Abstract
OBJECTIVE:
To determine factors associated with colonization by carbapenem-resistant Pseudomonas aeruginosa and multiresistant Acinetobacter spp.
METHODS:
Surveillance cultures were collected from patients admitted to the intensive care unit at admission, on the third day after admission and weekly until discharge. The outcome was colonization by these pathogens. Two interventions were implemented: education and the introduction of alcohol rubs. Compliance with hand hygiene, colonization pressure, colonization at admission and risk factors for colonization were evaluated.
RESULTS:
The probability of becoming colonized increased during the study. The incidence density of colonization by carbapenem-resistant P. aeruginosa and multiresistant Acinetobacter spp. and colonization pressure were different between periods, increasing gradually throughout the study. The increase in colonization pressure was due to patients already colonized at admission. The APACHE II score, colonization pressure in the week before the outcome and male gender were independent risk factors for colonization. Every 1% increase in colonization pressure led to a 2% increase in the risk of being colonized.
CONCLUSION:
Colonization pressure is a risk factor for carbapenem-resistant P. aeruginosa and multiresistant Acinetobacter spp. colonization. When this pressure reaches critical levels, efforts primarily aimed at hand hygiene may not be sufficient to prevent transmission.
Keywords: Colonization Pressure, Hand Hygiene, Pseudomonas Aeruginosa, Acinetobacter spp., Carbapenem Resistance
INTRODUCTION
Infections caused by multidrug-resistant organisms (MDROs) are a major public health concern due to the associated cost, morbidity and mortality. Hand hygiene is considered to be an effective measure to control the dissemination of MDROs within hospitals, but compliance remains a challenge (1-3). Several other strategies to decrease the transmission of MDROs have been studied, and colonization pressure (CP) appears to be an important measure that guides infection control interventions (4-7).
During a 28-month period, two interventions were implemented without success in a 12-bed neurology intensive care unit (NeICU) in an attempt to reduce colonization by carbapenem-resistant Pseudomonas aeruginosa (CRPA) and multiresistant Acinetobacter spp. (MRAC). The aims of this study were to reevaluate different occurrences during this period to identify factors associated with colonization by CRPA and MRAC, with special attention to CP, and to understand the reasons for the failure of the interventions.
METHODS
This study was an evaluation of data collected at a 12-bed NeICU in a 2,200-bed tertiary-care teaching hospital located in the city of São Paulo, Brazil, from April 2000 through July 2002.
Study design
A database was created during a 28-month period in which active surveillance and selected interventions were attempted to reduce the risk of CRPA and MRAC colonization in the NeICU without success.
Three periods were defined: Period 1: April to August 2000; Period 2: August 2001 to January 2002; and Period 3: February to July 2002. During each period, active surveillance cultures from the oropharyngeal, axillary and rectal sites were collected from the patients.
To increase hand hygiene compliance, two interventions were implemented throughout the evaluated period: healthcare personnel (HCP) education, followed by the introduction of alcohol hand rubs.
Educational intervention compliance with hand washing and the correct use of gloves during different patient care activities were evaluated among HCP for 6 hours per day over 1 week in the middle of Period 1, according to WHO recommendations (8). At the end of this observational week, a questionnaire on hand washing, isolation precautions and the spread of MDROs was administered to all HCP. Based on observations and the questionnaire, a theoretical and practical training program on hand hygiene and methods to avoid MDRO cross-transmission was implemented as an educational intervention over the last 4 months of Period 1.
Four months after the completion of the training program, another week of observations of HCP compliance with hand washing and the use of gloves was performed. Ten alcohol-rub dispensers were then installed in strategic locations in the NeICU, and HCP were encouraged to use these dispensers.
Eleven months following completion of the training program and 7 months following installation of the alcohol-rub dispensers, the HCP's hand hygiene practices were observed for one additional week.
The periods were named the pre-intervention baseline period (PI; Period 1), post-educational intervention assessment period (PE; Period 2) and post-alcohol hand-rub intervention assessment period (PA; Period 3).
