Abstract
Elevated levels of cyclooxygenase (COX)-derived prostaglandin E2 (PGE2) occur in inflamed tissues. To evaluate the potential links between inflammation and breast cancer, levels of urinary prostaglandin E-metabolite (PGE-M), a stable end metabolite of PGE2, were quantified. We enrolled 400 patients with breast cancer: controls with early breast cancer (n=200), lung metastases (n=100) and metastases to other sites (n=100). Patients completed a questionnaire, provided urine and had measurements of height and weight. Urinary PGE-M was quantified by mass spectrometry. Ever smokers with lung metastasis who had not been exposed to NSAIDs had the highest PGE-M levels. PGE-M levels were increased in association with elevated BMI (p<0.001), aging (p<0.001), pack-year smoking history (p=0.02), lung metastases (p=0.02) and recent cytotoxic chemotherapy (p=0.03). Conversely, use of NSAIDs, prototypic inhibitors of COX activity, was associated with reduced PGE-M levels (p<0.001). Based on the current findings, PGE-M is likely to be a useful biomarker for the selection of high risk subgroups to determine the utility of interventions that aim to reduce inflammation and possibly the development and progression of breast cancer, especially in overweight and obese women.
Keywords: Breast cancer, smoking, NSAIDs, inflammation, PGE2, lung metastases, BMI
Introduction
Worldwide, breast cancer is the leading cause of cancer-related death in women (1). Ultimately, breast cancer mortality is linked to the development of metastatic disease, which is dependent both on factors related to the tumor cell (seed) and the microenvironment (soil) (2). In general, the search for risk factors for distant metastases has focused on tumor-specific traits, including stage, grade, and expression of the estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) (3, 4). The link between tumor characteristics and site-specific metastases from breast cancer is well established. Strong expression of the ER and PR is associated with preferential development of bone metastases, whereas tumors that overexpress HER2 are associated with an increased risk of visceral metastases, including lung (5, 6). Tumor gene expression profiling has further enhanced our understanding of this process. In preclinical studies, transcriptomic analysis identified a set of genes including cyclooxygenase-2 (COX-2) that mediated breast cancer metastasis to the lung (7, 8). Clearly, specific features of tumor cells are linked to site-specific metastases, but the host microenvironment is also likely to be important. For example, in experimental models, pulmonary inflammation is a risk factor for metastases to the lung (9, 10). Cigarette smoking, a well established cause of pulmonary inflammation (11), has been associated with both breast cancer metastasizing to the lung and increased mortality in observational studies (12–14)
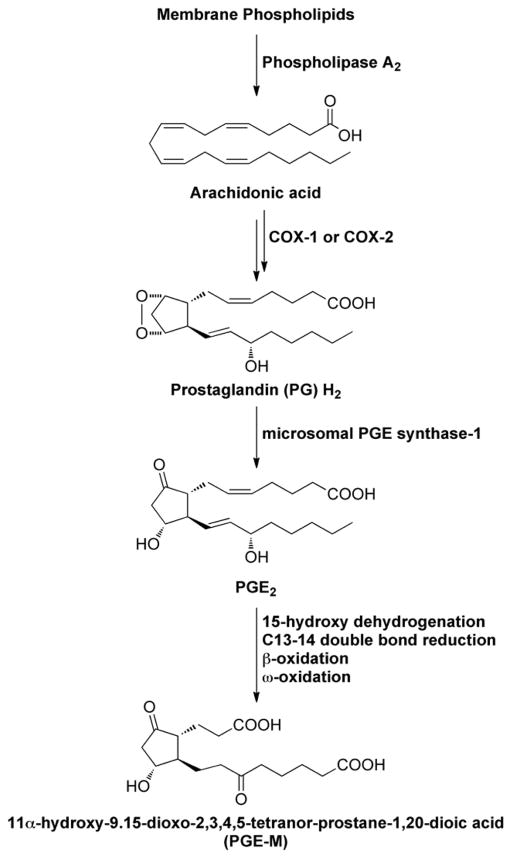
Lung inflammation is associated with increased levels of COX-2, a rate-limiting enzyme in the synthesis of prostaglandin E2 (PGE2) (15, 16). Elevated concentrations of PGE2 have been reported in the exhaled breath and sputum of COPD patients (15, 17). Catabolism of PGE2 results in a stable end metabolite (PGE-M), which is excreted in the urine (Fig. 1) and used as an index of systemic PGE2 levels (18). Previously, smokers were shown to have high urinary PGE-M levels due to increased COX-2 activity, findings that have been attributed to subclinical lung inflammation (19–21). One goal of the current cross-sectional study was to investigate whether smoking-related pulmonary inflammation manifested as increased levels of urinary PGE-M was associated with lung metastasis. Both obesity and aging are well established risk factors for inflammation and the development of breast cancer (22–24). Hence, two additional objectives of this study were to a) evaluate whether obesity was associated with elevated urinary PGE-M levels and (b) define the relationship between aging and levels of urinary PGE-M. We also postulated that use of nonsteroidal anti-inflammatory drugs (NSAIDs), prototypic inhibitors of COX, would be associated with reduced levels of urinary PGE-M. Overall our findings suggest a significant role for inflammation in the pathogenesis of breast cancer and highlight the potential utility of urinary PGE-M as a biomarker of inflammation that could prove valuable in future intervention studies.
Figure 1.
