Abstract
Ravuconazole is a new antifungal triazole with broad-spectrum activity and a long half-life in plasma. We studied the antifungal efficacy, safety, and pharmacokinetics of ravuconazole lysine phosphoester in escalating dosages for the treatment of invasive pulmonary aspergillosis due to Aspergillus fumigatus in persistently neutropenic rabbits. Treatment groups consisted of rabbits treated with ravuconazole at 2.5 (RVC2.5), 5 (RVC5), and 10 (RVC10) mg/kg of body weight/day, rabbits treated with amphotericin B (AMB) at 1 mg/kg/day, or untreated controls. There was a dose-dependent reduction of pulmonary residual fungal burden (CFU per gram) in RVC5-, RVC10-, and AMB-treated rabbits in comparison to untreated controls (P < 0.01, P < 0.001, and P < 0.01, respectively). These findings correlated with progressive galactomannan antigenemia in untreated controls and the RVC2.5-treated rabbits, a lower galactomannan index (GMI) in RVC5- and RVC10-treated rabbits, and a similarly low GMI in AMB-treated rabbits (P < 0.01). Rabbits treated with RVC5, RVC10, and AMB also showed a reduction of organism-mediated pulmonary injury, as measured by infarct scores and lung weights, in comparison to untreated controls (P < 0.001). These results were supported by decreased pulmonary infiltrates detected by computed tomography in RVC5- and RVC10-treated rabbits in comparison to untreated controls (P < 0.05). Survival throughout the entire study was achieved in 95% of RVC5-treated rabbits (P < 0.001), 85% of RVC10-treated rabbits (P < 0.001), and 50% of AMB-treated rabbits (P < 0.05) in comparison to none of the untreated controls. Ravuconazole showed linear plasma pharmacokinetics and a large volume of distribution while maintaining concentrations in plasma above the MIC throughout the dosing interval. There was no evidence of hepatotoxicity or nephrotoxicity among ravuconazole-treated animals. Intravenously administered ravuconazole lysine phosphoester showed dose-dependent efficacy and an excellent safety profile for the treatment of invasive pulmonary aspergillosis in persistently neutropenic rabbits.
Invasive pulmonary aspergillosis is an important cause of infectious morbidity and mortality in immunocompromised patients (1, 7, 19, 25, 27, 31, 34, 42, 45). Conventional amphotericin B (AMB) is active for the primary treatment of invasive pulmonary aspergillosis, but its use is limited by dose-dependent nephrotoxicity (17, 39). Voriconazole also is effective for the primary treatment of aspergillosis (21). However, the use of voriconazole may be limited by hepatotoxicity, cutaneous reactions, and drug interactions (8, 44), and the current overall response rate for the treatment of invasive aspergillosis remains at approximately 50%. Moreover, the cyclodextrin solution of voriconazole may preclude administration of the intravenous (i.v.) solution to patients with renal impairment. Thus, there is a critical need for safe and effective antifungal compounds for the treatment of this life-threatening infection. The next generation of antifungal triazoles offers potential therapeutic and safety benefits for the treatment of invasive aspergillosis.
Ravuconazole (BMS-207147; ER-30346) is a novel antifungal triazole that has a potent and relatively broad spectrum of antifungal activity (3, 10, 11, 15, 22, 23, 33, 40, 47). In vitro studies have demonstrated potent activity of ravuconazole against Candida albicans, C. dubliniensis (including fluconazole- and itraconazole-resistant isolates), C. tropicalis, C. parapsilosis, C. krusei, and Cryptococcus neoformans (16, 36, 37, 49). Additional studies have demonstrated that ravuconazole is active in vitro against clinical isolates of filamentous fungi, including Aspergillus spp., Fusarium spp., Scedosporium apiospermum, and Trichosporon spp. (4, 9, 20, 28, 32, 35).
Preclinical and clinical studies have indicated that ravuconazole has a relatively long half-life in comparison to other triazoles. Most human trials thus far have investigated dosages of ravuconazole of 100 to 200 mg/day (BMS-207147 investigator brochure; Bristol-Myers Squibb Pharmaceutical Research Institute, 1998). Orally administrated ravuconazole also has shown encouraging activity in transiently immunocompromised animal models of disseminated aspergillosis, candidiasis, cryptococcosis, and histoplasmosis (5, 6, 20, 24, 38).
Little is known, however, about the activity and safety of ravuconazole against primary pulmonary aspergillosis in persistently neutropenic hosts, particularly at higher dosages. Most neutropenic and immunocompromised patients with invasive aspergillosis are seriously ill and require an i.v. administered antifungal agent. The lysine phosphoester conjugate of ravuconazole (BMS-379224) was synthesized to address this important need. We therefore investigated the antifungal efficacy, plasma pharmacokinetics, and safety of ravuconazole lysine phosphoester for the treatment of experimental pulmonary aspergillosis in persistently neutropenic rabbits.
MATERIALS AND METHODS
Organisms.
Aspergillus fumigatus NIH 4215 (ATCC MYA-1163), NIH 97-2025, and NIH 97-2350 were clinical isolates obtained from fatal cases of pulmonary aspergillosis. All three isolates were used in all in vitro and in vivo experiments. The organisms were subcultured from a frozen isolate (stored at −70°C) on Sabouraud dextrose slants (BBL, Cockeysville, Md.) and incubated for 24 h at 37°C. The slants were then allowed to grow at room temperature for an additional 5 days before conidia were harvested.
MICs against A. fumigatus were determined according to NCCLS standard M38-A microdilution methods (13, 29). A suspension of A. fumigatus was first diluted in normal saline (NS) (Quality Biological, Inc., Gaithersburg, Md.) to an optical density (OD) of 80 to 82% transmittance for a final inoculum of 0.5 × 106 to 1.0 × 106 CFU/ml. The suspension was then diluted in RPMI 1640 (BioWhittaker, Walkersville, Md.) at 1:50. This dilution corresponds to twice the density needed for the inoculum. Aliquots of 0.1 ml of serially diluted drug and 0.1 ml of the inoculum suspension were inoculated into microtiter wells and incubated for 48 h at 35°C. The final concentrations of ravuconazole (Bristol-Myers Squibb Company, Princeton, N.J.) ranged from 16 to 0.3 μg/ml. MICs were also determined by substituting antibiotic medium 3 (National Institutes of Health Media Unit, Bethesda, Md.) for RPMI 1640. The MIC was defined as 80% inhibition of the growth in the control well. The minimal fungicidal concentration (MFC) was then determined by plating 20 μl from wells showing no turbidity (12). The lowest concentration of drug that allowed the growth of fewer than three colonies was designated the MFC. The MICs of ravuconazole for A. fumigatus strains 4215, 97-2025, and 97-2350 in RPMI 1640 were 1.0, 0.5, and 0.25 μg/ml, respectively. The MICs of AMB for A. fumigatus strains 4215, 97-2025, and 97-2350 in RPMI 1640 were 1.0, 0.5, and 0.5 μg/ml, respectively. The MFCs of AMB for all strains in antibiotic medium 3 and in RPMI 1640 were 1.0 and 1.0 μg/ml, respectively. The MFCs of ravuconazole in RPMI 1640 for A. fumigatus strains 4215, 97-2025, and 97-2350 were 1.0, 1.0, and 4.0 μg/ml, respectively.
