Abstract
During endochondral bone formation, chondrocytes and osteoblasts synthesize and mineralize the extracellular matrix through a process that initiates within matrix vesicles (MVs) and ends with bone mineral propagation onto the collagenous scaffold. pH gradients have been identified in the growth plate of long bones, but how pH changes affect the initiation of skeletal mineralization is not known. Tissue-nonspecific alkaline phosphatase (TNAP) degrades extracellular inorganic pyrophosphate (ePPi), a mineralization inhibitor produced by ectonucleotide pyrophosphatase/ phosphodiesterase-1 (NPP1), while contributing Pi from ATP to initiate mineralization. TNAP and NPP1, alone or combined, were reconstituted in dipalmitoylphosphatidylcholine (DPPC) liposomes to mimic the microenvironment of MVs. The hydrolysis of ATP, ADP, AMP and PPi was studied at pH 8 and 9 and compared to the data determined at pH 7.4. While catalytic efficiencies in general were higher at alkaline pH, PPi hydrolysis was maximal at pH 8 and indicated a preferential utilization of PPi over ATP, at pH 8 versus 9. In addition, all proteoliposomes induced mineral formation when incubated in a synthetic cartilage lymph (SCL) containing 1 mM ATP as substrate and amorphous calcium phosphate (ACP) or calciumphosphate- phosphatidylserine complexes (PS-CPLX) as nucleators. Propagation of mineralization was significantly more efficient at pHs 7.5 and 8 than at pH 9. Since a slight pH elevation from 7.4 to 8 promotes considerably more hydrolysis of ATP, ADP and AMP primarily by TNAP, this small pH change facilitates mineralization, especially via upregulated PPi hydrolysis by both NPP1 and TNAP, further elevating the Pi/PPi ratio, thus enhancing bone mineralization.
Keywords: biomineralization, alkaline pH, microenvironment, proteoliposomes, pyrophosphate, ATP
During endochondral bone formation, chondrocytes and osteoblasts synthesize and mineralize the extracellular matrix [1] through a process that starts within matrix vesicles (MVs) as the sites of initiation of hydroxyapatite (HA) deposition and ends with bone mineral propagation onto the collagenous scaffold. The phosphatases tissue-nonspecific alkaline phosphatase (TNAP, EC 3.1.3.1) and ectonucleotide pyrophosphatase/ phosphodiesterase-1 (NPP1, EC 3.6.1.9) have been implicated in MV-mediated calcification [2].
TNAP is confined to the cell surface of osteoblasts and chondrocytes, including the membranes of their shed MVs [3]. Several studies have proposed a phosphate-generating function for TNAP producing inorganic phosphate (Pi) needed for HA crystallization [4], while others proposed that TNAP’s role is to hydrolyze the mineralization inhibitor PPi [5] to facilitate mineral precipitation and growth [6], a conclusion supported by our own work [7,8]. Hence, by hydrolyzing extracellular PPi, a proper concentration of this mineralization inhibitor is maintained to allow normal bone mineralization.
PPi is generated by the ectonucleotide pyrophosphatase/phosphodiesterase (NPP) family of isozymes. NPP1 is plasma membrane-bound, whereas autotaxin (NPP2) is secreted and B10 (NPP3) is abundant in intracellular spaces [9]. All three isozymes are expressed in a wide variety of tissues, including bone and cartilage [10], and they have the common ability to hydrolyze diesters of phosphoric acid into phosphomonoesters. NPP1 is highly abundant on the surfaces of osteoblasts and chondrocytes as well as on the membrane of their MVs and it has a role in inhibiting HA precipitation by its PPigenerating property [11]. However, NPP1 can also act as a phosphatase [12], and we have shown that it can generate Pi from both ATP and PPi in the MV microenvironment [13,14].
Initiation of biomineralization occurs in a microenvironment which determines the biological properties and physiological interplay between MV-associated enzymes and proteins. Recent data [15] suggest that the location of TNAP on the membrane of MVs plays a role in determining substrate selectivity in this microcompartment, suggesting that assays of TNAP bound to MVs or to liposome systems might be more relevant than assays done with solubilized enzymes, particularly when studying the hydrolysis of organophosphate substrates. The ability of synthetic or natural vesicles [16,17] to mimic the structure and function of biomembranes makes these structures an advantageous and convenient model to help us advance our understanding of MV-mediated calcification. Simão et al. [14] reconstituted either TNAP, NPP1 or both together into liposome membranes and evaluated their synergistic and/or antagonistic activity, at physiological pH, when presented with physiological substrates relevant to the biomineralization process.
In addition to biophysical factors, it has been shown that also the pH may play a regulatory role during the mineralization process [18–20]. Since the hydrolysis of ATP, ADP, AMP and PPi by TNAP and NPP1 critically contributes to bone formation and since these enzymes show an alkaline pH optimum [14,21,22], we have studied the hydrolysis of these phosphosubstrates by proteoliposomes harboring TNAP and/or NPP1 at slightly alkaline pH (pH 8 vs. 9) and compared our findings with data obtained at physiological pH. Our results show that even modest pH elevations have a strong impact on the phosphosubstrates hydrolysis rate.
Previous studies discovered a nucleational core that drives mineral formation by native MVs [23,24]. Components identified to be essential were amorphous calcium phosphate (ACP) and its combined form with phosphatidylserine (PS), the calcium-phosphate-lipid complexes (PS-CPLX). Here we have also examined the effects of ACP and PS-CPLX for their ability to modulate mineral formation induced by proteoliposomes containing either TNAP, NPP1 or both together when incubated in a synthetic cartilage lymph (SCL) carefully constructed to contain physiological levels of electrolytes and substrates.
Materials and Methods
Cell Culture and Preparation of Membrane Fractions Rich in TNAP and NPP1
Cells were prepared and cultured according to Simão et al. [25]. A plasmid containing rat NPP2 Nterminal signal peptide (33 residues) connected to the residues of mouse NPP1 (residues 85–905) was kindly provided by Dr. Bollen [26]. A glycosylphosphatidylinositol (GPI)-anchored form of mouse NPP1 was produced and expressed as described [14]. Membrane-bound TNAP and NPP1 were obtained from 14-day primary osteoblast cultures or from transfected COS-1 cells, respectively, as described [14].
Solubilization and Partial Purification of GPI-anchored Enzymes with Polyoxyethylene-9-Lauryl Ether (Polidocanol)
Membrane-bound enzymes (0.2 mg/ml total protein) were solubilized with 1% polidocanol (w/v) (final concentration) for 2 h, with constant stirring, at 25 °C. After centrifugation at 100,000×g for 1 h at 4 °C, detergent-free solubilized enzymes were obtained using 200 mg of Calbiosorb resin and 1 ml of polidocanol-solubilized enzyme (~0.03 mg of protein/ml), as previously described [27]. All protein concentrations were estimated in the presence of 2% (w/v) SDS [28]. Bovine serum albumin was used as a standard.
Liposome Preparation and Incorporation of GPI-anchored TNAP and NPP1 into Liposomes
Dipalmitoyl phosphatidylcholine (DPPC) liposomes and DPPC proteoliposomes containing TNAP, NPP1 or TNAP + NPP1 were prepared as previously described [14].
Enzymatic Assays
For ATP, ADP, AMP, and PPi hydrolysis, the phosphomonohydrolase activities were assayed discontinuously, at 37 °C, by measuring the amount of inorganic phosphate liberated, as before [29], adjusting the assay medium to a final volume of 0.5 ml. The reaction was initiated by the addition of the enzyme and stopped with cold 30% trichloracetic acid (TCA) at appropriate time intervals. The reaction mixture was centrifuged at 4000×g, and phosphate was quantified in the supernatant according to the procedure described by Heinonen and Lahti [30]. Standard assay conditions were 50 mM 2-amino-2- methyl-propan-1-ol (AMPOL) buffer, pH 9, containing 2 mM MgCl2 and substrate, or 50 mM Tris-HCl buffer, pH 8, containing 2 mM MgCl2 and substrate. Initial velocities were constant for at least 90 min, provided that less than 5% of substrate was hydrolyzed. Controls without added enzyme were included in each experiment to correct for non-enzymatic hydrolysis of substrate. One enzyme unit (1 unit/mg) is defined as the amount of enzyme hydrolyzing 1.0 nmol of substrate/min/mg of protein. Maximum velocity (Vmax), apparent dissociation constant (K0.5), and Hill coefficient (n) obtained from substrate hydrolysis were calculated as described [31]. Data were reported as the mean of triplicate measurements of three different enzyme preparations. Statistically significant differences were defined as p ≤ 0.05.