Schematic showing PGE-M Synthesis
Materials and Methods
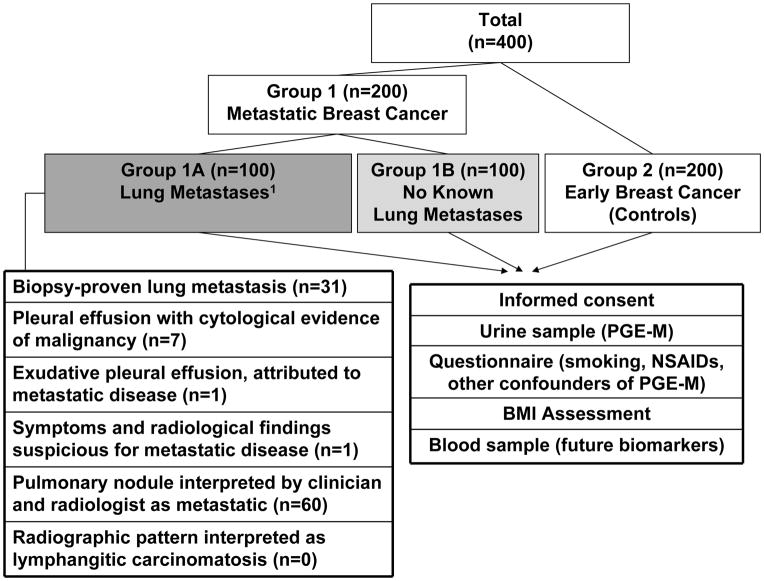
This study was approved by the Institutional Review Board of Memorial Sloan-Kettering Cancer Center. Women aged ≥18 years, with histologically proven breast cancer were approached during routine clinic visits. After informed consent, all patients gave a urine sample, completed a questionnaire and provided a blood sample for future studies. The total planned accrual was 400 patients. For the primary endpoint, urinary PGE-M was compared between the following groups (Fig. 2): group #1 consisted of patients with metastatic breast cancer, diagnosed on biopsy or imaging study. Histological confirmation of stage IV disease was not an eligibility requirement. These patients were subdivided into those with lung metastases (group #1A; n=100) and patients with no known lung metastases (group #1B; n=100). The definition of lung metastases (or their absence) was made at study entry as previously described by Murin et al (25). Patients were considered to have lung metastases if any one (or more) of six criteria were met, otherwise patients with metastatic disease were assigned to group #1B “no known lung metastases” (Fig. 2). Group #2 (controls, n=200) were made up of women who had been treated for early breast cancer and had no clinical evidence of disease. According to the most recent guidelines from the American Society of Clinical Oncology, patients with a history of early breast cancer should be followed up with history and clinical examination and routine imaging is not recommended (26). Therefore, all patients in group 2 were clinically assessed for the absence of metastatic disease within 1 month of study entry.
Figure 2.
Study Schema
Due to concerns about the unknown but possible confounding effects on PGE-M, patients who had recently received breast/chest wall radiotherapy (within three months), systemic chemotherapy/trastuzumab (within three months) and corticosteroids (within 4 weeks) were excluded. Furthermore, given the rarity of male breast cancer, men were also excluded. However, due to slower than expected accrual in patients with metastatic disease, the study was amended after the first 256 patients were enrolled, so that women receiving chemotherapy and trastuzumab were eligible. Administered therapy was considered as a possible confounder.
The questionnaire was developed for the study and included detailed information about inflammatory conditions of the lung (appendix 1). Data from the questionnaire detailed smoking exposure and included questions related to possible confounders of PGE-M such as nonsteroidal anti-inflammatory drug (NSAID) use and other chronic inflammatory conditions. Due to the importance of detailing confounders relative to the timing of the urine sample, patients were required to complete the questionnaire in-person on the study day. In general, patients completed the questionnaire with the consenting physician but this was not required. Electronic medical records and medication lists, which are routinely updated by patients and physicians at clinic visits, were reviewed to cross-reference data from the questionnaire. Where conflicts existed, any single documentation was taken as proof of the presence of this condition/receipt of these medicines. Patients undergoing chemotherapy all have height and weight measured yielding body mass index (BMI) results. Patients for whom this information was not available underwent height and weight measurement on the study day. All patients who signed a valid consent form and gave urine were considered evaluable.
Definitions
Using standard definitions, ever and never smokers were defined according to whether patients had smoked at least 100 cigarettes or not in their life-time. Former smokers were defined as patients with ≥100 cigarette smoking history and who quit ≥12 months before enrollment. Current smokers were defined as patients with ≥100 cigarette smoking history who had smoked at least one cigarette in the last year. Total smoking exposure was estimated in pack years. Patients were classified as “regular” NSAID users if they admitted to daily NSAID use (or if their regular medications listed an NSAID). Otherwise patients were classified as “occasional” users. Subjects also reported days from last NSAID use.
Urine Samples
A single void urine (<100mls) specimen was collected from each patient, aliquoted into 2mL cryovials, anonymized, and stored at −80°C. Upon completion of patient enrollment, urine was analyzed for PGE-M using mass spectrometry as previously described (18–20), without knowledge of any clinical characteristics. To account for variations in hydration status, PGE-M was normalized to urinary creatinine.
Biostatistics
The associations between urinary PGE-M and covariates were first examined in a univariate analysis. For categorical covariates with two or more categories, the non-parametric Wilcoxon rank-sum test and Kruskal-Wallis test respectively were used. For continuous covariates, the Spearman’s rank correlation coefficient was quantified and compared to the null hypothesis (no correlation). To identify potential confounders of the association between PGE-M and the study groups, the strength of association between covariates and study groups was examined using the Kruskal-Wallis and Chi-squared tests for continuous and categorical covariates, respectively. Variables that showed evidence of association with PGE-M (p<0.25) and those that were notably different across study groups (p<0.25) were included in a multivariate model, using multiple linear regression analysis on log transformed PGE-M data. An AIC based backward variable selection procedure was then used to identify a set of covariates that best fit the PGE-M data.
