Abstract
OBJECTIVE:
To determine the validity of alpha-1-acid glycoprotein as a novel biomarker for mortality in patients with severe sepsis.
METHODS:
We prospectively included patients with severe sepsis or septic shock at the emergency department at a single tertiary referral teaching hospital. All of the patients were enrolled within the first 24 hours of emergency department admission, and clinical data and blood samples were obtained. As the primary outcome, we investigated the association of serum levels of alpha-1-acid glycoprotein and 96-hour mortality with logistic regression analysis and generalized estimating equations adjusted for age, sex, shock status and Acute Physiology and Chronic Health Evaluation II score.
RESULTS:
Patients with septic shock had lower alpha-1-acid glycoprotein levels at the time of emergency department admission compared to patients without shock (respectively, 149.1±42.7 vs. 189.8±68.6; p = 0.005). Similarly, non-survivors in the first 96 hours were also characterized by lower levels of alpha-1-acid glycoprotein at the time of emergency department admission compared to survivors (respectively, 132.18±50.2 vs. 179.8±61.4; p = 0.01). In an adjusted analysis, alpha-1-acid glycoprotein levels ≤120 mg/dL were significantly associated with 96-hour mortality (odds ratio = 14.37; 95% confidence interval = 1.58 to 130.21).
CONCLUSION:
Septic shock patients exhibited lower circulating alpha-1-acid glycoprotein levels than patients without shock. Alpha-1-acid glycoprotein levels were independently associated with 96-hour mortality in individuals with severe sepsis.
Keywords: Alpha-1-acid Glycoprotein, Acute-Phase Protein, Severe Sepsis, Shock, Emergency Department, Mortality
INTRODUCTION
The primary cause of death in septic patients is related to organ dysfunction (1). Although the pathogenesis of multiple organ dysfunction syndrome (MODS) is not completely understood, the role of neutrophils and endothelial cells is thought to be crucial (2,3).
In the evolution of sepsis, the initial exacerbated pro-inflammatory response is counter-regulated through a phenomenon known as the compensatory anti-inflammatory response syndrome (CARS) (4). Acute-phase proteins (APPs) are important components of this last phenomenon and act by dampening the side-effects of inflammatory reactions, promoting the adequate resolution of inflammation and restoring homeostasis (5).
Alpha-1-acid glycoprotein (AGP), also known as orosomucoid, is one of the major APPs; its serum concentration increases two- to five-fold during an acute-phase response, such as sepsis (6). AGP is primarily synthesized by hepatocytes, although extra-hepatic synthesis has also been reported (6). Although the precise biological function of this protein is not completely understood, AGP has been reported to exhibit immunomodulatory functions in several cells types, including leukocytes, platelets and endothelial cells (7). In sepsis, AGP has recently been demonstrated to be required for the maintenance of capillary permeability and to be partially protective in several rodent models of shock, in which it acts to maintain the perfusion of vital organs (8). However, AGP could potentially inhibit neutrophil migration to the infectious focus in experimental models of sepsis, thus leading to inadequate bacterial clearance and resulting in an increased death rate (9).
Because minimizing the time-to-treatment is a crucial goal for reducing mortality, admitting septic patients to the emergency department (ED) could reduce the variation in the time-to-treatment that serves as a potential confounding variable. This factor is important because the accurate determination of the time of sepsis onset is difficult (i.e., there is no reliable clinical marker, such as chest pain in acute coronary syndromes) and intensive care unit (ICU) admission can be delayed for hours after a patient arrives in the ED, especially in developing countries. ED-based studies could be highly relevant for determining the accuracy and reliability of tools used to estimate disease severity and to predict mortality risk in septic patients (10-12). Therefore, the aims of this study were to investigate the serum levels of AGP in patients with severe sepsis in the ED and to determine the utility of this glycoprotein in predicting mortality in patients with sepsis in this setting.