During the three periods, a set of three surveillance cultures (oropharyngeal, axillary and rectal swabs) were collected from every patient admitted to the NeICU on the day of admission, on the third day post-admission and weekly until discharge from the ICU.
Surveillance cultures were collected from patients until their first positive result, at which point the patients were put under contact precautions, or until discharge from the unit or death if the results were negative during the entire ICU stay. There were no individual rooms for patients under contact precautions, and patient cohorting was not implemented.
Oropharyngeal samples were collected by swabbing both the posterior pharynx and deep inside the throat. Axillary swabs were collected bilaterally. Rectal samples were collected by inserting the swab 5 cm inside the rectum. After collection, the swabs were separately placed in Stuart media. Their microbiological processing focused on the identification of MRAC and CRPA. The swabs were plated on MacConkey agar, and isolates were phenotypically identified by conventional methods and an automated system (Vitek, BioMérieux, Hazelwood, MO). Antimicrobial susceptibility was evaluated using the automated system (Vitek, BioMérieux, Hazelwood, MO) and the disk diffusion method, both in accordance with NCCLS guidelines (now CLSI) (9).
Evaluation of the database
All patients admitted to the NeICU were included in the analysis of incidence density, except for patients who were colonized by CRPA or MRAC at NeICU admission and patients who had only one set of surveillance cultures collected (because their stay in the NeICU was shorter than 3 days). The origin of patients already colonized at admission to the NeICU was also evaluated. A patient was considered colonized if a clinical or surveillance culture was positive.
Patient data included the following: age; gender; surgery as a cause of admission; severity of illness, as measured by the APACHE II score (10); and the number of days in the hospital before admission to the NeICU.
Colonization rates and incidence density rates
To evaluate the time elapsed between admission and MRAC or CRPA colonization, Kaplan-Meyer curves for which the endpoint was “becoming colonized” by either of these pathogens and for which the censors were “discharge from NeICU or death” were constructed. Incidence density rates of MRAC or CRPA colonization for each period were calculated as the number of new colonizations during the period X1000/the number of patient-days until exit from the cohort (colonization, discharge from ICU or death). MRAC or CRPA CP was calculated weekly (the number of colonized patient-days during each week X100/the number of patient-days in that same week).
Compliance with hand hygiene
Compliance with hand hygiene before and after patient contact and the correct use of gloves were evaluated in each period. The weekly incidences of new CRPA or MRAC colonization of patients were also calculated for each period.
The rate of MRAC or CRPA colonization at admission in each period and the origin of patients colonized at admission were evaluated.
Data analysis
Patient age, APACHE II scores and number of days in the hospital prior to NeICU admission were compared between periods with analysis of variance (ANOVA). Patient gender and surgery prior to admission were analyzed between periods with Pearson's chi-square test, as were observations of HCP practices. Patients who became colonized were compared with patients who were not colonized using the Kruskal-Wallis test, Pearson's chi-square test and Student's t test. The log-rank test was used to compare Kaplan-Meier curves.
Stata v.7 (StataCorp, College Station, TX) and SPSS v.11 (SPSS, Chicago, IL) were used for data analysis.
RESULTS
A total of 457 patients were evaluated, with 174 patients in the PI, 142 in the PE and 141 in the PA.
Patient characteristics are presented in Table 1. Significantly more patients underwent surgery during the first period; no other statistically significant differences were observed between periods.
Table 1.