Results
Baseline Characteristics
From September 2010 to June 2011, 400 women of median age 58 years (range 24–88) enrolled. All patients completed the study questionnaire, provided a urine sample and had BMI measured objectively by a clinician. Patients with lung metastases were older and had a greater number of sites of metastatic disease than patients in other groups (Table 1). Elevated BMI was associated with metastasis (p=0.035). Despite not being an eligibility requirement, most patients in group 1 had a biopsy of at least one metastatic site consistent with the diagnosis of breast cancer (Supplemental Table 1). As expected, stage distribution at presentation was significantly different across study groups (p<0.001). The group of patients with lung metastases was enriched for HER2 positive tumors (p=0.02). In total, 68 (19%) women had at least one known inflammatory lung condition, and this proportion was similar across study groups (Table 1). Consistent with differences in tumor phenotype between groups, there were significant differences in the breast cancer treatments received in the last month between groups (Supplemental Table 1). As noted above, accrual was slower in patients with metastatic disease prior to the study amendment which allowed patients receiving chemotherapy and trastuzumab to participate (Supplemental Table 2)
Table 1.
Baseline Patient Characteristics
| Lung Metastases (group 1A) n=100 | No Known Lung Metastases (group 1B) n=100 | Controls (group 2) n=200 | P value | |
|---|---|---|---|---|
|
| ||||
| N (%) | N (%) | N (%) | ||
|
| ||||
| Age (years), Median (Range) | 62 (33–88) | 57 (24–85) | 56 (30–87) | 0.006 |
|
| ||||
| BMI, Median (Range) | 26.8 (16.9–54.6) | 26.8 (16.0–41.7) | 25.4 (17.3–51.4) | 0.035 |
|
| ||||
| Time from Diagnosis to Participation1 (months), Median (Range) | 109 (1–423) | 75 (0–371) | 39 (5–299) | <0.001 |
|
| ||||
| Sites of Metastatic Disease | ||||
|
| ||||
| Lung | 100 (100) | 0 (0) | 0 (0) | <0.001 |
| Bone | 49 (49) | 69 (69) | 0 (0) | 0.006 |
| Lymph Node | 36 (36) | 23 (23) | 0 (0) | 0.06 |
| Liver | 17 (17) | 20 (20) | 0 (0) | 0.72 |
| Brain | 5 (5) | 2 (2) | 0 (0) | 0.45 |
| Other2 | 11 (11) | 12 (12) | 0 (0) | 1 |
|
| ||||
| Number of Sites of Metastatic Disease | <0.001 | |||
|
| ||||
| 0 | 0 (0) | 0 (0) | 200 (100) | |
| 1 | 21 (21) | 77 (77) | 0 (0) | |
| 2 | 43 (43) | 20 (20) | 0 (0) | |
| 3 | 33 (33) | 3 (3) | 0 (0) | |
| 4 | 3 (3) | 0 (0) | 0 (0) | |
|
| ||||
| Tumor Phenotype | 0.02 | |||
|
| ||||
| ER/PR Positive HER23 Negative | 71 (71) | 82 (82) | 141 (70) | |
| HER2 Positive | 22 (22) | 12 (12) | 23 (12) | |
| Triple4 Negative | 6 (6) | 3 (3) | 21 (11) | |
| Unknown (missing HER2 status) | 1 (1) | 3 (3) | 15 (8) | |
|
| ||||
| Received Breast/Chest Radiotherapy | 53 (53) | 35 (35) | 133 (77) | <0.001 |
|
| ||||
| Inflammatory Conditions of the Lung | ||||
|
| ||||
| Asthma | 12 (12) | 13 (13) | 22 (11) | 0.88 |
| COPD | 6 (6) | 6 (6) | 7 (4) | 0.50 |
| Other5 | 9 (9) | 9 (9) | 11 (6) | 0.40 |
|
| ||||
| Breast Cancer Treatment in Last Month | 83 (83) | 88 | 120 | |
|
| ||||
| Time Since Last Treatment | <0.001 | |||
|
| ||||
| Day of Study | 56 (56) | 58 (58) | 119 (60) | |
| Within One Month | 31 (31) | 32 (32) | 3 (20) | |
| None Recent | 13 (13) | 10 (10) | 78 (39) | |
|
| ||||
| Treatment Received | ||||
|
| ||||
| Chemotherapy/Targeted Therapy | 44 (44) | 31 (31) | 0 | <0.001 |
| Endocrine Therapy | 44 (44) | 66 (66) | 120 (60) | 0.004 |
|
| ||||
| Smoking History | 0.42 | |||