MTT hyphal damage assays.
A rapid hyphal damage assay with a tetrazolium salt (methylthiazoldiphenyl tetrazolium bromide [MTT]) (26) was conducted to evaluate the effects of ravuconazole on the hyphae of A. fumigatus. Yellow MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Sigma, St. Louis, Mo.) is cleaved by dehydrogenases of metabolically active fungi, which reduce it to its purple MTT-formazan derivative; the latter is then quantitated by spectrophotometry after alcohol extraction from the hyphae. Hyphal damage was assessed at 2, 6, and 24 h following exposure to concentrations of ravuconazole ranging from 0.1 to 25 μg/ml; results were reported as the mean percent damage at each concentration. Absorbance readings were obtained with a microplate spectrophotometer (Multiscan MMC/340; Titertek, Huntsville, Ala.) at the dual wavelengths of 570 and 690 nm. Hyphal damage was determined as follows: percent hyphal damage = 1 − [(OD of control wells − OD of test wells)/OD control wells] × 100.
Animals.
A total of 113 healthy female New Zealand White rabbits (Hazleton Research Products, Inc., Denver, Pa.) weighing 2.1 to 3.6 kg at the time of inoculation were used in all experiments. These studies were approved by the Animal Care and Use Committee of the National Cancer Institute. All rabbits were housed and monitored under humane care and use in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and according to National Institutes of Health guidelines for animal care and guidelines of the National Research Council (30). Rabbits were individually housed and maintained with water and standard rabbit feed ad libitum.
Vascular access was established in each rabbit by the surgical placement of a silastic tunneled central venous catheter as previously described (48). The silastic catheter permitted nontraumatic venous access for repeated blood sampling for studies of biochemical and hematological parameters, plasma pharmacokinetics, serum galactomannan levels, and administration of parenteral agents. Serum samples were drawn from all rabbits at the initiation of immunosuppression, during the course of pulmonary aspergillosis, and before death. Death was not used as an end point. Rabbits were euthanatized according to Animal Care and Use Committee-approved prespecified humane end points by i.v. administration of pentobarbital (65 mg of pentobarbital sodium/kg of body weight; pentobarbital sodium was in the form of 0.5 ml of Beuthanasia-D special [euthanasia solution]; Schering-Plough Animal Health Corp., Union, N.J.) at the end of each experiment, 24 h after the administration of the last dose of the study drug. All animals were included in the data analysis.
Inoculation.
Pulmonary aspergillosis was established as previously described (14). For each experiment, the inoculum of A. fumigatus was prepared from Sabouraud dextrose slants inoculated as described above. Conidia were harvested under a laminar airflow hood with a solution of 10 ml of 0.025% Tween 20 (Fisher Scientific, Fair Lawn, N.J.) in NS, transferred to a 50-ml conical tube, washed, and counted with a hemacytometer. The concentration was adjusted in order to give each rabbit a predetermined inoculum of 1.0 × 108 to 1.25 × 108 conidia of A. fumigatus in a volume of 250 to 350 μl. The concentrations of the inocula were confirmed by culturing serial dilutions on Sabouraud glucose agar.
Inoculation was performed on day 2 of the experiments while the animals were under general anesthesia. Each rabbit was anesthetized i.v. with 0.5 to 0.6 ml of a 2:1 (vol/vol) mixture of ketamine (100 mg/ml; Ketaset; Phoenix Scientific, Inc., St. Joseph, Mo.) and xylazine (20 mg/ml; Rompun, Bayer Corp., Agriculture Division, Animal Health, Shawnee Mission, Kans.). Once satisfactory anesthesia was obtained, a Flagg O straight-blade laryngoscope (Welch-Allyn Inc., Skaneateles Falls, N.Y.) was inserted into the oral cavity until the vocal cords were clearly visible. The A. fumigatus inoculum was then administered intratracheally with a tuberculin syringe attached to a 5.25-in. 16-gauge Teflon catheter (Becton Dickinson Infusion Therapy Systems Inc., Sandy, Utah).
Immunosuppression and maintenance of neutropenia.
To simulate the conditions of persistent neutropenia, i.v. treatment with cytarabine (Ara-C) (Cytosar-U; The Upjohn Company, Kalamazoo, Mich.) was initiated 1 day before endotracheal inoculation of the animals. Profound and persistent neutropenia (a granulocyte concentration of <100 granulocytes/μl) was achieved with an initial course of 525 mg of Ara-C per m2 for five consecutive days. A maintenance dose of 484 mg of Ara-C per m2 was administered for four additional days on days 8, 9, 13, and 14 of the experiment to maintain persistent neutropenia. Concomitant thrombocytopenia was present at a range of 30,000 to 50,000 platelets/μl. Methylprednisolone (Abbott Laboratories, North Chicago, Ill.) at a dose of 5 mg/kg of body weight/day was administered on days 1 and 2 of the experiment to inhibit macrophage activity against conidia in order to facilitate the establishment of infection.
Ceftazidime (75 mg/kg given i.v. twice daily; Glaxo, Inc., Research Triangle Park, N.C.), gentamicin (5 mg/kg given i.v. every other day; Elkins-Sinn, Inc., Cherry Hill, N.J.), and vancomycin (15 mg/kg given i.v. daily; Abbott Laboratories) were administered from day 4 of immunosuppression until study completion to prevent opportunistic bacterial infections during neutropenia. In order to prevent antibiotic-associated diarrhea due to Clostridium spiriforme, all rabbits continuously received 50 mg of vancomycin per liter of drinking water.
Total leukocyte counts and percentages of granulocytes were monitored twice weekly with a Coulter Counter (Coulter Corporation, Miami, Fla.), peripheral blood smears, and differential counts.
Antifungal compounds and treatment regimens.