Characteristics of patients and compliance with hand hygiene and the use of gloves during the three study periods.
| Pre-intervention (PI) | Post-education (PE) | Post-alcohol (PA) | p-value | |
| Male gender | 64 (50%) | 43 (41%) | 38 (41%) | 0.29 |
| Surgery | 120 (94%) | 86 (82%) | 82 (89%) | 0.02 |
| Age (years) | ||||
| mean (SD) | 40 (15) | 44 (18) | 41 (18) | 0.25 |
| median (range) | 42 (2-73) | 43 (5-92) | 45 (5-71) | |
| APACHE II score (points) | ||||
| mean (SD) | 9 (6) | 10 (6) | 10 (6) | 0.08 |
| median (range) | 8 (0-25) | 9 (0-31) | 9 (0-27) | |
| Length of stay in the hospital prior to NeICU admission (days) | ||||
| mean (SD) | 11 (24) | 8 (9) | 8 (12) | 0.29 |
| median (range) | 5 (0-182) | 5 (0-55) | 4 (0-85) | |
| Compliance of HCP with hand hygiene and the use of gloves | ||||
| Hand hygiene before patient care | 97 (20%) | 95 (37%) | 22 (27%) | PI×PE: p<0.001 PE×PA: p = 0.102 PI×PA: p = 0.163 |
| Hand hygiene after patient care | 141 (30%) | 112 (45%) | 18 (23%) | PI×PE: p<0.001 PE×PA: p<0.001 PI×PA: p = 0.166 |
| Correct use of gloves during patient care | 242 (69%) | 213 (80%) | 61 (74%) | PI×PE: p = 0.001 PE×PA: p = 0.245 PI×PA: p = 0.301 |
SD: standard deviation; APACHE: Acute Physiology and Chronic Health Disease Classification System; HCP: healthcare personnel.
Colonization by MRAC and/or CRPA
In the PI, 174 patients were admitted to the NeICU. Of these patients, 128 were included in the cohort; 3 patients were excluded because they were positive at admission, and 43 had only one set of cultures collected. Three patients were colonized by MRAC, and 15 were colonized by CRPA.
In the PE, 142 patients were admitted to the NeICU. Of these patients, 105 were included in the cohort; 23 patients were excluded because they were positive at admission, and 14 had only one set of cultures collected. Two patients were colonized by CRPA, and 21 were colonized by MRAC.
In the PA, 141 were patients admitted to the NeICU. Of these patients, 92 were included in the cohort; 24 patients were excluded because they were positive at admission, and 25 had only one set of cultures collected. Seven patients were colonized by CRPA, and 19 were colonized by MRAC.
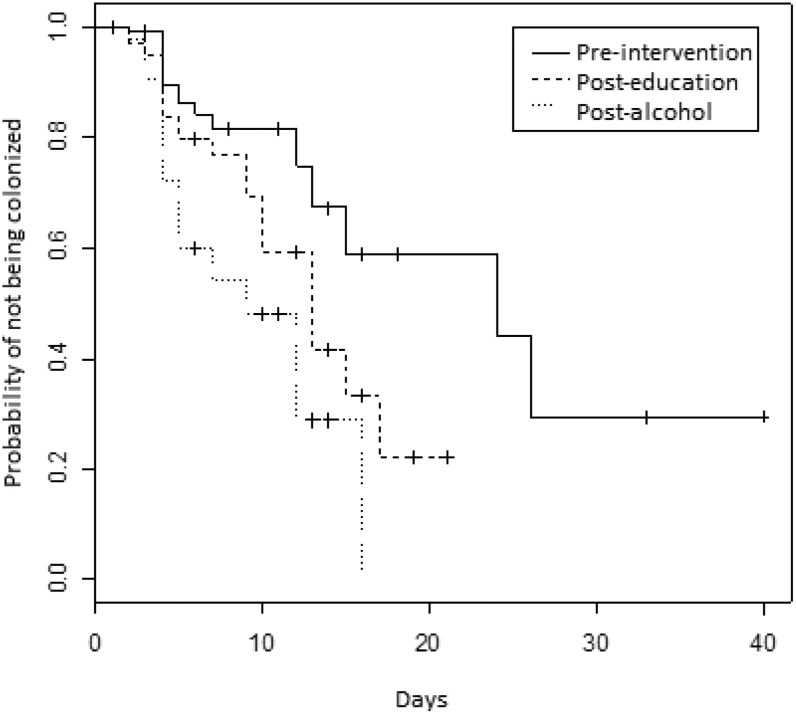
The time to colonization during the NeICU stay was shorter in the PA compared to the PI (p<0.001) (Figure 1. Comparing the PI and the PE, there was a trend toward an increased incidence density in the PE, although it was not statistically significant (p = 0.07). The incidence densities are shown in Table 2. When controlling for surgery and the APACHE II score, the difference between the first and the third periods remained statistically significant (p<0.001).
