Abstract
Stem cell-based cell replacement of lost midbrain dopamine (mDA) neurons is a potential therapy for Parkinson’s disease (PD). Toward this goal, it is critical to optimize various aspects of cell transplantation and to assess functional recovery through behavioral tests in validated animal model(s) of PD. At present, cell transplantation studies are being done almost exclusively in neurotoxin-based animal models, because few genetic models of PD exhibit robust mDA neuronal loss. Here, we used a genetic model of PD, the aphakia mouse, which demonstrates selective degeneration of mDA neurons in the substantia nigra. We systematically investigated the functional effects of transplanting embryonic stem cell derived cells at different stages of in vitro differentiation; embryoid body (EB), neural progenitor (NP), and neuronal differentiated (ND) stages. We found that transplantation of NP cells yielded the best outcomes for both survival and behavioral improvement while transplantation of EB and ND cells resulted in high teratoma-like tumor formation and poor survival, respectively. In behavioral paradigms specific to basal ganglia, the NP cells group prominently improved motor behavioral defects 1 and 2 months post transplantation. Furthermore, we found that NP cells transplantation also improved cognitive impairments of aphakia mice, as examined by the passive avoidance task. Importantly, these graft-induced functional improvements well correlated with survival of tyrosine hydroxylase-positive DA neurons. Taken together, we propose that the aphakia mouse can serve as a novel and useful platform for cell transplantation studies to assess both neurological and cognitive improvements and that NP stage cells represent an optimal stage for transplantation.
Keywords: Parkinson’s disease; Dopaminergic neurons; Embryonic stem cells; 6-hydroxydopamine; Aphakia; Tyrosine hydroxylase; L-3,4-dihydroxyphenylalanine
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder affecting approximately 1% of the population over age 65 (33,34). At present, there are no treatments that can halt or slow down PD’s progress. Given that a major characteristic of PD is selective loss of a specific cell type, i.e., midbrain dopaminergic (mDA) neurons in the substantia nigra (SN), a potential therapeutic approach is stem cell-based replacement therapy. Indeed, clinical transplantation trials showed that grafting human fetal mesencephalic tissues could, in some patients, dramatically improve PD symptoms for a long period (26,44). These successful clinical studies demonstrated the proof-of-principle supporting cell-based replacement therapy of PD. However, fetal cell transplantation has significant technical and practical limitations, including the limited and controversial availability of transplantable human fetal cells. In addition, more recent double blind, placebo-controlled clinical trials found no significant behavioral benefits in primary endpoints, representing a major setback for the field (25,43). These rather poor clinical outcomes may have been caused by many possible factors including the lack of a standardized cell source, different transplantation procedures, unfavorable cell composition (e.g., too many serotonergic neurons), and the clinical status of transplanted PD patients (most of them had previously been treated with L-3,4-dihydroxyphenylalanine (L-DOPA)) (36,37,49). Therefore, there is an unmet and enormous demand for a standardized and unlimited cell source in order to further develop cell-based therapy of PD, which will require more systematic and extensive studies of cell transplantation in appropriate animal models.
First established in mice and then in humans, blastula-derived embryonic stem cells (ESCs) due to their unlimited in vitro proliferation and pluripotency represent a promising cell source for transplantation therapy of PD (21,38,51). To evaluate the potential of ESC-derived cells as a transplantable cell source, many laboratories investigated in vitro neuronal differentiation followed by transplantation and observed that mouse ESCs can generate neural progenitor/precursor (NP) cells and then functional mDA neurons which can ameliorate motor dysfunction in animal models of PD (3,8,27,28,31,32,35,46). Notably, these studies used different stages of in vitro differentiated cells derived from mouse ESCs, including undifferentiated ESCs (8), NPs (15,16), or differentiated neurons (3,28,32,40,46). In addition, human ESC-derived cells have also been shown to generate mDA neurons and improve motor dysfunction in animal models of PD, mostly using differentiated neuronal cells (6,11,14,47).
To date, the assessment of therapeutic effects of ESC transplantation has been based on limited behavioral assays, such as observing a reduction of turning behavior in 6-hydroxy dopamine (6-OHDA)-lesioned rodents(9,41). While this behavioral model is very useful for assessing the behavioral outcomes of stem cell transplantation, it is desirable to develop additional genetic models of PD that can examine various behavioral aspects related to PD symptoms including non-motor behaviors such as cognitive impairments. We and others previously demonstrated that Pitx3-deficient aphakia (ak) mice display prominent and selective loss of mDA neurons in the SN and show defects of the nigrostriatal pathway (29,30,42,50,52), suggesting that ak mice can be used as a novel and useful genetic model of PD. Indeed, although initial studies did not reveal any PD-like motor deficits by measuring gross motor activity (29), we found that ak mice exhibit motor deficits in nigrostriatal pathway-sensitive behavioral tests, which was reversed by treatment with L-dopa (30). In addition, ak mice displayed “DA denervation supersensitivity”, which is another prominent feature observed both in animal models and in PD patients. Furthermore, ak mice were found to be impaired in striatum-dependent cognitive tasks such as rotarod learning, t-maze, and inhibitory avoidance tasks (2), indicating that some neuropsychiatric aspects of PD can also be tested in this unique model. In the present study, we sought to test transplantation of mouse ESC-derived cells at different stages of differentiation in ak mice. Since a high number of animals with the same level of mDA neuronal loss can be easily obtained, the use of this genetic model makes it possible to test different conditions of cell transplantation. In particular, using the 5-stage in vitro differentiation procedure (16,17,35), we attempted to test the effects of transplantation of different stage cells derived from mouse ESCs, e.g., embryonic bodies (EBs), neural progenitors (NPs), and differentiated neuronal cells (ND), using ak mice treated with saline and L-DOPA as negative and positive controls, respectively. Based on our recent study showing cognitive impairments in ak mice (2), we also addressed whether transplantation of ESC-derived cells can ameliorate these non-motor deficits as well.
Materials and Methods
Animals
Homozygous aphakia/aphakia (ak/ak) mice were originally obtained from Jackson Labs (Bar Harbor, ME, USA) (strain B6×C57BLKS-ak; JR942). Several breeding pairs were expanded and maintained in the Animal Care Facility at McLean Hospital, as previously described (2,30). Mice homozygous for the retinal degeneration 1 (rd1) mutation (B6.C3-Pde6brd1; The Jackson Laboratory) were used as a blind mouse control to exclude the possibility that any potential changes that we observed were due to ak mice’s blindness (2,30). 2–3 month old mice were used for the following assays. The use of animals was in accordance with McLean’s Institutional Animal Care and Use Committee and followed National Institutes of Health guidelines.
ESC Culture and Differentiation
Early passage mouse J1 ES cells (with a passage number lower than 12) were used in this study. J1 cells were maintained and differentiated according to the five-stage in vitro differentiation protocol, as described previously (17). Briefly, embryonic stem cells (ESCs) (Stage 1) were differentiated into embryoid bodies (EB; Stage 2) on nonadherent bacterial dishes for 4 days in EB medium, and plated onto an adhesive tissue culture surface. NP cells were selected and expanded in insulin, transferrin, selenium and fibronectin (ITSFn) media (neural progenitor/precursor (NP); Stage 3 and 4), and then basic Fibroblast Growth Factor (bFGF) was removed to induce neuronal differentiation (17,35). During this neuronal differentiation stage (differentiated neuronal cells (ND); Stage5), some cells started to exhibit neuronal morphology at day 3 and the vast majority of them became neuronal cells at day 7. To systematically investigate the therapeutic potential of different stage cells derived from ESCs, EB (undifferentiated), NPs (multipotent) and ND (differentiated) cells were transplanted into the striatum of ak mice (n=6, n=20, and n=20, respectively).
Immunocytochemistry and immunohistochemistry
Immunocytochemistry and immunohistochemistry assays were performed as described previously (15,17). Using 4% formaldehyde (Electron Microscopy Sciences, Ft. Washington, PA), cells were fixed for 30 min and then incubated with blocking buffer (PBS, 10% normal donkey serum, NDS) for 10 minutes. For the BrdU staining, samples were treated for 30 min in 1N HCl, to denature DNA, and sequentially incubated with sodium borate solution (pH 8.0) for 15 min. Following incubation of cells with primary antibodies in Phosphate buffered saline (PBS) including 2% NDS at 4°C, mouse anti-nestin (Rat401, 1 µg/ml; Developmental Studies Hybridoma Bank, Iowa City, IA), rabbit anti-β-tubulin type III (Tuj1) (1:2000; Covance, Princeton, NJ), and sheep anti-tyrosine hydroxylase (TH, 1:200; Pel-Freez.com) were used. For fluorescence staining, cells seeded on coverslips were further incubated with fluorescent-labeled secondary antibodies (Cy2- or Rhodamine Red-X-labeled donkey immunoglobulin G; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) in PBS with 2% NDS for 30 minutes at room temperature. Each coverslip was counterstained with 1 µg/ml DAPI (4,6-diamidino-2-phenylindole) and then examined using a Leica TCS/NT confocal microscope (Leica, Heerbrugg, Switzerland) equipped with krypton, krypton/argon, and helium lasers. For immunohistochemistry, mice grafted with ESC-derived cells were terminally anesthetized using an intraperitoneal overdose of pentobarbital (150 mg/kg; Sigma). Brains perfused with 4% formaldehyde and fixed with 30% sucrose overnight were cryo-sectioned into 30-µm thick slices. Every 6th section of the striatum was used to analyze graft size, to count the number of surviving cells and to characterize specific cell types. Brains were incubated with the following primary antibodies: rabbit anti-TH (1:2000 for 3,3'-diaminobenzidine (DAB) staining and 1:200 for immunofluorescence (Pel-Freeze, Rodgers, AR)); sheep anti-TH (1:250 for immunofluorescence) (Pel-Freeze, Rodgers, AR); mouse anti-TH (Chemicon, Temecula, CA)(1:200 for immunofluorescence); rabbit anti-Pitx3 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA and 1:00, Millipore); mouse anti-nestin (Rat401, 1 µg/ml; Developmental Studies Hybridoma Bank, Iowa City, IA), rabbit anti-β-tubulin (1:2000; Covance, Princeton, NJ), mouse anti-GFAP (1:500, Chemicon), Doublecortin (DCX; 1:200, Cell Signaling), NeuN (1:1000, Chemicon), Girk2 (1:200, Abcam or 1:80, Alomone Labs, Jerusalem, Israel), Nurr-1 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA), anti-rat BrdU (AbD serotec, Oxford, UK; 1:500), and Corin (1:200, Nobus biologicals). For immunofluorescence analysis, each brain section was incubated with Alexa Fluor 488 or 594-conjugated secondary antibodies (Molecular Probes, Carlsbad, CA). To measure graft size and to count TH+ cells within each graft (n=6 for each experimental group), every sixth section was collected and stained for TH. Stereological analysis was performed with DAB stained sections using an integrated Axioskop 2 microscope (Carl Zeiss) and Stereo Investigator image capture equipment and software (Microbright Field, Williston, VT).