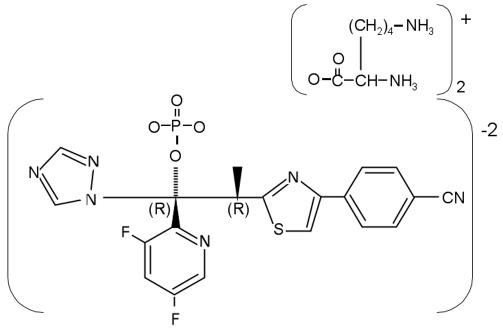
Rabbits were grouped to receive ravuconazole or desoxycholate AMB (Fungizone Apothecon; Bristol-Myers Squibb) for the treatment of established invasive pulmonary aspergillosis or no drug (untreated controls). Ravuconazole was provided as a phosphoester lysine salt (BMS-379224) (Fig. 1) and dissolved in 5% dextrose injection solution (Abbott Laboratories) to produce a stock solution (37.5 mg/ml) that was maintained at +4°C. Prior to use, ravuconazole was freshly diluted with 5% dextrose injection solution to a 9.375-mg/ml solution for the 2.5-mg/kg/day dosage (RVC2.5) or to an 18.75-mg/ml solution for the 5-mg/kg/day dosage (RVC5). Reconstituted ravuconazole was administered at an ambient temperature as a slow i.v. bolus over 1 min. AMB was diluted with sterile water to a concentration of 1 mg/ml and administered i.v. at 1 mg/kg/day slowly (0.1 ml every 10 s).
FIG. 1.
Structure of ravuconazole lysine phosphoester.
Antifungal therapy with RVC2.5, RVC5, or ravuconazole at 10 mg/kg/day (RVC10) or with AMB at 1 mg/kg/day i.v. was administered for 12 days starting 24 h after endotracheal inoculation of A. fumigatus conidia. The effects of the loading dose of ravuconazole were studied with the following loading and maintenance doses, respectively: 10 and 2.5 (RVC10-2.5), 10 and 5 (RVC10-5), 20 and 10 (RVC20-10), 30 and 10 (RVC30-10), and 40 and 10 (RVC40-10).
Outcome variables.
The following panel of outcome variables was used to assess antifungal efficacy: residual fungal burden (log CFU per gram), pulmonary infarct score, lung weight, survival, computed tomography (CT) score, and galactomannan index (GMI). Pulmonary infarct score, lung weight, and CT scan were used as measures of organism-mediated pulmonary injury.
Histopathological analysis.
Pulmonary lesions were excised and fixed in 10% neutral buffered formalin. Paraffin-embedded tissue sections were sectioned and stained with either periodic acid-Schiff or Grocott-Gomori methenamine-silver stain. Tissues were microscopically examined for pulmonary injury and structural changes in Aspergillus hyphae.
Fungal cultures.
Lung tissues from each rabbit were sampled and cultured by standard excision of tissue from each lobe. Each tissue sample was weighed, placed in a sterile polyethylene bag (Tekmar Corp., Cincinnati, Ohio), and homogenized with sterile saline for 30 s (Stomacher 80; Tekmar Corp., Cincinnati, Ohio) (43). Lung homogenate dilutions (10−1 and 10−2) were prepared in sterile NS. Aliquots (100 μl) from homogenates and homogenate dilutions were plated on Sabouraud glucose agar plates, incubated at 37°C for the first 24 h, and then left at room temperature for another 24 h. Carryover of the drug was controlled by serial dilution and by streaking of a small aliquot (100 μl) onto a large volume of agar (one full agar plate per 100-μl aliquot). The number of CFU of A. fumigatus was counted and recorded for each lobe, and the CFU per gram was calculated. A finding of one colony of A. fumigatus was considered positive.
Pulmonary lesion scores.
The entire heart-lung block was carefully resected at autopsy. The heart was dissected away from the lungs, leaving the tracheobronchial tree and lungs intact. The lungs were weighed and inspected by at least two observers, who were unaware of the treatment group and who recorded hemorrhagic infarct lesions (if any) in each lobe. Hemorrhagic infarcts were dark red consolidated lesions that corresponded histologically to coagulative necrosis and intra-alveolar hemorrhage. The number of positive lobes was added together, and the mean value for all positive lobes was calculated for each treatment group.
Survival.
The survival time in days postinoculation was recorded for each rabbit in each group. Surviving rabbits were euthanatized by sodium pentobarbital anesthesia on day 13 postinoculation, 24 h after the last dose of the study drug.
CT.
Serial CT scans of the lungs were obtained during all experiments in order to monitor the effects of antifungal therapy on organism-mediated pulmonary injury during the course of infection. Briefly, rabbits were sedated with ketamine and xylazine and then placed prone, head first, on the scanning couch. CT was performed with an ultrafast electron-beam CT scanner (model CE 0459 HiSpeed CT/I; GE Medical Systems, Milwaukee, Wis.) as previously described (46). With the high-resolution, table-incremented, volume acquisition mode, 3-mm-thick ultrafast CT scans were obtained every 4 s. A small scan circle and a 9.6-cm-diameter reconstruction circle with a matrix of 512 by 512 were used; these parameters resulted in a pixel size of less than 1 mm. Scan parameters were 80 kV and 120 mA, and the scan duration was 0.8 s. In virtually all cases, 30 slices were sufficient to scan the entire thorax of the rabbit. Images were photographed by using lung windows with a level of −600 Hounsfield units and a width of 1,800 Hounsfield units.
Galactomannan assay.
Blood was collected every other day from each rabbit for the determination of serum galactomannan concentrations. Serum galactomannan concentrations were determined by using the Platelia Aspergillus EIA (Genetic Systems/Sanofi Diagnostic Pasteur, Redmond, Wash.) one-stage immunoenzymatic sandwich microplate assay method, which was performed according to the manufacturer's directions (Platelia Aspergillus 62797; immunoenzymatic detection of galactomannan antigen of Aspergillus in serum; Sanofi Diagnostic Pasteur). The assay uses rat monoclonal antibody EB-A2, which is directed against Aspergillus galactomannan (41). The monoclonal antibody is used to sensitize the wells of the microplate and to bind the antigen. Peroxidase-linked monoclonal rat antibody is used as the detector antibody. The optical absorbances of specimens and controls are determined by using a microplate spectrophotometer equipped with 450- and 620-nm filters (Multiscan MMC/340).
Enzyme immunoassay data were expressed as the serum GMI plotted over time. The GMI for each test serum is equal to the OD of a sample divided by the OD of a threshold serum provided in the test kit. Sera with GMIs of less than 1 were considered negative. Sera with GMIs of greater than 1.5 were considered positive. Sera with GMIs of between 1 and 1.5 were considered indeterminate. Serial serum galactomannan levels were plotted over time of administration of the antifungal compound.
Toxicity studies.
Blood was collected from each rabbit every other day, starting from the first day after inoculation and continuing throughout the treatment. Plasma samples were stored in tubes (Sarstedt Inc., Newton, N.C.) at −70°C until all samples were processed simultaneously. Chemical determinations of potassium, aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, urea nitrogen, and total bilirubin concentrations in serum were performed with the penultimate sample drawn from each rabbit.
Pharmacokinetic studies.
The pharmacokinetics of ravuconazole in plasma following the administration of BMS-379224 were investigated with 5 to 13 infected animals each per dosage cohort by optimal plasma sampling. Time points for minimal plasma sampling were determined on the basis of full plasma drug concentration profiles obtained for healthy rabbits following the administration of similar dosages. Plasma sampling was performed on day 6 of antifungal therapy. Blood samples were drawn at 0.25, 2, 6, 12, and 24 h postdosing. Blood samples were collected in heparinized syringes. All plasma samples were immediately centrifuged and stored at −70°C until assayed.