Measurement of Nucleotide Hydrolysis by HPLC
Hydrolysis of ATP and ADP by proteoliposomes was determined at 37 °C in 50 mM AMPOL buffer, pH 9, containing 2 mM MgCl2 and substrate. Reactions were started by the addition of enzyme and, at predetermined intervals, samples were removed and immediately quantified. Nucleotides were separated and quantified by HPLC, injecting a 20-µl aliquot of the sample into a C18 reversed-phase column (Shimadzu) and eluting it at 1.5 ml/min, with the mobile phase consisting of 50 mM potassium-phosphate buffer (pH 6.4), 5 mM tetrabutylammonium hydrogen sulfate and 18% (v/v) methanol. A260nm was continuously monitored and nucleotide concentrations were determined from the area under the absorbance peaks [14].
Synthesis of ACP and PS-CPLX
The synthesis of ACP and PS-CPLX was done according to the method described by Genge et al. [32]. An emulsion of PS was prepared by drying 1.25 mg of PS in chloroform under N2 to form a thin film in a test tube. Then 2 ml of the Pi-rich intracellular phosphate (ICP) buffer was added. This buffer, which modeled the electrolyte composition of ultrafiltrates of the intracellular fluid of growth plate chondrocytes [33], contained 106.7 mM K+, 45.1 mM Na+, 1.5 mM Mg2+, 115.7 mM Cl−, 23.0 mM Pi, 10 mM HCO3−, 1.5 mM SO4 2−, and 3.1 mM N3− as a preservative; its total molarity was 153.3 mM, and its pH was 7.2. The tube was sonicated for 2 min at 25 °C to form a uniform translucent emulsion of small unilamellar vesicles. For example, to make the nucleators, 17.5 µl of 100 mM CaCl2 was added to 1 ml of the PS emulsion with rapid stirring. On the addition of Ca2+ to ICP buffer, ACP instantly forms because of the high Ca2+×Pi ion product, and during this formative period, nascent ACP combines with the PS liposomes to form the insoluble PS-CPLX, which is then harvested by centrifugation for 5 min at approximately 15,000×g. The pellets were resuspended in 1 ml of 16.5 mM Tris buffer, pH 8 by brief sonication to yield uniform suspensions. In some experiments, PS was omitted and the 100 mM CaCl2 stock was added into the ICP buffer with rapid stirring to form ICP-based ACP. These procedures model the processes that occur in growth plate chondrocytes, where intracellular Ca2+ levels increase dramatically at sites of MV formation [34].
Microplate Mineralization Assay with Proteoliposomes
TNAP-, NPP1-, and TNAP plus NPP1-proteoliposomes were incubated in SCL containing ACP or PS-CPLX, at three different pHs (7.5, 8 and 9). SCL contained 2 mM Ca2+ and 1 mM ATP as the Pi source, in addition to 104.5 mM Na+, 133.5 mM Cl−, 63.5 mM sucrose, 16.5 mM Tris, 12.7 mM K+, 5.55 mM glucose, 1.83 mM HCO3−, and 0.57 mM Mg sulfate [35]. Empty liposomes without incorporated enzymes were used as control. Mineral formation was measured by turbidity (i.e., absorbance) at 340 nm (A340nm) using a multiwell microplate assay system described by Genge and coworkers [36]. Triplicate samples (100 µl) of each were successively distributed into wells of a 96-well microplate. Turbidity measurements were made after brief (10 s) agitation, after 48 h of incubation at 37°C, using a Molecular Devices M3 microplate reader. All measurements were performed in triplicate. Statistical comparisons were done by one-way ANOVA followed by Tukey’s test. P values less than 0.05 are considered significant.
Results
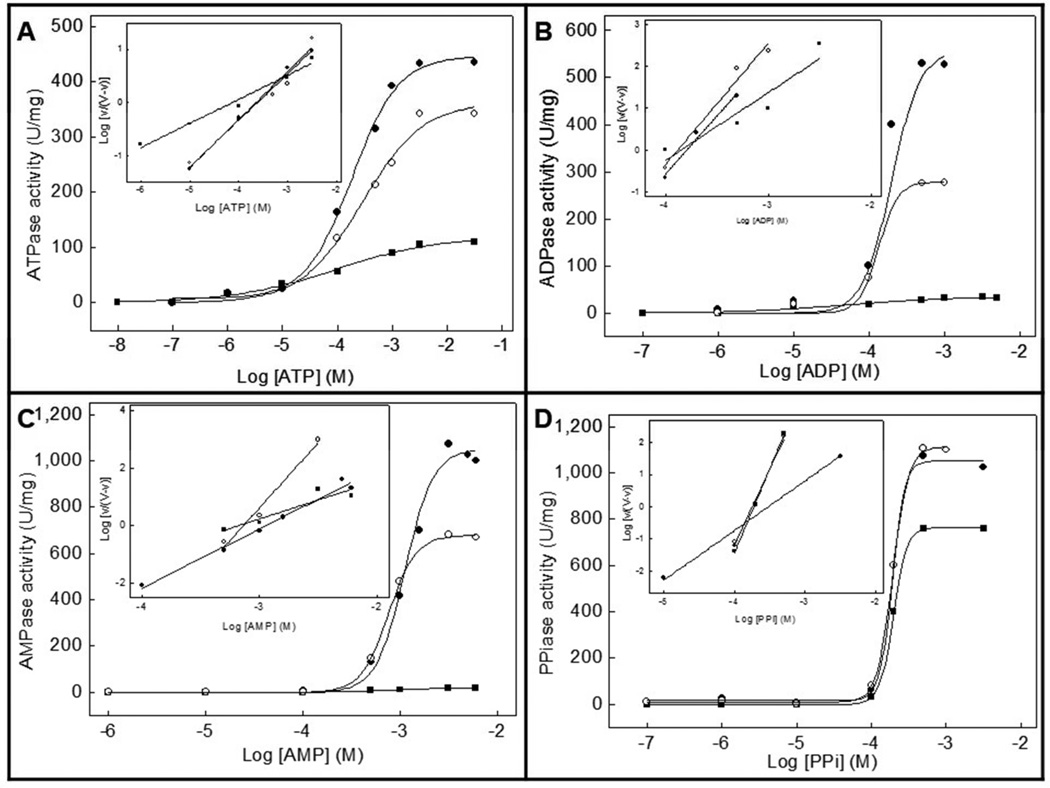
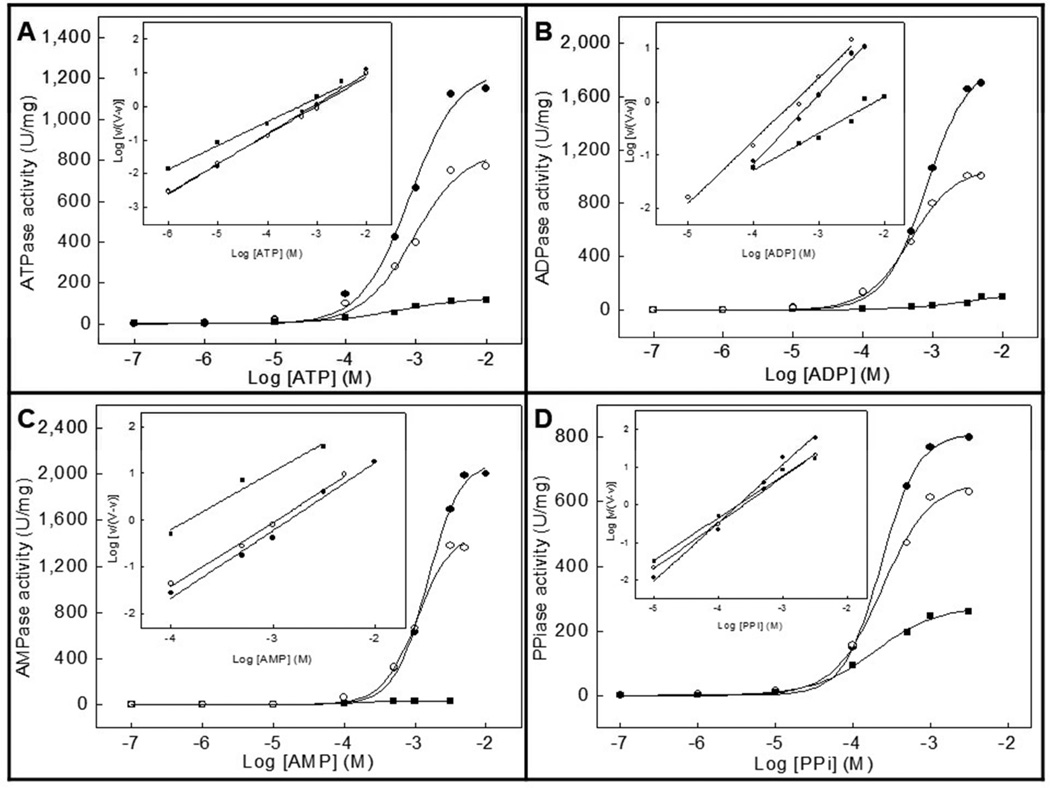
Since the optimum pH for the activity of TNAP and NPP1 is alkaline [14,21,22], we have compared the effects of slightly alkaline pH (pH 8 vs. 9) with those obtained at physiological pH [14] on the hydrolysis of the physiological substrates ATP, ADP, AMP and PPi by proteoliposomes harboring TNAP alone, NPP1 alone or both together. Table 1 and Table 2 summarize the kinetic parameters obtained at pH 8 and 9, respectively. Fig. 1 and Fig. 2 illustrate how the activities depend on substrate concentration, at pH 8 and 9, respectively.
