Abstract
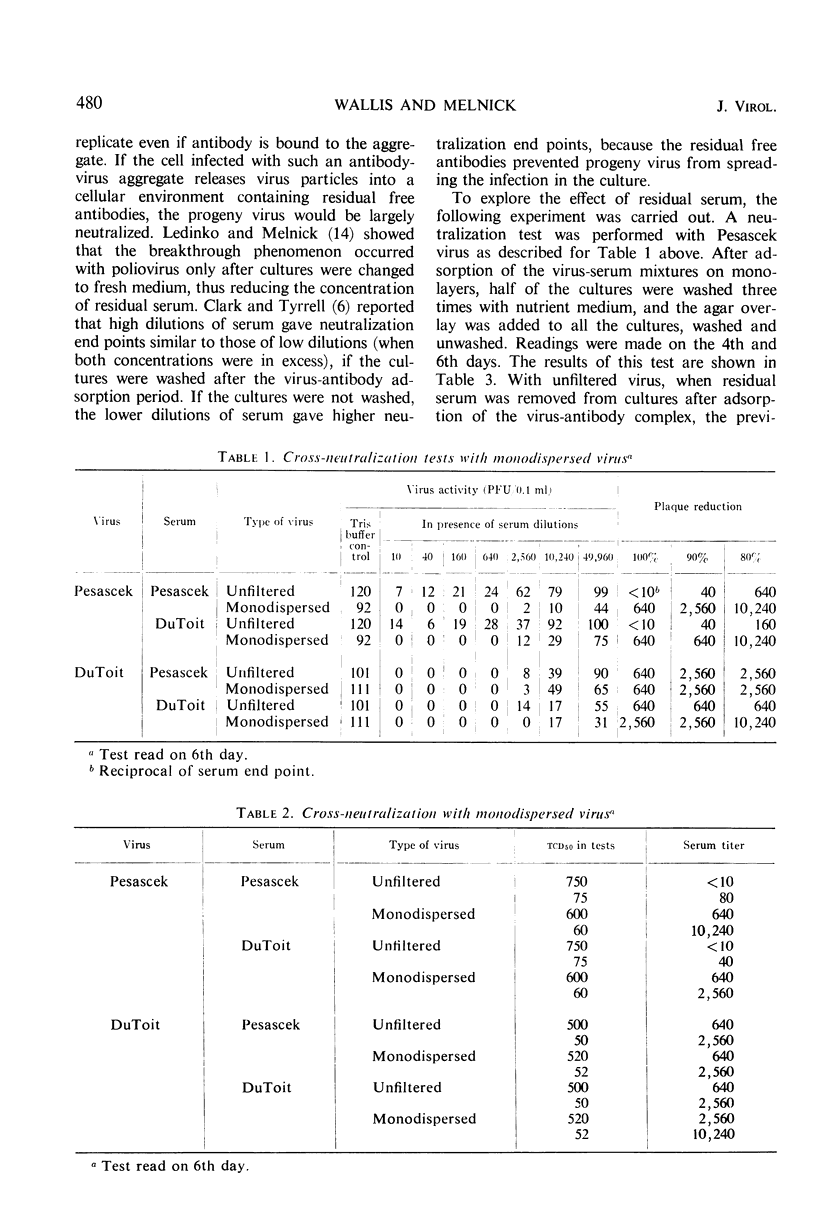
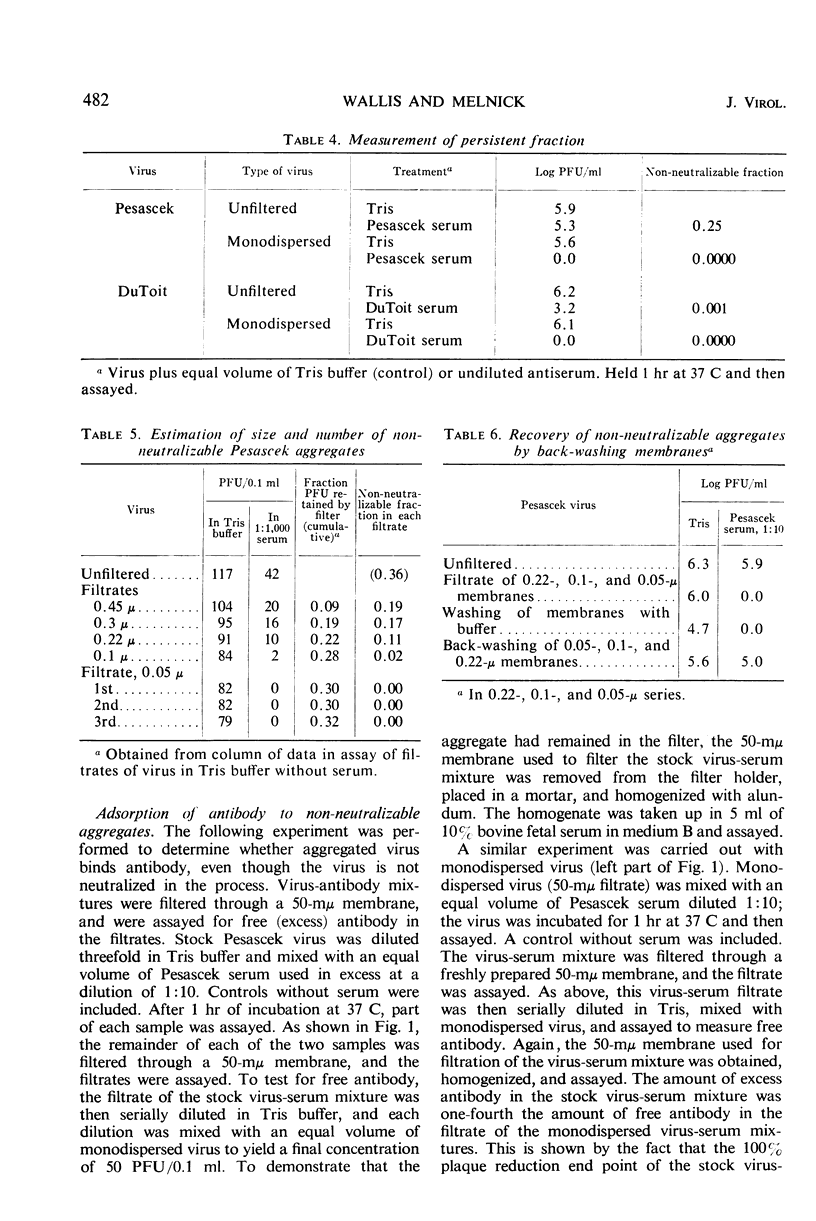
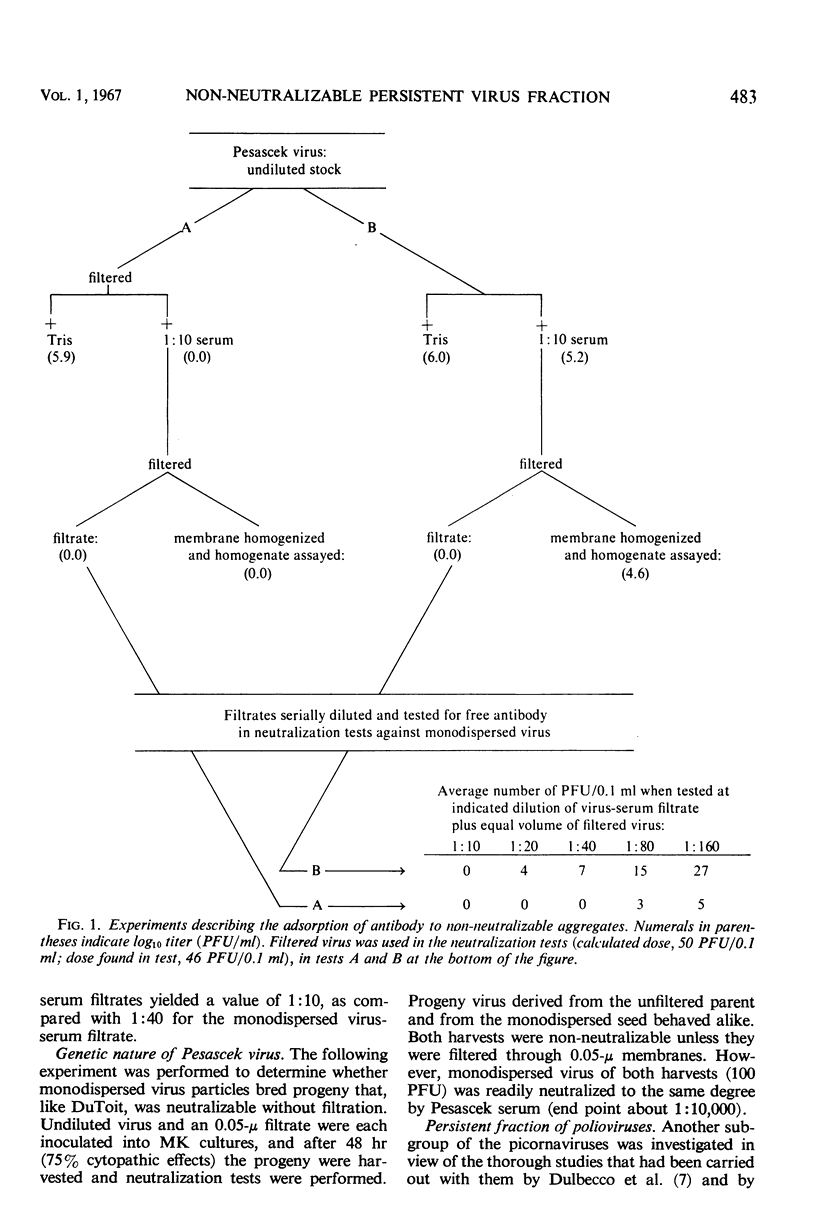
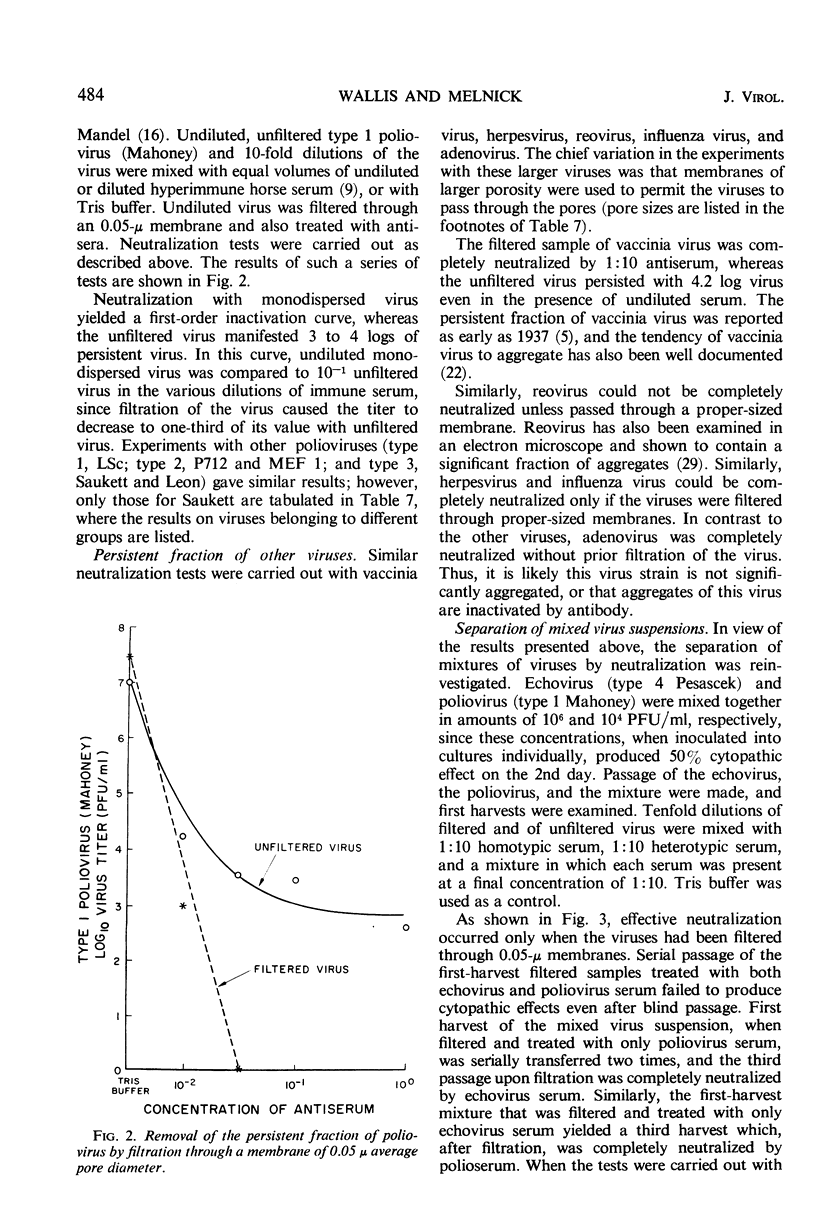
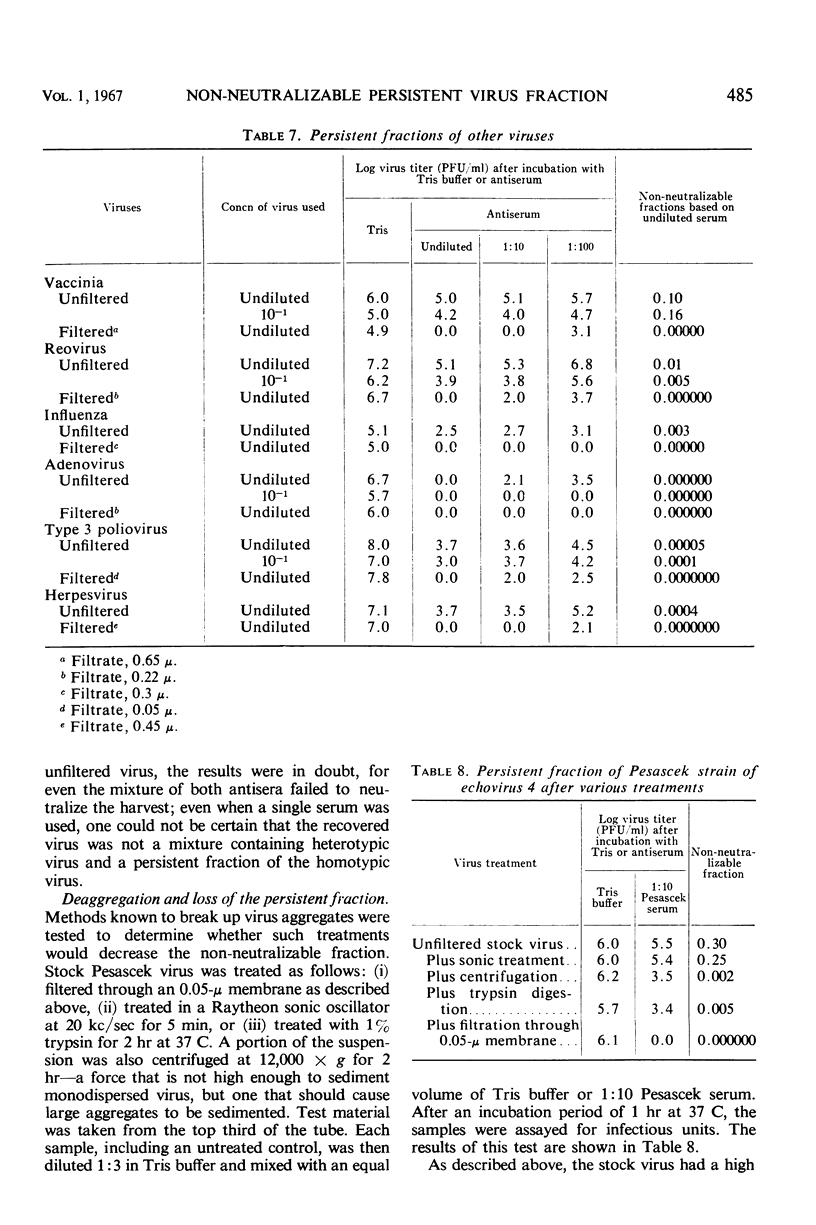
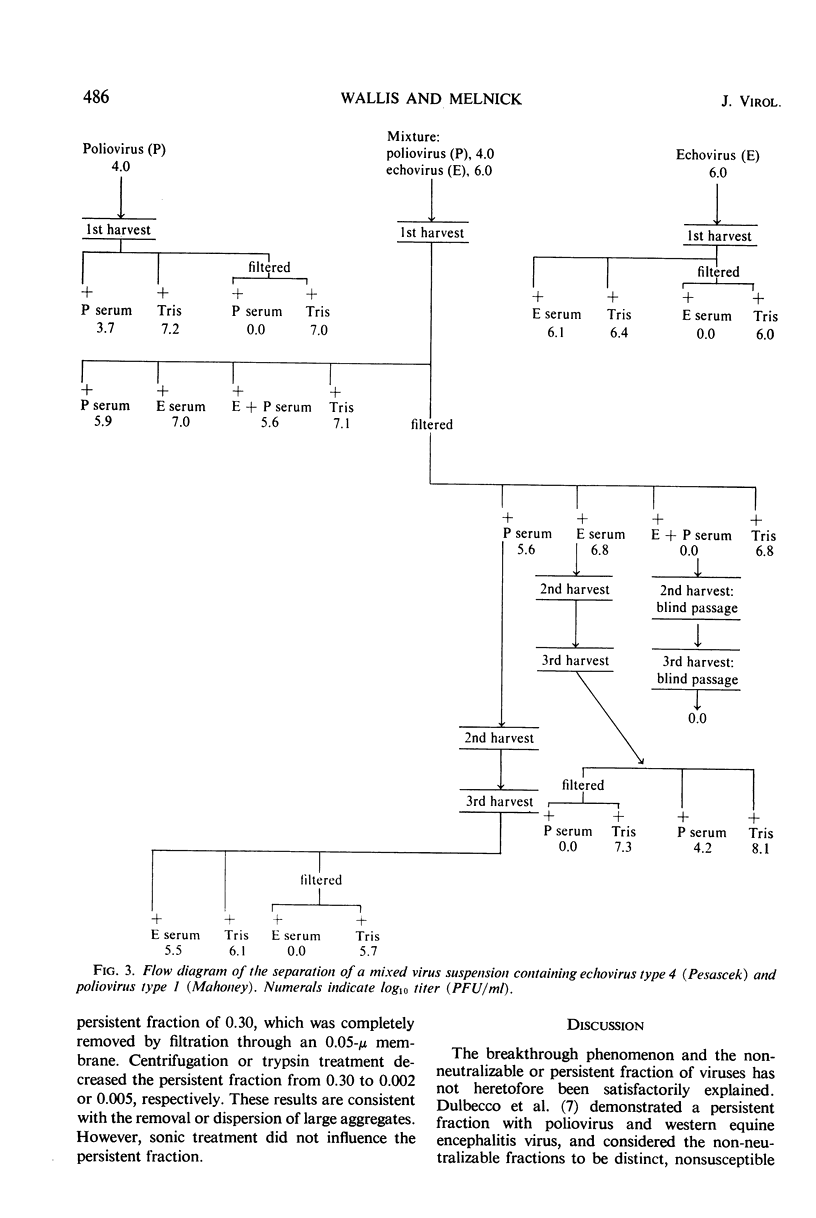
The non-neutralizable or persistent fraction of virus populations has been found to be caused by aggregated virus. Detailed investigation was performed with the prototype strain of echovirus type 4 (Pesascek), as this virus is notorious for its large non-neutralizable fraction. When Pesascek virus was clarified by low-speed centrifugation, homologous antiserum hardly neutralized the virus. However, when the virus was filtered through membranes having a porosity only twice the diameter of the virus, monodispersed virus was obtained which was efficiently neutralized. Serum titers were up to 1,000 times higher if the neutralization test was carried out with monodispersed virus. Virus in non-neutralizable aggregates was found to constitute 30% of the infective units of unfiltered Pesascek virus