Transplantation Procedure
Preanesthesia, mice were injected i.p. with acepromazine (3.3 mg/kg, PromAce, Fort Dodge, IA) and atropine sulfate (0.2 mg/kg, Phoenix Pharmaceuticals, St. Joseph, MO) followed by anesthesia with ketamine/xylazine (60 mg/kg and 3 mg/kg, respectively, i.p.). A Kopf stereotaxic frame (Kopf Instruments, Tujunga, CA) was used for transplantation. Postoperatively, buprenorphine (0.032 mg/kg; i.p.) was administered. In the 24 hours post operation, buprenorphine (0.032 mg/kg; i.p.; Sigma) was administrated twice. Based on our preliminary results showing that grafted cells survived equally well with or without treatment with immunosuppressant (data not shown). On injection day, 2 µl of cell suspension were prepared by trypsinization and suspension in PBS. Each animal (two months old) received an injection of 1 µl (0.25 µl/min) of cell suspension on each side of the striatum (from the bregma: anterior-posterior + 0.05, lateral ± 0.18, ventral − 0.30, coordinated according to the atlas of Frankline and Paxinos) using a 22-gauge, 2.5 µl Hamilton syringe. Based on previous studies from this and other laboratories (8,15,16,32) and our preliminary tests (data not shown), animals were given 2,000 EB cells, 250,000 NP cells, or 250,000 ND cells per microliter in each hemisphere. To track injected cells, donor cells were labeled with BrdU (10µmol/L) 24 hours before transplantation. Before removing the needle, a 5-min waiting period was given to prevent cell reflux. Animals did not receive immunosuppressants in this study. The L-Dopa group received 12.5 mg/kg benserazide 20 min prior to administration of 25mg/kg L-Dopa followed by behavioral assessment at 10 min intervals. The L-Dopa group was given L-Dopa once prior to behavioral assessment for a total of two injections.
Behavioral Tests
One hour before testing, all animals were allowed to acclimate to the dimly lit testing room (illuminated by a red 25 watt incandescent bulb). Each behavioral task (30) was performed at two time points, one month and two months post-transplantation using the same transplanted animals. All sessions were videotaped and coded in slow motion by two independent raters blind to the mouse groups.
Behavioral Experimental groups: There were six experimental groups for each behavioral test: (1) sham-operated ak group (negative control; n=10), (2) L-Dopa-treated ak group (positive control; n=10), (3) sham-operated rd1 group (blind control; n=10), (4) EB cells transplanted ak group (n=6), (5) NP cells transplanted ak group (n=10), (6) ND cells transplanted ak group (n=10), and (7) WT control (n=8). Animals received saline or L-Dopa one day before the test and ESC-derived cells were transplanted one or two months prior to administering behavioral tests.
Locomotor Activity: A polycarbonate cage 44 cm × 22 cm × 15 cm surrounded by photobeam detectors (transected by a 4 × 8 horizontal infrared beam grid) was used for the locomotor (ambulatory) activity test. Either consecutive horizontal or vertical photobeam breaks were considered locomotor activity and were recorded at 1-hour intervals for 24 hours for statistical analysis. Each mouse was returned to its home cage. In this test, wild type mice were included as additional control.
Cylinder Test: Spontaneous movement was measured using a small transparent cylinder (height, 15.5 cm; diameter, 12.7 cm). Mice were placed in the cylinder for 3 mins and the number of rears was measured. A rear was defined as an animal's vertical movement with both forelimbs and immediately touching the wall of the cylinder after removing both limbs from the ground.
Beam Test: Another motor function was measured using the beam test in which a 1 m length beam was used. The starting width of the beam was 3.5 cm and it gradually narrowed to 0.5 cm in 1-cm increments. Animals were given 2 days training sessions before testing. During the training period, a mesh grid normally covering the beam was removed. On the day of the test, a mesh grid (1 cm square) was added to the beam covering the beam surface and leaving a ~1 cm room between the grid and the beam surface. In each session, animals ran three trials. 1) The mean number of steps taken by each animal and 2) the mean time to traverse across three trials were collected and used for statistical analysis.
Pole Test: Pole test was performed using a vertical wooden pole 50 cm in length and 1 cm in diameter. Each mouse was gently placed head upwards on top of the pole attached to each animal's home cage. All animals ran tree trials for each session and received 2 training sessions. On the third day, animals ran three trials, and 1) the time to orient downward and 2) the total travel time were measured for analysis.
Passive Avoidance: Passive avoidance test was performed to measure mice’s associative learning and memory. The Gemini Avoidance system (San Diego Instruments) was used for this test. The equipment consists of two compartments (each side 25cm × 20cm × 20cm) split by an automated metal door. The dark side compartment has a shock source on a grid floor that can be electrified and a bright side compartment not connected to the shock. On the first day of testing, defined as the learning or acquisition day, each mouse was placed individually in one compartment in which there was no shock source and the chamber door was closed outside. The trial began after a 30 second adaptation period, in which the mouse was allowed to freely explore the compartment. After adaptation, the house light then automatically turned on and the metal door separating the two compartments automatically went up. The gate closed when either the animal entered the dark compartment, or 500 seconds elapsed, whichever came first. Immediately after the animal entered the dark side, the gate closed and the animal was given a 2-second shock (0.02 mA). On the first day, the latency to enter the dark side was automatically recorded. The animal stayed in the dark side for 10000 milliseconds after the shock was delivered and then was returned to its home cage. The experimenter counted the number of recorded animal’s vocalizations to ensure they received an electrical shock. On the second day, defined as the memory or retention day, mice were placed on the same side, bright compartment using the same parameters as during training. However, contrary to the first day, on the second day no shock is administered. If the animal remembered receiving a shock upon entering the dark compartment, then the animal would be reluctant to enter the dark compartment and prefer to stay in the bright compartment. If the animal had no memory of receiving a shock upon entering the dark compartment, no hesitation to enter the dark compartment would ensue. Therefore, the latency difference between the two testing days is considered a measurement of retention or memory.
Statistical Methods
Statistical analyses were performed using the statistical Analysis System (Version 9.1; SAS institute, Cary, NC) on a Cornell University mainframe computer. Performance measurements were analyzed using the PROC Mixed program or the PROC GLIMMIX program, a generalized linear mixed models procedure for conducting repeated measures analyses of non-normal data (53). Means were calculated for each animal for each testing condition, defined by the following variables (where appropriate): groups and time-block. Each time block consisted of 5 minutes, thus dividing the 30 minutes into six blocks for the locomotor activity task. For the beam, pole, and cylinder tests, the first month after transplantation was analyzed. In addition, the means for each testing condition, defined by the groups and time values, were compared for the transplanted groups. The time variable consisted of two time-points: 1 and 2 months after transplantation. For the passive avoidance test, the means of the values obtained by calculating the latency difference between day 1 and day 2 (d2–d1) were compared using two-way ANOVA. For all of post-hoc contrast analysis, fisher's Least Significant Difference test (LSD) that the SAS programs automatically provided was used for specific comparisons. Correlations between surviving TH+ cells in the host striatum and motor function scores from the cylinder test were analyzed using Pearson’s correlation coefficient.