Table 1.
Kinetic parameters for the hydrolysis of substrates by TNAP-, NPP1-, or TNAP + NPP1-proteoliposomes. Kinetic analysis was performed in 50 mM Tris-HCl buffer, pH 8, containing 2 mM MgCl2 and substrate.
| Substrates | Kinetic parameters |
Proteoliposomes | ||
|---|---|---|---|---|
| TNAP | NPP1 | TNAP + NPP1 | ||
| ATP | Vm (U/mg) | 446 ± 37 | 120 ± 15 | 363 ± 24 |
| K0.5 (mM) | 0.19 ± 0.01 | 0.095 ± 0.01 | 0.28 ± 0.02 | |
| n | 0.90 ± 0.07 | 0.45 ± 0.03 | 0.87 ± 0.05 | |
| kcat/K0.5 (M−1.s−1) | 4,522 | 2,741 | - | |
| PPi | Vm (U/mg) | 1,053 ± 83 | 765 ± 41 | 1,113 ± 98 |
| K0.5 (mM) | 0.19 ± 0.01 | 0.20 ± 0.008 | 0.19 ± 0.007 | |
| n | 1.5 ± 0.08 | 5.2 ± 0.4 | 4.7 ± 0.3 | |
| kcat/K0.5 (M−1.s−1) | 11,084 | 8,300 | - | |
| ADP | Vm (U/mg) | 558 ± 42 | 35 ± 2.8 | 279 ± 20 |
| K0.5 (mM) | 0.20 ± 0.02 | 0.053 ± 0.004 | 0.13 ± 0.01 | |
| n | 2.8 ± 0.1 | 1.6 ± 0.09 | 2.9 ± 0.2 | |
| kcat/K0.5 (M−1.s−1) | 5,580 | 1,433 | - | |
| AMP | Vm (U/mg) | 1,051 ± 73 | 19 ± 1.7 | 680 ± 43 |
| K0.5 (mM) | 1.1 ± 0.08 | 0.71 ± 0.05 | 0.76 ± 0.03 | |
| n | 2.0 ± 0.1 | 4.2 ± 0.2 | 4.5 ± 0.3 | |
| kcat/K0.5 (M−1.s−1) | 1,911 | 58 | - | |
Table 2.
Kinetic parameters for the hydrolysis of substrates by TNAP-, NPP1-, or TNAP + NPP1-proteoliposomes. Kinetic analysis was performed in 50 mM AMPOL buffer, pH 9, containing 2 mM MgCl2 and substrate.
| Substrates | Kinetic parameters |
Proteoliposomes | ||
|---|---|---|---|---|
| TNAP | NPP1 | TNAP + NPP1 | ||
| ATP | Vm (U/mg) | 1,243 ± 37 | 131 ± 10 | 849 ± 73 |
| K0.5 (mM) | 0.82 ± 0.07 | 0.54 ± 0.04 | 0.93 ± 0.08 | |
| n | 0.90 ± 0.05 | 0.71 ± 0.02 | 0.86 ± 0.05 | |
| kcat/K0.5 (M−1.s−1) | 3,033 | 526 | - | |
| PPi | Vm (U/mg) | 813 ± 61 | 276 ± 15 | 660 ± 58 |
| K0.5 (mM) | 0.23 ± 0.02 | 0.19 ± 0.02 | 0.24 ± 0.03 | |
| n | 1.5 ± 0.07 | 1.1 ± 0.01 | 1.2 ± 0.08 | |
| kcat/K0.5 (M−1.s−1) | 7,066 | 3,151 | - | |
| ADP | Vm (U/mg) | 1,856 ± 95 | 179 ± 20 | 1,069 ± 97 |
| K0.5 (mM) | 0.82 ± 0.06 | 6.7 ± 0.14 | 0.49 ± 0.04 | |
| n | 1.3 ± 0.04 | 0.69 ± 0.02 | 1.2 ± 0.03 | |
| kcat/K0.5 (M−1.s−1) | 4,527 | 58 | - | |
| AMP | Vm (U/mg) | 2,114 ± 84 | 30 ± 2.7 | 1,503 ± 140 |
| K0.5 (mM) | 1.5 ± 0.2 | 0.15 ± 0.01 | 1.1 ± 0.2 | |
| n | 1.4 ± 0.09 | 1.2 ± 0.03 | 1.4 ± 0.06 | |
| kcat/K0.5 (M−1.s−1) | 2,818 | 435 | - | |
Fig 1.
Effect of increasing concentrations of (a) ATP, (b) ADP, (c) AMP and (d) PPi on the Pi-generating activity of DPPC proteoliposomes containing: (●) TNAP, (■) NPP1 or (○) TNAP plus NPP1. Assays were done at 37 °C and buffered with 50 mM AMPOL, pH 8, containing 2 mM MgCl2 and substrate and released Pi measured
Fig 2.
Effect of increasing concentrations of (a) ATP, (b) ADP, (c) AMP and (d) PPi on the Pi-generating activity of DPPC proteoliposomes containing: (●) TNAP, (■) NPP1 or (○) TNAP plus NPP1. Assays were done at 37 °C and buffered with 50 mM AMPOL, pH 9, containing 2 mM MgCl2 and substrate and released Pi measured
The highest hydrolysis rate (Vm) for TNAP- and for NPP1-proteoliposomes was found for AMP at pH 9 and for PPi at pH 8, respectively. At pH 9, similarly to what happens at pH 7.4 [14], the K0.5 values were quite different (Table 2). The catalytic efficiencies (kcat/K0.5) were also lower for NPP1-proteoliposomes compared with TNAP-proteoliposomes for all substrates at both pHs, pointing to a major role of TNAP in the hydrolysis of these substrates (Tables 1 and 2). Hence, the activity patterns of TNAP-, NPP1-, and TNAP + NPP1-proteoliposomes at alkaline pHs suggested a predominant activity of TNAP in mixed vesicles when compared with NPP1. The catalytic efficiencies at pH 9 were only higher for the hydrolysis of AMP, largely due to the lower K0.5 at this pH (Table 2). The catalytic efficiencies of ATP, ADP and PPi hydrolysis by NPP1 were considerably higher at pH 8 (Table 1).
When the hydrolysis rates, affinities and catalytic efficiencies were reported relative to the corresponding values at physiological pH (Table 3), we could calculate a pH 8/pH 7.4 and pH 9/pH 7.4 ratio for each parameter, allowing normalized efficiency comparisons, relative to the previously determined values [14]. Affinity constants were minimally affected from pH 7.4 to pH 8, but increased considerably when the pH was increased to pH 9 (Table 3). Despite relative higher hydrolysis rates (Vm) at pH 9 compared to pH 8, the catalytic efficiencies were considerably higher at pH 8, relative to the values at pH 7.4. Catalysis was non-cooperative during ATP hydrolysis at both alkaline pHs by all proteoliposomes (Tables 1 and 2), in contrast to the positive cooperativities observed at physiologic pH [14].