METHODS
Patients
The study protocol was reviewed and approved by our institutional ethics committee. Informed written consent was obtained from all enrolled patients or substitute decision makers. This study involved a retrospective analysis of prospectively collected data. Patients older than 18 years who were admitted to the ED of the School of Medicine of Ribeirão Preto of the University of São Paulo (Brazil) between May 28, 2009 and October 25, 2010 and fulfilled the diagnostic criteria for severe sepsis were included in this study as a convenience sample within 24 hours after admission. We defined severe sepsis as sepsis plus at least one organ dysfunction according to the Sequential Organ Failure Assessment (SOFA) score (13). Any value of the SOFA score ascribed for organ dysfunction secondary to sepsis defined a patient as having severe sepsis. Patients who met any of the following criteria or had any of the following conditions were excluded from the study: liver cirrhosis, hepatitis, a low neutrophil count (<1,000 cells/mm3), a hematologic or solid malignancy, acquired immunodeficiency disease syndrome, chronic use of corticoids, a recent blood transfusion (within the past 10 days) and pregnancy.
To calibrate the commercial AGP kit, 16 healthy volunteers (each of whom provided a signed consent form) were tested using the AGP kit as controls (mean age±SD: 33.6±9.6, 60% male). These assays verified that the values generated were within the range described by the manufacturer.
Clinical data collection
Clinical data, including demographics and comorbidities, and laboratory data, including the levels of C-reactive protein (CRP), sodium, creatinine, lactate and total bilirubin; the ratio of arterial partial pressure of oxygen to the fraction of inspired oxygen (PaO2/FiO2); the number of white blood cells and platelets; and all other data required to calculate severity-of-illness scores, were prospectively collected. All of the patients were monitored until the time of death or hospital discharge. SOFA and Acute Physiology and Chronic Health Evaluation II (APACHE II) (14) scores were calculated for the first 24 hours after arrival at the ED. Investigators who collected the clinical data were blinded to the biological assay results. Septic shock was defined as severe sepsis requiring the use of a vasopressor drug (a SOFA score of 3–4).
AGP measurements
Peripheral blood was obtained from patients and collected in a dry tube. All samples were stored at -70°C until further analysis. The minimum and maximum times between storage and measurement of samples were one month and eight months, respectively. Serum levels of AGP were measured in our institution's biochemistry lab using a commercially available kit based on immunoturbidimetric methods (Quibasa Química Básica) according to the manufacturers' instructions.
Statistical analysis
Data were entered into a Microsoft Access database (Microsoft, Seattle, WA), and we used Stata version 9.2 software (StataCorp, College Station, TX) for statistical analyses. Clinical data were summarized using means±SD or medians with interquartile ranges (IQRs) for continuous variables; percentages were used for categorical data. The comparisons between groups for continuous variables were performed using t-tests or equivalent nonparametric tests. We used a chi-square or Fisher exact test, as appropriate, for categorical variables. A Spearman's rank correlation coefficient (ρ) was calculated to describe correlations between AGP levels and clinical variables. Receiver-operator characteristic (ROC) curves were constructed to evaluate the capacities of the exposures (AGP levels, APACHE II and SOFA scores) to predict the outcome (96-hour mortality). The area under the curve (AUC) was obtained and employed for comparison using a log-rank test. The primary outcome was early mortality, which was defined as death for any reason during the first 96 hours after ED admission. For comparison, we defined an AGP cut-off level of >120 mg/dL, which is the upper normal level in normal adults (i.e., 50 to 120 mg/dL as specified by the manufacturer of our kit). An assessment of the cutoff values for the two biomarkers of early mortality was undertaken. The best cutoff values for the SOFA and APACHE II score were defined as the values that maximized the Youden index (15).
To examine the prognostic value of AGP levels at the time of ED admission, Kaplan-Meier survival curves were constructed stratifying AGP levels as either ≤120 mg/dl or >120 mg/dl. We used multiple logistic regression for evaluating the association of the outcome (death within 96 hours) and the exposure (AGP levels) while controlling for possible confounders. We used forward stepwise regression that considered demographics, clinical status (shock) and level of organ dysfunction (APACHE II). These variables were chosen because they were representative and respected the conditions for multivariate analysis. Moreover, our sample size limited the inclusion of other variables that were not so strongly associated with the outcome. The odds ratios and corresponding 95% confidence intervals were calculated as measures of the clinical impact of variables. All analyses were two-tailed with p-values<0.05 denoting statistical significance.
