Abstract
Purpose
Molecular targeting of cellular signaling pathways is a promising approach in cancer therapy, but often fails to achieve sustained benefit because of the activation of collateral cancer cell survival and proliferation pathways. We tested the hypothesis that a combination of targeted agents that inhibit compensatory pathways would be more effective than single agents in controlling pancreatic cancer cell growth. We investigated whether everolimus, an mTOR inhibitor, and sorafenib, a multi-kinase inhibitor, would together inhibit growth of low-passage, patient-derived pancreatic cancer xenografts in mice more efficaciously than either agent alone.
Methods
Tumor volume progression was measured following treatment with both drugs as single agents, in combination, and at multiple doses. Pharmacokinetics in tumors and other tissues was also assessed. Pharmacodynamic interactions were evaluated quantitatively.
Results
A 5-week regimen of daily oral doses of 10 mg/kg sorafenib and 0.5 mg/kg everolimus, alone and in combination, did not achieve significant tumor growth inhibition. Higher doses (20 mg/kg of sorafenib and 1 mg/kg of everolimus) inhibited tumor growth significantly when given alone and caused complete inhibition of growth when given in combination. Tumor volume progression was described by a linear growth model, and drug effects were described by Hill-type inhibition. Using population modeling approaches, dual-interaction parameter estimates indicated a highly synergistic pharmacodynamic interaction between the two drugs.
Conclusions
The results indicate that combinations of mTOR and multi-kinase inhibitors may offer greater efficacy in pancreatic cancer than either drug alone. Drug effects upon tumor stromal elements may contribute to the enhanced anti-tumor efficacy.
Keywords: Everolimus, Sorafenib, Xenografts, Non-competitive interaction model, PBPK-PD, Pancreatic cancer
Introduction
Pancreatic cancer is the fourth-leading cause of cancerrelated deaths in the United States [1]. The standard of care treatment includes chemotherapy with gemcitabine, which yields a median survival of 5.4 months in advanced disease. Addition to gemcitabine-based therapy of erlotinib, an inhibitor of the epidermal growth factor receptor (EGFR)-associated tyrosine kinase yields a slight improvement in pancreatic cancer survival [2]. Signal transduction pathways involved in tumor cell proliferation or angiogenesis often are mutated in solid tumors, and molecularly targeted agents that interdict these pathways represent a promising approach. Given the poor prognosis for advanced and gemcitabine-refractory disease in pancreatic cancer, there is an urgent clinical need for improved therapeutic strategies, and agents that target additional signaling pathways in cancer cells may hold promise.
Somatic mutations are common in pancreatic cancer, and 80 % of tumors have KRAS mutations that lead to the hyper-activation of the mitogen-activated protein kinase (MAPK) signaling pathway [3]. Sorafenib (Nexavar, BAY 43-9006) inhibits Raf, vascular endothelial growth factor receptors (VEGFR), and platelet-derived growth factor receptor-β (PDGF-β). By inhibition of these targets, sorafenib decreases tumor microvessel density and demonstrates in vitro and in vivo anti-proliferative activity against a variety of solid tumor models, including pancreatic cancer [4, 5].
The PI3 k-Akt-mTOR pathway is another signaling pathway implicated in tumor progression and treatment resistance and is a target of interest. Inhibition of the mTOR pathway leads to cell cycle arrest by abrogation of proliferative signals and decreased VEGF expression [6, 7]. Everolimus is an mTOR inhibitor that has shown in vivo and in vitro activity in pancreatic cancer models [8, 9].
To date, sorafenib and everolimus have failed to achieve significant clinical benefit as single agents in pancreatic cancer patients [10, 11], but a combination of both agents has not been explored in the disease. The lack of efficacy of these agents individually may be due to the activation of compensatory pathways through cross talk between the MAPK and PI3 k-Akt-mTOR pathways [12–14]. However, reversal of sorafenib resistance by the use of an AKT inhibitor was reported in hepatocellular carcinoma cells [15]. Thus, our hypothesis is that simultaneous inhibition of collateral and compensatory signaling pathways will result in synergistic effects of sorafenib and everolimus, leading to improved efficacy in pancreatic cancer.
Low-passage, patient-derived primary pancreatic adenocarcinomas explants [16] were used to investigate the effects of everolimus and sorafenib alone and in combination. Drug effects on the intended signaling pathway targets were analyzed and used to identify biologically effective doses. We quantified pharmacokinetics, drug effects, and developed a semi-mechanistic population pharmacodynamic model to explore the nature of the PK/ PD interaction of the two drugs. Tumor biomarkers were also quantified in order to complement our pharmacodynamic investigation.
Materials and methods
Preparation of sorafenib and everolimus formulations
A microemulsion of 2 % w/v everolimus (RAD001; obtained from Pharmaceutical Development, Novartis Pharma AG, Basel, Switzerland) was diluted with pyrogenfree water to the required concentration. Sorafenib was prepared weekly by dissolving its p-toluenesulfonate salt (purchased from LC laboratories, Woburn, MA) in Cremophor EL and ethanol 1:1 (vol:vol) at a fourfold higher concentration than required for administration and stored at room temperature with protection from light. The solution was prepared 1 h before dosing by diluting the stock solution with water.
Patient-derived primary pancreatic cancer explant model
An explant model employing low-passage, patient-derived, histopathologically verified, pancreatic adenocarcinomas in SCID (severe combined immunodeficient) mice was used, as described earlier [16]. Tumors were initiated in CB-17 SCID mice (4–6 weeks age) by inserting 2×2×2 mm pieces of donor tumors subcutaneously adjacent to the abdominal wall. All procedures were carried out under a laminar flow hood using aseptic conditions and were approved by the Institutional Animal Care and Use Committees of the University at Buffalo and Roswell Park Cancer Institute.
Experimental design
Two different patient-derived pancreatic adenocarcinomas were selected for investigation and are designated Tumor1 (TP17862) and Tumor2 (TP17624) [16]. Three sets of experiments were performed (Supplemental Information, Table S1). For each experiment, animals were randomized into groups based upon tumor volume and treatment was initiated approximately 6–8 weeks after tumor implantation, when tumors reached a volume of 100–200 mm3. Both drugs were given in a volume of 10 mL/kg by oral gavage using a bead-tipped curved gavage needle under aseptic conditions. Animals were dosed for 5 weeks according to a regimen of 5 consecutive daily doses followed by two drug-free days. Tumors were measured along 3 axes every other day using vernier calipers, and the volume was calculated by the formula , where l is the length of the longest axis (mm), and w and h are perpendicular axes in the other two dimensions [17]. Body weights were measured every other day throughout the duration of the study.