Table 3.
Comparison between the kinetic parameters obtained at different pHs for the hydrolysis of the indicated substrates by DPPC proteoliposomes containing TNAP, NPP1 or TNAP + NPP1.
| Substrate | Kinetic parameters |
pH 8.0/ pH 7.4a Ratios for Proteoliposomes |
pH 9.0/ pH 7.4a Ratios for Proteoliposomes |
||||
|---|---|---|---|---|---|---|---|
| TNAP | NPP1 | TNAP + NPP1 | TNAP | NPP1 | TNAP + NPP1 | ||
| ATP | Vm | 27 | 36 | 24 | 76 | 39 | 56 |
| K0.5 | 1.6 | 5.9 | 1.8 | 6.8 | 34 | 5.8 | |
| kcat/K0.5 | 12 | 6.1 | - | 7.9 | 1.2 | - | |
| PPi | Vm | 37 | 209 | 80 | 29 | 75 | 47 |
| K0.5 | 0.27 | 2.9 | 1.0 | 0.32 | 2.7 | 1.3 | |
| kcat/K0.5 | 29 | 73 | - | 18 | 28 | - | |
| ADP | Vm | 25 | 7.2 | 20 | 83 | 37 | 76 |
| K0.5 | 0.71 | 0.74 | 0.87 | 2.9 | 93 | 3.3 | |
| kcat/K0.5 | 27 | 9.7 | - | 22 | 0.4 | - | |
| AMP | Vm | 45 | 4.0 | 41 | 90 | 6.3 | 91 |
| K0.5 | 1.5 | 3.6 | 1.5 | 2.1 | 0.75 | 2.2 | |
| kcat/K0.5 | 0.31 | 1.1 | - | 0.46 | 8.5 | - | |
Data at pH 7.4 are described in [14].
Hydrolysis of PPi showed no cooperative regulation at pH 9, but positive cooperativity was observed at pH 8 for proteoliposomes harboring NPP1 (Tables 1 and 2). K0.5 values were quite similar at alkaline pHs (Tables 1 and 2), but higher than the values at pH 7.4 [14]. Surprisingly, an increase in the hydrolysis rates and catalytic efficiencies was observed for NPP1-proteoliposomes at pH 8, compared to pH 7.4 [14] (Table 3).
Thus, comparing the differences in the kinetic parameters obtained for the different substrates (Tables 1, 2 and 3), we observed that the hydrolysis rates (Vm) increase significantly at alkaline pHs for both enzymes and their combination, but since the affinities (K0.5) hardly changes and the catalytic efficiencies (kcat/K0.5) are much higher for PPi when compared with ATP, ADP and AMP, a small pH elevation to pH 8 induces a relative advantage for PPi hydrolysis by NPP1 over phosphonucleotides hydrolysis, an advantage less clear at pH 9 (Table 3). Hence, these experiments illustrate that a small elevation in the pH considerably influences enzyme catalysis by TNAP and NPP1 differently, speeding up PPi hydrolysis, resulting in an elevation of the Pi/PPi ratio.
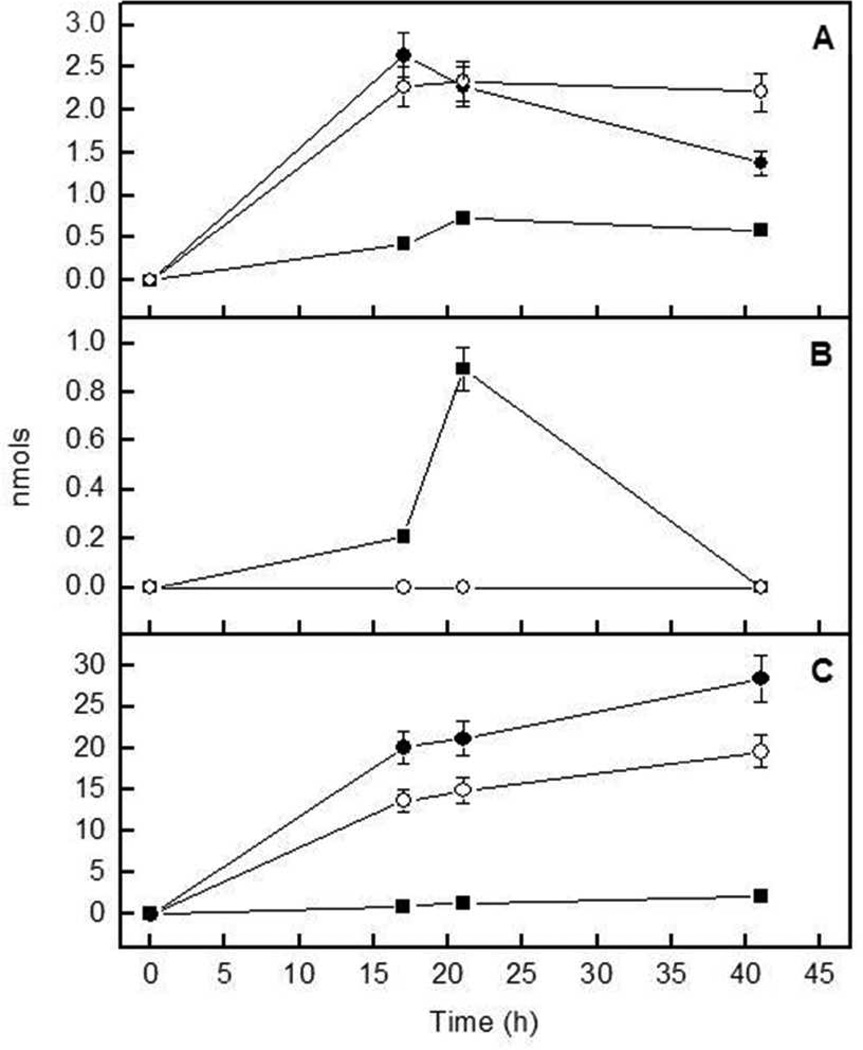
Because our data suggested a balanced hydrolysis for the various substrates, we also investigated the nucleotide hydrolysis at pH 9, monitoring the formation of reaction intermediates as a function of time during hydrolysis of ATP and ADP, separating and quantifying the products by HPLC. The amounts of ADP, AMP and/or adenosine produced for the different proteoliposomes are shown for the hydrolysis of ATP (Fig. 3) and ADP (Fig. 4).
Fig 3.
Progression of the formation of (a) ADP, (b) AMP and (c) adenosine during hydrolysis of 100 nmol of ATP by (●) TNAP-proteoliposomes, (■) NPP1-proteoliposomes and (■) TNAP plus NPP1-proteoliposomes. Hydrolysis was determined in 50 mM AMPOL buffer, pH 9, containing 2 mM MgCl2 and 1 mM ATP. Nucleotide concentrations were monitored by HPLC
Fig 4.