Results
Ak mice exhibit no impairments in overall spontaneous locomotor activity
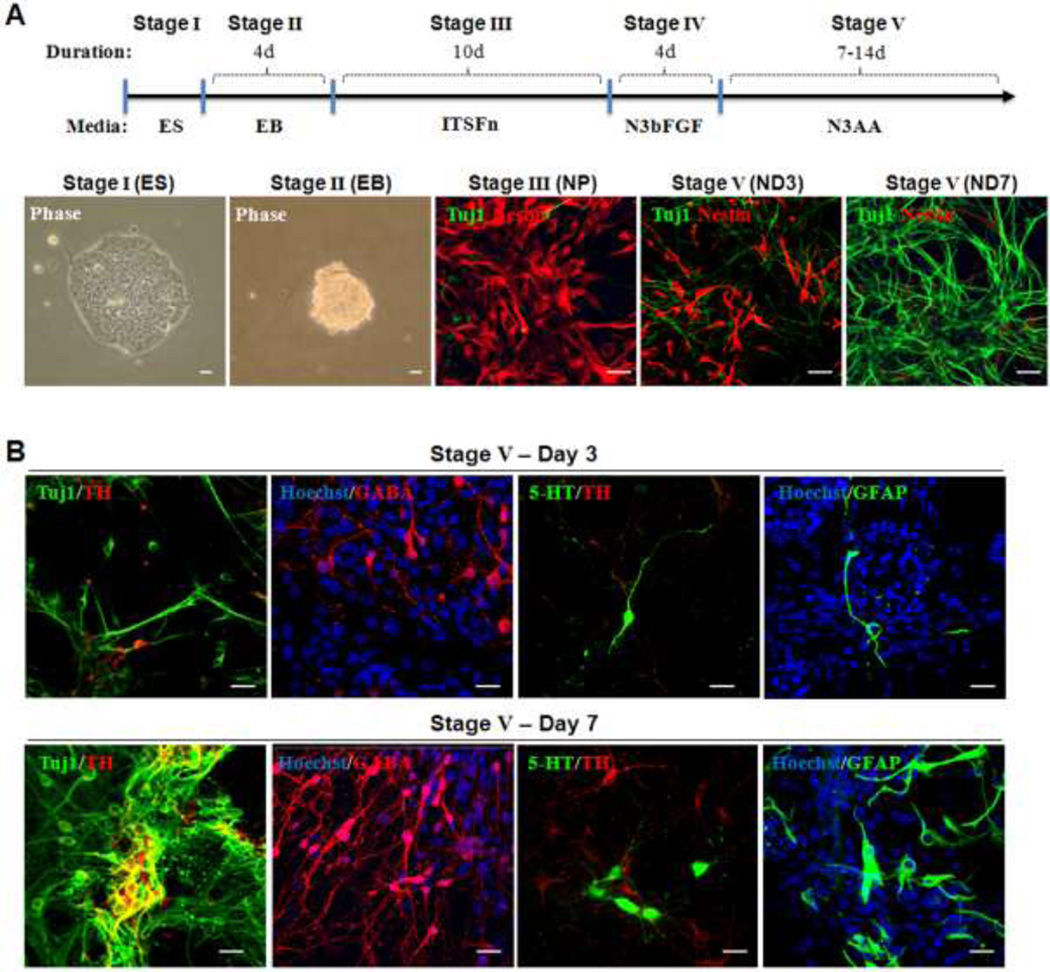
In the present study, using a genetic model of PD (the ak mouse), we tested the effect of transplantation of cells at three different stages derived from mouse ESCs (i.e., EB, multipotent NP, and differentiated ND cells) (Fig. 1A). Notably, the vast majority of NP stage cells were Nestin+ with a minor population of Tuj1+ cells. In contrast, ND stage cells were mostly Tuj1+. Since late ND stage cells could not survive in host brains following transplantation, we used early ND stage cells (ND3) for transplantation, having a significant proportion of remaining Nestin+ cells (Fig. 1). These ND stage cells contained different neural cell types, including DA, GABA, serotonergic neurons and astrocytes, demonstrating the multipotency of NP cells (Fig. 1B).
Figure 1.
(A) The 5 stage in vitro differentiation of mouse ESCs: Representative pictures of each stage cells (upper panel) and a schematic diagram of the 5 stage in vitro differentiation of mouse ESCs are shown. Embryoid bodies (EBs; stage II) were derived from undifferentiated cells (ESCs; stage I). NP cells were induced using defined medium (stage III) and then expanded in the presence of basic fibroblast growth factor (bFGF; stage IV), in which a highly homologous nestin+ cells are evident. Next, NP cells were differentiated into Tuj1+ cells by mitogen removal (ND; stage V). At the early ND stage (e.g., ND3), a significant proportion of cells are still nestin+, while the vast majority become Tuj1+ neuronal cells at a later stage (e.g., ND7 or later). (B)Neuronal marker expression at early and late stage of ND cells: Differentiated ND stage cells were further analyzed for their lineage-specific marker expression by immunocytochemical analyses. Immunofluorescent analyses showed the presence of general neuronal (Tuj1), GABAergic (GABA), serotonergic (5HT) neurons, and astrocytes (GFAP) in early (ND3; upper panel) and late stages (ND7; lower panel) cells. Notably, greater proportions of total cells showed these lineage cell types at ND7 than at ND3. Size bar: 50 µm, each scale bar is adjusted accordingly
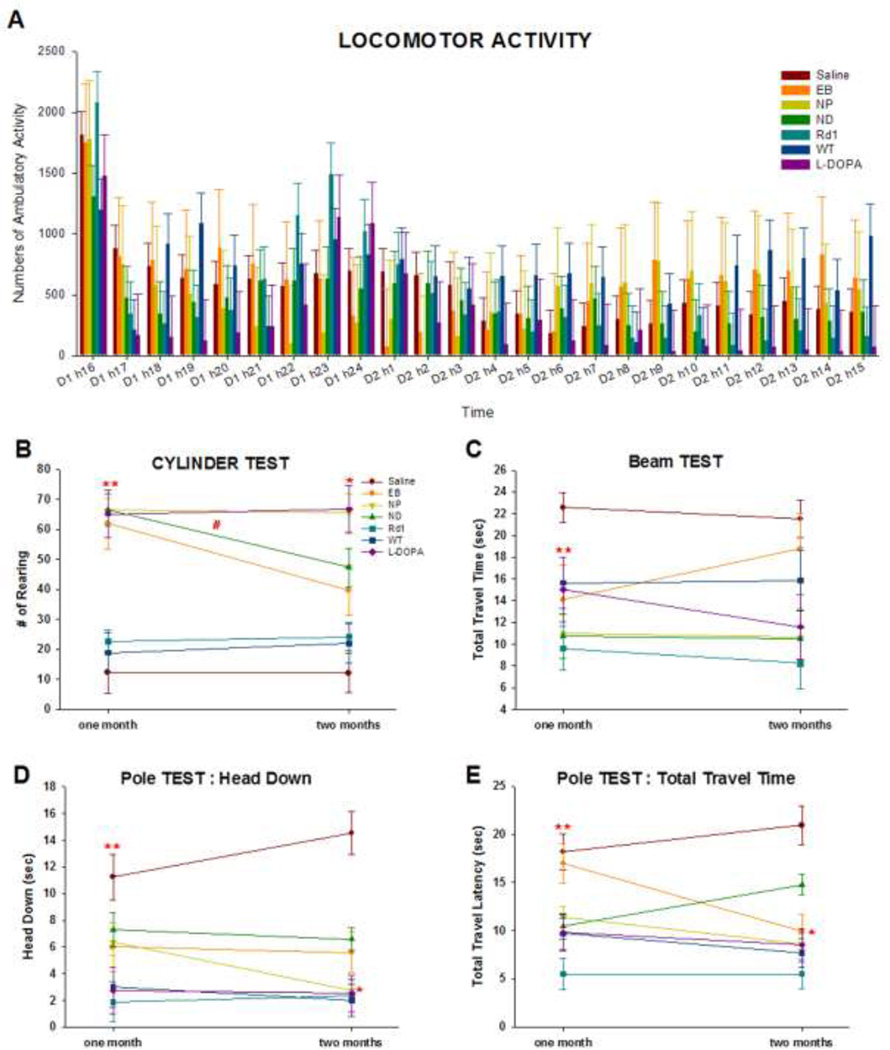
In addition to three cell-treated ak groups, four control groups were employed: 1) a sham-operated group as negative control, 2) an L-Dopa-treated group as positive control, 3) wild type control (WT), and 4) an rd1 group (rd1) as a blinded positive control group to assure that any potential changes that we observed were not due to ak mice’s blindness, but rather were related to the loss of mDA neurons (2,30). We first tested if the cell transplanted groups showed any difference in overall spontaneous locomotor activity at 1 month post-transplantation. As shown in Figure 2A, there were no statistically significant differences between the six experimental groups in overall spontaneous locomotor activity. This finding is consistent with our previous results (30) and further showed that basic motor activity of cell-transplanted ak mice was indistinguishable from that of control groups such as sham-operated ak, WT, and rd mice. Most groups showed slightly increased activity during night time (Fig. 2A). The ability of ak mice to display normal movement in non-demanding tasks is important because it suggests that these animals can be further tested in a series of more demanding and specific behaviors for the assessment of motor and cognitive functions.
Figure 2.
(A) Locomotor Activity: General locomotor activity is not altered in transplanted ak mice. There were no statistically significant differences between the seven experimental groups in overall spontaneous locomotor activity measured in the automatic locomotor box for 24 hours. In this test, mice were treated with L-Dopa and saline one day before the test, while cell transplantation was performed one month prior to the test.
(B) Cylinder Test: Spontaneous exploratory activity was measured in the cylinder for 3 minutes at 1 and 2 months post-transplantation. Sham-operated ak mice, WT and rd1 groups displayed a significantly lower number of rears than ak mice transplanted with EB, NP, or ND cells and L-DOPA-injected ak mice (p < 0.001). There were no differences between groups with the exception of sham-operated ak mice. In the assessment of performance 1 and 2 months post-transplantation, only ak mice transplanted with NP cells showed a long-term effect (p < 0.05). While EB and ND transplanted ak mice displayed a decrease in rearing 2 months post-transplantation compared with 1 month post-transplantation (p < 0.01, slope difference compared with performance between1 and 2 months), ak mice transplanted with NP cells showed constantly high numbers of rearing 2 months post-transplantation. At 2 months, the WT and rd1 groups maintained lower rearing number.
** indicates p < 0.001 compared with sham-operated, WT and rd1 groups
# indicates p < 0.05 compared with EB, ND groups
* indicates p < 0.01, slope difference compared with performance between 1 and 2 months post-transplantation
(C)Challenging Beam Test: ak mice transplanted with EB, NP, or ND cells or treated with L-Dopa, WT, and rd1 groups showed significantly faster time to travel the challenging beam than the ak sham-operated group (p < 0.001). There were no statistically significant interactions between groups and time (p = 0.46). The interaction pattern, however, indicated that traverse time in ak mice transplanted with EB cells increased 2 months post-transplantation, further demonstrating that ak mice transplanted with NP and ND cells, but not EB cells, exhibited long-term beneficial gains.