|
| ||||
| Current | 3 (3) | 8 (8) | 20 (10) | |
| Former | 41 (41) | 40 (40) | 84 (42) | |
| Ever | 44 (44) | 48 (48) | 104 (52) | |
| Never | 56 (56) | 52 (52) | 96 (48) | |
|
| ||||
| Smoking Exposure6, Median (Range) | 24 (1–126) | 27 (1–150) | 20 (1–150) | 0.98 |
|
| ||||
| Second Hand Smoking Exposure | 0.38 | |||
|
| ||||
| Ever | 54 (54) | 63 (63) | 122 (61) | |
| Never | 46 (46) | 37 (37) | 78 (39) | |
|
| ||||
| NSAID Use | 0.94 | |||
|
| ||||
| Regular | 33 (33) | 31 (31) | 65 (33) | |
| Occasional | 66 (66) | 69 (69) | 135 (68) | |
| Missing Data | 1 (1) | 0 (0) | 0 (0) | |
|
| ||||
| Time since NSAID (days) Median (Range) | 2.5 (0–713) n= 68 | 1 (0–1008) n=69 | 1 (0–4309) N = 142 | 0.77 |
|
| ||||
| NSAID Exposure within Seven Days | 50 (50) | 47(47) | 95 (48) | 0.90 |
BMI: Body Mass Index
Defined as time from first invasive breast cancer to day of study consent
Include soft tissue, adrenal and peritoneum
HER2= human epidermal growth factor receptor 2
Defined as ER=0%, PR=0% and HER2 Negative (Immunohistochemistry 0/1+ or FISH <2.0)
Including bronchiectasis, interstitial lung disease, granulomatous disease, pulmonary fibrosis and sarcoidosis
Defined in Pack Years among ever smokers
Urinary PGE-M by Patient Subgroup
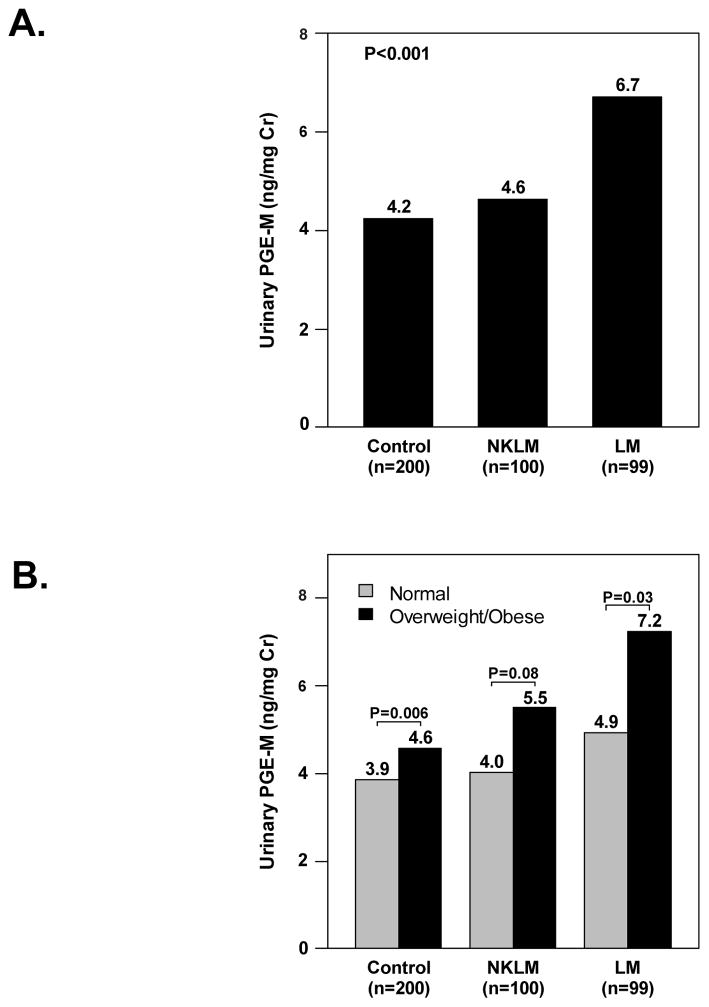
For technical reasons, urinary PGE-M could not be measured on one urine sample from a patient with lung metastases. Therefore, PGE-M results were available on 399 patients. The median PGE-M level was 5.47ng/mg creatinine (range 0.68 –43.4) in patients with metastatic disease (group1), which was significantly higher than the median PGE-M of 4.23ng/mg creatinine (range 0.86–62.6) in controls (group 2) (p<0.001). In addition, PGE-M levels were significantly different across the three preplanned study groups (p<0.001, Fig. 3A). Specifically, the median PGE-M was 6.7ng/mg creatinine (range 0.7–43.4) among patients with lung metastases, which was significantly higher than 4.6ng/mg creatinine (range 0.7–26.8) in patients with no known lung metastases (p=0.003) and 4.2ng/mg creatinine (range 0.9–62.6) in controls (p<0.001). Upon careful examination of the effect of other sites of metastasis on PGE-M levels, it was observed that patients with liver metastasis with or without lung metastasis also showed higher PGE-M levels compared to the controls (p=0.01 and <0.001, respectively). There was no detectable difference in PGE-M levels between patients with no known lung metastases and controls (p=0.34) or between patients with no known lung/liver metastases and controls (p=0.99).
Figure 3.
A, PGE-M levels were significantly different across study groups (P<0.001). Specifically, patients with lung metastasis (LM) had significantly higher urinary PGE-M levels [median (range)] (ng/mg creatinine) compared to patients with no known lung metastasis (NKLM) and the controls [6.7(0.7, 43.4) vs. 4.6 (0.7, 26.8) vs. 4.2 (0.9, 62.6), P=0.003 and P<0.001, respectively]. Urinary PGE-M levels were not significantly different between NKLM patients and the controls (P=0.34). B, PGE-M levels were significantly associated with increasing BMI (Spearman’s ρ=0.24, P<0.001). In each study group, urinary PGE-M levels [median (range)] (ng/mg creatinine) were higher in overweight/obese subjects compared to normal weight subjects [Controls: 4.6 (1.0, 62.6) vs. 3.9 (0.9, 26.8), P=0.006; Patients with NKLM: 5.5 (0.7, 26.7) vs. 4.0 (1.3, 26.8), P=0.08; Patients with LM: 7.2 (0.7, 43.4) vs. 4.9 (0.8, 22.7), P=0.03].