Progression of the formation of (a) AMP and (b) adenosine during hydrolysis of 100 nmol of ADP by (●) TNAP-proteoliposomes, (■) NPP1-proteoliposomes and (○) TNAP plus NPP1-proteoliposomes. Hydrolysis was determined in 50 mM AMPOL buffer, pH 9, containing 2 mM MgCl2 and 1 mM ADP. Nucleotide concentrations were monitored by HPLC
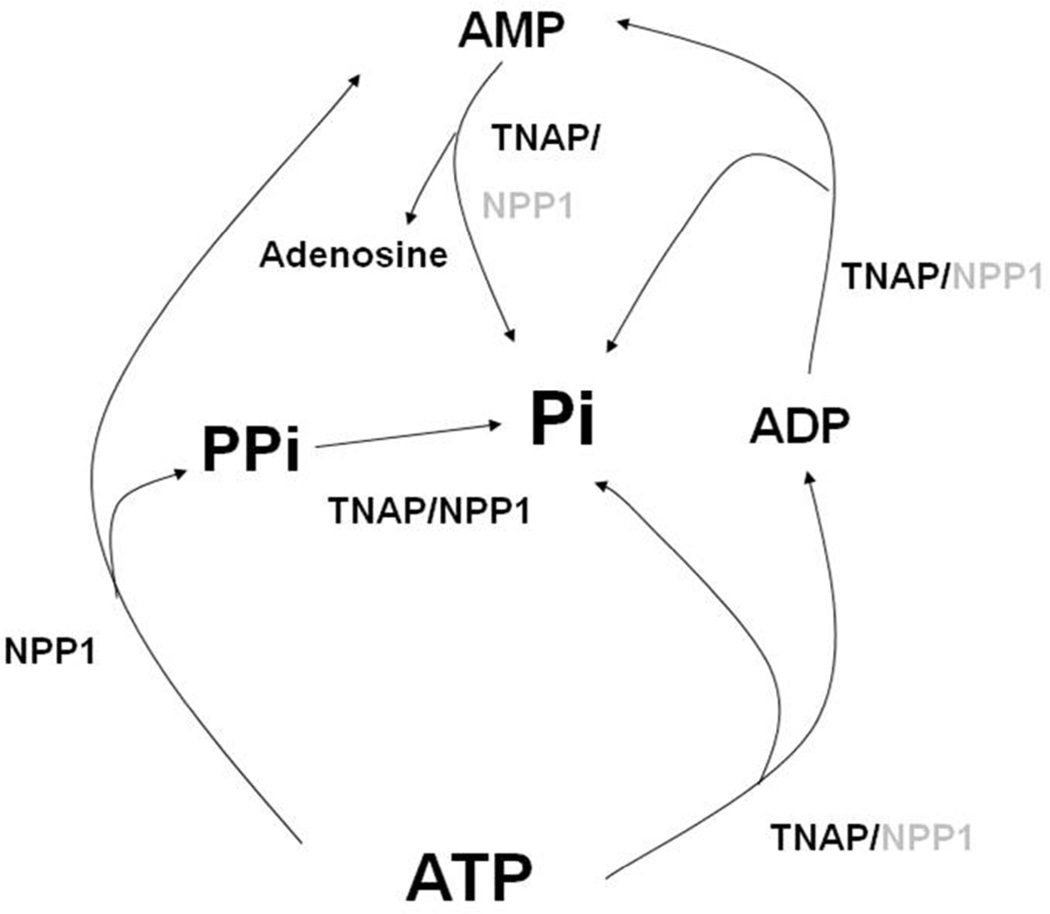
Hydrolysis of ATP resulted in ADP, AMP and adenosine formation by the various proteoliposomes with different efficiencies, as predicted by Table 2 (Fig. 3). Nonetheless, the time-dependent catalysis of ATP is more pronounced in TNAP-proteoliposomes than in the liposomes containing NPP1, confirming the major role of TNAP in the hydrolysis of this substrate. In accordance with the predominant role of TNAP in the TNAP-proteoliposomes, the concentration of the reaction intermediate ADP was higher for TNAP- and TNAP + NPP1-proteoliposomes (Fig. 3a). AMP only accumulated to low levels because it was hydrolyzed with high catalytic efficiency to adenosine and phosphate (Table 2). Adenosine accumulated to a higher degree upon incubations with TNAP- and TNAP + NPP1-proteoliposomes (Fig. 3c). The larger adenosine accumulation by these proteoliposomes reflects the higher activity of TNAP during ADP formation and its further breakdown to AMP and adenosine compared with NPP1, which generates AMP and PPi directly, in addition to behaving as a phosphatase (Table 2 and Fig. 5).
Fig 5.
Representation of all the possible simultaneous enzymatic reactions during catalysis of substrates by TNAP and NPP1 at alkaline pH. Indicated in black and gray are the enzymes with higher and lower catalytic efficiencies, respectively, for the indicated substrates
When ADP was used, the highest catalysis rate was observed (Fig. 4) again for TNAP-proteoliposomes. However, catalysis of ADP by NPP1-proteoliposomes (Table 2 and Fig. 4) confirmed that this enzyme can behave as a phosphatase.
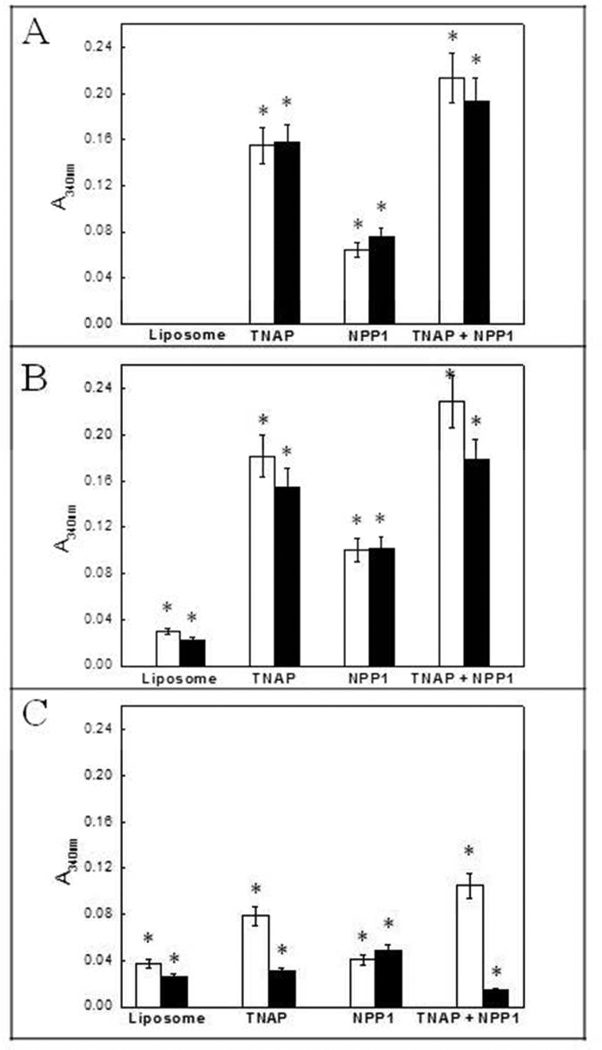
Compared to control incubations with empty liposomes, all proteoliposomes induced mineral formation when incubated in SCL containing 1 mM ATP as substrate, but the induction of mineralization was equivalent at pH 7.5 and 8, and considerably less at pH 9 (Fig. 6). When comparing the effects of ACP and PS-CPLX in modulating mineral formation, both complexes did not present significant differences at pHs 7.5 and 8, but at pH 9 PS-CPLX had a more pronounced effect on mineral formation by proteoliposomes containing TNAP when compared to the proteoliposomes containing NPP1 (Fig. 6). Yet, TNAP + NPP1 proteoliposomes triggered significantly more extensive Pi-dependent mineralization than TNAP proteoliposomes, despite the lower content in the former proteoliposomes [14], suggestive of additive hydrolytic activities for NPP1 and TNAP.
Fig 6.
Effect of TNAP-, NPP1-, and TNAP plus NPP1-proteoliposomes on mineralization of ACP and PS-CPLX-seeded SCL, at pH 7.5 (A), 8 (B) and 9 (C), in the presence of 1 mM ATP, after 48 h of incubation at 37°C. White bars: PS-CPLX-seeded SCL. Black bars: ACP-seeded SCL. The ACP and PS-CPLX nucleators were prepared and mineralization was monitored in the microplate, as described in Materials and Methods. Empty liposomes without incorporated enzymes were used as control, and the bars show the increment in absorbencies over 48h.
Discussion
Endochondral calcification is a complex process in which Ca2+ and Pi in solution are processed and brought together to induce precipitation of a mineral phase that ultimately forms HA, the principal mineral of bone. One of the essential features of mineralization-inducing MVs is the presence of a nucleation core [23,24] composed of ACP, complexed in part with PS to form calcium-phosphate-lipid complexes (PS-CPLX) capable of inducing mineral formation when incubated in a SCL [36,37,38]. It is important to recognize that ACP is a kinetically unstable mineral that forms only when both Ca2+ and Pi are present in high (millimolar) levels [39,40]. Thus, the presence of ACP provides important clues to the process of MV formation. Although Pi-containing PS-CPLXs can induce HA formation, in vivo many factors appear to be involved in regulating its formation and activity [41]. PS-CPLXs are present at the early stages of almost all calcifying tissues, as growth plate cartilage [42], bone [43], and MVs [44].
