Abstract
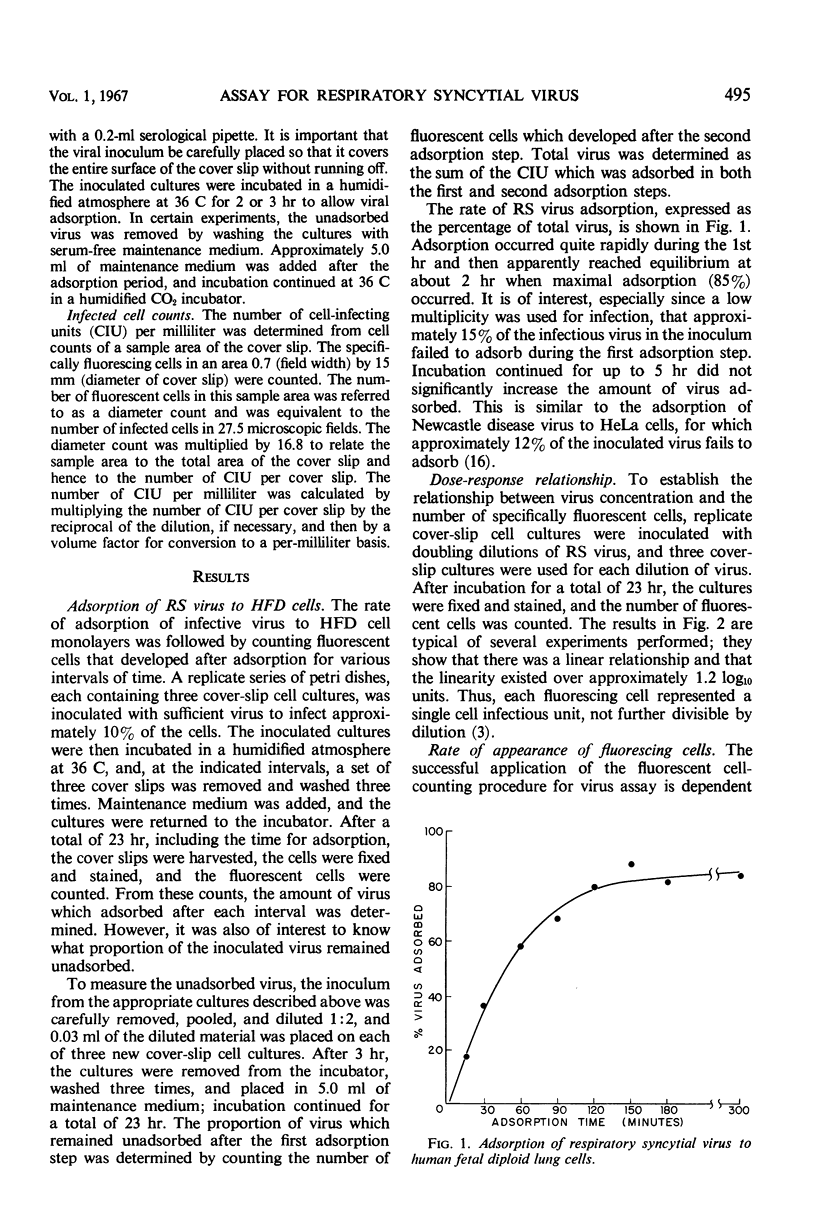
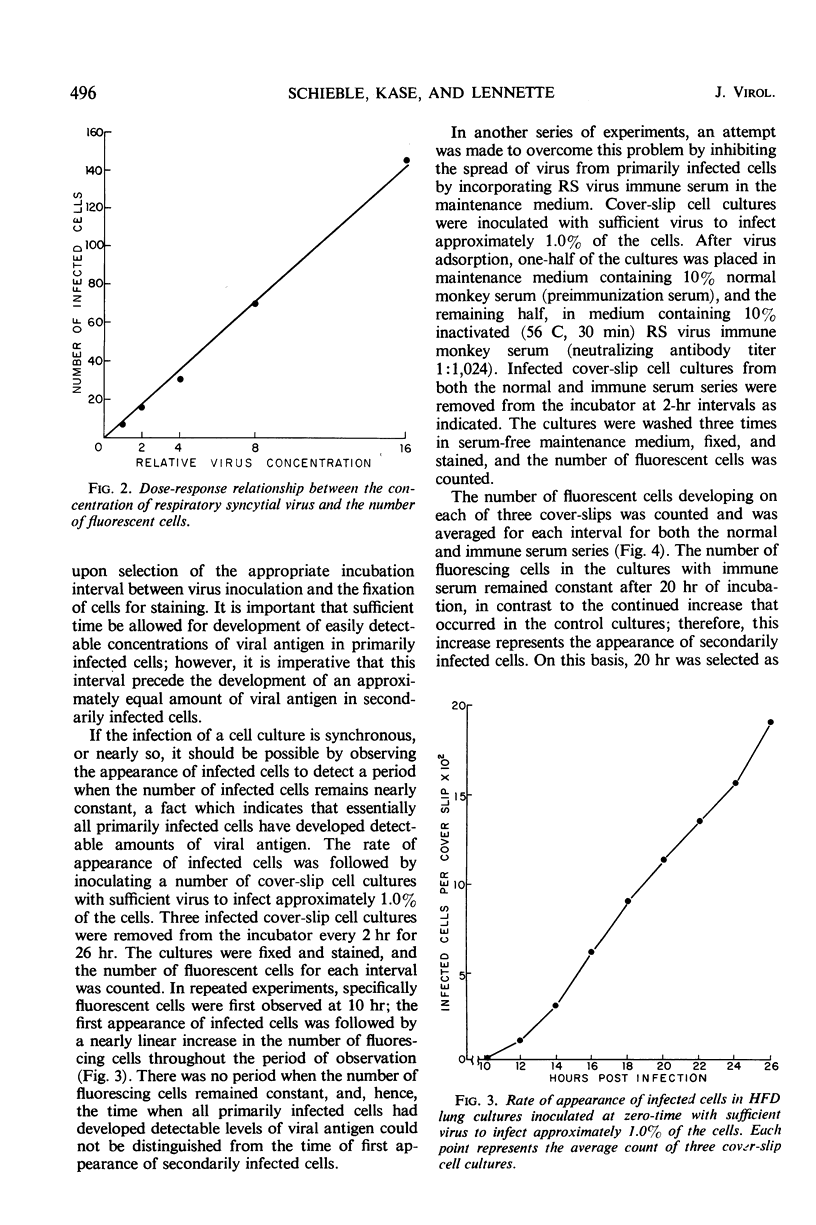
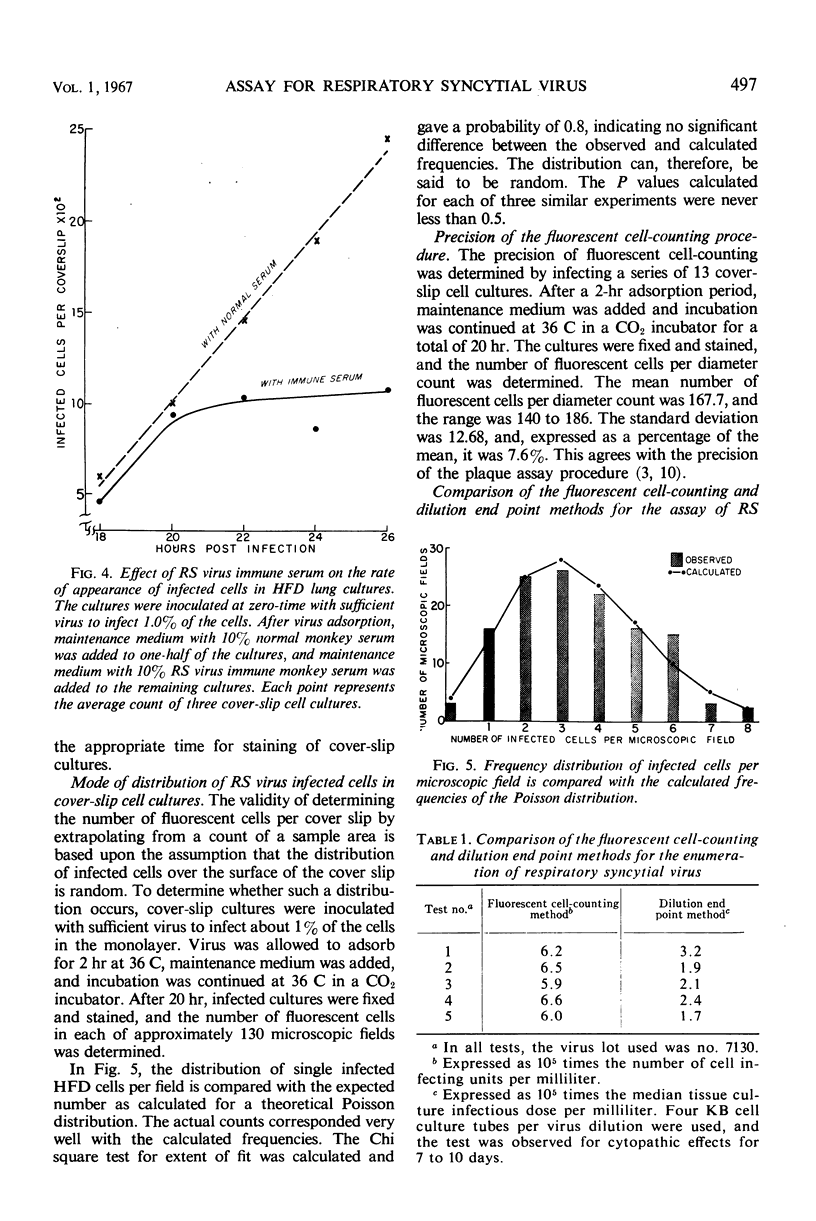
The fluorescent cell-counting technique was applied to the enumeration of cell-infecting units of respiratory syncytial (RS) virus in human fetal diploid (HFD) cover-slip cell cultures; it was a sensitive, precise, and rapid assay method. Approximately 2 hr was required for maximal adsorption of RS virus to HFD cell monolayers. However, about 15% of the infectious virus in the inoculum remained unadsorbed; this percentage was not significantly reduced even when the adsorption period was extended to 5 hr. A linear relationship between virus concentration and the number of fluorescent cells existed over a range of 1.2 log10 units. Variation of the mean of replicate determinations in a single experiment was approximately 7.5%. The distribution of single infected HFD cells on cover-slip cell cultures corresponded with the calculated frequencies of the Poisson distribution. The Chi square test for the extent of fit was calculated for several experiments, and the value of P was never less than 0.5. The addition of immune serum after virus adsorption effectively inhibited the development of detectable levels of viral antigen in secondarily infected cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENNETT C. R., Jr, HAMRE D. Growth and serological characteristics of respiratory syncytial virus. J Infect Dis. 1962 Jan-Feb;110:8–16. doi: 10.1093/infdis/110.1.8. [DOI] [PubMed] [Google Scholar]