Smoking and NSAID Exposure
Approximately half of the patients in all subgroups were classified as ever smokers (Table 1). There was no difference in the proportion of ever smokers between groups (p=0.42) although the total numbers of current smokers was low (Table 1). The absolute numbers of current smokers by subgroup were three (3%), eight (8%) and 20 (10%) in groups 1A, 1B and 2, respectively. In addition to cigarettes, five patients, all of whom were ever smokers, admitted to other tobacco/non-tobacco (e.g. marijuana) smoking exposure. Therefore, considering this did not lead to the reclassification of any never as ever smokers.
There were no differences between groups in terms of NSAID exposure within seven days of participation (p=0.94; Table 1).
PGE-M and Smoking Exposure
The association between urinary PGE-M and smoking exposure was examined in univariate analyses. Smoking exposure was considered as 1) a continuous variable (pack years), 2) a categorical variable with three categories (current/former/never smoker), and 3) a categorical variable with two categories (ever vs. never smoker). As a continuous variable, extent of smoking exposure was associated with PGE-M levels (Spearman’s ρ=0.125, p=0.01). As expected, current smokers had higher PGE-M levels than former smokers and never smokers (Supplemental Table 3), although this difference was not statistically significant (p=0.22). For the primary study endpoint, levels of PGE-M were compared between ever and never smokers by patient subgroup (Table 2). The difference in PGE-M levels between never smokers and ever smokers with lung metastases was not statistically significant (p=0.54) (Table 2). No evidence of a correlation between PGE-M and second-hand smoke exposure was seen.
Table 2.
PGE-M by Smoking Status and NSAID Use
| Median (Range)† | Median (Range)† | P value | |
|---|---|---|---|
| Smoking Status | Never smokers | Ever smokers | |
| Lung Metastases (group 1A) n=99 | 6.1 (0.7–43.4) n=56 | 7.4 (0.8–28.9) n=43 | 0.54 |
| No Known Lung Metastases (group 1B) n=100 | 4.8 (0.7–26.8) n=52 | 4.6 (1.3–26.7) n=48 | 0.96 |
| Controls (group 2) n=200 | 4.0 (0.9–62.6) n=96 | 4·5 (1.0–26.8) n=104 | 0.45 |
| Overall NSAID Use | ≤7 days since NSAID | > 7days since NSAID | |
| Lung Metastases (group 1A) n=99 | 5.4 (0.7–19.3) n=49 | 7.4 (1.8–43.4) n=50 | 0.005 |
| No Known Lung Metastases (group 1B) n=100 | 5.2 (0.7–26.7) n=47 | 4.1 (1.3–26.8) n=53 | 0.22 |
| Controls (group 2) n=200 | 3.8 (1.0–62.6) n=95 | 4.5 (0.9–30.8) n=105 | 0.04 |
| Interaction Between Smoking, NSAIDs and PGE-M | |||
| Ever smokers | ≤7 days since NSAID | > 7days since NSAID | |
| Lung Metastases (group 1A) n=99 | 5.2 (0.8–19.3) n=25 | 10.6 (2.9–28.9) n=18 | 0.01 |
| No Known Lung Metastases (group 1B) n=100 | 5.5 (1.3–26.7) n=21 | 3.6 (1.4–12.5) n=27 | 0.08 |
| Controls (group 2) n=200 | 3.8 (1.0–26.8) n=51 | 5.2 (1.5–24.2) n=53 | 0.03 |
| Never smokers | ≤7 days since NSAID | > 7days since NSAID | |
| Lung Metastases (group 1A) n=99 | 5.6 (0.7–19.1) n=24 | 6.7 (1.8–43.4) n=32 | 0.13 |
| No Known Lung Metastases (group 1B) n=100 | 4.9 (0.7–19.6) n=26 | 4.6 (1.3–26.8) n=26 | 0.83 |
| Controls (group 2) n=200 | 3.8 (1.2–62.6) n=44 | 4.0 (0.9–30.8) n=52 | 0.43 |
normalized to urinary creatinine and expressed as ng/mg creatinine
PGE-M and NSAID Use
Patients with lung metastases who had not taken NSAIDs, prototypic inhibitors of COX activity, in the seven days before urine collection had the highest PGE-M levels (median 7.4 ng/mg creatinine; range 1.8–43.4) (Table 2). Significantly lower PGE-M levels were seen in patients who had taken NSAIDS within seven days compared to those who had not among patients with lung metastases (p=0.005) and controls (p=0.04). Consistent with the hypothesized role of inflammation in mediating lung metastases, the highest levels of PGE-M were seen among ever smokers with lung metastases who had not used NSAIDs within seven days of the urine collection (median 10.6 ng/mg creatinine). Among ever smokers with lung metastases, lower PGE-M levels were seen in patients who had taken NSAIDs in the last seven days compared to those who had not (p=0.01).
Effect of Other Variables on PGE-M
Consistent with prior evidence that obesity causes subclinical inflammation including elevated COX-2 levels, increasing BMI was associated with increased PGE-M both as a continuous variable (ρ=0.24, p< 0.001) and as a categorical variable (p<0.001). Similar correlations between PGE-M and BMI were also seen in each patient subgroup (Fig 3B.). No significant differences were seen in the median PGE-M between the 68 women with inflammatory lung conditions (median 5.04 ng/mg creatinine, range 0.81–28.07) and the 331 women without these conditions (median 4.74 ng/mg creatinine, range 0.68–62.6, p=0.26). Time from breast cancer diagnosis to study participation was associated with elevated PGE-M (p=0.02) as was the number of metastatic sites (p<0.001). Elevated PGE-M levels were seen in patients who had received cytotoxic chemotherapy (p<0.001), anti-HER2 therapy (p<0.001), and bevacizumab (p=0.08) in the previous month in univariable analysis. No significant effect on PGE-M levels was seen for receipt of endocrine therapy (p=0.32), or for different classes of chemotherapy (p=0.33). No association between PGE-M and receipt of radiation was seen (p=0.5)).