RESULTS
Study population
In this study, a total of 70 patients were prospectively included. The mortality rate within the first 96 hours after ED admission was 15.71% (n = 11 patients). The global mortality at 28 days was 31.43% (n = 22 patients). Baseline clinical and laboratory parameters for survivors and non-survivors at the 96-hour time point are summarized in Table 1. Non-survivor patients (n = 11) exhibited higher SOFA (p = 0.02) and APACHE II (p<0.001) scores and lower PaO2/FiO2 ratios (p = 0.04) and were more likely to present with septic shock (p = 0.02) than survivors (n = 59). The primary source of infection for most patients was the respiratory tract followed by the urinary tract (Table 1.
Table 1.
Demographic and clinical parameters of survivors and non-survivors in the first 96 hours after emergency department admission.
| Survivors (n = 59) | Non-survivors (n = 11) | p-value | |
| Male sex, n (%) | 31 (52.5%) | 5 (45.4%) | 0.66 |
| Age*) (years) | 64 (55 to 76) | 68 (59 to 82) | 0.22 |
| Diabetes mellitus, n (%) | 18 (30.5%) | 6 (54.5%) | 0.12 |
| Site of infection | 33 (55.9%) | 5 (45.5%) | |
| Lung, n (%) | |||
| Genitourinary tract, n (%) | 16 (27.1%) | 4 (36.3%) | 0.78 |
| Other, n (%) | 10 (17.0%) | 2 (18.2%) | |
| PaO2/FiO2¶) ratio, median±SD | 209±120 | 131±101 | 0.04 |
| Creatinine (mg/dl), median±SD | 1.98±1.65 | 2.80±2.04 | 0.14 |
| Bilirubin# (mg/dl), median±SD | 1.67±3.03 | 3.73±7.25 | 0.16 |
| Lactemia§ (mg/dl), median±SD | 3.14±1.88 | 5.00±4.26 | 0.08 |
| Platelet count (103/mm3), median±SD | 194±115 | 189±112 | 0.88 |
| C-reactive protein¥ (mg/dl), median±SD | 19.1±10.44 | 13.4±8.84 | 0.13 |
| Septic shock, n (%) | 22 (37.3%) | 8 (72.7%) | 0.029 |
| APACHE II score*) | 19 (13 to 27) | 38 (24 to 41) | <0.001 |
| SOFA score*) | 7 (4 to 11) | 11 (8 to 14) | 0.02 |
| AGP (mg/dl), median±SD | 179.8±61.4 | 132.18±50.2 | 0.01 |
Variable expressed as median (25th to 75th percentiles);
n = 68 patients (57 survivors×11 non-survivors); #n = 58 patients (50 survivors×8 non-survivors); §n = 69 patients (58 survivors×11 non-survivors); ¥n = 62 patients (52 survivors×10 non-survivors).
APACHE, Acute Physiology and Chronic Health Evaluation; Pa02/FI02, arterial partial pressure of oxygen/fraction of inspired oxygen ratio; SD: standard deviation; SOFA, Sequential Organ Failure Assessment score.
No significant differences in AGP serum levels were observed between genders or based on diabetes mellitus status. However, patients with septic shock had lower AGP levels at ED admission when compared to patients without shock (respectively, 149.1±42.7 vs. 189.8±68.6; p = 0.005). Similarly, non-survivors in the first 96 hours were also characterized by lower levels of AGP at ED admission compared with survivors (respectively, 132.18±50.2 vs. 179.8±61.4; p = 0.01) (Table 1.
AGP levels were significantly lower in the 30 septic shock patients (149.1±42.7) compared to the 40 patients without shock (189.8±68.6; p<0.01). In addition, non-survivors exhibited lower AGP levels both 96 hours (p = 0.018) and 28 days after ED admission (p = 0.03).