Histology and biochemical analysis
Tumors were harvested at predetermined time points at the end of the treatment regimen. For PK analysis, tumors were homogenized with 0.75 mL methanol/water 50/50; for biomarker analysis by ELISA, tumors were homogenized in a lysis buffer (below). For histological analysis, tumors were fixed in paraformaldehyde for 24 h. The fixed tumors were then paraffin embedded, sectioned, and stained with hematoxylin and eosin (H and E). Images of tissue sections were acquired using a Zeiss Axiovert 200 M microscope (Carl Zeiss, Inc., Thornwood, NY) with a 209/0.75 Apochromat lens and digital color camera. Representative fields were selected for analysis.
ELISA analysis
Tumor samples were weighed and 50 µg of sample was washed with cold phosphate buffered saline (PBS) and then lysed in cell extraction buffer (Invitrogen, Camarillo, CA Cat. #FNN0011). ELISA kits (Invitrogen) were used to measure total ERK and phosphorylated ERK (pERK), and total AKT and phosphorylated AKT (pAKT). VEGFR2 was measured using a mouse-specific ELISA kit (Quantikine, R&D Systems Inc., Minneapolis, MN). Samples were diluted using standard diluent buffer (0.1 % sodium azide) prior to the assay. All assays were conducted according to manufacturer’s instructions.
Drug quantification
At the end of the treatment regimen, three animals were sacrificed at each of a series of time points over 24 h. At the time of sacrifice, animals were weighed, anesthetized using ketamine/xylazine, and sacrificed by harvesting blood from the abdominal aortic artery into syringes containing ethylenediaminetetraacetic acid (EDTA) as an anticoagulant. Plasma was prepared from blood by centrifugation (2,000g for 15 min at 4 °C) and frozen at −20 °C until further analysis. Blood, lungs, muscle, brain, adipose tissue, kidneys, liver, pancreas, skin, spleen, gut, and tumor were collected. Tissue samples were prepared by homogenization in methanol/water (50:50 vol:vol). Drug concentrations in plasma and tissues were measured by liquid chromatography/tandem mass spectrometry using published methods [18, 19]. Drug concentrations for tumor were converted from ng/mL tissue extract to ng/g tissue by assuming a tissue density of 1 g/mL.
Data analysis
The area under the effect curve (AUEC) describing tumor volume progression versus time was estimated by the trapezoidal rule using WinNonlin, version 5.2.1 (Pharsight Corporation, Palo Alto, CA). Data were analyzed statistically by one-way ANOVA with post hoc Tukey tests or with the two-tail student’s t test. A value of P < 0.05 was considered significant.
Mathematical model
Pharmacokinetics
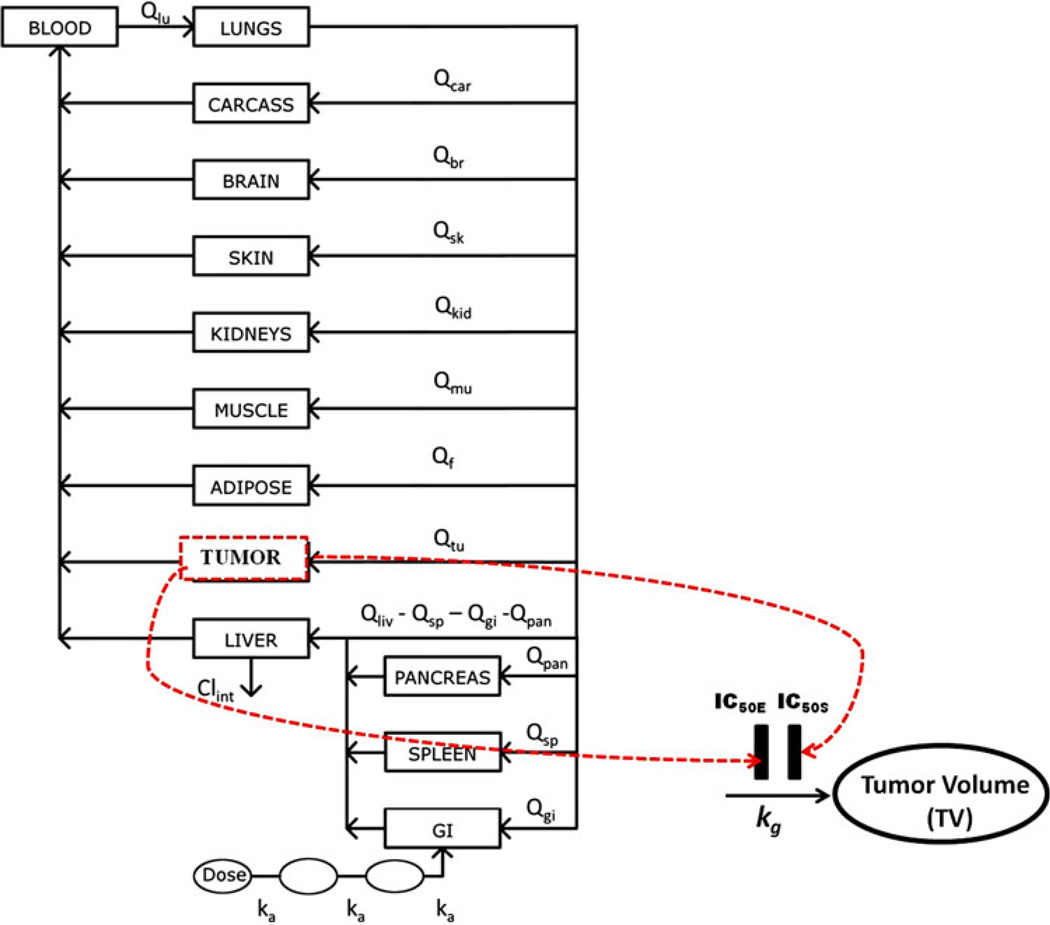
A physiologically based pharmacokinetic (PBPK) model was applied to describe the kinetics of everolimus and sorafenib in tumor and multiple tissues of mice [20]. This model was used to estimate drug concentration (CD) in the tumor as a function of time. A diagram of the PK/PD model is shown in Fig. 1. The plasma and tissue concentrations versus time were fitted simultaneously. The pharmacokinetic parameters were then fixed in the subsequent pharmacodynamic analysis.