One of the most effective ways to destroy the ability of the nucleational core to induce mineral formation is simple exposure to isosmotic, pH 6 citrate buffer [23]. This reveals that it is highly sensitive to even mildly acidic conditions. On the other hand, at pH 8, the nucleational core is highly stable and insoluble. Past studies on the formation of mineral have shown that the nucleational activity of the MVs and the nucleational core is operative only within a very narrow pH range – between 7.4–7.8 [45]. Either below or above this range, its ability to nucleate mineral formation was very reduced. But in the studies reported by Wu et al. [46], the pH range in which rapid mineral formation occurred was broader (pH 7.4– 8.0). This is in agreement with our data, since the induction of mineralization by our proteoliposomes, in the absence of pyrophosphate was more efficient at pHs 7.5 and 8 and already much less at pH 9, although the optimum pH for the activities of TNAP and NPP1 is alkaline. This difference may be due to the fact that substrate hydrolysis occurs in a lipidic microenvironment while the process of nucleation/mineralization takes place in a different microenvironment, thus the optimum pH for substrate hydrolysis may be distinct from the optimum pH for mineralization, which, moreover, depends on the biophysical properties of the calcium-phosphate complexes and their stability, both pH dependent processes [47].
Proteoliposomes can be obtained by mechanical dispersion, sonication, extrusion, solvent dispersion, co-solubilization with detergents, reverse phase evaporation and direct insertion after detergent removal [17,48,49]. Our long term goal is to reconstitute more complex proteoliposomes containing TNAP, NPP1, PHOSPHO1 [50], Pit-1/2, ANK, Annexins, etc. [51], that will recapitulate the key events leading to initiation of MV-mediated calcification in vitro, since the lipid membrane plays an important role as a nucleation agent in the biomineralization process [52], as a protective and/or activation agent [53].
In order to understand the physiological interplay between important MV-associated enzymes in the initiation of biomineralization, it is important to keep in mind the microenvironment in which these enzymes function, which can have a profound effect on their biological properties, since the lipid membrane plays an important role in the initiation of the biomineralization process [54,55]. Thus, the ability of synthetic or natural vesicles [16,17] to mimic the structure and function of biomembranes makes these structures an advantageous and convenient model to reconstitute either TNAP, NPP1 or both together, since the modulation of enzymes activities by the lipid microenvironment seems to be common between enzymes that have a glycosylphosphatidylinositol (GPI) anchor. Lehto and Sharom [56] reported a reduction in the catalytic efficiency of the enzyme 5’-nucleotidase upon insertion of the protein into liposomes with different lipid composition, and the activity could be restored upon enzyme release from the membranes after treatment of the proteoliposomes with GPI-specific PLC. They reported that the degree of activity reduction was dependent on the lipid bilayer where the GPI anchor was inserted, suggesting that different lipids affect the activity of the enzyme in different ways, maybe through alteration in protein conformation transmitted from the membrane to the protein solely through the GPI anchor [57]. We have previously documented that although the enzymatic efficiency (kcat/Km) remained comparable between polidocanol-solubilized and membrane-bound TNAP for all substrates used, the value of kcat/Km for the GPI-specific PLC-solubilized enzyme increased ~ 108-, 56-, and 556-fold for pNPP, ATP, and PPi, respectively, compared with the membrane-bound enzyme [15].
Bone homeostasis is profoundly affected by local pH. The skeleton contains a large reserve of alkaline mineral (hydroxyapatite), which is ultimately available to neutralize metabolic H+ if acid-base balance is not maintained within narrow limits [20]. Bone cells are extremely sensitive to the direct effects of pH: acidosis inhibits mineral deposition by osteoblasts but activates osteoclasts to resorb bone and other mineralized tissues [20]. These responses act to maximize the availability of OH− ions from HA in solution, where they can buffer excess H+. The mechanisms by which bone cells sense pH changes are likely to be complex, involving ion channels and receptors in the cell membrane, and direct intracellular effects [20].
Most of the studies correlating pH with different aspects of the biomineralization process are done at pHs close to physiological pH, although pH appears to play a regulatory role during the mineralization process [18–20]. Wu et al. [19] reported numerous focal elevations in pH (> 8.0) in central regions of the maturing and early hypertrophic chondrocytes, with lower pH (6.5–7.2) near the cell periphery of the late hypertrophic and calcifying cells, suggesting that this pattern of cytoplasmic alkalinization and subsequent acidification contributes to loading of Ca2+ and Pi into MVs during their formation by the chondrocytes. Chakkalakal et al. [18] reported that the pH of repair tissue fluids may play a regulatory role in the healing and mineralization of bone, suggesting that the process of mineralization and bone repair is tissue pH-dependent. Even when the formation of HA was investigated in the pH range 9.5–12.0 [58], the influence of mild pH fluctuations on the players implicated in the biomineralization process is less well known.
Pi transport into MVs appears to take place by at least two pathways: the first being mediated by a Pi transporter, e.g., the type-III Na+-dependent phosphate transporter (NaPiT) [59], and a second not strictly Na+-dependent [60]. This second category has been shown to be alkaline pH-specific. So, an alkaline pH would favor mineralization by promoting Pi transport to the inner of MVs. Besides, Balcerzak et al. [61] characterized the proteome of MVs isolated from chicken embryo bones and cartilage, and reported the presence of H+-ATPase, making it possible to establish a pH gradient, allowing a more alkaline pH in the neighborhood of this pump.
The hydrolysis rates were quite different at pH 7.4 and alkaline pHs (Table 3), being relatively slower at physiological pH for all substrates. But analysis of K0.5 values (Table 3) for pHs 8 and 9 shows that the affinities for PPi are comparable for the combined enzymes proteoliposomes, such not being the case for ATP. Also, at alkaline pHs, the catalytic efficiencies are much higher for both TNAP- and NPP1-proteoliposomes for PPi hydrolysis than for ATP hydrolysis. Since at pH 8, NPP1 becomes such an efficient enzyme in catalyzing PPi, our findings suggest that mild pH elevations will convert PPi into a preferred substrate, for both TNAP and NPP1. A further elevation of the pH to 9 does not further drive this differentiation, since the catalytic efficiencies for PPi conversion by NPP1 drop again. Likewise, due to the high K0.5 values at pH 9, catalysis of ATP, ADP and AMP does not further improve. Increasing pH also impacts on the solubility of Ca (pyro)phosphate [47], strongly reducing the solubility of calciumphosphate, at pH > 8. This is clearly illustrated in our present study, where poor mineralization occurred at pH 9. In view of the limited solubility of Mg pyrophosphate at physiological pH (around 40 µM), it is not surprising that a gain for the PPi hydrolysis benefits from a narrow pH range only. Further pH elevation beyond pH 8 will affect the solubility of Ca and Mg pyrophosphate, i.e. will no longer have a beneficial effect on bone mineralization.
Our data and those of others have conclusively demonstrated that a major role of TNAP is to restrict the extracellular pool of PPi to allow controlled calcification to proceed [7,8]. The ability of TNAP to use ATP as substrate to initiate calcification has also been documented by many investigators [5,62], although the details of how ATP-derived Pi participates in calcification remain to be established. Our own work has confirmed that purified TNAP and MVs efficiently hydrolyze ATP as well as its metabolites ADP and AMP [13]. It is well known that pH plays an important role in determining the behavior of TNAP, and also of NPP1, as a pyrophosphatase or as an ATPase. Our work shows that a slight elevation in pH from 7.4 to 8 significantly increases the hydrolysis of PPi by NPP1 and the hydrolysis of ATP by TNAP. This implies that small pH fluctuations will facilitate bone formation by elevating the Pi/PPi ratio at least in the very narrow pH zone where the nucleational core is operative, up to a maximum of pH 8 [45].
The use of proteoliposomes suggests that TNAP activity and specificity in phosphosubstrate hydrolysis can be enhanced by slight local pH elevations in the neighborhood of calcifying MVs, affecting biomineralization. A slightly alkaline pH outside the MVs could favor two mechanisms: provide larger amounts of Pi during nucleation of the first HA crystals and reduce PPi more efficiently, enough to support crystals growth along collagen fibrils. The critical balance between these reactions (Fig. 5) is believed to establish a proper Pi/PPi ratio, conductive to biomineralization.