Prognostic values of AGP, APACHE II and SOFA score
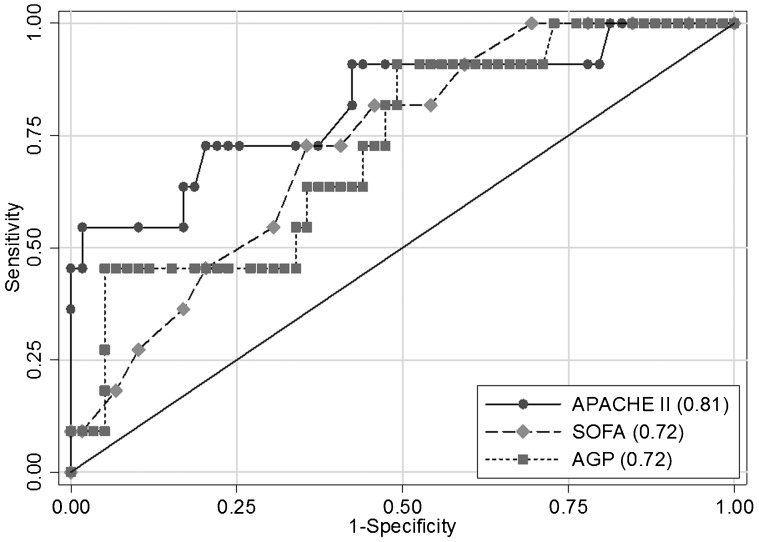
AUCs for AGP (AUC = 0.72; 95% CI = 0.56 to 0.88; sensitivity 45.5%; specificity 88.1%; accuracy 81.4%), SOFA (AUC = 0.72 for a value of 11; 95% CI = 0.58 to 0.87; sensitivity 54.5%; specificity 69.5%; accuracy 67.1%) and APACHE II (AUC = 0.81 for a value of 24; 95% CI = 0.65 to 0.97; sensitivity 81.8%; specificity 57.6%; accuracy 61.4%) are compared in Figure 1. While we made a somewhat arbitrary decision to use an AGP cut off of 120 mg/dl, optimization using the Youden index allowed us to obtain the most accurate cut-off value of 103 mg/dl (sensitivity 45.4%; specificity 93.2%; accuracy 85.7%). All three of these markers had a similar capacity to predict this significant outcome, and no statistically significant differences were observed between the markers (p>0.05).
Figure 1.
Receiver operation characteristic analysis using AGP serum levels, APACHE II and SOFA as predictors of 96-hour mortality.
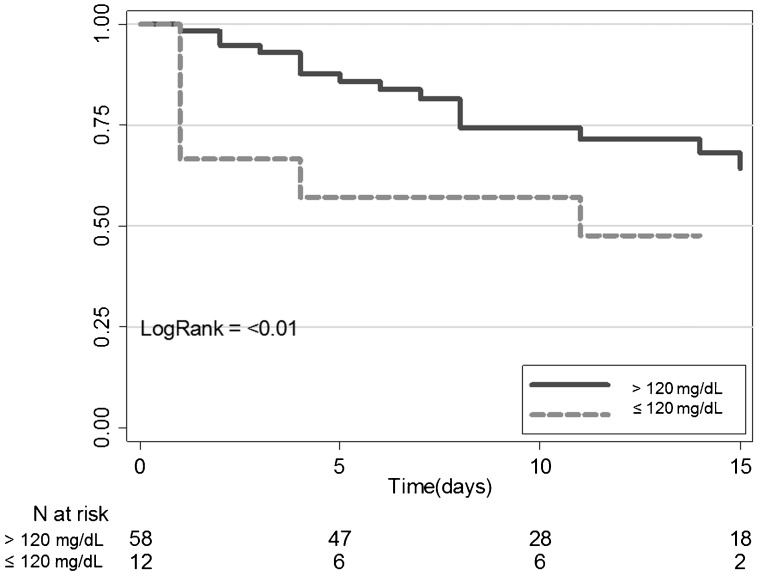
Patients with AGP serum levels of ≤120 mg/dL at the time of ED admission exhibited higher mortality within the first 96 hours than those with higher AGP levels (> 120 mg/dl; Chi-square: 7.36; p<0.01). Figure 2 shows the Kaplan-Meier 96-hour survival curves classified by AGP levels at the time of admission (above or below 120 mg/dL). The log-rank test confirmed the statistical significance of the AGP effect (p<0.01). Logistic regression analysis indicated that, in a model adjusted for age, gender, shock status and APACHE II score, an AGP serum level ≤120 mg/dL was still associated with increased likelihood of death within the first 96 hours after ED admission (Table 2.