Multivariate Model for PGE-M
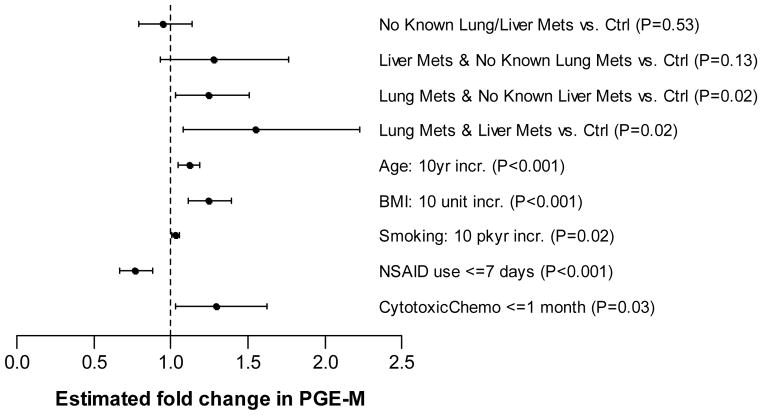
A multivariate model was developed to include variables that showed evidence of association with PGE-M (p<0.25) and those that were notably different across study groups (p<0.25). Notably, time from diagnosis and extent of metastatic disease did not meet these criteria. In the final multiple linear regression model (Fig. 4), PGE-M levels were significantly different across study groups after adjusting for other important covariates. In this final analysis, ever smokers with lung metastases had the highest covariate-adjusted PGE-M levels. Specifically, PGE-M levels were significantly higher in patients with lung metastases (regardless of liver metastasis status) compared to controls (p=0.02). However, the difference in PGE-M levels between patients with no known lung/liver metastases and controls was not statistically significant (p=0.53) In addition, several other factors were independently associated with increased PGE-M levels including pack year smoking history (p=0.02), BMI (p<0.001), age (p<0.001) and receipt of cytotoxic chemotherapy within the last month (p=0.03)). Notably, cytotoxic chemotherapy was the only administered therapy significantly associated with urinary PGE-M levels in multivariable analysis. Conversely, receipt of NSAIDs within seven days was strongly associated with significantly lower levels of PGE-M (p<0.001).
Figure 4.
Multivariate Model for PGE-M. Estimated fold change in PGE-M (and 95% CI) with respect to covariates in the multivariable linear regression model (an indicator variable of statin use was also included in the final model but its effect was not statistically significant).
Discussion
The highest levels of urinary PGE-M, a biomarker of inflammation, were found in ever smokers with lung metastases. While this is consistent with the hypothesis that pulmonary inflammation predisposes to breast cancer metastasizing to the lung, we acknowledge that there are other potential explanations. Because both smoking and lung metastasis were each independent determinants of elevated urinary PGE-M, the high PGE-M levels in smokers with lung metastasis could reflect an additive effect, rather than suggesting a causal link between inflammation and metastasis. At the same time, our data do not exclude the hypothesis that smoking-induced lung inflammation increases risk of metastasis and are consistent with this possibility. A prospective trial would need to be carried out to further investigate a potential link between lung inflammation and breast cancer metastases to the lung.
Additional observations were made regarding elevated levels of urinary PGE-M and specific sites of metastases. In comparison to patients with histories of breast cancer without evidence of active disease, levels of urinary PGE-M were increased in women with metastases to the lung. This can potentially be explained by one or more mechanisms. Overexpression of COX-2 in tumor cells within the lung metastases can explain this finding (27, 28). In support of this possibility, Massagué and colleagues showed in preclinical models that COX-2 was one of the genes that mediate breast cancer metastasis to the lung (7, 8, 29). It’s possible, therefore, that selective elevation of urinary PGE-M in patients with metastasis to the lung reflects increased COX-2 levels in tumor cells as predicted by their work. In addition to overexpression of COX-2 in tumor cells, another possibility is that inflammation occurs in response to lung metastasis leading to increased local production of PGE2. Finally, the potential role of PGE2 catabolism in the lung should be considered. The normal lung plays a critical role in the catabolism of PGE2 and contributes to the short half-life of PGE2 in blood (30). Because PGE-M is a catabolic product of PGE2 (Fig. 1), active lung catabolism in combination with overproduction of PGE2 by tumor or inflammatory cells may explain the increased levels of urinary PGE-M found in patients with breast cancer metastases to the lung. Based on these findings, studies to determine whether other tumor types that cause lung metastases also lead to elevated levels of urinary PGE-M are warranted. Ultimately, if COX-2 contributes to the elevated levels of urinary PGE-M in patients with breast cancer metastases to the lung, it is possible that this important subset of patients would benefit from treatment with an inhibitor of COX-2, perhaps as a component of a multi-agent approach (31).
Obesity is linked to both the development and progression of breast cancer possibly through the development of inflammation (22, 23, 32). Recently, we showed in both mouse models of obesity and human breast white adipose tissue that obesity-related inflammation was associated with increased levels of COX-2 and PGE2 (33–35). In the current study, obesity was associated with both metastasis and elevated levels of urinary PGE-M. These findings fit with both prior evidence that inflammation plays a role in obesity-related breast cancer progression, and the elevated PGE2 levels found in inflamed white adipose tissue (35–37). In addition to being of mechanistic interest, these results have potentially significant practical implications for the field of cancer prevention. For example, urinary PGE-M has the potential to be a valuable biomarker for assessing the utility of behavioral, nutritional or pharmacological interventions that aim to reduce obesity-related inflammation and possibly the development and progression of breast cancer. Studies are underway to test this possibility.