Concentrations of ravuconazole in plasma were determined after protein precipitation with acetonitrile (1:2 [vol/vol]) by a reversed-phase high-performance liquid chromatography- UV method. The mobile phase consisted of acetonitrile-water (58:42 [vol/vol]). Separation was achieved by using a C18 ODS-AQ column (inside diameter, 150 by 4.6 mm; particle size, 5 μm; part no. AQ12S051546WT; YMC Inc., Wilmington, N.C.). Ravuconazole was detected with UV (wavelength, 284 nm). Quantitation was based on the peak height concentration response of the reference standard, BMS-207147. Ten-point standard curves (range of concentrations, 0.05 to 20 μg/ml) were linear, with r2 values of greater than 0.996. Samples containing concentrations exceeding the upper limits of the standard curves were assayed after dilution with the mobile phase after the determination of concentration-response linearity. The lower limit of quantitation in plasma was 0.10 μg/ml. Accuracies were within ±12%, and intra- and interday variabilities (precision) were <10%; at the lower limit of quantitation, accuracies and precision were within 16 and 13%, respectively.
Pharmacokinetic parameters for ravuconazole were determined by model-independent analysis. The following pharmacokinetic parameters were determined: maximum concentrations in plasma (Cmax); concentrations at 24 h after dosing (Cmin); the area under the plasma concentration-time curve (AUC) from 0 to 24 h (AUC0-24), calculated by trapezoidal estimation; and dose linearity, determined on the basis of the mean dose-normalized AUC from 0 h to infinity (AUC0-∞). Plasma drug clearance (CL), apparent volume of distribution at steady state (Vss), and half-life were calculated by using standard equations (18).
Statistical analysis.
Comparisons between groups were performed by using the Kruskal-Wallis test (nonparametric analysis of variance [ANOVA]) or the Mann-Whitney U test, as appropriate, for treatment groups versus untreated controls. A two-tailed P value of <0.05, which had already been adjusted for multiple comparisons by Bonferroni's method, was considered to be statistically significant. Survival was plotted by Kaplan-Meier analysis. Differences in survival between treatment groups and untreated controls were analyzed by the log rank test. Values are expressed as means and standard errors of the means (SEMs).
RESULTS
MTT hyphal damage assays.
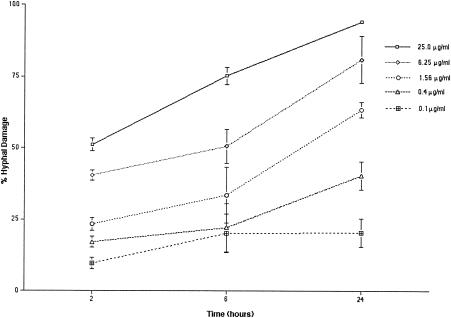
Concentration-dependent and time-dependent effects of ravuconazole on the hyphae of A. fumigatus were noted (Fig. 2). At ravuconazole concentrations ranging from 0.1 to 25 μg/ml, there was significant (P ≤ 0.002) concentration-dependent damage to hyphae. After 2 h, a ravuconazole concentration of 0.1 μg/ml produced a percentage (mean and SEM) of hyphal damage of 9.65% ± 1.92%, whereas a ravuconazole concentration of 25 μg/ml produced a percentage of hyphal damage of 51.2% ± 2.21% (Fig. 2). After 6 h, a ravuconazole concentration of 0.1 μg/ml produced a percentage of hyphal damage of 20.2% ± 6.63%, whereas a ravuconazole concentration of 25 μg/ml produced a percentage of hyphal damage of 75.3% ± 3.02%. There was no significant time-dependent effect on hyphae with further incubation for 24 h at a ravuconazole concentration of 0.1 μg/ml (percentage of hyphal damage, 20.4% ± 5%), whereas a ravuconazole concentration of 25 μg/ml produced a percentage of hyphal damage of 94.3% ± 0.46% after 24 h. This in vitro concentration-dependent effect of ravuconazole correlated with in vivo dose-dependent efficacy (Fig. 3).
FIG. 2.
A. fumigatus hyphal damage caused by ravuconazole, as determined by MTT assays. In vitro MTT assays revealed significant (the P value, as determined by ANOVA, was <0.002) concentration-dependent hyphal damage. Values are given as means and SEMs (error bars). Because the SEMs were small for several time points, the error bars may not always be apparent in the hyphal damage curves.
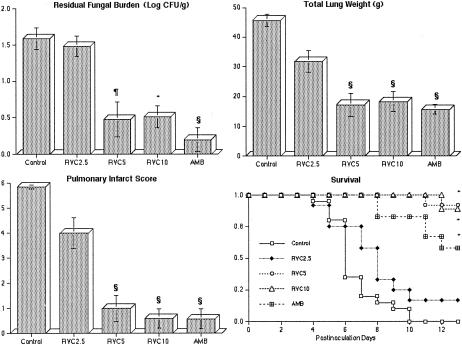
FIG. 3.
Response of primary pulmonary aspergillosis in persistently neutropenic rabbits to antifungal therapy, as measured by the mean concentration of residual organisms in pulmonary tissue (residual fungal burden), mean lung weight, mean pulmonary infarct score, and survival in untreated controls (n = 20) and rabbits treated with ravuconazole (RVC2.5 [n = 12], RVC5 [n = 10], or RVC10 [n = 10]) or AMB (1 mg/kg/day) (n = 12). Values are given as means and SEMs (error bars). P values are indicated as follows (comparisons of the indicated groups to the untreated control group, calculated by using the Kruskal-Wallis test [nonparametric ANOVA]): *, P < 0.05; §, P < 0.01; and ¶, P < 0.001. For the measure of survival, the values on the y axis are the probability of survival plotted by Kaplan-Meier analysis. Survival of rabbits in treatment groups differed significantly from that of control rabbits, as determined by the log rank test (*, P < 0.05).
Antifungal therapy.
There was a significant dose-dependent reduction of the residual A. fumigatus burden in lung tissues from rabbits treated with ravuconazole in comparison to lung tissues from untreated controls. Rabbits treated with RVC2.5 showed minimal benefit, while those treated with RVC5 and RVC10 showed reductions approaching that obtained with AMB at 1 mg/kg/day (Fig. 3).
There also was a significant reduction of organism-mediated pulmonary injury, as measured by total lung weight and pulmonary infarct score, in rabbits treated with ravuconazole, and this effect was comparable to that obtained with AMB (Fig. 3). The mean lung weights in rabbits treated with RVC5 and RVC10 but not RVC2.5 were significantly lower than those in the untreated controls (P < 0.001). Rabbits treated with RVC5 and RVC10 but not RVC2.5 also showed a significant reduction of the mean pulmonary infarct score in comparison to the untreated controls (P < 0.001).