Fig. 1.
PBPK-PD model of drug effects on tumor volume progression. The PBPK model is a representative model for everolimus and sorafenib. The dotted lines represent transduction of drug concentrations into inhibition of tumor growth. Symbols are defined in Table 1
Tumor pharmacodynamics
In the model, the growth of unperturbed tumor (TV) is described as
| (1) |
where kg is the zero-order net rate constant and TV0 is the tumor volume when dosing started.
Based on the mechanisms of sorafenib and everolimus, their inhibitory effect on tumor growth is given by
| (2) |
where Imax is the maximum inhibition due to the drug and IC50 is the concentration of drug (CD) in tumor required for 50 % of maximal effect.
The effect of the combination of the two drugs is given by
| (3) |
where Ψ is the drug interaction term, which represents the magnitude of change in IC50 when given in combination. The interaction term can be added to either IC50 value. This equation is adapted from previously described mechanistic models that evaluated indirect effects of two drugs in combination [21, 22]. The Imax for both drugs was assumed to equal 1.
A population analysis was performed to resolve kg, TV0 and IC50 The between-subject variability (BSV) was estimated for kg, TV0, and Ψ using an exponential error model. Analysis was performed by nonlinear mixed effects modeling with simultaneous fitting using NONMEM (VI level 1.1, ICON Development Solutions, Ellicott City, MD). NONMEM was compiled using Intel Visual Fortran v9.0 (Intel Corporation, Santa Clara, CA). All estimations were carried out using the first-order conditional estimation method with interaction option (FOCEI). Runs were executed using Wings for NONMEM (Version 6.11, http://wfn.sourceforge.net).
Results
Antitumor activity of everolimus and sorafenib in patient-derived primary pancreatic cancer explants
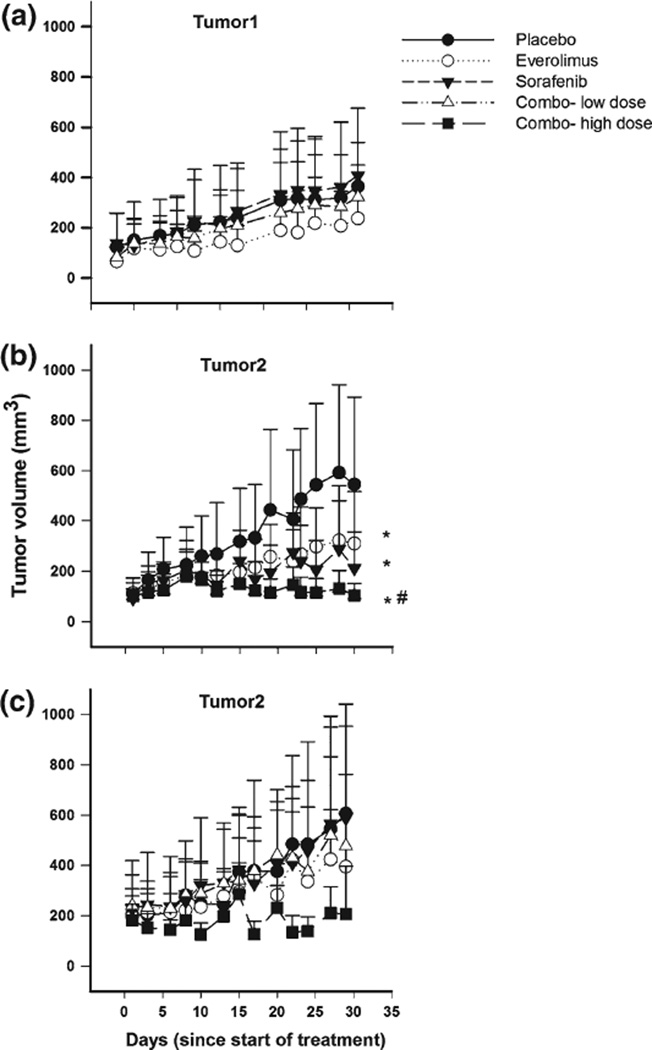
In order to investigate the antitumor efficacy of everolimus and sorafenib as single and combined agents on pancreatic cancer explants, groups of 9 mice bearing 75–200 mm3 tumors were treated orally with everolimus at doses of 0.5 and 1 mg/kg, sorafenib at 10 and 20 mg/kg, and with a combination of both at the low or high dose of each. The dosing regimen was 5 daily doses followed by two drugfree days, repeated for 5 weeks (Supplemental Information, Table S1). Control animals were treated with drug-free vehicle. Tumor responses to treatment are shown in Fig. 2.
Fig. 2.