Aging was another correlate of increased urinary PGE-M levels. Elevated levels of multiple proinflammatory mediators, known inducers of COX-2 and PGE2 synthesis, occur during aging and may contribute to this finding (38). It’s also possible that reduced levels of estrogen, a molecule with known anti-inflammatory properties, contribute to aging-related increases in urinary PGE-M levels. Our results support the possibility that inflammation is one of the mechanisms by which aging predisposes to cancer. Alternatively, it is possible that the age-related increase in PGE-M simply reflects the presence of atherosclerosis or other age-related inflammatory conditions (39).
Tissue injury is associated with the induction of both COX-2 and PGE2 (40) and several proinflammatory cytokines induce COX-2 transcription and the synthesis of this prostaglandin. Previously, we demonstrated that treatment with cytotoxic chemotherapy led to increased levels of COX-2 and PGE2 in tumor tissue (41). Here we note that treatment with cytotoxic chemotherapy was associated with elevated levels of urinary PGE-M, fitting with these prior findings. Whether the cytotoxic chemotherapy-mediated increases in urinary PGE-M levels correlate with either tumor response or the development of side effects is unknown and deserves further investigation. Finally, recent use of NSAIDs was associated with a significant reduction in levels of urinary PGE-M. This result is consistent with prior studies in which either dual inhibitors of COX-1/COX-2 or selective COX-2 inhibitors were shown to suppress PGE-M levels (18–20). Because NSAIDs, including aspirin, are commonly used by patients for a variety of reasons a critical aspect of the conduct of future studies of urinary PGE-M concerns the need to obtain an accurate medication history or the utility of urinary PGE-M as an informative biomarker will be severely compromised. If a prospective trial is to be done to test an intervention that may attenuate obesity-related inflammation, it will be best to exclude regular users of NSAIDs and aspirin if urinary PGE-M is to be used as a biomarker.
In summary, this study provides new insights into the link between inflammation and breast cancer and highlights the potential utility of urinary PGE-M as a clinically relevant biomarker. Such a marker could prove useful in future studies that aim to reduce the risk of obesity-related cancers or possibly those due to other chronic inflammatory conditions.
Supplementary Material
Supplemental Table 1. Additional patient characteristics
Supplemental Table 2. Patient enrollment by timing of study amendment
Supplemental Table 3. PGE-M levels in current vs. former vs. never smokers
Acknowledgments
We would like to thank the Behavioral Research Methods Core Facility at MSKCC for assisting in the design of the study and in developing the study questionnaire.
Grant Support
Funding was received from the Clinical and Translational Science Center through the NIH UL1RR024996 (Morris and Zhou), 2011 Conquer Cancer Foundation of ASCO Young Investigator Award (Morris), Flight Attendants Medical Research Institute (Dannenberg), Breast Cancer Research Foundation (Hudis and Dannenberg), Botwinick-Wolfensohn Foundation (Dannenberg) (in memory of Mr. and Mrs. Benjamin Botwinick), the Metastases Research Center of MSKCC and the Alan and Sandra Gerry Metastasis Research Initiative (Hudis). This work was presented in part at the 2012 ASCO Annual Meeting, resulting in a 2012 Conquer Cancer Foundation of ASCO Merit Award (Morris).
Footnotes
Previously presented at the Annual Meeting of the American Society of Clinical Oncology 2012 ASCO meeting, Chicago, IL, 2012
Conflicts of Interest
The authors have no conflicts of interest to declare
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–3. [PubMed] [Google Scholar]
- 3.Morris PG, McArthur HL, Hudis CA. Therapeutic options for metastatic breast cancer. Expert Opin Pharmacother. 2009;10:967–81. doi: 10.1517/14656560902834961. [DOI] [PubMed] [Google Scholar]
- 4.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–7. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992;89:10578–82. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris PG, McArthur HL, Hudis CA. Breast Metastasis. In: Welch D, Lyden D, Psaila B, editors. Cancer Metastasis: Biologic Basis and Therapeutics. Cambridge University Press; 2010. [Google Scholar]
- 7.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–24. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–70. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 9.Taranova AG, Maldonado D, 3rd, Vachon CM, Jacobsen EA, Abdala-Valencia H, McGarry MP, et al. Allergic pulmonary inflammation promotes the recruitment of circulating tumor cells to the lung. Cancer Res. 2008;68:8582–9. doi: 10.1158/0008-5472.CAN-08-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stathopoulos GT, Sherrill TP, Han W, Sadikot RT, Yull FE, Blackwell TS, et al. Host nuclear factor-kappaB activation potentiates lung cancer metastasis. Mol Cancer Res. 2008;6:364–71. doi: 10.1158/1541-7786.MCR-07-0309. [DOI] [PubMed] [Google Scholar]
- 11.Martey CA, Pollock SJ, Turner CK, O’Reilly KM, Baglole CJ, Phipps RP, et al. Cigarette smoke induces cyclooxygenase-2 and microsomal prostaglandin E2 synthase in human lung fibroblasts: implications for lung inflammation and cancer. Am J Physiol Lung Cell Mol Physiol. 2004;287:L981–91. doi: 10.1152/ajplung.00239.2003. [DOI] [PubMed] [Google Scholar]
