Abstract
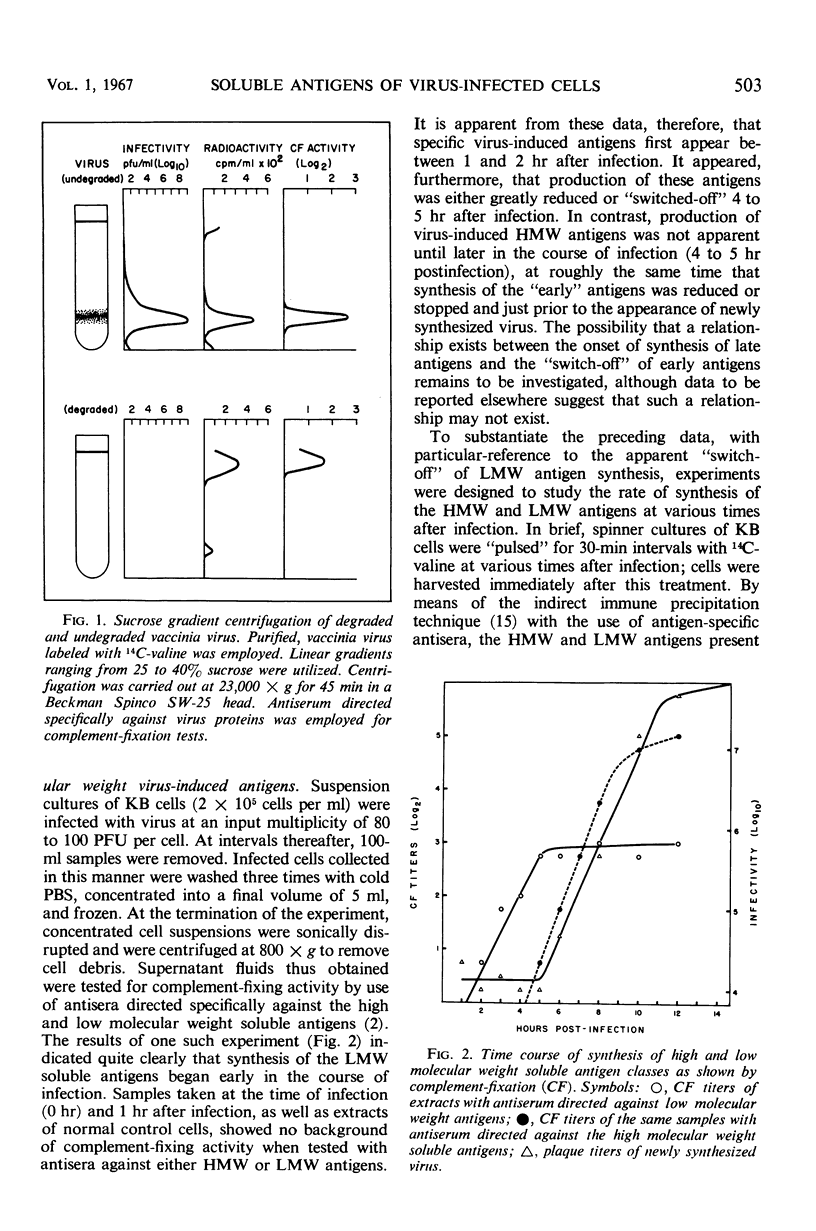
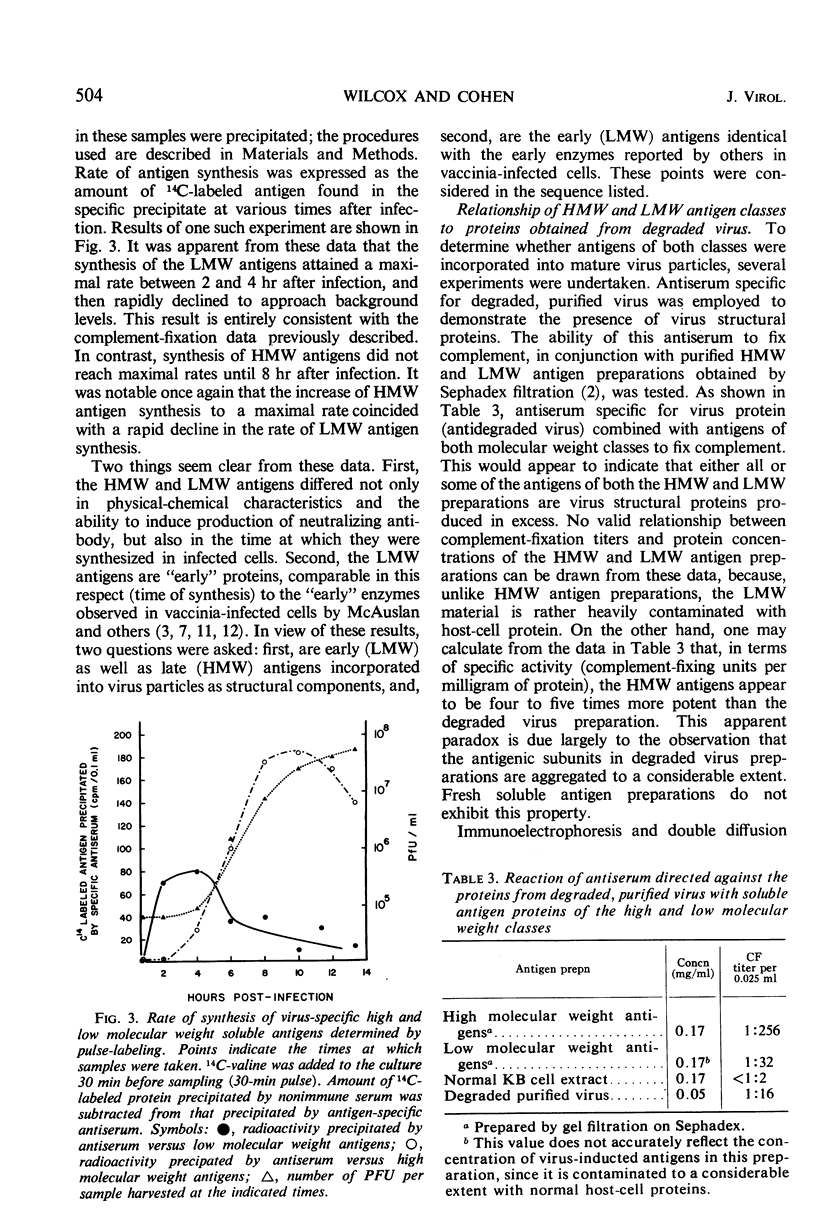
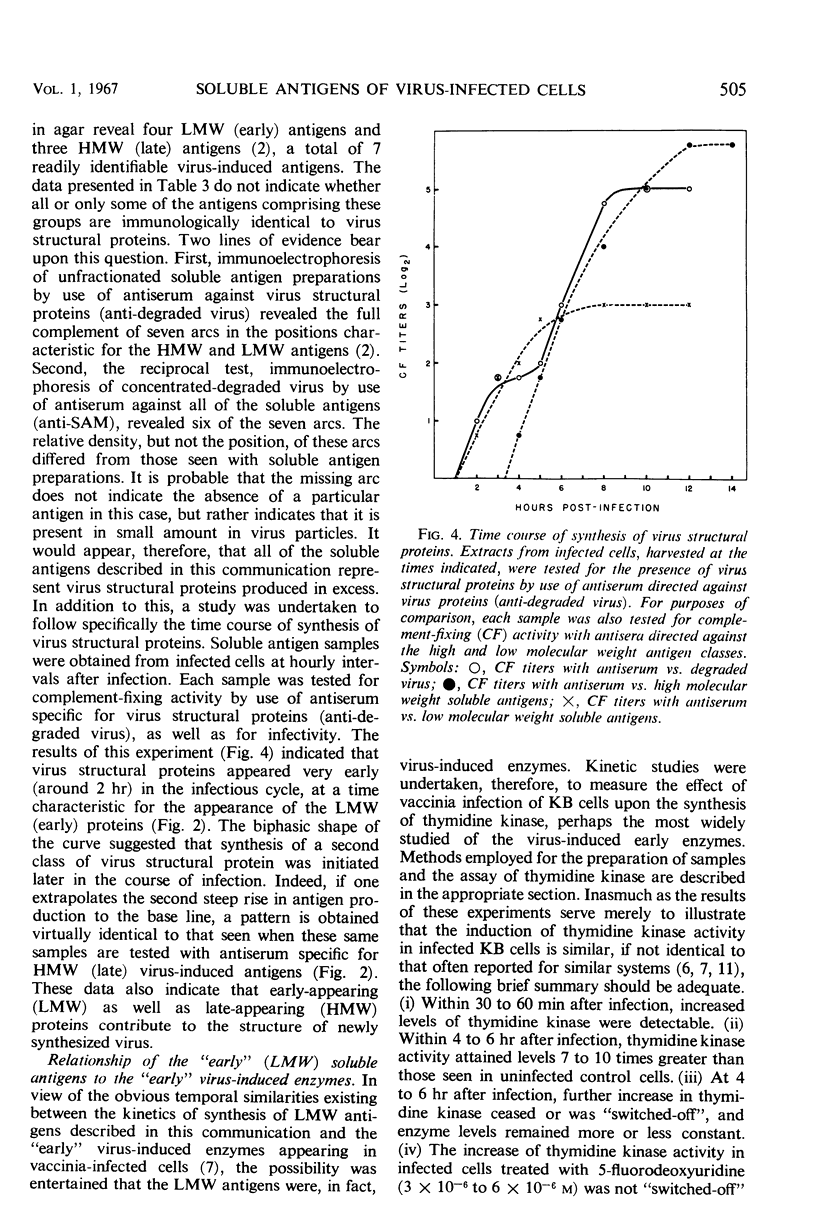
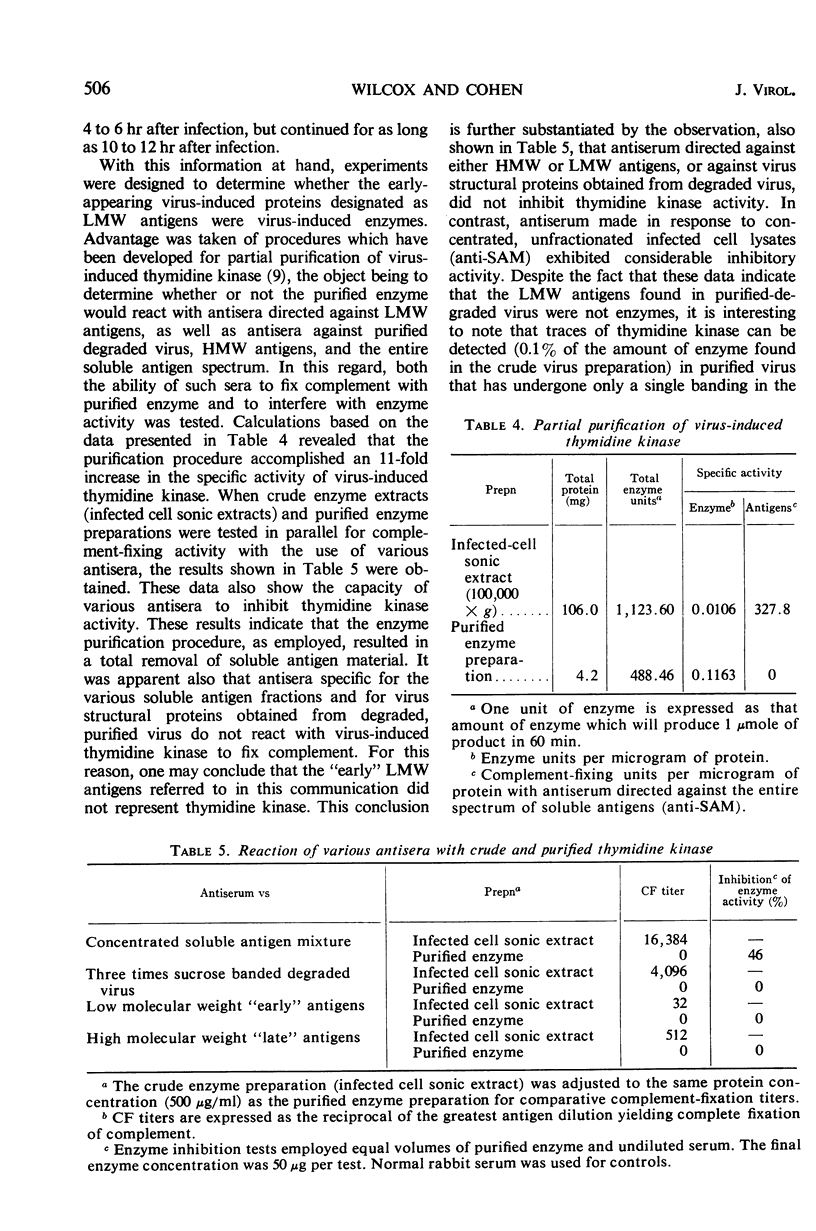
Virus-induced soluble antigens produced in mammalian cells after infection with vaccinia virus can be divided into two classes on the basis of molecular weight. Synthesis of the low molecular weight antigens begins early in the course of infection (1 to 2 hr), and is switched-off rather abruptly 4 to 5 hr after infection in a manner similar to that reported for the early enzymes characteristic of this same system. It was demonstrated, however, that these antigens do not include virus-induced thymidine kinase, a major virus-induced enzyme, nor is it likely that the low molecular weight antigens described here share identity with any of the virus-induced enzymes. A portion of the low molecular weight antigens appear to be incorporated into the structure of newly synthesized virus, probably