Abstract
Comparing the origins of AIDS and malaria may provide insight for gauging the prospect of future pathogen transmissions from apes to humans.
Two of the most widespread and virulent diseases of modern humans appear to have originated in our closest relatives, the African apes. There is evidence that the parasite Plasmodium falciparum, the cause of malaria in humans, is of gorilla origin (1, 2), whereas the pandemic form of human immunodeficiency virus type 1 (HIV-1), the cause of acquired immune deficiency syndrome (AIDS), stems from a lentivirus infection of chimpanzees (3). Is their great ape origin a coincidence, or are there important parallels that could provide insight into the prospects of future zoonoses in which other pathogens from the great apes might successfully colonize humans?
Pathogens must interact with a large number of host proteins to replicate in infected cells and thus tend to spread more readily between closely related hosts (4). Hence, the precursors of HIV-1 and P. falciparum that originated in great apes were likely better suited to infect humans than lentiviruses or malaria parasites from other mammals. However, despite substantial exposure and possibly numerous cross-species transfers, humans appear to have been successfully colonized by each of these pathogens only once. This implies that even ape pathogens have to overcome substantial adaptive hurdles to become established in the human host. For example, it has long been assumed that a human-specific mutation in the sialic acid biosynthetic pathway protects humans from infection by the species of Plasmodium that infect apes (5). However, not all P. falciparum parasites use sialic acids to invade erythrocytes, whereas an interaction between a parasite protein and the Ok blood group antigen basigin appears to be an obligatory step in this process (6). The ape homologs of basigin are quite divergent, and so it is likely that the gorilla precursor of P. falciparum had to adapt to spread efficiently in the human host. Similarly, lentiviruses, such as HIV-1 and its chimpanzee precursor—a strain of simian immunodeficiency virus called SIVcpz—are susceptible to various host-specific restriction factors, including tetherin, a protein that inhibits the egress of virions from infected cells. There are four groups of HIV-1 (called M, N, O, and P), which each arose from an independent ape-to-human transmission event; however, only group M viruses have achieved full anti-tetherin adaptation, and only this group has become pandemic (7).
That only a small subset of ape pathogens has successfully colonized humans highlights another point. Although two subspecies of chimpanzees, Pan troglodytes troglodytes and P. t. schweinfurthii, are each endemically infected by distinct forms of SIVcpz, only strains from the former have been identified in humans (8) (see the figure). Similarly, the ancestor of P. falciparum represents just one of at least six distinct Plasmodium species within the subgenus Laverania that infect either western gorillas or chimpanzees (1). Thus, at least in these two cases, African apes harbor a much greater variety of related pathogens than have been found in humans, raising the question: Are the seemingly unsuccessful pathogens incapable of infecting humans, or do they have the same potential to cross-infect and adapt, but have not yet had the opportunity? The latter possibility has obvious public health implications.
Figure 1. Figure Ape reservoir.
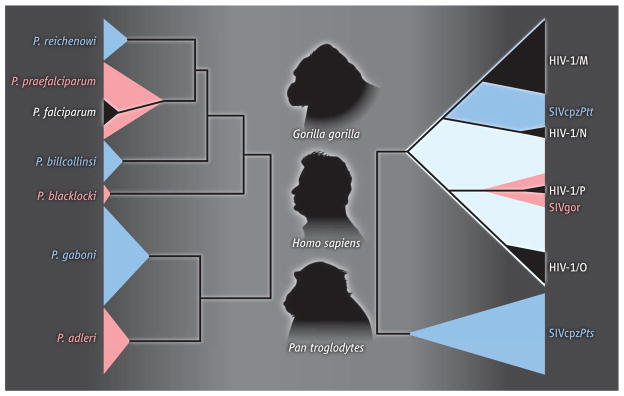
(Left) The subgenus Laverania contains six Plasmodium species, three that infect chimpanzees (blue) and three that infect gorillas (pink); only one of these has given rise to a human parasite (P. falciparum; black) (12). (Right) There are two major SIVcpz lineages that infect the central (upper blue clade) and eastern (lower blue clade) subspecies of chimpanzees, respectively. SIVcpzPtt strains from central chimpanzees have crossed the species barrier to humans, generating HIV-1 groups M and N (black), and to gorillas, generating SIVgor (pink). Group P is of likely gorilla origin, whereas the ape precursor of group O has not yet been defined (light blue) (8). Despite widespread distribution among eastern chimpanzees, SIVcpzPts has not been found in humans.