The highest survival rates were achieved with RVC5 and RVC10. There were significant improvements in the survival of rabbits treated with RVC5 (9 of 10 [90%]; P < 0.001), RVC10 (8 of 9 [88.9%]; P < 0.001), and AMB (7 of 12 [58.3%]; P < 0.001) in comparison to that of untreated controls (Fig. 3). Of the rabbits treated with RVC2.5, only 2 of 12 (16.7%) survived, and none of the 20 untreated controls survived (Fig. 3).
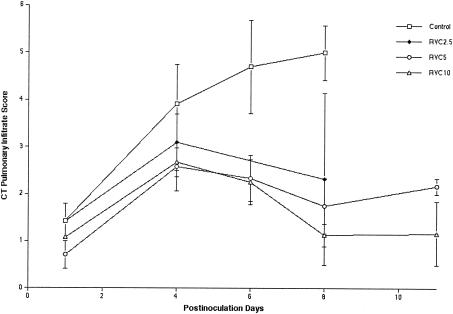
Consistent with the dose-dependent reduction of organism-mediated pulmonary injury, ultrafast CT scans demonstrated the resolution of pulmonary infiltrates in rabbits treated with ravuconazole (Fig. 4). There was a significant dose-related effect in the reduction of CT-measured pulmonary injury in RVC5- and RVC10-treated rabbits in comparison to untreated controls (P < 0.05). During the first 4 days of treatment, there was an increase in the pulmonary infiltrates in rabbits treated with RVC2.5, RVC5, and RVC10. However, the magnitude of the pulmonary infiltrate scores in rabbits treated with RVC5 and RVC10 within the first 4 days was lower than that in RVC2.5-treated rabbits or untreated controls. Following day 4 of treatment, there was a reduction of the number of infiltrates in rabbits treated with RVC5 and RVC10.
FIG. 4.
Pulmonary infiltrate scores determined by image analysis of serial CT scans of untreated control rabbits and ravuconazole-treated rabbits from all groups. Animals treated with ravuconazole showed significant resolution of pulmonary infiltrates in comparison to untreated controls (the P value, as determined by ANOVA, was <0.05). There were insufficient numbers of surviving rabbits to conduct CT scans for the untreated control group and the RVC2.5 group beyond day 8. Values are expressed as means ± SEMs.
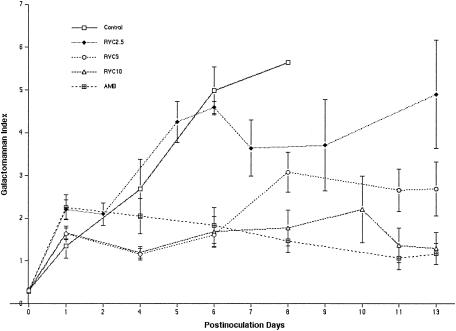
As a surrogate marker of the antifungal therapeutic response, the levels of galactomannan antigenemia (expressed as GMIs) in untreated control rabbits and RVC2.5-treated rabbits increased through day 6, correlating with the progression of invasive pulmonary aspergillosis (Fig. 5). The GMI in RVC2.5-treated rabbits declined after 6 days of therapy in surviving animals. The patterns of the GMIs in rabbits treated with RVC5, RVC10, and AMB were similar, with an early reduction of the GMIs in comparison to those in untreated controls and RVC2.5-treated rabbits. By day 8, a dose-dependent hierarchy was evident, with a higher GMI in RVC2.5-treated rabbits and lower GMIs in RVC5-, RVC10-, and AMB-treated rabbits (P ≤ 0.001 for differences between the GMI in untreated controls and the GMIs in RVC5-, RVC10-, and AMB-treated rabbits).
FIG. 5.
Galactomannan antigenemia (expressed as the GMI) in persistently neutropenic rabbits with pulmonary aspergillosis. By day 8, a dose-dependent hierarchy was evident, with GMIs being higher in the RVC2.5 group and lower in the RVC5, RVC10, and AMB groups (the P value for differences between the GMI in controls and the GMI in the RVC5, RVC10, or AMB group was ≤0.001). There were insufficient numbers of surviving rabbits to conduct GMI determinations for controls beyond day 8. Values are expressed as means ± SEMs.
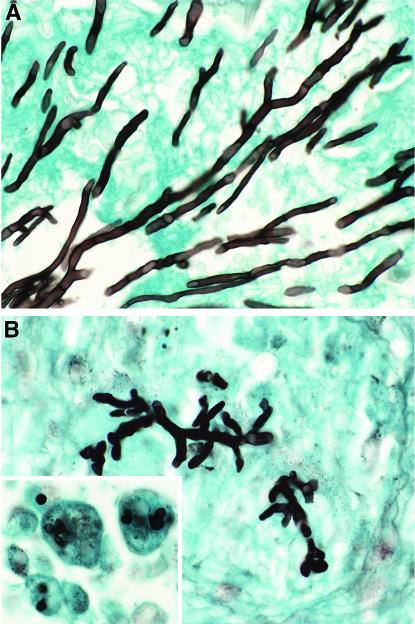
Also consistent with the antifungal efficacy of ravuconazole against pulmonary aspergillosis was its effect on hyphal morphology (Fig. 6). RVC5 treatment either fully eradicated hyphae from tissue or altered hyphal morphology. RVC5 treatment resulted in truncated, irregular, and disrupted hyphal structures sparsely distributed throughout the lungs. Other sections of lungs from RVC5-treated rabbits showed nongerminated conidia of A. fumigatus within pulmonary alveolar macrophages. Germination to hyphae was apparently suppressed by ravuconazole treatment. Tissue from RVC10-treated rabbits had no demonstrable fungal elements.
FIG. 6.
Effects of ravuconazole in vivo on hyphal structures of A. fumigatus in experimental pulmonary aspergillosis. All specimens were stained with Grocott-Gomori methenamine-silver stain. (A) Lung tissue from an untreated control rabbit, with typical slender dichotomously branching septate hyphae. (B) Reduction in length and distortion of hyphal elements in a representative section of lung tissue from an RVC5-treated rabbit. The insert in panel B shows nongerminated conidia within macrophages from an RVC5-treated rabbit. Magnification, approximately ×794.
We further assessed the potential benefits of loading doses of ravuconazole. Rabbits treated with RVC10-2.5 showed a significant reduction of organism-mediated pulmonary injury, as measured by total lung weight and pulmonary infarct score, in comparison to untreated controls (P ≤ 0.01). Rabbits treated with RVC10-5, RVC20-10, RVC30-10, and RVC40-10 showed a significant reduction of pulmonary fungal burden, total lung weight, and pulmonary infarct score in comparison to untreated controls (P ≤ 0.001). On the other hand, there were no significant benefits of treatment with the loading dose and maintenance regimen over treatment with only the maintenance regimen.
Plasma pharmacokinetics.