Tumor volume as a function of time in control and treated mice. a Tumor1 after dosing with everolimus (0.5 mg/kg), sorafenib (10 mg/ kg), and the combination (everolimus 0.5 mg/kg and sorafenib 10 mg/ kg). b Tumor2 after dosing with everolimus (1 mg/kg), sorafenib (20 mg/kg), and combination (everolimus 1 mg/kg and sorafenib 20 mg/kg) (Tumor volume measured up to day 30). c Tumor2 after dosing with everolimus (0.5 mg/kg), sorafenib (10 mg/kg), combo-low (everolimus 0.5 mg/kg and sorafenib 10 mg/kg), and combo-high (everolimus 1 mg/kg and sorafenib 20 mg/kg) (Tumor volume measured up to day 28). *p <0.05, compared to the control; #p <0.05, compared to individual treatments (p = 0.03—Placebo vs. everolimus treatment, p value = 0.05—Placebo vs. sorafenib treatment, p value = 0.05—Placebo vs. combination treatment)
After 5 weeks of treatment of animals bearing Tumor1 with 0.5 mg/kg everolimus and 10 mg/kg sorafenib, there was no effect of either drug, alone or in combination, upon volume progression (Fig. 2a). The lack of efficacy could be a result of either intrinsic resistance of Tumor1 to the drugs or insufficient drug doses. Therefore, doses were escalated to 1 mg/kg everolimus and 20 mg/kg sorafenib and the efficacy was evaluated using Tumor2. After 5 weeks of treatment, the AUEC of animals treated with either drug alone was approximately 40 % smaller than of control animals (Fig. 2b), and the difference was statistically significant (p < 0.05). In animals treated with the two drugs combined, tumor growth was inhibited almost completely. The Tumor/ Control (T/C) ratio calculated for the mean tumor volumes at the end of treatment was 0.6 for treatment with everolimus alone, 0.5 for treatment with sorafenib alone, and 0.2 for the combination. Multiple-dose levels were evaluated with Tumor2 to investigate its sensitivity to everolimus and sorafenib. After 5 weeks of treatment, the higher dose combination of 1 mg/kg everolimus and 20 mg/kg sorafenib showed sustained control of tumor volume for Tumor2. Notably, the lower doses 0.5 mg/kg everolimus and 10 mg/ kg sorafenib, which were inefficacious on Tumor1, also were inefficacious on Tumor2 (Fig. 2c), alone or in combination.
Based upon body weight, both drugs were well tolerated alone or in combination at the higher dose levels. For all treatment groups, body weight was within 7–10 % of control animals, except for the high-dose combination group, in which body weight was within 12 % of controls after the third week of dosing (Supplemental Information, Fig. S1). No drug lethality was observed for any treatment regimen.
Pharmacokinetics
The concentrations of everolimus and sorafenib were measured in plasma and tumor over a 24-h period following the last dose of the 5-week treatment regimen and the values are reported elsewhere [20]. In plasma, oral everolimus exhibited an absorption phase followed by a biexponential decline (not shown). The tumor concentrations of everolimus followed a trend similar to that of plasma elimination, and therefore, a perfusion-rate-limited model for tumor captured the tumor concentrations reasonably well. The model fitting yielded a partition coefficient between plasma and tumor of 0.5 for everolimus. Sorafenib plasma concentration versus time profiles exhibited a delayed absorption (not shown), which was described in the sorafenib PBPK model using a series of transit absorption compartments (Fig. 1). The tumor concentrations of sorafenib followed a similar trend but exhibited a greater-than-proportional increase in tumor deposition at the higher dose compared to the lower dose, which may result from the action of saturable drug efflux transporters [20]. Tumor concentrations were described using a perfusion-rate-limited model. By employing different partition coefficients for the two doses, the model captured the concentration–time profiles reasonably. The model fitting yielded a sorafenib partition coefficient between plasma and tumor of 0.74 at the lower dose and 2.12 at the higher dose. The data, details of the PBPK model, its implementation, and parameter estimates are described elsewhere [20].
Tumor growth dynamics
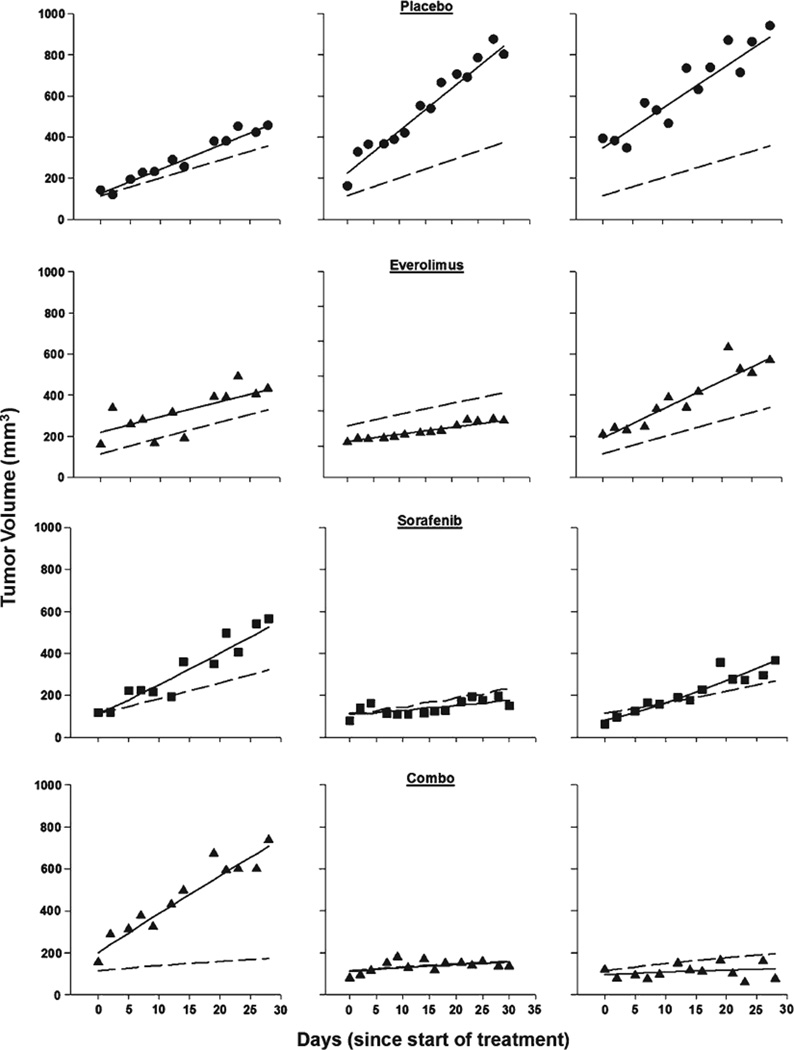
The pharmacodynamic model described in Eq. 1–Eq. 3 was used to integrate sorafenib and everolimus pharmacokinetics quantitatively into a pharmacodynamic model of tumor response. The tumors of control animals receiving placebo grew in linear fashion (as shown in Fig. 3), and data were captured satisfactorily by the proposed model (Eq. 1). The Hill-type inhibitory model for single drug effect (Eq. 2) reasonably described the data for both agents. The Imax for both drugs was fixed to 1, reflecting the assumption that sufficiently high doses of each drug would cause 100 % inhibition of tumor growth. A non-competitive interaction model (Eq. 3) satisfactorily described the complete tumor growth inhibition observed for the twodrug combination. Representative individual predictions for animals undergoing each treatment in each study are shown in Fig. 3. Goodness-of-fit plots (not shown) indicated that model predictions were in reasonable agreement with the observed tumor volumes. The weighted residuals did not reflect any systematic deviations.