but only 0.1% of the antigenically related DuToit strain. This explains why DuToit strain has been a more satisfactory indicator strain for detecting type 4 antibodies, regardless of the echo 4 strain used for inducing the antibodies. Clarified suspensions and ultrafiltrates of viruses belonging to the picorna-, reo-, myxo-, adeno-, herpes-, and poxvirus groups were studied. Clarified suspensions yielded persistent fractions of 0.005% for poliovirus, of 0.1% for reovirus, of 0.6% for influenza virus, of <0.001% for adenovirus, of 0.06% for herpesvirus, and of 10 to 30% for vaccinia virus. In all cases the persistent fractions were removed by membrane filters which had a pore diameter no larger than twice that of the virus under test, and the high concentration of virus in each ultrafiltrate was completely neutralized by antiserum.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashe W. K., Notkins A. L. Neutralization of an infectious herpes simplex virus-antibody complex by anti-gamma-globulin. Proc Natl Acad Sci U S A. 1966 Aug;56(2):447–451. doi: 10.1073/pnas.56.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRON A. L., KARZON D. T. Characteristics of ECHO 4 (Shropshire) virus isolated during epidemic of aseptic meningitis. J Immunol. 1961 Nov;87:608–615. [PubMed] [Google Scholar]
- BRADISH C. J., FARLEY J. O., FERRIER H. E. Studies on the nature of the nature of the neutralization reaction and the competition for neutralizing antibody between components of the virus system of foot-and-mouth disease. Virology. 1962 Nov;18:378–400. doi: 10.1016/0042-6822(62)90029-6. [DOI] [PubMed] [Google Scholar]
- DE FAZEKAS S. T., GROTH S., WATSON G. S., REID A. F. The neutralization of animal viruses. I. A model of virus-antibody interaction. J Immunol. 1958 Mar;80(3):215–224. [PubMed] [Google Scholar]
- DULBECCO R., VOGT M., STRICKLAND A. G. A study of the basic aspects of neutralization of two animal viruses, western equine encephalitis virus and poliomyelitis virus. Virology. 1956 Apr;2(2):162–205. doi: 10.1016/0042-6822(56)90017-4. [DOI] [PubMed] [Google Scholar]