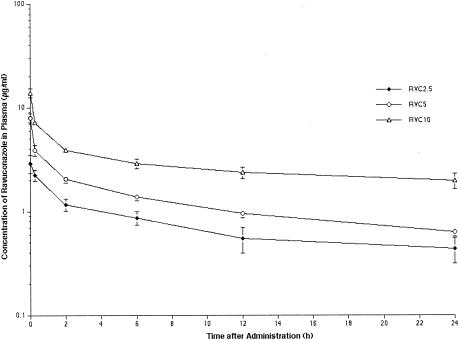
The observed plasma concentration-time profiles for RVC2.5, RVC5, and RVC10 are depicted in Fig. 7, and the corresponding pharmacokinetic parameters are listed in Table 1.
FIG. 7.
Plasma ravuconazole concentration profiles following i.v. administration of the prodrug, BMS-379224. All values represent the means and SEMs for six or more rabbits per group.
TABLE 1.
Pharmacokinetic parameters for ravuconazole at various dosages over 6 days in rabbits with invasive pulmonary aspergillosis
| Treatment group (no. of rabbits) | Mean ± SEMa:
|
|||||
|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Cmin (μg/ml) | AUC0-24 (μg · h/ml) | AUC0-∞/dose (h/ml) | Vss (liters/kg) | CL (liters/h/kg) | |
| RVC2.5 (16) | 2.7 ± 0.38 | 0.54 ± 0.11 | 20.27 ± 3.12 | 2.86 ± 0.40 | 9.65 ± 2.57 | 0.146 ± 0.03 |
| RVC5 (12) | 7.96 ± 0.74 | 0.62 ± 0.05 | 28.56 ± 3.19 | 2.36 ± 0.20 | 5.02 ± 0.70 | 0.178 ± 0.01 |
| RVC10 (11) | 13.88 ± 1.37 | 1.97 ± 0.33 | 68.66 ± 7.58 | 2.53 ± 0.28 | 3.50 ± 0.33 | 0.162 ± 0.01 |
P values indicating the degree of significance of differences in pharmacokinetic parameters across the dosage range of 2.5, 5.0, and 10 mg/kg/day were determined by ANOVA and found to be <0.001 (Cmax), <0.001 (Cmin), <0.001 (AUC0-24), 0.5185 (AUC0-∞/dose), <0.0035 (Vss), and 0.4818 (CL).
RVC2.5, RVC5, and RVC10 resulted in escalating peak levels in plasma that ranged from 2.78 ± 0.38 to 13.9 ± 1.37 μg/ml, exceeding the MICs for the experimental isolates. With RVC5 and RVC10, the mean concentrations in plasma remained at or above the median MIC of 0.5 μg/ml throughout the dosing interval. Ravuconazole exhibited a comparatively large Vss that exceeded that of total body water. Ravuconazole showed linear plasma pharmacokinetics, as evidenced by an unchanged CL and an unchanged dose-normalized AUC0-∞ across the investigated dosage ranges.
The administration of a loading dose at day 1 led to an increase in the mean AUC0-24 after the fifth dose (to 108 and 119% for RVC10-2.5 and RVC10-5 and to 114, 124, and 97% for RVC20-10, RVC30-10, and RVC40-10, respectively), but this increase was not statistically significant.
Safety.
Table 2 shows that rabbits treated with AMB had significantly higher levels of creatinine and urea nitrogen in serum than did rabbits treated with ravuconazole or untreated controls (P < 0.001). There was no significant elevation of the levels of bilirubin or hepatic transaminases in serum in any of the treatment groups.
TABLE 2.
Effects of ravuconazole and AMB on levels in serum of creatinine, urea nitrogen, ALT, AST, and bilirubin in persistently neutropenic rabbits with pulmonary aspergillosis
| Treatment group (no. of rabbits) | Mean ± SEM level in serum of:
|
||||
|---|---|---|---|---|---|
| Creatinine (mg/dl) | Urea nitrogen (mg/dl) | ALT (U/liter) | AST (U/liter) | Bilirubin (mg/dl) | |
| Controls (10) | 0.71 ± 0.06 | 19.90 ± 1.73 | 31.67 ± 3.63 | 19.67 ± 4.48 | 0.17 ± 0.06 |
| RVC5 (10) | 0.72 ± 0.04 | 17.00 ± 0.91 | 48.90 ± 8.87 | 27.40 ± 6.41 | 0.27 ± 0.11 |
| RVC10 (10) | 0.84 ± 0.06 | 18.90 ± 2.61 | 24.90 ± 3.02 | 11.00 ± 1.62 | 0.10 ± 0.00 |
| AMB (6) | 2.03 ± 0.33a | 95.83 ± 16.62a | 16.50 ± 2.83 | 7.67 ± 0.71 | 0.12 ± 0.17 |
The P value, as determined by ANOVA with Bonferroni's correction for multiple comparisons, was <0.001.
DISCUSSION
Ravuconazole administered i.v. to persistently neutropenic rabbits with primary pulmonary aspergillosis showed potent dose-dependent in vivo antifungal activity, reduced the residual fungal burden, improved survival, resolved galactomannan antigenemia, and reduced organism-mediated pulmonary injury, as measured by lung weights, pulmonary infarct scores, and CT scans. These antifungal effects of ravuconazole were comparable to those of AMB. In correlation with these in vivo activities, ravuconazole had concentration-dependent and time-dependent effects on hyphae, as measured by MTT hyphal damage assays. There was no evidence of hepatic or renal toxicity due to ravuconazole therapy at the dosages studied. In comparison, AMB administered at a dosage of 1 mg/kg/day caused significant renal impairment. Ravuconazole administered at dosages of 2.5 to 10 mg/kg/day showed linear plasma pharmacokinetics, and concentrations in plasma were maintained above the MIC throughout most of the 24-h dosing interval at dosages of ≥2.5 mg/kg/day. To our knowledge, this is the first reported laboratory investigation of the parenteral formulation of ravuconazole.
The development of a parenteral formulation of an antifungal compound is critical for its use in the treatment of invasive pulmonary aspergillosis in persistently neutropenic and other profoundly immunocompromised hosts. The novel structural modification of ravuconazole phosphorylates the hydroxyl group to serve as the diester bridge for the two lysine residues. The four amino groups of the two lysine residues confer a net positive polar charge to allow solubility in an aqueous solution. The phosphoester bonds are rapidly metabolized by hepatic alkaline phosphatase following i.v. administration. Infusion of ravuconazole lysine phosphoester was well tolerated by the animals, with no infusion-related toxicity. The parenteral solution offers potential for the treatment of immunocompromised patients who are unable to tolerate an oral formulation. Unlike the parenteral formulations of voriconazole and itraconazole, where the cyclodextrins will accumulate in renal insufficiency, the lysine and phosphate compounds of ravuconazole are readily cleared from the circulation.