Fig. 3.
Representative tumor volume–time profiles from each study for control and treated mice. Symbols represent experimental data, solid lines are the individual predictions, and dashed lines are the population predictions
All data were fit simultaneously to the equations and final parameters were obtained. The parameter means and inter-individual variability (IIV) for these studies are summarized in Table 1. The mean population parameter estimate for the baseline tumor volume TV(0) was 115 mm3 (IIV 68.5), for kg the growth rate was 0.36 mm3/h (IIV 87.8), the mean estimate for IC50E was 153 ng/mL and for IC50S was 734 ng/mL. The estimate for Ψ (sorafenib increasing the efficacy of everolimus) was 0.321. The precision on all parameters was reasonable. An estimated value of Ψ < 1 signifies a synergistic interaction between the two drugs. Ψ is an empirical term here, but it reflects a change in IC50 when the two drugs are combined, and the value obtained suggests an increase in tumor sensitivity to the drug combination. We tested other models with and without variability in the IC50’s and Ψ, and also whether Ψ affected the IC50 of sorafenib. Similar results were obtained (data not shown). All the models indicated a synergistic interaction between the two drugs. Fittings from a model in which Ψ affected the IC50E (for everolimus) were indistinguishable from the model in which Ψ affected IC50S (for sorafenib). However, the results of in vitro drug interaction studies [22] indicated that the model hypothesizing Ψ affecting IC50E was more stable and therefore was used as the final model (Eq. 3). The residual proportional error for the predicted tumor volumes was 18 %, and the additive error was 12 mm3.
Table 1.
Population parameter estimates from the PD model
| Parameter (units) | Parameter description | Estimate (% RSE) |
|---|---|---|
| TV(0) (mm3) | Baseline tumor volume | 115 (7.1) |
| kg (mm3/h−) | Net tumor growth rate constant | 0.37 (10.6) |
| IC50E (ng/mL) | Inhibition constant everolimus | 153 (67.3) |
| IC50S (ng/mL) | Inhibition constant sorafenib | 734 (35.7) |
| Ψ | Interaction term | 0.321 (102) |
| IIV TV(0) | Inter-individual variability in baseline tumor volume | 68.5 (18.7) |
| IIV kg | Inter-individual variability in Net tumor growth rate constant | 87.8 (17.4) |
| Res Err 1 (%) | Proportional residual error | 18.0 (13.8) |
| Res Err 2 (mm3) | Additive residual error | 12 (51.1) |
The visual predictive checks (VPC) revealed that the final model predictions were in reasonable agreement with the observed tumor volumes. In the VPC (Supplemental Information, Fig. S2), measured tumor volumes were overlaid with the 90 % prediction intervals for modelpredicted tumor volumes stratified by drug treatment. The 5th percentile, median, and 95th percentile were predicted adequately with no apparent bias. The results suggest that model-simulated tumor volumes were reasonably close to the observed tumor volumes that were used for model building.
Histology
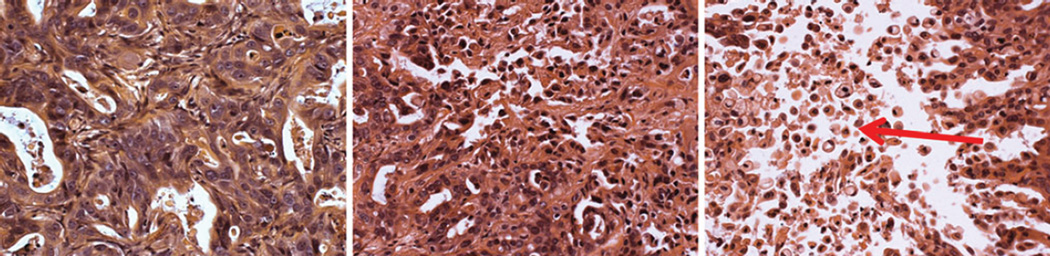
Histological analysis of tumors at the end of 5 weeks of treatment with the two-drug combination revealed a dosedependent increase in the proportion of apoptotic cells compared to placebo-treated controls (Fig. 4). Tumors also appeared progressively less organized than control tumors with the highest dose combination.
Fig. 4.
Effect of combination of everolimus and sorafenib on tumor histological characteristics at the completion of the 5-week dosing regimen as assessed by H and E staining. Tumors are from (a) placebo-treated animal, (b) animal treated with low dose combination, and (c) animal treated with high-dose combination. Arrow: apoptotic cells. Magnification ×20
Biomarker analysis
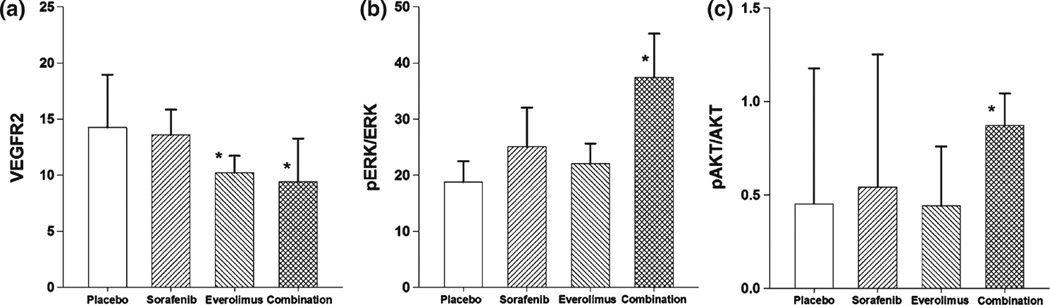
Tumor samples were obtained from Tumor2 at the end of the 5-week, multiple-dose study and biomarkers of drug effects were evaluated. The drug treatments are listed in Supplemental Information, Table S1. The analysis revealed significant changes only in tumor tissue taken from animals receiving the higher, efficacious dose. In groups treated with 1 mg/kg everolimus alone or in combination with 20 mg/kg sorafenib, VEGFR2 expression was significantly lower than in placebo-treated controls or the equivalent sorafenib-alone group (Fig. 5a). Sustained treatment with either everolimus alone or the two-drug combination resulted in lower overall tumor VEGFR2 expression.