** indicates p < 0.001 compared with sham-operated
(D)Pole Test, Head Down: ak mice showed a significantly longer latency to orient themselves downwards than any other group (p < 0.003). Mice transplanted with cells from each of the three stages showed decreased times to achieve downwards orientation compared with untreated ak mice (p < 0.0001). The transplanted groups performances were comparable with that of the L-DOPA WT and rd1 control groups. A significant interaction between groups and time (p < 0.05) demonstrated that time to orient the head downwards in ak mice transplanted with NP cells was much faster than in the groups transplanted with cells from the EB or ND stages 2 months post-transplantation.
** indicates p < 0.001 compared with sham-operated group
* indicates p < 0.05, compared with EB, ND, and sham-operated groups at 2 months
(E)Pole Test, Total Travel Time: the total time to travel downward to the animals’ home cage was tested. Sham-operated ak mice showed longer times to travel down the pole than NP, ND, WT, rd1, and L-DOPA-treated mice (p < 0.003). In this test, ak mice transplanted with EB cells showed longer time to return to the their home cage compared with mice from the NP, ND, Rd1, and L-DOPA groups. Analysis of the long-term effect of transplanted cells revealed a significant interaction between groups and time (p < 0.05).
** indicates p < 0.003 compared with sham-operated group
* indicates p < 0.05, compared with ND, and sham-operated groups at 2 months
Transplantation of ESC-derived cells reverses motor behavior deficits that are sensitive to the nigrostriatal pathway
Gross motor activity tests did not show the presence of motor deficits in any of the experimental groups (Fig. 2A). Most of groups showed increased activity during night time (Fig. 2A). We further performed a battery of behavioral tests specific for nigrostriatal impairment, including assessments of spontaneous exploratory activity (the cylinder test) and sensory motor coordination (the challenging beam test and the pole test) (23,24).
On the cylinder test, spontaneous exploratory activity was measured in a transparent cylinder. Remarkably, when tested 1 month post transplantation, sham-operated ak mice displayed a significantly lower number of rears (less than 6-fold) than any of L-Dopa or ESC-derived cell transplanted groups (F(6,106) = 22.33, p < 0.0001). Specifically, ak mice transplanted with EB, NP, or ND cells and L-Dopa-injected ak mice exhibited a significantly higher number of rearing behaviors during the 3 minutes of testing than the sham-operated group (p < 0.001) (Fig. 2B). There were no differences between the groups with the exception of the sham-operated group, WT group and rd1 group, suggesting that transplantation of all three stage ESC-derived cells generated prominent therapeutic effects that were comparable with L-Dopa treatment. To further investigate long-term effect of cell transplantation, separate experiments were performed 2 months post transplantation. Interestingly, we found that only ak mice transplanted with NP cells showed a longer-term behavioral effect, as indicated by a significant interaction between groups and time (F (6, 67) = 10.96, p < 0.0001) (Fig. 2B). With the exception of ak mice transplanted with NP cells, all cell treated groups displayed a decrease in rearing 2 months post-transplantation compared with 1 month post-transplantation. In contrast, ak mice transplanted with NP cells maintained an increased rearing 2 months post-transplantation (Fig. 2B). 2 months post-transplantation acute injection of L-DOPA increased rearing activity (p < 0.0001). Consistent with 1-month behaviors, the WT and rd1 groups did not differ from the sham-operated ak group, suggesting that the cylinder test is not sensitive enough to differentiate the normal groups from ak mice.
On the challenging beam test, the sham-operated ak group displayed significantly longer latencies to traverse the beam than any of the other groups (F(6, 106) = 8.26, p < 0.0001) (Fig. 2C). Post-hoc comparisons confirmed that ak mice transplanted with EB, NP, or ND cells or treated with L-DOPA, WT and rd1 groups traversed the beam significantly faster than the sham-operated ak group (p < 0.001). The impairment in beam traversal shown by the sham-operated group mimics the slower movements observed in PD patients. Our results indicate that ak mice transplanted with ESC-derived cells (EB, NP, or ND cells) displayed significant motor improvements in beam traversal time. Furthermore, the level of improvement resulting from cell transplantation was comparable with that obtained with L-Dopa administration. We also performed the challenging beam test 2 months post-transplantation. There is a significant interaction between groups and time was not significant (F (6,47.5) = 3.41, p = 0.007). The interaction pattern indicated that traverse time in ak mice transplanted with EB cells was worse than those transplanted with NP- or ND cells 2 months post-transplantation, further suggesting that transplanted EB cells did not show long-term behavioral effect while that of NP or ND cells showed long-term effects (Fig. 2C).
We next performed the pole test, which had been used to assess basal ganglia-related movement behavior in mice (30,47). When tested 1 month post-transplantation, sham-operated ak mice placed heads facing upwards at the top of a vertical pole showed a longer latency to orient themselves downwards than mice in the other groups (F(6,72) = 4.15, p < 0.001, Fig. 2D). Mice with transplanted cells from all three stages showed a reduction in the time required for downward orientation (p < 0.01), indicating that transplanted cells ameliorated impaired motor function seen in ak mice. However, it took more time for mice from transplanted groups for downward orientation compared with rd1, WT controls and L-Dopa-treated ak mice, indicating that motor improvement in this test was partial 1 month post-transplantation. When we further examined this behavior two months post-transplantation, we observed a significant interaction between groups and time (F (6, 72) = 9.66, p < 0.0001, Fig. 2D) and found that the time to orient the head downwards was significantly faster in NP-transplanted ak mice than EB- or ND-transplanted ak mice. The total time to travel down to the home cage also showed consistent results similar to those of the head orientation measurement. At one month post-transplantation, the time to travel down was longer in sham-operated ak mice than those from the NP, ND, rd1, WT, and L-Dopa treated groups (F(6,74.4) = 10.29, p < 0.0001, Fig. 2E). ak mice transplanted with EB cells took longer time to come down into the home cage compared with the NP, ND, rd1, and L-Dopa groups. At two months post-transplantation, we again observed a significant interaction between groups and time (F (6, 71.1) = 3.51, p < 0.004, Fig. 2E). ak mice transplanted with NP cells showed further improved behavior.
Taken together, the above results indicate that transplantation of ak mice with ESC-derived cells significantly improves motor behaviors specific to the nigrostriatal pathway and that transplantation of NP-stage cells resulted in better and/or longer term improvements than that of other stages in these motor tests.
Associative learning deficit of ak mice is reversed by ESC-derived cell transplantation
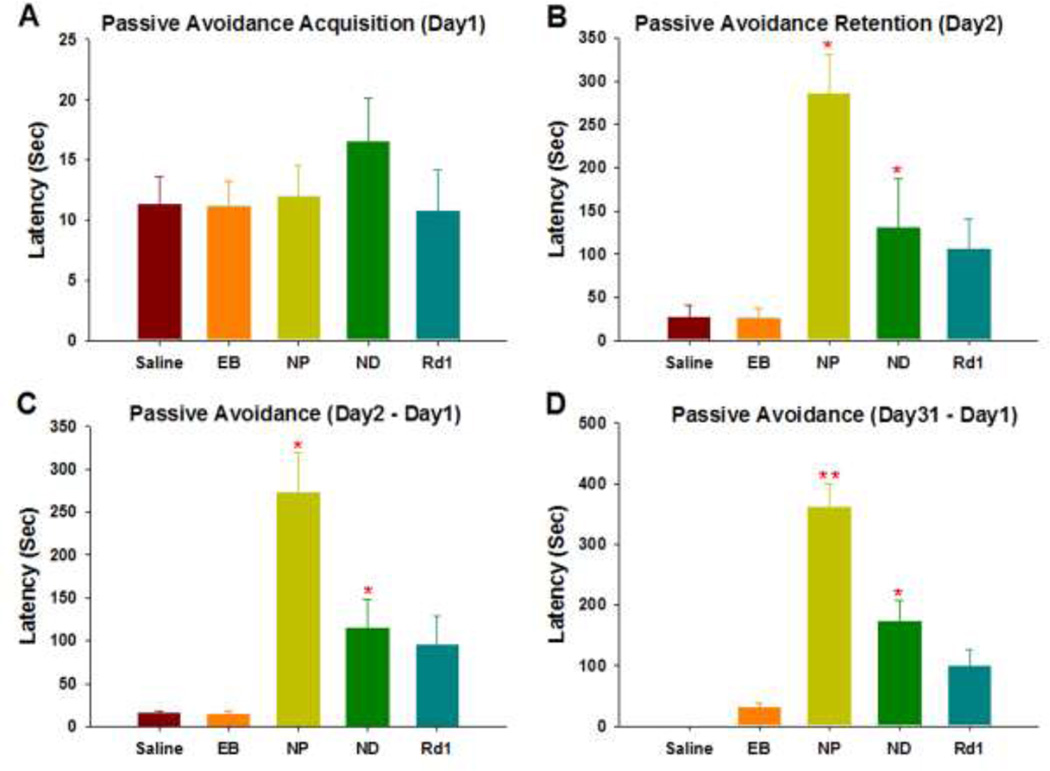
Inhibitory avoidance learning is an operant associative learning task that blind mice can readily perform (19,22). Indeed, the inhibitory avoidance task acquisition trial showed no difference between ak, and rd1 mice (data not shown). When we tested ak groups transplanted with NP or ND cells and the rd1 group at 1 month post-transplantation (which is designated Day1 in Fig. 3A), they showed no difference compared to the sham-operated group in the latency step into the shock paired side, that is in line with the observation that the baseline locomotor activity of animals was similarly intact (Fig. 2A). On day 2, 24 hours after receiving a shock, ak mice transplanted with NP and ND cells and the rd1 group showed strikingly increased latencies, often more than 10-fold the latencies of day 1. In sharp contrast, the latencies of sham-operated ak mice and EB cells-transplanted ak mice increased only slightly if at all on day 2, indicating memory impairment. Comparison of the five groups with respect to change in inhibitory avoidance, which is the change in latency due to learning, showed that sham-operated ak mice were significantly different from ak mice transplanted with NP (p < 0.05) or ND (p < 0.0001), and the rd1 group (p < 0.05) whereas ak mice transplanted with EB did not show a group difference compared with the sham-operated ak group and showed significantly shorter latency compared with ak mice transplanted with NP (p < 0.05) or ND (p < 0.05), and the rd1 group (p < 0.05) (Fig. 3C). There was no difference between ak mice transplanted with NP or ND in any of the measurements. To assess the long-term survival of transplanted cells, passive avoidance tests were performed 2 months after transplantation. The two transplanted groups and the rd1 group exhibited increased latencies, while sham-operated and EB transplanted ak mice did not show any increase (ps < 0.05, Fig. 3D). There was a significant difference between NP and ND transplanted ak mice indicating that NP showed long lasting effect on passive avoidance (p < 0.001). Interestingly, the NP group also showed significantly increased latencies compared with the blind normal control ( the rd1 group). To rule out differences in shock sensitivity, vocalizations were measured, but no statistically significant differences were observed (data not shown).
