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale–Revised. New York, NY: Psychological Corporation; 1981. [Google Scholar]
- Wicherts JM, Dolan CV, Hessen DJ, Oosterveld P, van Baal GCM, Boomsma DI, Span MM. Are intelligence tests measurement invariant over time? Investigating the nature of the Flynn effect. Intelligence. 2004;32:509–537. [Google Scholar]