Fig. 5.
Biomarker expression in tumors at the end of the 5-week dosing regimen of everolimus (1 mg/kg), sorafenib (20 mg/kg), the 2-drug combination (everolimus 1 mg/kg and sorafenib 20 mg/kg), or placebo. a VEGFR2. b Ratio of pERK to total ERK. c Ratio of pAKT to total AKT
Ratios of pERK versus total ERK were also measured in Tumor2 (Fig. 5b). Sustained treatment with the two-drug combination administered at the higher, efficacious dose showed that the pERK/ERK ratio in tumors was elevated significantly compared to placebo-treated controls. Neither of the drugs as single agents resulted in a significant alteration of the pERK/ERK ratio. An examination of the data for pERK and ERK revealed that the elevation of the pERK/ERK ratio resulted from an overall decline in tumor in total ERK expression (not shown), perhaps reflecting the drastic changes in tumor architecture and cellularity that resulted from the sustained treatment with the highly efficacious regimen (Fig. 4).
The ratio of pAKT to total AKT was also measured (Fig. 5c). Compared to controls, there was no statistical difference in the pAKT/AKT ratio between sorafenib and everolimus as single agents. However, there was a significant increase in the pAKT/AKT ratio at the end of 4 weeks of combination treatment with the higher sorafenib and everolimus doses compared to controls (Fig. 5c).
Discussion
The present work investigates the hypothesis that simultaneous inhibition of compensatory growth and survival pathways, the MAPK and PI3 k-Akt-mTOR pathways in cancer cells by molecularly targeted agents will result in synergistic inhibition of tumor growth. The combination of MAPK inhibitors with inhibitors of the PI3 k-Akt-mTOR pathway in solid tumors has been speculated as both feasible and efficacious [23–26] and has been effective in human melanoma and hepatocellular carcinoma (HCC) [27–30]. Our results show that the combination of the mTOR inhibitor everolimus and the multikinase inhibitor sorafenib is more efficacious than either drug alone in a patient-derived primary pancreatic cancer explant model. The lower sorafenib dose level was selected based on its reported activity against cell line-based pancreatic cancers in mice [31]. The higher sorafenib dose was selected to match the steady-state systemic exposure in humans at clinically relevant doses [32, 33]. For everolimus, the lower dose level was selected based on its activity in syngeneic murine pancreatic tumors [8], which was greater than clinically relevant dose [34]. The higher everolimus dose was selected by doubling the lower dose.
The comparison of drug exposures in mice and humans is summarized in Supplemental Information, Table S2. At the efficacious higher doses, tumor growth suppression by the two-drug combination is supra-additive and exceeds the effect of each drug alone. However, in these patient-derived pancreatic cancer explants, the doses that were efficacious were higher than those reported to exert synergy in cell line-based pancreatic tumors or in xenografts of other cancers such as hepatocellular carcinoma [5, 8, 35]. Based upon an analysis of published human exposure data (Table S2) and additional pharmacometric extrapolations (not shown), pharmacokinetic profiles equivalent to those demonstrating efficacy in tumor-bearing mice are unlikely to be achievable for this two-drug combination in clinical settings.
A semi-mechanistic, population-based model was developed to describe quantitatively the tumor suppressive effects of everolimus and sorafenib at two doses, alone and in combination. This represents the first semi-mechanistic model describing the pharmacodynamic interactions of these drugs. The final model assumed nonlinear inhibition of tumor growth by each drug, with the IC50 estimated for each drug reflecting quantitatively the tumor concentrations required for half-maximal inhibition of tumor growth rate. The effect of the combination was described using a dual drug inhibition approach [21] wherein the interaction term Ψ provides a quantitative estimate of the magnitude by which the IC50 of one drug is altered by the other.
In the model, the effect of drug upon tumor growth kinetics links drug concentration or exposure to tumor response. The estimated tumor growth rate constant kg suggests that the tumor grows at a mean rate of 0.3 mm3 per hour regardless of the original tumor size. The interindividual variability (IIV) of estimates for kg reflects the different rates of tumor growth in the experimental subjects, which were somewhat heterogeneous. The IIV in the starting tumor volume TV(0) reflects the moderate-to-high variability in tumor volume at the initiation of treatment. The IIV in these parameters and in the range of individual tumor responses to treatment suggest an advantage of employing patient-derived tumor explants, in that they may reflect the heterogeneous characteristics of cells growing within the complex microenvironment of the tumor.
All pharmacodynamic models employed to analyze the data obtained indicated a synergistic interaction between the two drugs. The variability of the estimated IC50 values and Ψ could not be determined with good precision, because only two doses of each drug were used in the studies, and therefore were not estimated in the final model.
The evaluation of biomarkers of drug response yielded interesting insights into the pharmacological and physiological responses of the tumor to sustained, 5-week dosing with efficacious doses and combinations. The elevation of tumor pAkt at the end of dosing suggests the activation of a feedback mechanism that increases the AKT survival signaling [36]; therapies employing inhibitors of AKT activation would be postulated to abolish this feedback response and could sustain the arrest of tumor progression as the tumor adapts to this therapeutic combination. The two-drug combination also resulted in an increased ratio of pERK/ERK after protracted treatment with the combination, which further investigation revealed to represent a decrease in total tumor ERK, rather than increased activation via up-regulated pERK. Thus, efficacious treatment may select for tumor cells that are less dependent upon ERK signaling. Finally, the decrease in VEGFR2 receptors that resulted from the prolonged high-dose combination treatment appeared to result from everolimus exposure rather than sorafenib exposure. This result may appear surprising, given that sorafenib is a well-known VEGF inhibitor [5]. Similar up-regulation of VEGF has been observed previously in HCC [35] for sorafenib when combined with everolimus or other tyrosine kinase inhibitors [37, 38] and can be attributed to a feedback response to suppression of VEGF receptor signaling. Interestingly, the tumor samples used for these studies were collected at intervals over 24 h after the last dose, but biomarker responses were not strongly time dependent. This finding would argue in favor of the interpretation that the prolonged treatment regimen at efficacious dose levels resulted in tumor adaptation to therapy and the selection of tumor properties that may better resist therapy. Elimination of residual disease would presumably require follow-on therapies that exploit the adaptation and evolution of the tumor as treatment progresses.