Figure 1.
Kaplan-Meier curves indicating the probability of not becoming colonized by carbapenem-resistant P. aeruginosa and/or multiresistant Acinetobacter spp. during each of the three study periods. Neurology Intensive Care Unit, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, Brazil, April 2000 to July 2002.
Table 2.
Incidence density of colonization, colonization pressure and rate of colonization at ICU admission during the three study periods.
| Incidence density of colonization | Colonization pressure (range) (95% confidence interval) | Rate of colonization at admission | |
| Pre-intervention | 24.8 per 1000 patient-days | 14.7 (0-28.1) | 2% |
| (10-20) | |||
| Post-education | 38.2 (0-83.3) | 16% | |
| 43.3 per 1000 patient-days | (27-49) | ||
| Post-alcohol | 53.3 (16.7-70) | 18% | |
| 67.5 per 1000 patient-days | (47-59) |
Observation of HCP practices
There were significant increases in the use of gloves, hand hygiene before patient care and hand hygiene after patient care during the PE (Table 1.
The installation of dispensers of alcohol hand rubs (the second intervention) did not increase compliance with hand hygiene or the use of gloves. Compliance with hand hygiene after patient care in the PA was even lower than in the PI, despite the availability of alcohol hand-rub dispensers.
CP
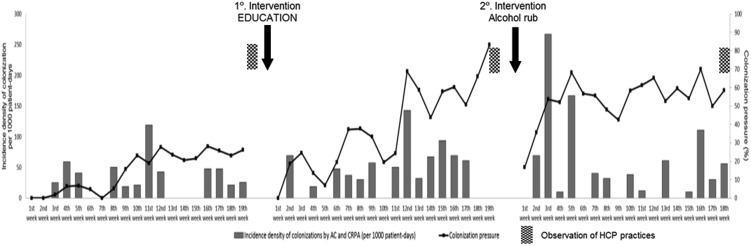
MRAC and CRPA CP differed between the periods. There was an increase in CP from the PI through the PE, and values >50% were reached in the PA (Figure 2. The rate of MRAC or CRPA colonization at admission to the NeICU was 2% in the PI, 16% in the PE and 18% in the PA (Table 2. Most of the patients already colonized at admission were admitted from the emergency room (53%).
Figure 2.
Weekly colonization pressure and incidence density of multiresistant Acinetobacter spp. or carbapenem-resistant Pseudomonas aeruginosa colonization in each of the three study periods. Neurology Intensive Care Unit, Hospital das Clínicas da Universidade de São Paulo, Brazil, April 2000 to July 2002. CRPA: carbapenem-resistant P. aeruginosa; AC: multiresistant Acinetobacter spp.; HCP: healthcare personnel.
Risk factors for colonization
The 67 patients who became colonized during the three study periods were compared with the 240 patients who were not colonized. A bivariate analysis demonstrated that the APACHE II score, CP in the NeICU in the week before an outcome and male gender were significantly associated with colonization (Table 3. Surgery prior to admission was inversely associated with colonization. In the multivariate analysis (Table 4, the APACHE II score and CP in the week before an outcome were significantly associated with colonization by MRAC and CRPA. Every 1% increase in CP in the week before an outcome led to a 2% increase in the risk of being colonized. Male gender and surgery prior to admission also remained associated with colonization.
Table 3.