Figure 2.
Survival curves at 96 hours for patients grouped according to their AGP serum levels (≤or>120 mg/dL).
Table 2.
Multiple logistic regression analysis of variables to 96-hour mortality.
| Odds ratio* | CI 95% | p-value | |
| AGP | 6.19 | 1.48–25.74 | 0.012 |
| AGP+age | 9.34 | 1.89–46.10 | 0.006 |
| AGP+age+gender | 10.53 | 2.02–54.81 | 0.005 |
| AGP+age+gender+status of shock | 8.60 | 1.58–46.60 | 0.013 |
| AGP+age+gender+status of shock+APACHE II | 14.37 | 1.58–130.21 | 0.018 |
AGP, Alpha-1 acid glycoprotein is expressed as a dichotomized variable (> or ≤120 mg/dL); APACHE II, Acute Physiology and Chronic Health Evaluation II; *odds ratio of AGP serum levels ≤120 mg/dL; all variables were independently associated with mortality.
Correlation of AGP and organ dysfunction
AGP levels were inversely correlated with lactemia (ρ = −0.43; p = 0.0002) and SOFA scores (ρ = −0.26; p = 0.029). In contrast, AGP levels were directly correlated with both CRP levels (ρ = 0.47; p = 0.0001) and PAO2/FiO2 ratios (ρ = 0.33; p = 0.006). However, while they were statistically significant, these correlations were weak. No significant associations with creatinine, bilirubin, age or APACHE II scores were observed.
DISCUSSION
For the first time, this study demonstrates that among severe sepsis patients, low serum levels of AGP at ED admission are associated with increased mortality at 96 hours independent of age, gender, shock status and APACHE II score.
Death of septic patients primarily results from MODS and refractory shock (1). The pathogenesis of organ dysfunction is not completely understood, but several mechanisms have been identified, including an impaired neutrophil migration to the infectious focus combined with an inappropriate sequestration of these cells in secondary organs, together with endothelial dysfunction (18-20).
Several potential mechanisms could explain why AGP levels were decreased in non-survivors: 1) the binding of AGP to soluble or cell molecules, such as those presented on the endothelium, could diminish its serum bioavailability; 2) an increase in the clearance of AGP from the circulation could result in low serum AGP levels (i.e., different glycoforms of AGP could have different half-lives) (16); and 3) AGP synthesis could be impaired. Given that we excluded subjects with cirrhosis or hepatitis and because the serum levels of CRP and bilirubin were not significantly different between the groups, we did not favor this latter hypothesis.
Our study indicates that, among severe sepsis patients who arrived at the ED, those who presented with septic shock displayed significantly lower serum AGP levels than those without shock. Previous studies have reported beneficial effects of AGP in models of shock (8). This outcome may result from the high affinity of the AGP glycan moiety to E-selectin on the surfaces of endothelial cells, which contributes to enhanced capillary barrier function and reduced leukocyte adhesion, thus maintaining organ perfusion (17). We also identified a significant correlation between AGP levels and other markers of organ dysfunction; however, the correlations with these other markers were weak and difficult to interpret.
AGP has been shown to inhibit the rolling/adhesion and migration of leukocytes and the adhesion and aggregation of platelets (18). These functions are also related to the direct interaction of its glycan moiety, especially its sialyl Lewis X (sLeX) residue, with selectins present in leukocytes and platelets (17). Therefore, the reduction of AGP serum levels during severe sepsis may contribute to increased leukocyte accumulation in several organs, which is a key event leading to organ dysfunction (19). A previous study demonstrated that non-surviving septic patients displayed a marked elevation of fucosylated AGP and increased expression of SLeX groups on AGP, suggesting that these changes in AGP glycosylation may provide prognostic value in sepsis (20). Additionally, in agreement with our data, Seidelin et al. has shown that the soluble levels of L-selectin (sL-selectin) are lower in septic patients who die in the hospital than in healthy controls or in septic patients who survive (21). It is likely that high levels of sL-selectin can competitively disrupt the L-selectin-mediated binding of leukocytes to endothelial cells (21).