- Hampil B., Melnick J. L., Wallis C., Brown R. W., Braye E. T., Adams R. R., Jr Preparation of antiserum to enteroviruses in large animals. J Immunol. 1965 Nov;95(5):895–908. [PubMed] [Google Scholar]
- ITOH H., MELNICK J. L. The infection of chimpanzees with ECHO viruses. J Exp Med. 1957 Nov 1;106(5):677–688. doi: 10.1084/jem.106.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMISON R. M., MAYOR H. D., MELNICK J. L. Studies on ECHO 4 virus (Picornavirus group) and its intracellular development. Exp Mol Pathol. 1963 Apr;2:188–202. doi: 10.1016/0014-4800(63)90052-2. [DOI] [PubMed] [Google Scholar]
- Jamison R. M., Mayor H. D. Comparative study of seven picornaviruses of man. J Bacteriol. 1966 May;91(5):1971–1976. doi: 10.1128/jb.91.5.1971-1976.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAFFERTY K. J. THE INTERACTION BETWEEN VIRUS AND ANTIBODY. II. MECHANISM OF THE REACTION. Virology. 1963 Sep;21:76–99. doi: 10.1016/0042-6822(63)90306-4. [DOI] [PubMed] [Google Scholar]
- LEDINKO N., MELNICK J. L. Poliomyelitis viruses in tissue culture. V. Reaction of virus and antibody; variables of the quantitative neutralization test. Am J Hyg. 1953 Sep;58(2):223–247. [PubMed] [Google Scholar]
- MALHERBE H., HARWIN R., SMITH A. H. An outbreak of aseptic meningitis associated with ECHO virus type 4. S Afr Med J. 1957 Dec 14;31(50):1261–1264. [PubMed] [Google Scholar]
- MELNICK J. L. Application of tissue culture methods to epidemiological studies of poliomyelitis. Am J Public Health Nations Health. 1954 May;44(5):571–580. doi: 10.2105/ajph.44.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIFKIND R. A., GODMAN G. C., HOWE C., MORGAN C., ROSE H. M. Structure and development of viruses as observed in the electron microscope. VI. ECHO virus, type 9. J Exp Med. 1961 Jul 1;114:1–12. doi: 10.1084/jem.114.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBIN H., FRANKLIN R. M. On the mechanism of Newcastle disease virus neutralization by immune serum. Virology. 1957 Feb;3(1):84–95. doi: 10.1016/0042-6822(57)90025-9. [DOI] [PubMed] [Google Scholar]