Bivariate analysis of factors potentially associated with colonization by MRAC and CRPA in a neurology intensive care unit.
| Patients who became colonized (N = 67) | Patients who were not colonized during their stay in the unit (N = 258) | p-value | |
| Age (years) | 0.83 | ||
| mean (SD) | 44 (17) | 41 (17) | |
| median (range) | 45 (2-73) | 42 (5-92) | |
| Male gender | 41 (59%) | 104 (41%) | 0.02 |
| Surgery prior to admission | 55 (82%) | 229 (91%) | 0.03 |
| APACHE II score (points) | 0.004 | ||
| mean (SD) | 12(5.9) | 9 (5.8) | |
| median (range) | 12 (2-23) | 8 (0-31) | |
| Colonization pressure in the week before an outcome (colonization, discharge or death) | <0.001 | ||
| mean (SD) | 35.7% (20.5) | 29.5% (19.2) | |
| median (range) | 36.5% (0-70) | 24.4% (0-70) |
Table 4.
Multivariate analysis of factors associated with colonization by MRAC and CRPA in a neurology intensive care unit.
| Outcome | Odds ratio | 95% confidence interval | p-value |
| Colonization pressure in the week before the outcome | 1.02 | 1.01-1.04 | 0.008 |
| APACHE II score | 1.11 | 1.06-1.16 | <0.001 |
| Male gender | 2.24 | 1.24-4.05 | 0.008 |
| Surgery prior to admission | 0.29 | 0.13-0.65 | 0.003 |
CRPA: carbapenem-resistant P. aeruginosa; MRAC: multiresistant Acinetobacter spp.
DISCUSSION
Our study demonstrated that CP, APACHE II score and male gender were risk factors independently associated with nosocomial colonization by MRAC or CRPA and that surgery prior to admission was a protective factor.
During the period in which the database was generated and the interventions were applied, the educational intervention had a positive effect on hand hygiene compliance, but the incidence of new MRAC or CRPA colonization did not decrease, contrary to our expectations. In fact, colonization occurred earlier after each intervention, particularly during the third period. An analysis of the patient characteristics showed no differences between the three periods. These findings are difficult to explain.
We believe that CP rendered each period a distinct ecological situation. There was an increase in the prevalence of MRAC- or CRPA-colonized patients throughout the study, primarily due to the admission of patients who were already colonized and transferred from other areas of the hospital, particularly patients transferred from the emergency department (ED). Arvaniti et al. (4) found a correlation between the weekly acquisition of multiresistant Acinetobacter baumannii and the level of CP. In another study, during an outbreak of A. baumannii, being in the same room as a colonized patient was an independent risk factor for colonization (11). In a third study, antimicrobial use was not a risk factor for the acquisition of MRAC if CP exceeded 8.7% (6). A recent systematic review found a consistent relationship between CP and the risk of acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus spp. and Clostridium difficile in hospitals (5). Merrer et al. (12) found that an increase in CP from 10% to 40% increased the relative risk of being colonized by MRSA from 1 to 5.8. Another study showed that an increase in CP increased the risk of acquiring vancomycin-resistant enterococci (VRE) and found that when VRE CP reached values >50%, the effect of other risk factors became insignificant (13). In our study, CP in the week preceding the patients' exit from the cohort was an independent risk factor for colonization by MRAC and/or CRPA. Every 1% increase in CP led to a 2% increase in the risk of being colonized. In the PA in our study, CP was continuously >50%. The 39% increase in CP from the PI to the PA represented an increase in the risk of being colonized by 77%. It is possible that the high MRAC or CRPA CP in the second half of the PE and in the PA made the increase in hand hygiene compliance insufficient to prevent the spread of these pathogens.
The increase in the prevalence of MRAC- or CRPA-colonized patients in the PE and PA was due to the admission of already colonized patients to the NeICU. Compared with the PI, the rate of colonization at admission was 8-9-fold higher in the last two periods of the study. The identification of colonized patients at admission through active surveillance and contact precautions were not sufficient to avoid cross-transmission. As most patients colonized at admission came from the emergency room, interventions that included this unit might have been more successful. Our ED is an overcrowded area with patients who remain in the unit for days and occasionally in the corridors before a vacancy is found in another area of the hospital. In the ED, infection control practices are very often neglected.