Interestingly, in vitro studies of the combination of everolimus and sorafenib on pancreatic cancer cell lines did not show synergistic interaction between the two drugs [22] in striking contrast to the synergistic interaction observed in vivo. This finding suggests that effects of the drug combination upon non-malignant cells within the hyperplastic tumor stroma may contribute to the observed synergy.
In summary, this study investigated for potentially beneficial pharmacological interactions of combinations of everolimus and sorafenib and demonstrated synergistic inhibition of growth of pancreatic cancer xenografts by the two-drug combination. The doses of drugs required for treatment of pancreatic cancer were higher than would be expected based on previous results with other cancer models. The quantitative, systems mathematical model captured effects of drug upon tumor growth rate and captured the effects exerted by each drug, alone in combination, upon tumor growth dynamics in both control and treated animals. Quantitative assessment of promising drug combinations in low-passage, patient-derived pancreatic cancers may provide a valuable tool in developing more effective treatment regimens, which to date has been largely based upon qualitative observations of empirical data, often in tumor models that have little resemblance to the clinical disease.
Supplementary Material
Acknowledgments
We thank Nancy Pyszczynski, Ninfa Straubinger, Rose Pitoniak, and Yang Qu for excellent technical assistance. We are grateful to The Novartis Institutes for Biomedical Research Basel, Switzerland for providing everolimus for animal studies. This work was supported in part by the pilot studies program of the University at Buffalo Clinical and Translational Research Center and the Buffalo Translational Consortium and by grant GM57980 from the National Institutes of Health.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00280-013-2117-x) contains supplementary material, which is available to authorized users.
Conflict of interest None.
Contributor Information
Dipti K. Pawaskar, Department of Pharmaceutical Sciences, School of Pharmacy and Pharmaceutical Sciences, State University of New York at Buffalo, Buffalo, NY 14214, USA
Robert M. Straubinger, Department of Pharmaceutical Sciences, School of Pharmacy and Pharmaceutical Sciences, State University of New York at Buffalo, Buffalo, NY 14214, USA Department of Cancer Pharmacology and Therapeutics, Roswell Park Cancer Institute, Buffalo, NY 14263, USA.
Gerald J. Fetterly, Department of Medicine, Roswell Park Cancer Institute, Buffalo, NY 14263, USA
Bonnie H. Hylander, Department of Immunology, Roswell Park Cancer Institute, Buffalo, NY 14263, USA
Elizabeth A. Repasky, Department of Immunology, Roswell Park Cancer Institute, Buffalo, NY 14263, USA
Wen W. Ma, Department of Medicine, Roswell Park Cancer Institute, Buffalo, NY 14263, USA
William J. Jusko, Department of Pharmaceutical Sciences, School of Pharmacy and Pharmaceutical Sciences, State University of New York at Buffalo, Buffalo, NY 14214, USA, wjjusko@buffalo.edu
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 3.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 6.Semela D, Piguet AC, Kolev M, Schmitter K, Hlushchuk R, Djonov V, Stoupis C, Dufour JF. Vascular remodeling and antitumoral effects of mTOR inhibition in a rat model of hepatocellular carcinoma. J Hepatol. 2007;46:840–848. doi: 10.1016/j.jhep.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 7.Lane HA, Wood JM, McSheehy PM, Allegrini PR, Boulay A, Brueggen J, Littlewood-Evans A, Maira SM, Martiny-Baron G, Schnell CR, Sini P, O’Reilly T. mTOR inhibitor RAD001 (everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitor. Clin Cancer Res. 2009;15:1612–1622. doi: 10.1158/1078-0432.CCR-08-2057. [DOI] [PubMed] [Google Scholar]
- 8.Boulay A, Zumstein-Mecker S, Stephan C, Beuvink I, Zilbermann F, Haller R, Tobler S, Heusser C, O’Reilly T, Stolz B, Marti A, Thomas G, Lane HA. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res. 2004;64:252–261. doi: 10.1158/0008-5472.can-3554-2. [DOI] [PubMed] [Google Scholar]
- 9.Stracke S, Ramudo L, Keller F, Henne-Bruns D, Mayer JM. Antiproliferative and overadditive effects of everolimus and mycophenolate mofetil in pancreas and lung cancer cells in vitro. Transpl Proc. 2006;38:766–770. doi: 10.1016/j.transproceed.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Wolpin BM, Hezel AF, Abrams T, Blaszkowsky LS, Meyerhardt JA, Chan JA, Enzinger PC, Allen B, Clark JW, Ryan DP, Fuchs CS. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27:193–198. doi: 10.1200/JCO.2008.18.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iqbal S, Lenz HJ, Yang D, Ramanathan RK, Bahary N, Shibata S, Morgan RJ, Gandara DR. A randomized phase II study of BAY 43-9006 in combination with gemcitabine in metastatic pancreatic carcinoma: A California Cancer Consortium study (CCC-P) ASCO, American Society of Clinical Oncology,p. 2008:11802. [Google Scholar]
- 12.Campbell M, Allen WE, Sawyer C, Vanhaesebroeck B, Trimble ER. Glucose-potentiated chemotaxis in human vascular smooth muscle is dependent on cross-talk between the PI3 K and MAPK signaling pathways. Circ Res. 2004;95:380–388. doi: 10.1161/01.RES.0000138019.82184.5d. [DOI] [PubMed] [Google Scholar]
- 13.Hausenloy DJ, Mocanu MM, Yellon DM. Cross-talk between the survival kinases during early reperfusion: its contribution to ischemic preconditioning. Cardiovasc Res. 2004;63:305–312. doi: 10.1016/j.cardiores.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Naegele S, Morley SJ. Molecular cross-talk between MEK1/2 and mTOR signaling during recovery of 293 cells from hypertonic stress. J Biol Chem. 2004;279:46023–46034. doi: 10.1074/jbc.M404945200. [DOI] [PubMed] [Google Scholar]