- SALK J. E. Poliomyelitis vaccine in the fall of 1955. Am J Public Health Nations Health. 1956 Jan;46(1):1–14. doi: 10.2105/ajph.46.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D. G., Kim K. S. Multiplicity reactivation and radiation survival of aggregated vaccinia virus. Calculation of plaque titer based on MR and particle aggregation seen in the electron microscope. Virology. 1966 Jul;29(3):359–366. doi: 10.1016/0042-6822(66)90211-x. [DOI] [PubMed] [Google Scholar]
- TAKEMOTO K. K., FABISCH P. INFLUENCE OF ACID POLYSACCHARIDES ON PLAQUE FORMATION BY INFLUENZA A2 AND B VIRUSES. Proc Soc Exp Biol Med. 1963 Dec;114:811–814. doi: 10.3181/00379727-114-28806. [DOI] [PubMed] [Google Scholar]
- TYTELL A. A., TOROP H. A., McCARTHY F. J. Adenovirus plaque formation in grivet monkey kidney cells. Proc Soc Exp Biol Med. 1962 Apr;109:916–918. doi: 10.3181/00379727-109-27377. [DOI] [PubMed] [Google Scholar]
- WALLIS C., MELNICK J. L. INFECTIVITY OF TYPE 4 ECHOVIRUS-ANTIBODY COMPLEX. Virology. 1965 Jun;26:175–179. doi: 10.1016/0042-6822(65)90044-9. [DOI] [PubMed] [Google Scholar]
- WALLIS C., SMITH K. O., MELNICH J. L. REOVIRUS ACTIVATION BY HEATING AND INACTIVATION BY COOLING IN MGC12 SOLUTIONS. Virology. 1964 Apr;22:608–619. doi: 10.1016/0042-6822(64)90083-2. [DOI] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Concentration of enteroviruses on membrane filters. J Virol. 1967 Jun;1(3):472–477. doi: 10.1128/jvi.1.3.472-477.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L., Rapp F. Effects of pancreatin on the growth of reovirus. J Bacteriol. 1966 Jul;92(1):155–160. doi: 10.1128/jb.92.1.155-160.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Morales F., Powell J., Melnick J. L. Plaque enhancement of enteroviruses by magnesium chloride, cysteine, and pancreatin. J Bacteriol. 1966 May;91(5):1932–1935. doi: 10.1128/jb.91.5.1932-1935.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOHN D. S., HAMMON W. M. ECHO-4 viruses: improved methods and strain selection for identification and serodiagnosis. Proc Soc Exp Biol Med. 1960 Oct;105:55–60. doi: 10.3181/00379727-105-26007. [DOI] [PubMed] [Google Scholar]