- COATES H. V., KENDRICK L., CHANOCK R. M. Antigenic differences between two strains of respiratory syncytial virus. Proc Soc Exp Biol Med. 1963 Apr;112:958–964. doi: 10.3181/00379727-112-28221. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODHEART C. R., JAROSS L. B. Human cytomegalovirus. Assay by counting infected cells. Virology. 1963 Apr;19:532–535. doi: 10.1016/0042-6822(63)90047-3. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L., MOORHEAD P. S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- JORDAN W. S., Jr Growth characteristics of respiratory syncytial virus. J Immunol. 1962 May;88:581–590. [PubMed] [Google Scholar]
- KISCH A. L., JOHNSON K. M. A plaque assay for respiratory syncytial virus. Proc Soc Exp Biol Med. 1963 Mar;112:583–589. doi: 10.3181/00379727-112-28111. [DOI] [PubMed] [Google Scholar]
- KISCH A. L., JOHNSON K. M., CHANOCK R. M. Immunofluorescence with respiratory syncytial virus. Virology. 1962 Feb;16:177–189. doi: 10.1016/0042-6822(62)90293-3. [DOI] [PubMed] [Google Scholar]
- LEIBOVITZ A. THE GROWTH AND MAINTENANCE OF TISSUE-CELL CULTURES IN FREE GAS EXCHANGE WITH THE ATMOSPHERE. Am J Hyg. 1963 Sep;78:173–180. doi: 10.1093/oxfordjournals.aje.a120336. [DOI] [PubMed] [Google Scholar]