The use of alcohol for hand hygiene is a well-established, widely used measure to reduce MDRO colonization. During data collection, despite being statistically significant, the improvement in hand hygiene rates in the PE was modest and only reached 45% after patient care, and the incidence density of colonization increased. In the next period, the hand hygiene rate decreased. It is possible that compliance with hand hygiene will only have an impact when a certain compliance threshold is attained. Trick et al. (3) found this threshold to be 23-46%, and a mathematical model predicted that a hand hygiene compliance rate of at least 50% would be necessary to decrease VRE transmission (14). Our interventions failed to achieve a sustained, epidemiologically significant increase in hand hygiene compliance, and the increase observed in the PE may have been insufficient to control cross-transmission.
The APACHE II score was also an independent risk factor for colonization by MRAC and CRPA. It is probable that more severely ill patients have more “colonization opportunities” because these patients have more invasive devices, receive more intensive care and are submitted to more invasive procedures. We cannot explain the association between male gender and colonization or the protective effect of surgery prior to admission in the acquisition of these pathogens.
Our study has several limitations. The data collection was performed in 2000-2002. Since then, educational programs for hand hygiene and dispensers for alcohol hand rubs have been implemented in most hospitals throughout the world. However, we were interested in evaluating the data produced during that period to understand the reasons for the failure of the interventions. As an association between CP and gram-negative cross-transmission risk has only recently been introduced in the literature (6), we considered the analysis of such data to be relevant. A second limitation is that environmental contamination was not evaluated at that time, although it may have played a role in the observed results (15-18). Additionally, surveillance cultures may not provide adequate sensitivity (19). In our study, we used cultures collected at three different sites to increase sensitivity. An analysis of these cultures showed that the positivity of cultures obtained at each body site was low (20).
In conclusion, in hyperendemic units, when CP reaches critical levels, efforts primarily aimed at hand hygiene may not be sufficient to prevent pathogen transmission. Due to the dynamics of pathogen dissemination, interventions to control MDROs may need to be expanded beyond a single unit and may require a more extensive evaluation of other units.
ACKNOWLEDGMENTS
This study was supported by the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, Brazil, by FAPESP (grant # 2013/08308-4), and by the U.S. Centers for Disease Control and Prevention, Atlanta, GA, USA. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Diseases Registry.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Naikoba S, Hayward A. The effectiveness of interventions aimed at increasing handwashing in healthcare workers—a systematic review. J Hosp Infect. 2001;47(3):173–80. doi: 10.1053/jhin.2000.0882. [DOI] [PubMed] [Google Scholar]
- 2.Pittet D, Hugonnet S, Harbarth S, Mourouga P, Sauvan V, Touveneau S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Lancet. 2000;356(9238):1307–12. doi: 10.1016/s0140-6736(00)02814-2. [DOI] [PubMed] [Google Scholar]
- 3.Trick WE, Vernon MO, Welbel SF, Demarais P, Hayden MK, et al. Multicenter intervention program to increase adherence to hand hygiene recommendations and glove use and to reduce the incidence of antimicrobial resistance. Infect Control Hosp Epidemiol. 2007;28(1):42–9. doi: 10.1086/510809. [DOI] [PubMed] [Google Scholar]
- 4.Arvaniti K, Lathyris D, Ruimy R, Haidich AB, Koulourida V, Nikolaidis P, et al. The importance of colonization pressure in multiresistant Acinetobacter baumannii acquisition in a Greek intensive care unit. Crit Care. 2012;16(3):R–102. doi: 10.1186/cc11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajao AO, Harris AD, Roghmann MC, Johnson JK, Zhan M, McGregor JC, et al. Systematic review of measurement and adjustment for colonization pressure in studies of methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and Clostridium difficile acquisition. Infect Control Hosp Epidemiol. 2011;32(5):481–9. doi: 10.1086/659403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortaleza CMCB, Freitas FM, Lauterbach GP. Colonization pressure and risk factors for acquisition of imipenem-resistant Acinetobacter baumannii in a medical surgical intensive care unit in Brazil. Am J Infect Control. 2013;41(3):263–5. doi: 10.1016/j.ajic.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Apisarnthanarak A, Pinitchai U, Thongphubeth K, Yuekyen C, Warren DK, Fraser VJ, et al. A multifaceted intervention to reduce pandrug-resistant Acinetobacter baumannii colonization and infection in 3 intensive care units in a thai tertiary care center: A 3-year study. Clin Infect Dis. 2008;47(6):760–7. doi: 10.1086/591134. [DOI] [PubMed] [Google Scholar]
- 8.WHO guidelines on hand hygiene in health care. Geneva: World Health Organization; 2009. Available from: http://whqlibdoc.who.int/publications/2009/9789241597906_eng.pdf.