In addition to Laverania parasites, wild apes also harbor close relatives of P. vivax, P. malariae, and P. ovale (1, 9), raising the possibility that each of these human parasites was also acquired from apes. The case of P. vivax is particularly intriguing: The closest known relatives of P. vivax had been thought to be parasites infecting macaques (which are monkeys, not apes) in Southeast Asia, suggesting that P. vivax may have originated in this region (10). However, a mutation that confers protection against P. vivax by inhibiting expression of its receptor, the Duffy antigen present on the surface of red blood cells, is not found in Asian populations. This mutation has a frequency nearing 100% in human populations in west and central Africa, which has been taken to indicate that P. vivax is more likely to have originated there (11). That wild African apes harbor closely related Plasmodium strains is consistent with this interpretation (12). Thus, searching for pathogens related to known infectious agents could reveal additional potential zoonoses. A case in point is HIV-2, a lentivirus that originated in sooty mangabeys (monkeys found in west Africa), which was discovered to also cause human AIDS while screening for HIV-1 (8).
The ability of ape pathogens to colonize humans also appears to have depended on a number of historical (and even prehistorical) events. Timing the origin of P. falciparum in humans is problematic because of difficulties calibrating the rate of molecular evolutionary change in Plasmodium. Although it has been suggested that P. falciparum emerged out of Africa with modern humans, around 60,000 years ago (13), others have argued that P. falciparum could only have been sustained in the human population within the last 10,000 years, after the advent of agriculture and the concomitant changes in human (and mosquito) population density (14). By contrast, pandemic HIV-1 emerged much more recently, around a century ago (8). Again, this timing would reflect not the opportunity for cross-species transmission, but rather the potential for onward spread, provided in this case by social changes associated with colonial rule and the rapid growth of west central African cities (15). Thus, the emergence of both P. falciparum and HIV-1 were likely contingent upon changes in human demography and behavior that opened new niches for these parasites.
Although the circumstances that aided the initial expansions of P. falciparum and HIV-1 may no longer be relevant, other human-controlled factors may open new niches for ape-derived pathogens in the future. For example, efforts to eliminate P. falciparum malaria in Africa will undoubtedly reduce the density of human Plasmodium infections, but this may ease the competitive burden for any newly transferred parasites. The monitoring of human populations that are at a higher possible risk, such as hunters and forest dwellers, may detect new cross-species transmissions. Such studies require new diagnostic tools to detect emerging pathogens. An example is the macaque parasite P. knowlesi, which went unrecognized as a public health threat (although it was long known to infect humans) until molecular screening revealed hundreds of zoonotic transmissions each year (16). It will also be important to study the ape precursors of the human pathogens in their natural hosts. For SIVcpz such studies have already produced invaluable information about its susceptibility to host restriction factors, pathogenic potential, and adaptability (7, 8); as yet nothing is known about the course of Plasmodium infections in apes. Such studies may also address the intriguing question of why successful pathogen transfer from apes to humans is a rare event. And although it is difficult to predict whether or not newly discovered ape pathogens have the potential to infect humans, new diagnostic tools will place researchers in a better position to provide advance warning that could benefit any subsequent public health response.
References
- 1.Liu W, et al. Nature. 2010;467:420. doi: 10.1038/nature09442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayouba A, et al. Int J Parasitol. 2012;42:709. doi: 10.1016/j.ijpara.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keele BF, et al. Science. 2006;313:523. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies TJ, Pedersen AB. Proc Biol Sci. 2008;275:1695. doi: 10.1098/rspb.2008.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin MJ, Rayner JC, Gagneux P, Barnwell JW, Varki A. Proc Natl Acad Sci USA. 2005;102:12819. doi: 10.1073/pnas.0503819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crosnier C, et al. Nature. 2011;480:534. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans DT, et al. Trends Microbiol. 2010;18:388. doi: 10.1016/j.tim.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharp PM, Hahn BH. Cold Spring Harb Perspect Med. 2011;1:a006841. doi: 10.1101/cshperspect.a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duval L, et al. Proc Natl Acad Sci USA. 2010;107:10561. doi: 10.1073/pnas.1005435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escalante AA, et al. Proc Natl Acad Sci USA. 2005;102:1980. [Google Scholar]
- 11.Carter R. Trends Parasitol. 2003;19:214. doi: 10.1016/s1471-4922(03)00070-9. [DOI] [PubMed] [Google Scholar]
- 12.Rayner JC, Liu W, Peeters M, Sharp PM, Hahn BH. Trends Parasitol. 2011;27:222. doi: 10.1016/j.pt.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanabe K, et al. Curr Biol. 2010;20:1283. doi: 10.1016/j.cub.2010.05.053. [DOI] [PubMed] [Google Scholar]
- 14.Carter R, Mendis KN. Clin Microbiol Rev. 2002;15:564. doi: 10.1128/CMR.15.4.564-594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pepin J. The Origins of AIDS. Cambridge Univ. Press; Cambridge: 2011. [Google Scholar]
- 16.Kantele A, Jokiranta TS. Clin Infect Dis. 2011;52:1356. doi: 10.1093/cid/cir180. [DOI] [PubMed] [Google Scholar]