Aside from its beneficial role in the CARS phenomenon, AGP can also be harmful. For example, if high levels of AGP are present for extended periods of time, it is possible that this can lead to immunoparalysis, which is related to increased rates of nosocomial sepsis, MODS and death in critically ill patients (22). Previously, in murine models of sepsis, our group has demonstrated that administration of AGP can inhibit the migration of neutrophils to infectious foci, increasing the mortality of animals (9). Furthermore, we recently demonstrated that increased AGP concentrations observed during diabetes play a role in the high susceptibility to infection in this disease (23). These results are in apparent contradiction with our present data. However, it should be noted that the animals involved in both of these experimental studies did not receive antibiotics. As a result, even if AGP was protective against organ dysfunction in this setting, the animals were completely unprotected against pathogens because AGP also impairs the recruitment of neutrophils to infectious foci, an effect that would further jeopardize the immune responses of the animals. In humans, the main problem in severe sepsis is MODS, as it is possible to control bacterial load using antibiotics. In fact, circulating AGP can represent an important tool regulating inflammation.
Finally, the results of this study suggest that AGP functions as a predictor of early mortality with fair to good accuracy when measured in patients with severe sepsis at the time of ED presentation. Although AGP is not superior to APACHE II or SOFA scores as a predictor of early mortality, AGP levels ≤120 mg/dL are the most specific marker of this outcome.
This study has limitations. We examined a small sample from a single institution. In addition, we were unable to measure AGP concentrations sequentially to determine the exact time course of the changes in serum levels of AGP. Although the reported half-life of radiolabeled AGP is approximately 5 days, its pharmacokinetic properties may change in severe sepsis (24). Another potential problem with the study could be a lack of standardized treatment, but our institution has a protocol based on the work of Rivers et al., and thus immediate fluid infusion and antibiotics were similarly offered to both groups (25). Furthermore, one caveat of the study, which is applicable for all studies concerning sepsis, is that we cannot tell the precise moment when the patient developed sepsis. Nevertheless, it should be emphasized that the study was conducted at a tertiary referral academic ED, which is included in a referral system that prioritizes severe cases (26).
In conclusion, AGP levels are an independent predictor of short-term survival in patients with severe sepsis admitted to the ED. Because AGP levels predict prognosis and because AGP is relatively easy and inexpensive to measure, its employment as a biomarker has the potential for widespread utilization. A larger multi-center study should be conducted to validate our findings.
ACKNOWLEDGMENTS
This study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo and Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brasil.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Vincent JL, Nelson DR, Williams MD. Is worsening multiple organ failure the cause of death in patients with severe sepsis. Crit Care Med. 2011;39(5):1050–5. doi: 10.1097/CCM.0b013e31820eda29. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420(6917):885–91. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 3.Silva E, Passos RH, Ferri MB, de Figueiredo LF. Sepsis: from bench to bedside. Clinics. 2008;63(1):109–20. doi: 10.1590/s1807-59322008000100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Poll T, van Deventer SJ. Cytokines and anticytokines in the pathogenesis of sepsis. Infect Dis Clin North Am. 1999;13(2):413–26, ix. doi: 10.1016/s0891-5520(05)70083-0. [DOI] [PubMed] [Google Scholar]
- 5.Johnson HL, Chiou CC, Cho CT. Applications of acute phase reactants in infectious diseases. J Microbiol Immunol Infect. 1999;32(2):73–82. [PubMed] [Google Scholar]