Acknowledgments
We thank Professor Mathieu Bollen (Leuven, Belgium) for providing the NPP2/NPP1 expression construct. This work was supported in part by Fundaçã o de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and grants [DE12889], [AR53102] and [AR47908] from the National Institutes of Health (NIH), USA. AMSS was recipient of studentships from CAPES and FAPESP.
References
- 1.Boskey A. In: Dynamics of Bone and Cartilage Metabolism. Seibel, Robins, Biezikian, editors. Academic Press; 2006. pp. 201–212. [Google Scholar]
- 2.Millán JL. The Role of Phosphatases in the Initiation of Skeletal Mineralization. Calcif Tissue Int. 2012 doi: 10.1007/s00223-012-9672-8. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali SY, Sajdera SW, Anderson HC. Isolation and characterization of calcifying matrix vesicles from epiphyseal cartilage. Proc Natl Acad Sci USA. 1970;67:1513–1520. doi: 10.1073/pnas.67.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robison R. The possible significance of hexosephosphoric esters in ossification. Biochem J. 1923;17:286–293. doi: 10.1042/bj0170286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer JL. Can biological calcification occur in the presence of pyrophosphate? Arch Biochem Biophys. 1984;231:1–8. doi: 10.1016/0003-9861(84)90356-4. [DOI] [PubMed] [Google Scholar]
- 6.Rezende AA, Pizauro JM, Ciancaglini P, Leone FA. Phosphodiesterase activity is a novel property of alkaline phosphatase from osseous plate. Biochem J. 1994;301:517–522. doi: 10.1042/bj3010517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hessle L, Johnsson KA, Anderson HC, et al. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci USA. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harmey D, Hessle L, Narisawa S, et al. Concerted regulation of inorganic pyrophosphate and osteopontin by Akp2, Enpp1, and Ank. Am J Pathol. 2004;164:1199–1209. doi: 10.1016/S0002-9440(10)63208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terkeltaub RA. Inorganic pyrophosphate generation and disposition in pathophysiology. Am J Physiol Cell Physiol. 2001;281:C1–C11. doi: 10.1152/ajpcell.2001.281.1.C1. [DOI] [PubMed] [Google Scholar]
- 10.Huang R, Rosenbach M, Vaughn R, et al. Expression of the murine plasma cell nucleotide pyrophosphohydrolase PC-1 is shared by human liver, bone, and cartilage cells. Regulation of PC-1 expression in osteosarcoma cells by transforming growth factor-beta. J Clin Invest. 1994;94:560–567. doi: 10.1172/JCI117370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson K, Moffa A, Chen Y, et al. Matrix vesicle plasma cell membrane glycoprotein-1 regulates mineralization by murine osteoblastic MC3T3 cells. J Bone Miner Res. 1999;14:883–892. doi: 10.1359/jbmr.1999.14.6.883. [DOI] [PubMed] [Google Scholar]
- 12.Gijsbers R, Ceulemans H, Stalmans W, Bollen M. Structural and catalytic similarities between nucleotide pyrophosphatases/phosphodiesterases and alkaline phosphatases. J Biol Chem. 2001;276:1361–1368. doi: 10.1074/jbc.M007552200. [DOI] [PubMed] [Google Scholar]
- 13.Ciancaglini P, Yadav MC, Simão AMS, et al. Kinetic analysis of substrate utilization by native and TNAP-, NPP1-, or PHOSPHO1-deficient matrix vesicles. J Bone Miner Res. 2010;25:716–723. doi: 10.1359/jbmr.091023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simão AMS, Yadav MC, Narisawa S, et al. Proteoliposomes harboring alkaline phosphatase and nucleotide pyrophosphatase as matrix vesicle biomimetics. J Biol Chem. 2010;285:7598–7609. doi: 10.1074/jbc.M109.079830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciancaglini P, Simão AMS, Camolezi FL, et al. Contribution of matrix vesicles and alkaline phosphatase to ectopic bone formation. Braz J Med Biol Res. 2006;39:603–610. doi: 10.1590/s0100-879x2006000500006. [DOI] [PubMed] [Google Scholar]
- 16.Ierardi DF, Pizauro JM, Ciancaglini P. Erythrocyte ghost cell-alkaline phosphatase: construction and characterization of a vesicular system for use in biomineralization studies. Biochim Biophys Acta. 2002;1567:183–192. doi: 10.1016/s0005-2736(02)00615-6. [DOI] [PubMed] [Google Scholar]
- 17.Ciancaglini P, Simão AMS, Bolean M, et al. Proteoliposomes in nanobiotechnology. Biophys Rev. 2012;4:67–81. doi: 10.1007/s12551-011-0065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakkalakal DA, Mashoof AA, Novak J, et al. Mineralization and pH relationships in healing skeletal defects grafted with demineralized bone matrix. J Biomed Mater Res. 1994;28:1439–1443. doi: 10.1002/jbm.820281209. [DOI] [PubMed] [Google Scholar]
- 19.Wu LNY, Genge BR, Dunkelberger DG, et al. Physicochemical characterization of the nucleational core of matrix vesicles. J Biol Chem. 1997;272:4404–4411. doi: 10.1074/jbc.272.7.4404. [DOI] [PubMed] [Google Scholar]
- 20.Arnett TR. Acidosis, hypoxia and bone. Arch Biochem Biophys. 2010;503:103–109. doi: 10.1016/j.abb.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Bollen M, Gijsbers R, Ceulemans H, et al. Nucleotide pyrophosphatase/phophodiesterases on the move. Crit Rev Biochem Mol Biol. 2000;35:393–432. doi: 10.1080/10409230091169249. [DOI] [PubMed] [Google Scholar]
- 22.Millán JL. From biology to applications in medicine and biotechnology. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co; 2006. Mammalian alkaline phosphatases. [Google Scholar]
- 23.Wu LN, Yoshimori T, Genge BR, et al. Characterization of the nucleational core complex responsible for mineral induction by growth plate cartilage matrix vesicles. J Biol Chem. 1993;268:25084–25094. [PubMed] [Google Scholar]
- 24.Wu LN, Genge BR, Dunkelberger DG, et al. Physicochemical characterization of the nucleational core of matrix vesicles. J Biol Chem. 1997;272:4404–4411. doi: 10.1074/jbc.272.7.4404. [DOI] [PubMed] [Google Scholar]
- 25.Simão AMS, Beloti MM, Cezarino RM, et al. Membrane-bound alkaline phosphatase from ectopic mineralization and rat bone marrow cell culture. Comp Biochem Physiol A Mol Integr Physiol. 2007;146:679–687. doi: 10.1016/j.cbpa.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Gijsbers R, Ceulemans H, Bollen M. Functional characterization of the non-catalytic ectodomains of the nucleotide pyrophosphatase/phosphodiesterase NPP1. Biochem J. 2003;371:321–330. doi: 10.1042/BJ20021943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camolezi FL, Daghastanli KPR, Magalhães PP, et al. Construction of an alkaline phosphatase-liposome system: a tool for biomineralization study. Int J Biochem Cell Biol. 2002;1282:1–11. doi: 10.1016/s1357-2725(02)00029-8. [DOI] [PubMed] [Google Scholar]
- 28.Hartree EF. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972;48:422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- 29.Pizauro JM, Ciancaglini P, Leone FA. Characterization of the phosphatidylinositol-specific phospholipase C-released form of rat osseous plate alkaline phosphatase and its possible significance on endochondral ossification. Mol Cell Biochem. 1995;152:121–129. doi: 10.1007/BF01076074. [DOI] [PubMed] [Google Scholar]
- 30.Heinonen JK, Lahti RJ. A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal Biochem. 1981;113:313–317. doi: 10.1016/0003-2697(81)90082-8. [DOI] [PubMed] [Google Scholar]