Ravuconazole caused a dose-dependent increase in A. fumigatus hyphal damage, as measured by MTT hyphal damage assays. By the end of 24 h, the higher concentrations (6.25 and 25 μg/ml) had caused approximately 75 to 90% hyphal damage. These in vitro findings correlate with the dose-dependent properties of ravuconazole in vivo; i.e., RVC5 and RVC10 resulted in an approximate 10-fold decline in residual fungal burden (log CFU per gram) after 8 to 12 days of antifungal therapy. Dosages exceeding 5 mg/kg did not appear to confer any significant advantages in the reduction of residual fungal burden, organism-mediated pulmonary injury, or survival. Consistent with the dose-dependent antifungal effect of ravuconazole, organism-mediated pulmonary injury, as measured by the CT pulmonary infiltrate score, also declined in a dose-dependent manner.
The galactomannan antigenemia time curve also indicated a dose-dependent effect, with untreated controls showing progressively increasing GMIs in comparison to lower GMIs for RVC2.5 and the lowest GMIs for RVC5 and RVC10. Because the GMI serves as a surrogate marker for residual fungal burden, RVC5 and RVC10 appeared to achieve a more rapid reduction of residual A. fumigatus burden than did RVC2.5. In addition to correlating with a reduction of residual fungal burden, this earlier resolution of the GMI in infected rabbits correlated with a reduction of mortality and organism-mediated pulmonary injury.
Andes and colleagues recently reported that the AUC/MIC ratio was strongly predictive of treatment outcome in a murine model of disseminated candidiasis (2). Serum protein binding levels in mice and humans are 95.8 and 98%, respectively (BMS-207147 investigator brochure). There was a threshold of greater efficacy at dosages of ≥5 mg/kg/day than at dosages of ≤2.5 mg/kg/day. The AUC/MIC ratios for RVC2.5, RVC5, and RVC10 are approximately 40, 60, and 140, respectively, for A. fumigatus in this model. Thus, an effective AUC/MIC ratio for the treatment of pulmonary aspergillosis appears to be ≥60.
RVC5 and RVC10 given i.v. were more effective than was RVC2.5; however, little is known about the safety of these higher dosages in humans. Considerably more data on the safety of ravuconazole in human volunteers are available for RVC2.5. Trials with healthy volunteers and with patients are currently under way to assess the safety, tolerability, and plasma pharmacokinetics of dosages of ≥5 mg/kg/day in the oral and parenteral formulations. The findings from this study of the efficacy, safety, and plasma pharmacokinetics lay the foundation for future clinical trials of parenteral ravuconazole in persistently neutropenic and other immunocompromised patients with invasive pulmonary aspergillosis.
REFERENCES
- 1.Anaissie, E. 1992. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin. Infect. Dis. 14(Suppl. 1):S43-S53. [DOI] [PubMed] [Google Scholar]
- 2.Andes, D., K. Marchillo, T. Stamstad, and R. Conklin. 2003. In vivo pharmacodynamics of a new triazole, ravuconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:1193-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arikan, S., and J. H. Rex. 2002. Ravuconazole Eisai/Bristol-Myers Squibb. Curr. Opin. Investig. Drugs 3:555-561. [PubMed] [Google Scholar]
- 4.Carrillo, A. J., and J. Guarro. 2001. In vitro activities of four novel triazoles against Scedosporium spp. Antimicrob. Agents Chemother. 45:2151-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clemons, K. V., and D. A. Stevens. 2001. Efficacy of ravuconazole in treatment of mucosal candidosis in SCID mice. Antimicrob. Agents Chemother. 45:3433-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemons, K. V., M. Martinez, L. Calderon, and D. A. Stevens. 2002. Efficacy of ravuconazole in treatment of systemic murine histoplasmosis. Antimicrob. Agents Chemother. 46:922-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-805. [DOI] [PubMed] [Google Scholar]
- 8.Denning, D. W., P. Ribaud, N. Milpied, D. Caillot, R. Herbrecht, E. Thiel, A. Haas, M. Ruhnke, and H. Lode. 2002. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin. Infect. Dis. 34:563-571. [DOI] [PubMed] [Google Scholar]
- 9.Diekema, D. J., S. A. Messer, R. J. Hollis, R. N. Jones, and M. A. Pfaller. 2003. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J. Clin. Microbiol. 41:3623-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drugs R D. 1999. ER-30346. BMS 207147. Drugs R D. 1:170-171. [DOI] [PubMed] [Google Scholar]
- 11.Ernst, E. J. 2001. Investigational antifungal agents. Pharmacotherapy 21:165S-174S. [DOI] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff, A., A. Fothergill, J. Peter, M. G. Rinaldi, and T. J. Walsh. 2002. Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for Aspergillus spp.: NCCLS collaborative study. J. Clin. Microbiol. 40:3204-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espinel-Ingroff, A., M. Bartlett, R. Bowden, N. X. Chin, C. Cooper, Jr., A. Fothergill, M. R. McGinnis, P. Menezes, S. A. Messer, P. W. Nelson, F. C. Odds, L. Pasarell, J. Peter, M. A. Pfaller, J. H. Rex, M. G. Rinaldi, G. S. Shankland, T. J. Walsh, and I. Weitzman. 1997. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J. Clin. Microbiol. 35:139-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis, P., J. W. Lee, A. Hoffman, J. Peter, A. Francesconi, J. Bacher, J. Schelhamer, P. A. Pizzo, and T. J. Walsh. 1994. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar d-mannitol and serum galactomannan as markers of infection. J. Infect. Dis. 169:356-368. [DOI] [PubMed] [Google Scholar]
- 15.Fung-Tomc, J. C., E. Huczko, B. Minassian, and D. P. Bonner. 1998. In vitro activity of a new oral triazole, BMS-207147 (ER-30346). Antimicrob. Agents Chemother. 42:313-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fung-Tomc, J. C., T. C. White, B. Minassian, E. Huczko, and D. P. Bonner. 1999. In vitro antifungal activity of BMS-207147 and itraconazole against yeast strains that are non-susceptible to fluconazole. Diagn. Microbiol. Infect. Dis. 35:163-167. [DOI] [PubMed] [Google Scholar]
- 17.Gallis, H. A., R. H. Drew, and W. W. Pickard. 1990. Amphotericin B: 30 years of clinical experience. Rev. Infect. Dis. 12:308-329. [DOI] [PubMed] [Google Scholar]
- 18.Gibaldi, M., and D. Perrier.1982. Pharmacokinetics, 2nd ed., p. 55-459. Marcel Dekker, Inc., New York, N.Y.