Figure 3.
An operant associative learning task: (A) In the acquisition trial of the inhibitory avoidance task, there were no differences between sham-operated ak mice and the cell-treated and rd1 groups. (B) Retention trial: 24 hours after receiving the shock, ak mice transplanted with NP, ND cells, and rd1 mice showed increased latencies on day 2 (p < 0.05) compared with mice from the sham-operated and EB-treated groups. (C) When all five groups were compared with regard to change in inhibitory avoidance, both sham and EB transplanted ak groups were significantly different from ak mice transplanted with ND (p < 0.05), NP (p < 0.001), and rd1 mice (p < 0.05). (D) To assess long-term effects, the passive avoidance test was performed 2 months after transplantation. Two transplanted groups (NP and ND) and the rd1 group exhibited increased latencies, while there were no changes in both sham and EB-transplanted ak mice groups (p < 0.05).
* indicates p < 0.05 compared with either sham-operated ak group or EB ak group
** indicates p < 0.001 compared with either sham-operated ak group or EB ak group
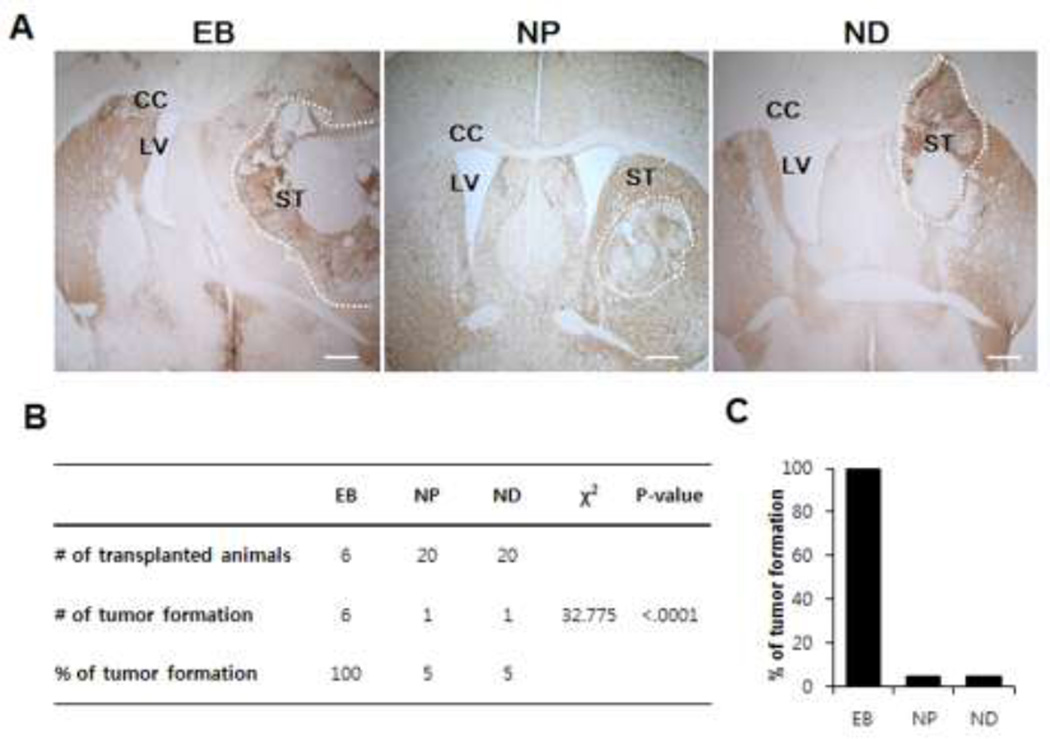
NPs efficiently generate DA neurons after in vivo transplantation into mouse striatum while EB transplantation resulted in teratoma generation in the host brain
At week 9 post-transplantation (after all behavioral tests were performed), we sacrificed transplanted ak mice and analyzed brain sections containing grafted cells. At this point, all 46 transplanted animals survived although all 6 ak mice transplanted with EB cells appeared to be pretty sick. Strikingly, when we analyzed their brains, we found that all six EB-transplanted ak mouse brains contained overgrown teratomas that disrupted host brain structure (Fig. 4A). In contrast, we found only one such teratoma out of 20 ak brains transplanted with NP or ND cells (Fig. 4B, C). Additional analysis using the Chi-squared test confirmed that the number of teratoma formation among groups were significantly different (χ2 = 32.775, p < 0.0001).
Figure 4.
Transplantation of EB cells resulted in teratoma formation in the host brains of all transplanted animals while NP or ND transplantation infrequently generated teratoma: When host brains were analyzed 9 weeks post transplantation, teratoma formation was found in all EB transplanted grafts without exception (A), while grafts of NP or ND cells generated tumor formation in only one out of 20 host brains (B). The dotted line marks the teratoma region. Size bar: 100 µm, Each scale bar is adjusted accordingly.
CC: corpus callosum, LV; lateral ventricle, ST; striatum
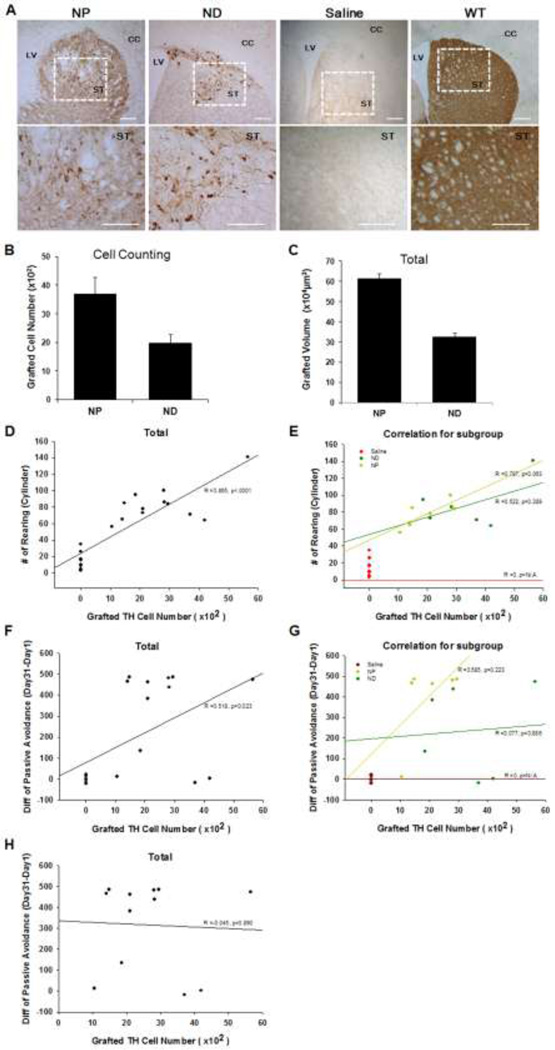
Due to severe brain structure damages and teratoma outgrowth in EB-transplanted ak mice, we further analyzed only NP- and ND-transplanted ak mice. In sharp contrast to sham-operated ak mice (containing no TH+ cell bodies), we observed the presence of large numbers of TH+ neurons in both NP- and ND-transplanted ak mice (Fig. 5A, upper panel, n = 6 for each group). These TH+ neurons residing in the grafts appeared to extend neurites into the host brain (Fig. 5A, bottom panel). The neurites density was much stronger than sham-operated ak group and comparable with the WT controls (Fig. 5A) . A statistically significant difference in the numbers of TH+ neurons and graft size was found in the striatum of NP- and ND-transplanted ak mice brains (Fig. 5B, C, p < 0.01, and p < 0.001, respectively). Taken together, our results indicate that differentiation and/or survival of DA neurons was optimal in NP cell transplantation, which corroborates with our behavioral outcomes. To evaluate the direct correlation between graft-induced functional improvement and surviving TH+ cells, we performed a correlation analysis of our behavioral results in the cylinder test using the Pearson correlation coefficient. Each dot indicates a mouse (Fig. 5D,E). Overall, there was a strong positive correlation between two variables (Pearson coefficient, r2 = 0.748, p<0.0001, Fig. 5D). When correlation was analyzed according to the treatment group, only the NP group exhibited a positive correlation between the two variables (Pearson coefficient, r2 = 0.619, p<0.05), but not the ND group (Pearson correlation coefficient, r2 = 0.273, p > 0.28 for the ND group) (Fig. 5E). As shown in the pattern of scattered dots, the NP group that exhibited an increased number of TH+ neurons tended to exhibit higher rears in the cylinder test. This suggests that the number of surviving TH+ cells in the striatum is positively associated with the recovery of impaired limb usage. Statistical analysis was not applicable for the sham group and EB transplanted group since there were no TH+ cells detected (the sham group) or the number of TH+ neurons could not be analyzed due to severe brain damage (the EB group). To further investigate whether improvement of cognitive function is dopamine-dependent, further examination of the correlation between DA neurons in the graft and the passive avoidance test was performed. There was a significant correlation of DA neurons in the graft and the performance of passive avoidance test (Fig. 5F, Pearson correlation coefficient, r2= 0.268, p < 0.02 for the total). However this positive correlation disappeared when the sham-operated group was removed (Fig. 5H, Pearson correlation coefficient, r2= 0.002, p > 0.89 for the total without Sham-operated group) (Fig. 5G). Furthermore, the correlation analysis of individual group did not show any positive correlation. Further investigations are warranted in order to identify the underlying mechanism by which impaired striatum dependent cognition was improved.