- 15.Chen KF, Chen HL, Tai WT, Feng WC, Hsu CH, Chen PJ, Cheng AL. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther. 2011;337:155–161. doi: 10.1124/jpet.110.175786. [DOI] [PubMed] [Google Scholar]
- 16.Hylander BL, Pitoniak R, Penetrante RB, Gibbs JF, Oktay D, Cheng J, Repasky EA. The anti-tumor effect of Apo2L/ TRAIL on patient pancreatic adenocarcinomas grown as xenografts in SCID mice. J Transl Med. 2005;3:22. doi: 10.1186/1479-5876-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh Y, Galviz G, Long BJ. Ultra-performance hydrophilic interaction liquid chromatography/tandem mass spectrometry for the determination of everolimus in mouse plasma. Rapid Commun Mass Spectrom. 2009;23:1461–1466. doi: 10.1002/rcm.4022. [DOI] [PubMed] [Google Scholar]
- 19.Jain L, Gardner ER, Venitz J, Dahut W, Figg WD. Development of a rapid and sensitive LC-MS/MS assay for the determination of sorafenib in human plasma. J Pharm Biomed Anal. 2008;46:362–367. doi: 10.1016/j.jpba.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawaskar D, Straubinger R, Fetterly G, Hylander B, Repasky E, Ma W, Jusko W. Physiologically based pharmacokinetic models for everolimus and sorafenib in mice. Cancer Chemother Pharmacol. 2013 doi: 10.1007/s00280-013-2116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Earp J, Krzyzanski W, Chakraborty A, Zamacona MK, Jusko WJ. Assessment of drug interactions relevant to pharmacodynamic indirect response models. J Pharmacokinet Pharmacodyn. 2004;31:345–380. doi: 10.1007/s10928-004-8319-4. [DOI] [PubMed] [Google Scholar]
- 22.Pawaskar DK, Straubinger RM, Fetterly GJ, Ma WW, Jusko WJ. Interactions of everolimus and sorafenib in pancreatic cancer cells. AAPS J. 2013;15:78–84. doi: 10.1208/s12248-012-9417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dancey JE, Chen HX. Strategies for optimizing combinations of molecularly targeted anticancer agents. Nat Rev Drug Discov. 2006;5:649–659. doi: 10.1038/nrd2089. [DOI] [PubMed] [Google Scholar]
- 24.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3 K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–137. doi: 10.3322/caac.20003. [DOI] [PubMed] [Google Scholar]
- 26.Konings IR, Verweij J, Wiemer EA, Sleijfer S. The applicability of mTOR inhibition in solid tumors. Curr Cancer Drug Targ. 2009;9:439–450. doi: 10.2174/156800909788166556. [DOI] [PubMed] [Google Scholar]
- 27.Lasithiotakis KG, Sinnberg TW, Schittek B, Flaherty KT, Kulms D, Maczey E, Garbe C, Meier FE. Combined inhibition of MAPK and mTOR signaling inhibits growth, induces cell death, and abrogates invasive growth of melanoma cells. J Invest Der-matol. 2008;128:2013–2023. doi: 10.1038/jid.2008.44. [DOI] [PubMed] [Google Scholar]
- 28.Molhoek KR, Brautigan DL, Slingluff CL., Jr Synergistic inhibition of human melanoma proliferation by combination treatment with B-Raf inhibitor BAY43-9006 and mTOR inhibitor Rapamycin. J Transl Med. 2005;3:39. doi: 10.1186/1479-5876-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newell P, Toffanin S, Villanueva A, Chiang DY, Minguez B, Cabellos L, Savic R, Hoshida Y, Lim KH, Melgar-Lesmes P, Yea S, Peix J, Deniz K, Fiel MI, Thung S, Alsinet C, Tovar V, Mazzaferro V, Bruix J, Roayaie S, Schwartz M, Friedman SL, Llovet JM. Ras pathway activation in hepatocellular carcinoma and anti-tumoral effect of combined sorafenib and rapamycin in vivo. J Hepatol. 2009;51:725–733. doi: 10.1016/j.jhep.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramakrishnan V, Timm M, Haug JL, Kimlinger TK, Halling T, Wellik LE, Witzig TE, Vincent Rajkumar S, Adjei AA, Kumar S. Sorafenib, a multikinase inhibitor, is effective in vitro against non-hodgkin lymphoma and synergizes with the mTOR inhibitor rapamycin. Am J Hematol. 2012;87:277–283. doi: 10.1002/ajh.22263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saber-Mahloogi H, Morse DE. Pharmacology Review. Rockville: Center for Drug Evaluation and Research; 2005. Pharmacology. [Google Scholar]
- 32.Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223–240. doi: 10.2165/00003495-200969020-00006. [DOI] [PubMed] [Google Scholar]
- 33.Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A, Hirte HW, Eder JP, Lenz HJ, Schwartz B. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–437. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 34.O’Donnell A, Faivre S, Burris HA3rd, Rea D, Papadimitrakopoulou V, Shand N, Lane HA, Hazell K, Zoellner U, Kovarik JM, Brock C, Jones S, Raymond E, Judson I. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–1595. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 35.Piguet AC, Saar B, Hlushchuk R, St-Pierre MV, McSheehy PM, Radojevic V, Afthinos M, Terracciano L, Djonov V, Dufour JF. Everolimus augments the effects of sorafenib in a syngeneic orthotopic model of hepatocellular carcinoma. Mol Cancer Ther. 2011;10:1007–1017z. doi: 10.1158/1535-7163.MCT-10-0666. [DOI] [PubMed] [Google Scholar]
- 36.Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, Baselga J, Guichard S, Rosen N. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov. 2011;1:248–259. doi: 10.1158/2159-8290.CD-11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drevs J. Soluble markers for the detection of hypoxia under antiangiogenic treatment. Anticancer Res. 2003;23:1159–1161. [PubMed] [Google Scholar]
- 38.Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, Sindhwani V, Blaszkowsky LS, Yoon SS, Lah-denranta J, Bhargava P, Meyerhardt J, Clark JW, Kwak EL, Hezel AF, Miksad R, Abrams TA, Enzinger PC, Fuchs CS, Ryan DP, Jain RK. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27:3027–3035. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.