- 31.Leone FA, Baranauskas JA, Furriel RPM, et al. SigrafW: An easy-to-use program for fitting enzyme kinetic data. Biochem Molec Educ. 2005;33:399–403. doi: 10.1002/bmb.2005.49403306399. [DOI] [PubMed] [Google Scholar]
- 32.Genge BR, Wu LN, Wuthier RE. Kinetic analysis of mineral formation during in vitro modeling of matrix vesicle mineralization: effect of annexin A5, phosphatidylserine, and type II collagen. Anal Biochem. 2007;367:159–166. doi: 10.1016/j.ab.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 33.Wuthier RE, Rice GS, Wallace JE, et al. In vitro precipitation of calcium phosphate under intracellular conditions: Formation of brushite from an amorphous precursor in the absence of ATP. Calcif Tissue Int. 1985;37:401–410. doi: 10.1007/BF02553710. [DOI] [PubMed] [Google Scholar]
- 34.Wu LNY, Wuthier MG, Genge BR, et al. In situ levels of intracellular Ca2+ and pH in avian growth plate cartilage. Clin Orthop Relat Res. 1997;335:310–324. [PubMed] [Google Scholar]
- 35.Wuthier RE. Electrolytes of isolated epiphyseal chondrocytes, matrix vesicles, and extracellular fluid. Calcif Tissue Res. 1977;23:125–133. doi: 10.1007/BF02012777. [DOI] [PubMed] [Google Scholar]
- 36.Genge BR, Wu LN, Wuthier RE. In vitro modeling of matrix vesicle nucleation: synergistic stimulation of mineral formation by annexin A5 and phosphatidylserine. J Biol Chem. 2007;282:26035–26045. doi: 10.1074/jbc.M701057200. [DOI] [PubMed] [Google Scholar]
- 37.Boskey AL, Goldberg MR, Posner AS. Calcium-phospholipid-phosphate complexes in mineralizing tissue. Proc Soc Exp Biol Med. 1978;157:590–593. doi: 10.3181/00379727-157-40103. [DOI] [PubMed] [Google Scholar]
- 38.Wu LN, Genge BR, Sauer GR, Wuthier RE. Characterization and reconstitution of the nucleational complex responsible for mineral formation by growth plate cartilage matrix vesicles. Connect Tissue Res. 1996;35:309–315. doi: 10.3109/03008209609029205. [DOI] [PubMed] [Google Scholar]
- 39.Termine JD, Peckauskas RA, Posner AS. Calcium phosphate formation in vitro: II. Effects of environment on amorphous crystalline transformation. Arch Biochem Biophys. 1970;140:318–325. doi: 10.1016/0003-9861(70)90072-x. [DOI] [PubMed] [Google Scholar]
- 40.Eanes ED. Thermochemical studies on amorphous calcium phosphate. Calcif Tissue Res. 1970;5:133–145. doi: 10.1007/BF02017543. [DOI] [PubMed] [Google Scholar]
- 41.Wuthier RE, Lipscomb GF. Matrix vesicles: structure, composition, formation and function in calcification. Front Biosci. 2011;16:2812–2902. doi: 10.2741/3887. [DOI] [PubMed] [Google Scholar]
- 42.Wuthier RE. Lipids of mineralizing epiphyseal tissues in the bovine fetus. J Lipid Res. 1968;9:68–78. [PubMed] [Google Scholar]
- 43.Boskey AL, Posner AS. Extraction of a calcium-phospholipid-phosphate complex from bone. Calcif Tissue Res. 1976;19:273–283. doi: 10.1007/BF02564010. [DOI] [PubMed] [Google Scholar]
- 44.Wuthier RE, Gore ST. Partition of inorganic ions and phospholipids in isolated cell, membrane and matrix vesicle fractions: evidence for Ca-Pi-acidic phospholipid complexes. Calcif Tissue Res. 1977;24:163–171. doi: 10.1007/BF02223311. [DOI] [PubMed] [Google Scholar]
- 45.Valhmu WB, Wu LN, Wuthier RE. Effects of Ca/Pi ratio, Ca2+ × Pi ion product, and pH of incubation fluid on accumulation of45Ca2+ by matrix vesicles in vitro. Bone Miner. 1990;8:195–209. doi: 10.1016/0169-6009(90)90105-o. [DOI] [PubMed] [Google Scholar]
- 46.Wu LN, Genge BR, Wuthier RE. Analysis and molecular modeling of the formation, structure, and activity of the phosphatidylserine-calcium-phosphate complex associated with biomineralization. J Biol Chem. 2008;283:3827–3838. doi: 10.1074/jbc.M707653200. [DOI] [PubMed] [Google Scholar]
- 47.Bennett RM, Lehr JR, McCarty DJ. Factors affecting the solubility of calcium pyrophosphate dihydrate crystals. J Clin Invest. 1975;56:1571–1579. doi: 10.1172/JCI108239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prasad R. Manual of membrane lipids. Berli Heidelderg: Springer-Verlag; 1996. [Google Scholar]
- 49.Simão AMS, Yadav MC, Ciancaglini P, Millán JL. Proteoliposomes as matrix vesicles' biomimetics to study the initiation of skeletal mineralization. Braz J Med Biol Res. 2010;43:234–241. doi: 10.1590/s0100-879x2010007500008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roberts S, Narisawa S, Harmey D, et al. Functional involvement of PHOSPHO1 in matrix vesicle-mediated skeletal mineralization. J Bone Miner Res. 2007;22:617–627. doi: 10.1359/jbmr.070108. [DOI] [PubMed] [Google Scholar]
- 51.Xiao Z, Camalier CE, Nagashima K, et al. Analysis of the extracellular matrix vesicle proteome in mineralizing osteoblasts. J Cell Physiol. 2007;210:325–335. doi: 10.1002/jcp.20826. [DOI] [PubMed] [Google Scholar]
- 52.Eanes ED. Biophysical aspects of lipid interaction with mineral: liposome model studies. Anat Rec. 1989;224:220–225. doi: 10.1002/ar.1092240211. [DOI] [PubMed] [Google Scholar]
- 53.Carruthers A, Melchior DL. How bilayer lipids affect membrane protein activity. TIBS. 1986;11:331–335. [Google Scholar]
- 54.Anderson HC, Hsu HH, Morris DC, et al. Matrix vesicles in osteomalacic hypophosphatasia bone contain apatite-like mineral crystals. Am J Pathol. 1997;151:1555–1561. [PMC free article] [PubMed] [Google Scholar]
- 55.Wu LN, Genge BR, Kang MW, et al. Changes in phospholipid extractability and composition accompany mineralization of chicken growth plate cartilage matrix vesicles. J Biol Chem. 2002;277:5126–5133. doi: 10.1074/jbc.M107899200. [DOI] [PubMed] [Google Scholar]
- 56.Lehto MT, Sharom FJ. Release of the glycosylphosphatidylinositol-anchored enzyme ecto-5'-nucleotidase by phospholipase C: catalytic activation and modulation by the lipid bilayer. Biochem J. 1998;332:101–109. doi: 10.1042/bj3320101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lehto MT, Sharom FJ. Proximity of the protein moiety of a GPI-anchored protein to the membrane surface: a FRET study. Biochemistry. 2002;41:8368–8376. doi: 10.1021/bi012038+. [DOI] [PubMed] [Google Scholar]
- 58.Lazic S. Microcrystalline hydroxyapatite formation from alkaline solutions. J Crystal Growth. 1995;147:147–154. [Google Scholar]
- 59.Guicheux J, Palmer G, Shukunami C, et al. A novel in vitro culture system for analysis of functional role of phosphate transport in endochondral ossification. Bone. 2000;27:69–74. doi: 10.1016/s8756-3282(00)00302-1. [DOI] [PubMed] [Google Scholar]
- 60.Wu LN, Sauer GR, Genge BR, et al. Effects of analogues of inorganic phosphate and sodium ion on mineralization of matrix vesicles isolated from growth plate cartilage of normal rapidly growing chickens. J Inorg Biochem. 2003;94:221–235. doi: 10.1016/s0162-0134(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 61.Balcerzak M, Malinowska A, Thouverey C, et al. Proteome analysis of matrix vesicles isolated from femurs of chicken embryo. Proteomics. 2008;8:192–205. doi: 10.1002/pmic.200700612. [DOI] [PubMed] [Google Scholar]
- 62.Hsu HH, Camacho NP, Anderson HC. Further characterization of ATP-initiated calcification by matrix vesicles isolated from rachitic rat cartilage. Membrane perturbation by detergents and deposition of calcium pyrophosphate by rachitic matrix vesicles. Biochim Biophys Acta. 1999;1416:320–332. doi: 10.1016/s0005-2736(98)00235-1. [DOI] [PubMed] [Google Scholar]