- 19.Groll, A. H., M. Kurtz, W. Schneider, V. Witt, H. Schmidt, M. Schneider, and D. Schwabe. 1999. Five-year-survey of invasive aspergillosis in a pediatric cancer center. Epidemiology, management and long-term survival. Mycoses 42:431-442. [DOI] [PubMed] [Google Scholar]
- 20.Hata, K., J. Kimura, H. Miki, T. Toyosawa, M. Moriyama, and K. Katsu. 1996. Efficacy of ER-30346, a novel oral triazole antifungal agent, in experimental models of aspergillosis, candidiasis, and cryptococcosis. Antimicrob. Agents Chemother. 40:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman, H. L., E. J. Ernst, and M. E. Klepser. 2000. Novel triazole antifungal agents. Exp. Opin. Investig. Drugs 9:593-605. [DOI] [PubMed] [Google Scholar]
- 23.Hossain, M. A., and M. A. Ghannoum. 2000. New investigational antifungal agents for treating invasive fungal infections. Exp. Opin. Investig. Drugs 9:1797-1813. [DOI] [PubMed] [Google Scholar]
- 24.Kirkpatrick, W. R., S. Perea, B. J. Coco, and T. F. Patterson. 2002. Efficacy of ravuconazole (BMS-207147) in a guinea pig model of disseminated aspergillosis. J. Antimicrob. Chemother. 49:353-357. [DOI] [PubMed] [Google Scholar]
- 25.Latgé, J.-P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levitz, S. M., and R. D. Diamond. 1985. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J. Infect. Dis. 152:938-944. [DOI] [PubMed] [Google Scholar]
- 27.McNeil, M. M., S. L. Nash, R. A. Hajjeh, M. A. Phelan, L. A. Conn, B. D. Plikaytis, and D. W. Warnock. 2001. Trends in mortality due to invasive mycotic diseases in the United States, 1980-1997. Clin. Infect. Dis. 33:641-647. [DOI] [PubMed] [Google Scholar]
- 28.Moore, C. B., C. M. Walls, and D. W. Denning. 2000. In vitro activity of the new triazole BMS-207147 against Aspergillus species in comparison with itraconazole and amphotericin B. Antimicrob. Agents Chemother. 44:441-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards.2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 30.National Research Council Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Commission on Life Sciences. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 31.Pannuti, C., R. Gingrich, M. A. Pfaller, C. Kao, and R. P. Wenzel. 1992. Nosocomial pneumonia in patients having bone marrow transplant: attributable mortality and risk factors. Cancer 69:2653-2662. [DOI] [PubMed] [Google Scholar]
- 32.Paphitou, N. I., L. Ostrosky-Zeichner, V. L. Paetznick, J. R. Rodriguez, E. Chen, and J. H. Rex. 2002. In vitro antifungal susceptibilities of Trichosporon species. Antimicrob. Agents Chemother. 46:1144-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patterson, T. F. 1999. Role of newer azoles in surgical patients. J. Chemother. 11:504-512. [DOI] [PubMed] [Google Scholar]
- 34.Patterson, T. F., W. R. Kirkpatrick, M. White, J. W. Hiemenz, J. R. Wingard, B. Dupont, M. G. Rinaldi, D. A. Stevens, and J. R. Graybill. 2000. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine (Baltimore) 79:250-260. [DOI] [PubMed] [Google Scholar]
- 35.Pfaller, M. A., S. A. Messer, R. J. Hollis, and R. N. Jones. 2002. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob. Agents Chemother. 46:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, G. V. Doern, M. E. Brandt, and R. A. Hajjeh. 1998. In vitro susceptibilities of Candida bloodstream isolates to the new triazole antifungal agents BMS-207147, Sch 56592, and voriconazole. Antimicrob. Agents Chemother. 42:3242-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaller, M. A., S. A. Messer, S. Gee, S. Joly, C. Pujol, D. J. Sullivan, D. C. Coleman, and D. R. Soll. 1999. In vitro susceptibilities of Candida dubliniensis isolates tested against the new triazole and echinocandin antifungal agents. J. Clin. Microbiol. 37:870-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts, J., K. Schock, S. Marino, and V. T. Andriole. 2000. Efficacies of two new antifungal agents, the triazole ravuconazole and the echinocandin LY-303366, in an experimental model of invasive aspergillosis. Antimicrob. Agents Chemother. 44:3381-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarosi, G. A. 1990. Amphotericin B: still the “gold standard” for antifungal therapy. Postgrad. Med. 88:151-166. [DOI] [PubMed] [Google Scholar]
- 40.Sheehan, D. J., C. A. Hitchcock, and C. M. Sibley. 1999. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 12:40-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stynen, D., J. Sarfati, A. Goris, M. C. Prevost, M. Lesourd, H. Kamphuis, V. Darras, and J. P. Latge. 1992. Rat monoclonal antibodies against Aspergillus galactomannan. Infect. Immun. 60:2237-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh, T. J., and A. H. Groll. 1999. Emerging fungal pathogens: evolving challenges to immunocompromised patients for the twenty-first century. Transpl. Infect. Dis. 1:247-261. [DOI] [PubMed] [Google Scholar]
- 43.Walsh, T. J., C. McEntee, and D. M. Dixon. 1987. Tissue homogenization with sterile reinforced polyethylene bags for quantitative culture of Candida albicans. J. Clin. Microbiol. 25:931-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walsh, T. J., I. Lutsar, T. Driscoll, B. Dupont, M. Roden, P. Ghahramani, M. Hodges, A. H. Groll, and J. R. Perfect. 2002. Voriconazole in the treatment of aspergillosis, scedosporiosis and other invasive fungal infections in children. Pediatr. Infect. Dis. J. 21:240-248. [DOI] [PubMed] [Google Scholar]
- 45.Walsh, T. J., J. W. Hiemenz, and E. Anaissie. 1996. Recent progress and current problems in treatment of invasive fungal infections in neutropenic patients. Infect. Dis. Clin. North Am. 10:365-400. [DOI] [PubMed] [Google Scholar]
- 46.Walsh, T. J., K. Garrett, E. Feuerstein, M. Girton, M. Allende, J. Bacher, A. Francesconi, R. Schaufele, and P. A. Pizzo. 1995. Therapeutic monitoring of experimental invasive pulmonary aspergillosis by ultrafast computerized tomography, a novel, noninvasive method for measuring responses to antifungal therapy. Antimicrob. Agents Chemother. 39:1065-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh, T. J., M. A. Viviani, E. Arathoon, C. Chiou, M. Ghannoum, A. H. Groll, and F. C. Odds. 2000. New targets and delivery systems for antifungal therapy. Med. Mycol. 38(Suppl. 1):335-347. [PubMed] [Google Scholar]
- 48.Walsh, T. J., P. Bacher, and P. A. Pizzo. 1988. Chronic silastic central venous catheterization for induction, maintenance, and support of persistent granulocytopenia in rabbits. Lab. Anim. Med. 38:467-470. [PubMed] [Google Scholar]
- 49.Yamazumi, T., M. A. Pfaller, S. A. Messer, A. Houston, R. J. Hollis, and R. N. Jones. 2000. In vitro activities of ravuconazole (BMS-207147) against 541 clinical isolates of Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:2883-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]