as internal proteins. In contrast, synthesis of the high molecular weight antigen class is initiated later in the course of infection (4 to 5 hr), just prior to the appearance of newly synthesized virus. Antiserum directed specifically against virus structural proteins forms precipitin bands with all of the high molecular weight antigens recognizable by immunoelectrophoresis. This evidence, coupled with the observation that the high molecular weight antigen fraction elicits production of specific virus-neutralizing antibody, strongly suggests that this antigen class represents virus structural subunits produced in excess.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEYARD G., ZWARTOUW H. T., WESTWOOD J. C. A PROTECTIVE ANTIGEN FROM THE POX-VIRUSES. I. REACTION WITH NEUTRALIZING ANTIBODY. Br J Exp Pathol. 1964 Apr;45:150–161. [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Wilcox W. C. Soluble antigens of vaccinia-infected mammalian cells. I. Separation of virus-induced soluble antigens into two classes on the basis of physical characteristics. J Bacteriol. 1966 Sep;92(3):676–686. doi: 10.1128/jb.92.3.676-686.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M. Studies on the biosynthesis of viral DNA. Cold Spring Harb Symp Quant Biol. 1962;27:219–235. doi: 10.1101/sqb.1962.027.001.022. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., BECKER Y. THE REPLICATION AND COATING OF VACCINIA DNA. J Mol Biol. 1964 Dec;10:452–474. doi: 10.1016/s0022-2836(64)80066-8. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Jungwirth C., Joklik W. K. Studies on "early" enzymes in HeLa cells infected with vaccinia virus. Virology. 1965 Sep;27(1):80–93. doi: 10.1016/0042-6822(65)90145-5. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. PROPERTIES OF DEOXYTHYMIDINE KINASE PARTIALLY PURIFIED FROM NONINFECTED MOUSE FIBROBLAST CELLS. Virology. 1965 May;26:16–27. doi: 10.1016/0042-6822(65)90021-8. [DOI] [PubMed] [Google Scholar]
- Kelloff G., Vogt P. K. Localization of avian tumor virus group-specific antigen in cell and virus. Virology. 1966 Jul;29(3):377–384. doi: 10.1016/0042-6822(66)90213-3. [DOI] [PubMed] [Google Scholar]
- LOH P. C., RIGGS J. L. Demonstration of the sequential development of vaccinial antigens and virus in infected cells: observations with cytochemical and differential fluorescent procedures. J Exp Med. 1961 Jul 1;114:149–160. doi: 10.1084/jem.114.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALZMAN N. P., SHATKIN A. J., SEBRING E. D. Viral protein and DNA synthesis in vaccinia virus-infected HeLacell cultures. Virology. 1963 Apr;19:542–550. doi: 10.1016/0042-6822(63)90049-7. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- SHATKIN A. J. The formation of vaccinia virus protein in the presence of 5-fluorodeoxyuridine. Virology. 1963 Jun;20:292–301. doi: 10.1016/0042-6822(63)90118-1. [DOI] [PubMed] [Google Scholar]