Figure 5.
(A) Transplantation of NP and ND stage cells into the striatum of ak mice resulted in the presence of large numbers of TH+ neurons in the grafts, while no TH+ cells were detected in the sham-operated group. The enlarged brain sections indicated by the boxes show the presence of TH + cell bodies in the host brain. Size bar: 100 µm, Each scale bar is adjusted accordingly. (B&C) Total numbers of TH + neurons and graft volume in the brain of NP-transplanted (n = 6 for each group) (B) and ND-transplanted ak mice (C). At 9 weeks post transplantation, total TH+ cells were counted. In the EB-transplanted group, quantification of TH+ cells was not feasible due to malformation of brain structure. There were statistically significant differences in the numbers of surviving TH+ neurons in the striatum of ak mice transplanted with NP and ND cells. Graft analysis revealed a larger volume in NP transplanted mice than in the ND transplanted group. (D&E) Correlation between graft-induced functional improvement and TH+ cells: Each dot indicates a mouse. There was a strong positive correlation between two variables (Pearson coefficient, r2 = 0.748, p<0.0001). The NP group exhibited a strong positive correlation between the two variables (Pearson coefficient, r2 = 0.619, p < 0.05, black color), but not in the ND group (Pearson correlation coefficient, r2 = 0.273, p > 0.28 for the ND group, N/A for the sham and EB group). (F) There was a positive correlation between two variables (Pearson coefficient, r2 = 0.268, p<0.02). (G & H) However, the positive correlation disappeared when individual groups were analyzed and sham-operated groups were removed.
NA indicates data were not applicable to compare among treatment groups
CC: corpus callosum, LV; lateral ventricle, ST; striatum
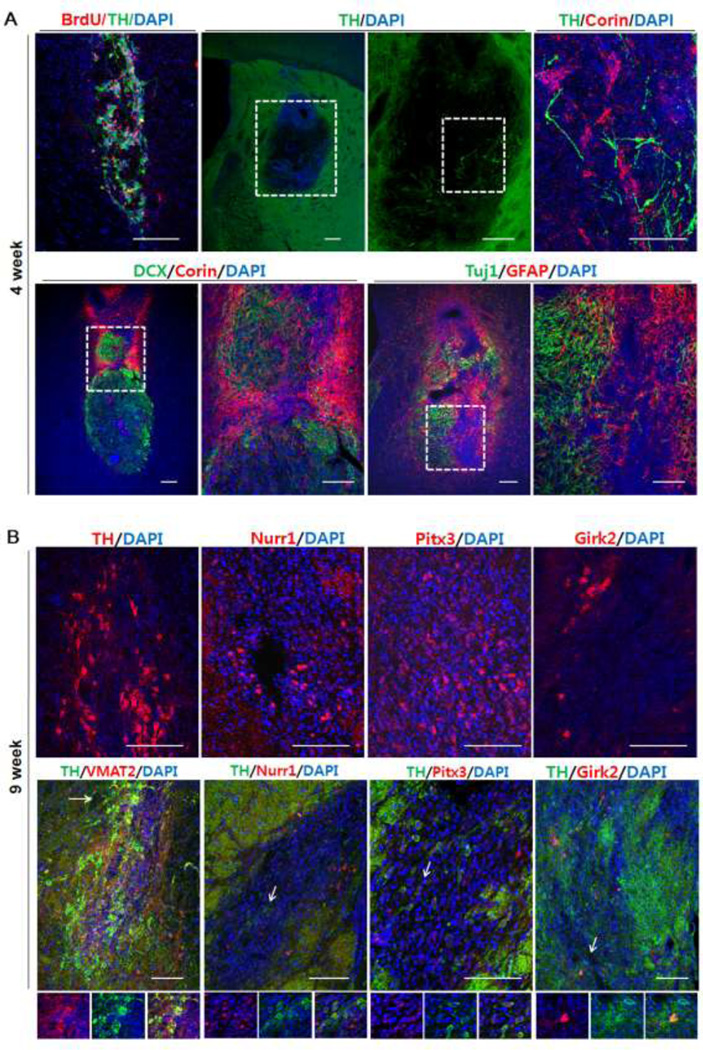
Next, we further examined the dopaminergic cell phenotype in ak mice transplanted with NPs at 9 weeks post transplantation (following all behavioral tests). In addition, at an earlier time point, we transplanted NPs in additional ak mice and analyzed the grafts at 4 weeks post transplantation. One month after transplantation, we found many Corin+ cells (a cell surface marker of floor plate cells) (44) but few TH+ cells at the injection site (Fig. 6A). BrdU labeled NPCs were co-localized with TH+ cells indicating that TH+ cells were differentiated from injected NPCs (Fig. 6A). In addition, we found that many doublecortin+ (DCX; a marker of migrating neuroblasts) cells were co-labeled with Corin+ cells at 4 weeks. DCX+ cells were clustered with Corin+ cells in the graft indicating that injected NPs may have the migration capacity.
Figure 6.
Marker gene expression in the grafts at 4 weeks and 9 weeks post transplantation: (A) BrdU labeled NPCs co-localized with TH+ cells. 4 weeks post-transplantation, a large number of Corin+ cells appeared in the graft, but few TH+ cells were observed. Many Corin+ cells coexpressed DCX. Many GFAP+ cells (astrocytes) and Tuj1+ cells (neurons) were clustered at the injection site. (B) 9 weeks post-transplantation, much more TH+ cells were detected, many of which co-expressed other mDA markers such as Pitx3, Nurr1, and Girk2. Arrows indicate co-expressing cells. Size bar: 100 µm, Each scale bar is adjusted accordingly
At 4 weeks post transplantation, GFAP+ cells and Tuj1+ cells were also clustered at the injection site (Fig. 6A). In comparison with the 4 weeks post-transplantation results, there were much more TH+ cells at 9 weeks post transplantation while Corin+ cells significantly decreased (Fig. 6B; data not shown). Furthermore, these TH+ cells appeared to have midbrain phenotype, as evidenced by co-expression of midbrain dopaminergic markers such as Pitx3 and Nurr1. In addition, some of these TH+ cells co-expressed the A9-specific marker, Girk2. Taken together, these data indicate that transplanted NP cells differentiated into midbrain-type DA neurons in the host brain.
Discussion
Since the main pathology of PD is selective degeneration of mDA neurons in the SN, stem cell-based cell replacement is a highly promising therapeutic approach. Indeed, several clinical trials of intrastriatal transplantation of human fetal mesencephalic tissues provided proof-of-principle that cell replacement therapy can work in PD patients (36,37,48). However, due to significant ethical, technical, and practical limitations of fetal cell transplantation, it is critical to develop additional cell sources by testing their behavioral effects in adequate animal models. Toward this goal, numerous laboratories have investigated a variety of stem cells using animal models from different species (e.g., mouse, rat, and monkey), which are mostly based on neurotoxin (e.g., 6-OHDA and MPTP) treatment to elicit nigrostriatal lesion (4,5,7,18). Since these neurochemical animal models are labor-intensive and prone to fluctuation in terms of the degree of lesion, it is challenging to use them for high throughput and systematic analyses of different aspects of cell transplantation therapy. With the long-term goal of establishing a genetic animal model(s) of PD for cell transplantation studies, the present study investigated the behavioral outcomes of transplantation of ESC-derived cells into the striatum of ak mice. While numerous genetic models of PD have been generated by knock out or overexpression of one or more familial PD genes in mice, they have fundamental limitations as host animals for stem cell transplantation studies because they neither display the selective loss of midbrain DA neurons nor display clear motor dysfunction (23,39). In this regard, the ak mouse is a promising candidate model because of the robust loss of midbrain DA neurons and motor deficits, although its severe early developmental deficit and potential compensatory action may not exactly mimic those seen in PD. Since ak mice can breed as homozygote pairs, large number of animals are readily available for systematic behavioral analyses with minimal individual fluctuations. This unique PD model also has the advantage to test both neurological as well as cognitive deficits. Thus, we transplanted, for the first time to our knowledge, cells at different stages of in vitro differentiation (i.e., pluripotent EB, multipotent NP, and differentiated ND cells) and compared the functional and behavioral outcomes. Based on previous findings that ak mice exhibit deficits in motor and cognitive behaviors (2,30), we also tested if stem cell transplantation can ameliorate cognitive impairments as well. This comprehensive study revealed several salient aspects of stem cell transplantation, as discussed below.