- 6.Fournier T, Medjoubi N, Porquet D. Alpha-1-acid glycoprotein. Biochim Biophys Acta. 2000;1482(1-2):157–71. doi: 10.1016/s0167-4838(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 7.Hochepied T, Berger FG, Baumann H, Libert C. Alpha(1)-acid glycoprotein: an acute phase protein with inflammatory and immunomodulating properties. Cytokine Growth Factor Rev. 2003;14(1):25–34. doi: 10.1016/s1359-6101(02)00054-0. [DOI] [PubMed] [Google Scholar]
- 8.Muchitsch EM, Auer W, Pichler L. Effects of alpha 1-acid glycoprotein in different rodent models of shock. Fundam Clin Pharmacol. 1998;12(2):173–81. doi: 10.1111/j.1472-8206.1998.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 9.Mestriner FL, Spiller F, Laure HJ, Souto FO, Tavares-Murta BM, Rosa JC, et al. Acute-phase protein alpha-1-acid glycoprotein mediates neutrophil migration failure in sepsis by a nitric oxide-dependent mechanism. Proc Natl Acad Sci U S A. 2007;104(49):19595–600. doi: 10.1073/pnas.0709681104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strehlow MC, Emond SD, Shapiro NI, Pelletier AJ, Camargo CA., Jr National study of emergency department visits for sepsis, 1992 to 2001. Ann Emerg Med. 2006;48(3):326–31. doi: 10.1016/j.annemergmed.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Rezende E, Silva JM, Jr, Isola AM, Campos EV, Amendola CP, Almeida SL. Epidemiology of severe sepsis in the emergency department and difficulties in the initial assistance. Clinics. 2008;63(4):457–64. doi: 10.1590/S1807-59322008000400008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Crit Care Med. 2007;35(8):1928–36. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 13.Vincent JL, Moreno R, Takala J, Willatts S, de MA, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 14.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. [PubMed] [Google Scholar]
- 15.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47(4):458–72. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto K, Nishi K, Kikuchi M, Watanabe H, Nakajou K, Komori H, et al. Receptor-mediated uptake of human alpha1-acid glycoprotein into liver parenchymal cells in mice. Drug Metab Pharmacokinet. 2010;25(1):101–7. doi: 10.2133/dmpk.25.101. [DOI] [PubMed] [Google Scholar]
- 17.Ceciliani F, Pocacqua V. The acute phase protein alpha1-acid glycoprotein: a model for altered glycosylation during diseases. Curr Protein Pept Sci. 2007;8(1):91–108. doi: 10.2174/138920307779941497. [DOI] [PubMed] [Google Scholar]
- 18.Costello M, Fiedel BA, Gewurz H. Inhibition of platelet aggregation by native and desialised alpha-1 acid glycoprotein. Nature. 1979;281(5733):677–8. doi: 10.1038/281677a0. [DOI] [PubMed] [Google Scholar]
- 19.Souto FO, Alves-Filho JC, Turato WM, Auxiliadora-Martins M, Basile-Filho A, Cunha FQ. Essential role of CCR2 in neutrophil tissue infiltration and multiple organ dysfunction in sepsis. 183(2):234–42. doi: 10.1164/rccm.201003-0416OC. Am J Respir Crit Care Med 2011 Jan 15. [DOI] [PubMed] [Google Scholar]
- 20.Brinkman-van der Linden EC, van Ommen EC, van DW. Glycosylation of alpha 1-acid glycoprotein in septic shock: changes in degree of branching and in expression of sialyl Lewis(x) groups. Glycoconj J. 1996;13(1):27–31. doi: 10.1007/BF01049676. [DOI] [PubMed] [Google Scholar]
- 21.Seidelin JB, Nielsen OH, Strom J. Soluble L-selectin levels predict survival in sepsis. Intensive Care Med. 2002;28(11):1613–8. doi: 10.1007/s00134-002-1501-5. [DOI] [PubMed] [Google Scholar]
- 22.Frazier WJ. Immunity, inflammation and sepsis: new insights and persistent questions. Crit Care. 2011;15(1):124. doi: 10.1186/cc10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiller F, Carlos D, Souto FO, de FA, Soares FS, Vieira SM, et al. alpha1-Acid Glycoprotein Decreases Neutrophil Migration and Increases Susceptibility to Sepsis in Diabetic Mice. Diabetes. 2012;61(6):1584–91. doi: 10.2337/db11-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Israili ZH, Dayton PG. Human alpha-1-glycoprotein and its interactions with drugs. Drug Metab Rev. 2001;33(2):161–235. doi: 10.1081/dmr-100104402. [DOI] [PubMed] [Google Scholar]
- 25.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 26.Adolfi Junior MS, Pallini FM, Pessotti H, Wolf CM, Patelli HT, Capeli RD, et al. Emergency medical coordination using a web platform: a pilot study. Rev Saude Publica. 2010;44(6):1063–71. doi: 10.1590/s0034-89102010000600011. [DOI] [PubMed] [Google Scholar]