- OSTERHOUT S., TAMM I. Measurement of herpes simplex virus by the plaque technique in human amnion cells. J Immunol. 1959 Oct;83:442–447. [PubMed] [Google Scholar]
- PHILIPSON L. Adenovirus assay by the fluorescent cell-counting procedure. Virology. 1961 Nov;15:263–268. doi: 10.1016/0042-6822(61)90357-9. [DOI] [PubMed] [Google Scholar]
- POSTLETHWAITE R. A plaque technique for the titration of vaccinia virus in chick embryo cells and some features of vaccinial infection in this system. Virology. 1960 Apr;10:466–482. doi: 10.1016/0042-6822(60)90130-6. [DOI] [PubMed] [Google Scholar]
- SPENDLOVE R. S., LENNETTE E. H., KNIGHT C. O., CHIN J. N. DEVELOPMENT OF VIRAL ANTIGEN AND INFECTIOUS VIRUS IN HELA CELLS INFECTED WITH REOVIRUS. J Immunol. 1963 Apr;90:548–553. [PubMed] [Google Scholar]
- Schieble J. H., Lennette E. H., Kase A. An immunofluorescent staining method for rapid identification of respiratory syncytial virus. Proc Soc Exp Biol Med. 1965 Oct;120(1):203–208. doi: 10.3181/00379727-120-30486. [DOI] [PubMed] [Google Scholar]
- TAYLOR-ROBINSON D., DOGGETT J. E. AN ASSAY METHOD FOR RESPIRATORY SYNCYTIAL VIRUS. Br J Exp Pathol. 1963 Oct;44:473–480. [PMC free article] [PubMed] [Google Scholar]
- WHEELOCK E. F., TAMM I. Enumeration of cell-infecting particles of Newcastle disease virus by the fluorescent antibody technique. J Exp Med. 1961 Feb 1;113:301–316. doi: 10.1084/jem.113.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]