- 9.NCCLS. 1999. Reference standards for antimicrobial susceptibility testing. Ninth informational supplement, 5th ed. NCCLS document M100-59. NCCLS, Wayne, Pa
- 10.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. [PubMed] [Google Scholar]
- 11.D'Agata EM, Thayer V, Schaffner W. An outbreak of Acinetobacter baumannii: the importance of cross-transmission. Infect Control Hosp Epidemiol. 2000;21(9):588–91. doi: 10.1086/501808. [DOI] [PubMed] [Google Scholar]
- 12.Merrer J, Santoli F, Appéré de Vecchi C, Tran B, De Jonghe B, Outin H. "Colonization pressure" and risk of acquisition of methicillin-resistant Staphylococcus aureus in a medical intensive care unit. Infect Control Hosp Epidemiol. 2000;21(11):718–23. doi: 10.1086/501721. [DOI] [PubMed] [Google Scholar]
- 13.Bonten MJ, Slaughter S, Ambergen AW, Hayden MK, van Voorhis J, Nathan C, Weinstein RA. The Role of “colonization pressure” in the spread of vancomycin-resistant Enterococci. Arch Intern Med. 1998;158(10):1127–32. doi: 10.1001/archinte.158.10.1127. [DOI] [PubMed] [Google Scholar]
- 14.Austin DJ, Bonten MJ, Weinstein RA, Slaughter S, Anderson RM. Vancomycin-resistant enterococci in intensive-care hospital settings: transmission dynamics, persistence, and the impact of infection control programs. Proc Natl Acad Sci U S A. 1999;96(12):6908–13. doi: 10.1073/pnas.96.12.6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karageorgopoulos DE, Falagas ME. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis. 2008;8(12):751–62. doi: 10.1016/S1473-3099(08)70279-2. [DOI] [PubMed] [Google Scholar]
- 16.Paterson DL. The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin Infect Dis. 2006 Sep 1;43 Suppl 2:S43–8. doi: 10.1086/504476. [DOI] [PubMed] [Google Scholar]
- 17.Blanc DS, Francioli P, Zanetti G. Molecular epidemiology of Pseudomonas aeruginosa in the intensive care units - a review. Open Microbiol J. 2007;1:8–11. doi: 10.2174/1874285800701010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces. A systematic review. BMC Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchaim D, Navon-Venezia S, Schwartz D, Tarabeia J, Fefer I, Schwaber MJ, Carmeli Y. Surveillance cultures and duration of carriage of multidrug-resistant Acinetobacter baumannii. J Clin Microbiol. 2007;45(5):1551–5. doi: 10.1128/JCM.02424-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalben MF, Oliveira MS, Garcia CP, Lobo RD, Costa SF, Toscano CM, et al. Swab cultures across three different body sites among carriers of carbapenem-resistant P. aeruginosa and Acinetobacter species: a poor surveillance strategy. J Hosp Infect. 2010;74(4):395–6. doi: 10.1016/j.jhin.2009.06.003. [DOI] [PubMed] [Google Scholar]