Transplantation of different stage cells result in different tumor formation frequencies in host animals
In order for stem cell transplantation to become a realistic treatment for PD, it is of the utmost importance that tumor formation be completely avoided. This is even more so because, albeit PD is a devastating disease, the life span of patients is not significantly compromised. Thus, it is critical to carefully assess tumor formation in transplantation studies using animal models and how to prevent it. In a previous study (8), transplantation of low numbers (1,000 to 2,000 cells per animal) of undifferentiated mouse ESCs in 6-OHDA lesioned rats resulted in direct differentiation into DA neurons and an improvement in motor function, while about 20% of transplanted animals developed teratoma-like tumors. Remarkably, such tumor formation was avoided when in vitro-differentiated mouse ESCs (stage 5 day 3) were used, resulting in efficient behavioral improvement (13). In addition, tumor formation could be avoided when purified NPs were transplanted using knock-in mouse ESCs at the Sox1 gene locus (16). However, these purified Sox1+ /GFP+ NPs inefficiently generated DA neurons, possibly due to different developmental origin. In the present study, we transplanted different stage cells from mouse ESCs into ak mice and compared functional and behavioral outcomes, including formation of teratoma-like tumors. Strikingly, all host animals transplanted with 4,000 EB cells (2,000 EB cells in each hemisphere) developed teratoma(s), which is quite different from the previous study showing 20% teratoma formation following transplantation with 1000 to 2,000 undifferentiated ESCs (8). Although exact reasons for these different results are not clear, it may be explained by the different experimental conditions used in these two studies. First, it should be noted that different species of host animals (rat and mouse) were used. Since the present study used isogenic cell transplantation, it may have caused a more striking formation of teratomas in the host brains. In support of this possibility, an interesting previous study demonstrated that, in the homologous mouse brain, transplantation of mouse ESCs generated teratomas with much higher frequencies (20). Second, different stage cells from mouse ESCs (stage 1 and 2) are used. Although both ES (stage 1) and EB (stage 2) cells have developmentally similar potential and are pluripotent, this difference may still contribute differently to teratoma formation. Third, the present study did not use immunosuppressant treatment because it was unnecessary due to their isogenic nature. Finally, this study used a smaller number of cells for transplantation compared to the 2002 study. Given that the mouse brain is much smaller than the rat brain, the cell dose of the present study was in fact much smaller, leading to a quite different outcome of teratoma formation. Another interesting finding is that teratoma formation was not completely prevented when differentiated cells were transplanted. It is possible that our cell preparation still contained some residual undifferentiated cells even at the stage 5, occasionally causing teratoma formation. Our finding strongly suggests that it is absolutely important to develop efficient methods/procedures to completely remove these remaining undifferentiated cells prior to clinical application of transplantation therapy. Alternatively, it is equally important to further develop efficient protocols to differentiate ESCs into functional cells and to purify them prior to transplantation.
Transplantation of NP-stage cells exhibit efficient and long-term behavioral recovery
Based on our previous studies showing selective degeneration of A9 mDA neurons in the SN and related motor behavior deficits in ak mice (29,30), we addressed how the transplantation of mouse ESCs-derived cells at each stage of differentiation affects these behaviors in comparison with genetic (rd) and drug (L-dopa)-treated control mice. Toward this goal, we tested various motor behavioral paradigms such as cylinder, beam, and pole tests one and two months post transplantation. These tasks are specific to basal ganglia function and involve: 1) letting them freely check the cylinder environment, 2) running them on the demanding beam, 3) elevating the animals by placing them on the pole, and 4) recording the frequency and direction of the specific behavior. Interestingly, in the cylinder test, grafted animals showed a significant increase in rearing compared to sham animals, indicating that transplantation of all stage cells exerted functional improvement. In the beam and pole tests, mice transplanted with NP or ND cells showed better improvements than those transplanted with EB cells one month post transplantation. Thus, mice transplanted with NP or ND cells exhibited better functional recovery than those transplanted with EB cells. Importantly, 2 months post-transplantation, NP-transplanted ak mice showed comparable or better improvement than EB- or ND-transplanted ak mice in all behavioral tests. Taken together, our results indicate that grafting of NP cells generated long-term and optimal functional integration in the host striatum. In line with this conclusion, a higher number of TH+ neurons was found in the striatum of NP- transplanted ak mice than ND-transplanted animals. Furthermore, there was a positive correlation between the number of TH+ neurons and the behavioral outcome (limb usage in the cylinder test) in the NP group.
Improved Cognitive Function by cell transplantation in ak mice
Up to 80% of PD patients exhibit a broad range of cognitive impairments, from dementia and executive dysfunction to more subtle memory loss (1,10,12,54). Thus, behavioral examination of cognitive dysfunction in PD animal models is crucial to assess the effectiveness of therapeutic approaches. Nevertheless, these cognitive/non-motor deficits in PD animal models have rarely been reported, largely due to the lack of appropriate animal models that can allow the assessment of such behavioral outcomes. Based on our previous study showing that ak mice exhibit cognitive impairments (2), we attempted to test whether transplantation of ESC-derived cells improves behavioral deficits in the passive avoidance task, a well-known assessment of learning. We found that ak mice transplanted with NP and ND cells showed prominently improved behavior, as evidenced by much prolonged latencies compared to the sham group. In particular, transplantation of NP cells increased the latency more than 10-fold, again suggesting that NP cells represent the best cell source for functional improvement. Furthermore, this cognitive improvement persisted 2 months post-transplantation. Further investigation is warranted to elucidate the mechanisms underlying the improvement in cognitive function. For instance, it will be of interest to investigate if restored DA neurotransmission or other neurotransmitter systems (e.g., serotonin or glutamate) is responsible for this improvement in this DA depleted animal model. In addition, it will be informative to perform similar transplantation experiments within other neuronal circuits that are known to underlie cognitive behaviors, preferentially using purified neuronal subtype populations.
Conclusions
Our findings strongly suggest that the ak mouse, a genetic PD model, is a useful animal model to investigate different aspects of PD such as motor deficits and cognitive impairments as well as therapeutic approaches such as cell transplantation and treatment with small molecules (e.g., L-dopa). The availability of large numbers of animals allow the systematic study of different aspect of cell transplantation therapy such as tumor formation in addition to behavioral improvements. In addition, our study emphasizes that grafting of pluripotent cells (e.g., EB or ESC) causes tumor formation and suggests that NP stage cells may represent the optimal stage cell source for functional integration and long-lasting behavioral improvement. However, NP cells can also cause teratoma formation if there are residual pluripotent cells, necessitating further strategy development to remove undifferentiated cells to completely avoid tumor formation prior to clinical application.
Acknowledgement
This study was supported by National Institute of Health grants (NS070577, MH048866, MH087903, KOSEF 2011-0029342, and KOSEF 2011-0013280).
Glossary
- PD
Parkinson’s disease
- DA
Dopamine
- ESCs
Embryonic stem cells
- 6-OHDA
6-hydroxyl Dopamine
- Ak
Aphakia
- EB
Embryonic body
- NP
Neural progenitor or neural precursor
- ND
Neuronal differentiated
- SN
Substantia nigra
- TH
Tyrosine hydroxylase
- L-DOPA
L-3,4-dihydroxyphenylalanine
Footnotes
Disclosures
The authors indicate no potential conflicts of interest.
References
- 1.Agid Y, Ruberg M, Dubois B, Pillon B, Cusimano G, Raisman R, Cash R, Lhermitte F, Javoy-Agid F. Parkinson's disease and dementia. Clin. Neuropharmacol. 1986;9(Suppl. 2):S22–S36. [PubMed] [Google Scholar]
- 2.Ardayfio P, Moon J, Leung KK, Youn-Hwang D, Kim KS. Impaired learning and memory in Pitx3 deficient aphakia mice: a genetic model for striatum-dependent cognitive symptoms in Parkinson's disease. Neurobiol. Dis. 2008;31(3):406–412. doi: 10.1016/j.nbd.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, Tabar V, Harrison NL, Beal MF, Moore MA, Studer L. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat. Biotechnol. 2003;21(10):1200–1207. doi: 10.1038/nbt870. [DOI] [PubMed] [Google Scholar]
- 4.Beal MF. Experimental models of Parkinson's disease. Nat. Rev. Neurosci. 2001;2(5):325–334. doi: 10.1038/35072550. [DOI] [PubMed] [Google Scholar]
- 5.Beal MF. Parkinson's disease: a model dilemma. Nature. 2010;466(7310):S8–S10. doi: 10.1038/466S8a. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Hur T, Idelson M, Khaner H, Pera M, Reinhartz E, Itzik A, Reubinoff BE. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells. 2004;22(7):1246–1255. doi: 10.1634/stemcells.2004-0094. [DOI] [PubMed] [Google Scholar]
- 7.Betarbet R, Sherer TB, Greenamyre JT. Animal models of Parkinson's disease. Bioessays. 2002;24(4):308–318. doi: 10.1002/bies.10067. [DOI] [PubMed] [Google Scholar]
- 8.Bjorklund LM, Sanchez-Pernaute R, Chung S, Andersson T, Chen IY, McNaught KS, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, Isacson O. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc. Natl. Acad. Sci. USA. 2002;99(4):2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blandini F, Cova L, Armentero MT, Zennaro E, Levandis G, Bossolasco P, Calzarossa C, Mellone M, Giuseppe B, Deliliers GL, Polli E, Nappi G, Silani V. Transplantation of undifferentiated human mesenchymal stem cells protects against 6-hydroxydopamine neurotoxicity in the rat. Cell Transplant. 19(2):203–217. doi: 10.3727/096368909X479839. [DOI] [PubMed] [Google Scholar]
- 10.Bosboom JL, Stoffers D, Wolters E. Cognitive dysfunction and dementia in Parkinson's disease. J. Neural. Transm. 2004;111(10–11):1303–1315. doi: 10.1007/s00702-004-0168-1. [DOI] [PubMed] [Google Scholar]
- 11.Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, Morizane A, Bergquist F, Riebe I, Nannmark U, Carta M, Hanse E, Takahashi J, Takahashi J, Sasai Y, Funa K, Brundin P, Eriksson PS, Li JY. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson's disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006;24(6):1433–1440. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]
- 12.Bronnick K, Aarsland D, Larsen JP. Neuropsychiatric disturbances in Parkinson's disease clusters in five groups with different prevalence of dementia. Acta Psychiatr. Scand. 2005;112(3):201–207. doi: 10.1111/j.1600-0447.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- 13.Chang JH, Choi JY, Chang JW, Park YG, Kim TS, Chung SS. Chronic epidural hematoma with rapid ossification. Childs Nerv. Syst. 2002;18(12):712–716. doi: 10.1007/s00381-002-0664-2. [DOI] [PubMed] [Google Scholar]
- 14.Cho MS, Lee YE, Kim JY, Chung S, Cho YH, Kim DS, Kang SM, Lee H, Kim MH, Kim JH, Leem JW, Oh SK, Choi YM, Hwang DY, Chang JW, Kim DW. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2008;105(9):3392–3397. doi: 10.1073/pnas.0712359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung S, Shin BS, Hedlund E, Pruszak J, Ferree A, Kang UJ, Isacson O, Kim KS. Genetic selection of sox1GFP-expressing neural precursors removes residual tumorigenic pluripotent stem cells and attenuates tumor formation after transplantation. J. Neurochem. 2006;97(5):1467–1480. doi: 10.1111/j.1471-4159.2006.03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung S, Shin BS, Hwang M, Lardaro T, Kang UJ, Isacson O, Kim KS. Neural precursors derived from embryonic stem cells, but not those from fetal ventral mesencephalon, maintain the potential to differentiate into dopaminergic neurons after expansion in vitro. Stem Cells. 2006;24(6):1583–1593. doi: 10.1634/stemcells.2005-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung S, Sonntag KC, Andersson T, Bjorklund LM, Park JJ, Kim DW, Kang UJ, Isacson O, Kim KS. Genetic engineering of mouse embryonic stem cells by Nurr1 enhances differentiation and maturation into dopaminergic neurons. Eur. J. Neurosci. 2002;16(10):1829–1838. doi: 10.1046/j.1460-9568.2002.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 19.Dyer RS, Hammond MA, Weldon DA, Booker TC. Influence of enucleation upon two-way avoidance behavior of rats, hamsters, chinchillas and BALB/cJ mice. Physiol. Behav. 1975;14(2):211–216. doi: 10.1016/0031-9384(75)90168-7. [DOI] [PubMed] [Google Scholar]
- 20.Erdo F, Buhrle C, Blunk J, Hoehn M, Xia Y, Fleischmann B, Focking M, Kustermann E, Kolossov E, Hescheler J, Hossmann KA, Hossmann KA, Trapp T. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J. Cereb. Blood Flow Metab. 2003;23(7):780–785. doi: 10.1097/01.WCB.0000071886.63724.FB. [DOI] [PubMed] [Google Scholar]
- 21.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 22.Farr SA, Banks WA, La Scola ME, Morley JE. Blind mice are not impaired in T-maze footshock avoidance acquisition and retention. Physiol. Behav. 2002;76(4–5):531–538. doi: 10.1016/s0031-9384(02)00749-7. [DOI] [PubMed] [Google Scholar]
- 23.Fleming SM, Fernagut PO, Chesselet MF. Genetic mouse models of parkinsonism: strengths and limitations. NeuroRx. 2005;2(3):495–503. doi: 10.1602/neurorx.2.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J. Neurosci. 2004;24(42):9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N. Engl. J. Med. 2001;344(10):710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 26.Hagell P, Schrag A, Piccini P, Jahanshahi M, Brown R, Rehncrona S, Widner H, Brundin P, Rothwell JC, Odin P, Wenning GK, Morrish P, Gustavii B, Björklund A, Brooks DJ, Marsden CD, Quinn NP, Lindvall O. Sequential bilateral transplantation in Parkinson's disease: effects of the second graft. Brain. 1999;122(Pt 6):1121–1132. doi: 10.1093/brain/122.6.1121. [DOI] [PubMed] [Google Scholar]
- 27.Hedlund E, Pruszak J, Ferree A, Vinuela A, Hong S, Isacson O, Kim KS. Selection of embryonic stem cell-derived enhanced green fluorescent protein-positive dopamine neurons using the tyrosine hydroxylase promoter is confounded by reporter gene expression in immature cell populations. Stem Cells. 2007;25(5):1126–1135. doi: 10.1634/stemcells.2006-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedlund E, Pruszak J, Lardaro T, Ludwig W, Vinuela A, Kim KS, Isacson O. Embryonic stem cell-derived Pitx3-enhanced green fluorescent protein midbrain dopamine neurons survive enrichment by fluorescence-activated cell sorting and function in an animal model of Parkinson's disease. Stem Cells. 2008;26(6):1526–1536. doi: 10.1634/stemcells.2007-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang DY, Ardayfio P, Kang UJ, Semina EV, Kim KS. Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res. Mol. Brain Res. 2003;114(2):123–131. doi: 10.1016/s0169-328x(03)00162-1. [DOI] [PubMed] [Google Scholar]
- 30.Hwang DY, Fleming SM, Ardayfio P, Moran-Gates T, Kim H, Tarazi FI, Chesselet MF, Kim KS. 3,4-dihydroxyphenylalanine reverses the motor deficits in Pitx3-deficient aphakia mice: behavioral characterization of a novel genetic model of Parkinson's disease. J. Neurosci. 2005;25(8):2132–2137. doi: 10.1523/JNEUROSCI.3718-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim DW, Chung S, Hwang M, Ferree A, Tsai HC, Park JJ, Nam TS, Kang UJ, Isacson O, Kim KS. Stromal cell-derived inducing activity, Nurr1, and signaling molecules synergistically induce dopaminergic neurons from mouse embryonic stem cells. Stem Cells. 2006;24(3):557–567. doi: 10.1634/stemcells.2005-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, McKay R. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418(6893):50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 33.Lang AE, Lozano AM. Parkinson's disease. First of two parts. N. Engl. J. Med. 1998;339(15):1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 34.Lang AE, Lozano AM. Parkinson's disease. Second of two parts. N. Engl. J. Med. 1998;339(16):1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- 35.Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat. Biotechnol. 2000;18(6):675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- 36.Lindvall O, Kokaia Z. Prospects of stem cell therapy for replacing dopamine neurons in Parkinson's disease. Trends Pharmacol. Sci. 2009;30(5):260–267. doi: 10.1016/j.tips.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441(7097):1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 38.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA. 1981;78(12):7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meredith GE, Kang UJ. Behavioral models of Parkinson's disease in rodents: a new look at an old problem. Mov. Disord. 2006;21(10):1595–606. doi: 10.1002/mds.21010. [DOI] [PubMed] [Google Scholar]
- 40.Morizane A, Takahashi J, Shinoyama M, Ideguchi M, Takagi Y, Fukuda H, Koyanagi M, Sasai Y, Hashimoto N. Generation of graftable dopaminergic neuron progenitors from mouse ES cells by a combination of coculture and neurosphere methods. J. Neurosci. Res. 2006;83(6):1015–1027. doi: 10.1002/jnr.20799. [DOI] [PubMed] [Google Scholar]
- 41.Nikkhah G, Rosenthal C, Falkenstein G, Roedter A, Papazoglou A, Brandis A. Microtransplantation of dopaminergic cell suspensions: further characterization and optimization of grafting parameters. Cell Transplant. 2009;18(2):119–133. doi: 10.3727/096368909788341324. [DOI] [PubMed] [Google Scholar]
- 42.Nunes I, Tovmasian LT, Silva RM, Burke RE, Goff SP. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc. Natl. Acad. Sci. USA. 2003;100(7):4245–4250. doi: 10.1073/pnas.0230529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, G M, Perl GM, Perl DP, Godbold J, Freeman TB. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann. Neurol. 2003;54(3):403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 44.Ono Y, Nakatani T, Sakamoto Y, Mizuhara E, Minaki Y, Kumai M, Hamaguchi A, Nishimura M, Inoue Y, Hayashi H, Takahashi J, Imai T. Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development. 2007;134(17):3213–3225. doi: 10.1242/dev.02879. [DOI] [PubMed] [Google Scholar]
- 45.Piccini P, Brooks DJ, Bjorklund A, Gunn RN, Grasby PM, Rimoldi O, Brundin P, Hagell P, Rehncrona S, Widner H, Lindvall O. Dopamine release from nigral transplants visualized in vivo in a Parkinson's patient. Nat. Neurosci. 1999;2(12):1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez-Gomez JA, Lu JQ, Velasco I, Rivera S, Zoghbi SS, Liow JS, Musachio JL, Chin FT, Toyama H, Seidel J, Green MV, Thanos PK, Ichise M, Pike VW, Innis RB, McKay RD. Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson disease. Stem Cells. 2007;25(4):918–928. doi: 10.1634/stemcells.2006-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat. Med. 2006;12(11):1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 48.Sedelis M, Schwarting RK, Huston JP. Behavioral phenotyping of the MPTP mouse model of Parkinson's disease; Behav. Brain Res. 2001;125(1–2):109–125. doi: 10.1016/s0166-4328(01)00309-6. [DOI] [PubMed] [Google Scholar]
- 49.Siddique J, Belin TR. Using an Approximate Bayesian Bootstrap to Multiply Impute Nonignorable Missing Data. Comput. Stat. Data Anal. 2008;53(2):405–415. doi: 10.1016/j.csda.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smidt MP, Smits SM, Bouwmeester H, Hamers FP, van der Linden AJ, Hellemons AJ, Graw J, Burbach JP. Early developmental failure of substantia nigra dopamine neurons in mice lacking the homeodomain gene Pitx3. Development. 2004;131(5):1145–1155. doi: 10.1242/dev.01022. [DOI] [PubMed] [Google Scholar]
- 51.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 52.van den Munckhof P, Luk KC, Ste-Marie L, Montgomery J, Blanchet PJ, Sadikot AF, Drouin J. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development. 2003;130(11):2535–2542. doi: 10.1242/dev.00464. [DOI] [PubMed] [Google Scholar]
- 53.Wolfinger R, O'Connell M. Generalized linear mixed models: a pseudo-likelihood approach. Journal of Statistical Computing Simulations. 1993;48:233–243. [Google Scholar]
- 54.Zgaljardic DJ, Foldi NS, Borod JC. Cognitive and behavioral dysfunction in Parkinson's disease: neurochemical and clinicopathological contributions. J. Neural. Transm. 2004;111(10–11):1287–1301. doi: 10.1007/s00702-004-0178-z. [DOI] [PubMed] [Google Scholar]