- 12.Scanlon EF, Suh O, Murthy SM, Mettlin C, Reid SE, Cummings KM. Influence of smoking on the development of lung metastases from breast cancer. Cancer. 1995;75:2693–9. doi: 10.1002/1097-0142(19950601)75:11<2693::aid-cncr2820751109>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Calle EE, Miracle-McMahill HL, Thun MJ, Heath CW., Jr Cigarette smoking and risk of fatal breast cancer. Am J Epidemiol. 1994;139:1001–7. doi: 10.1093/oxfordjournals.aje.a116939. [DOI] [PubMed] [Google Scholar]
- 14.Murin S, Pinkerton KE, Hubbard NE, Erickson K. The effect of cigarette smoke exposure on pulmonary metastatic disease in a murine model of metastatic breast cancer. Chest. 2004;125:1467–71. doi: 10.1378/chest.125.4.1467. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Chen P, Hanaoka M, Droma Y, Kubo K. Enhanced levels of prostaglandin E2 and matrix metalloproteinase-2 correlate with the severity of airflow limitation in stable COPD. Respirology. 2008;13:1014–21. doi: 10.1111/j.1440-1843.2008.01365.x. [DOI] [PubMed] [Google Scholar]
- 16.Taha R, Olivenstein R, Utsumi T, Ernst P, Barnes PJ, Rodger IW, et al. Prostaglandin H synthase 2 expression in airway cells from patients with asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:636–40. doi: 10.1164/ajrccm.161.2.9811063. [DOI] [PubMed] [Google Scholar]
- 17.Montuschi P, Kharitonov SA, Ciabattoni G, Barnes PJ. Exhaled leukotrienes and prostaglandins in COPD. Thorax. 2003;58:585–8. doi: 10.1136/thorax.58.7.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphey LJ, Williams MK, Sanchez SC, Byrne LM, Csiki I, Oates JA, et al. Quantification of the major urinary metabolite of PGE2 by a liquid chromatographic/mass spectrometric assay: determination of cyclooxygenase-specific PGE2 synthesis in healthy humans and those with lung cancer. Anal Biochem. 2004;334:266–75. doi: 10.1016/j.ab.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Gross ND, Boyle JO, Morrow JD, Williams MK, Moskowitz CS, Subbaramaiah K, et al. Levels of prostaglandin E metabolite, the major urinary metabolite of prostaglandin E2, are increased in smokers. Clin Cancer Res. 2005;11:6087–93. doi: 10.1158/1078-0432.CCR-05-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duffield-Lillico AJ, Boyle JO, Zhou XK, Ghosh A, Butala GS, Subbaramaiah K, et al. Levels of prostaglandin E metabolite and leukotriene E(4) are increased in the urine of smokers: evidence that celecoxib shunts arachidonic acid into the 5-lipoxygenase pathway. Cancer Prev Res (Phila Pa) 2009;2:322–9. doi: 10.1158/1940-6207.CAPR-09-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterz K, Scherer G, Ecker J. A simple and robust UPLC-SRM/MS method to quantify urinary eicosanoids. J Lipid Res. 2012;53:1026–36. doi: 10.1194/jlr.D023739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–42. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ewertz M, Jensen MB, Gunnarsdottir KA, Hojris I, Jakobsen EH, Nielsen D, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29:25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 24.Li CI, Daling JR, Malone KE. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J Clin Oncol. 2003;21:28–34. doi: 10.1200/JCO.2003.03.088. [DOI] [PubMed] [Google Scholar]
- 25.Murin S, Inciardi J. Cigarette smoking and the risk of pulmonary metastasis from breast cancer. Chest. 2001;119:1635–40. doi: 10.1378/chest.119.6.1635. [DOI] [PubMed] [Google Scholar]
- 26.Khatcheressian JL, Wolff AC, Smith TJ, Grunfeld E, Muss HB, Vogel VG, et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24:5091–7. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–93. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subbaramaiah K, Norton L, Gerald W, Dannenberg AJ. Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer: evidence for involvement of AP-1 and PEA3. J Biol Chem. 2002;277:18649–57. doi: 10.1074/jbc.M111415200. [DOI] [PubMed] [Google Scholar]
- 29.Minn AJ, Gupta GP, Padua D, Bos P, Nguyen DX, Nuyten D, et al. Lung metastasis genes couple breast tumor size and metastatic spread. Proc Natl Acad Sci U S A. 2007;104:6740–5. doi: 10.1073/pnas.0701138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes D, Otani T, Yang P, Newman RA, Yantiss RK, Altorki NK, et al. NAD+-dependent 15-hydroxyprostaglandin dehydrogenase regulates levels of bioactive lipids in non-small cell lung cancer. Cancer Prev Res (Phila) 2008;1:241–9. doi: 10.1158/1940-6207.CAPR-08-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dohadwala M, Yang SC, Luo J, Sharma S, Batra RK, Huang M, et al. Cyclooxygenase-2-dependent regulation of E-cadherin: prostaglandin E(2) induces transcriptional repressors ZEB1 and snail in non-small cell lung cancer. Cancer Res. 2006;66:5338–45. doi: 10.1158/0008-5472.CAN-05-3635. [DOI] [PubMed] [Google Scholar]
- 32.Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay JY. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br J Cancer. 2003;88:1721–6. doi: 10.1038/sj.bjc.6600956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila Pa) 2011;4:329–46. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–9. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, et al. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356–65. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL, et al. Epithelial-mesenchymal transition induced by TNF-alpha requires NF-kappaB-mediated transcriptional upregulation of Twist1. Cancer Res. 2012;72:1290–300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinicrope FA, Dannenberg AJ. Obesity and breast cancer prognosis: weight of the evidence. J Clin Oncol. 2011;29:4–7. doi: 10.1200/JCO.2010.32.1752. [DOI] [PubMed] [Google Scholar]
- 38.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–6. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Wang M, Zukas AM, Hui Y, Ricciotti E, Pure E, FitzGerald GA. Deletion of microsomal prostaglandin E synthase-1 augments prostacyclin and retards atherogenesis. Proc Natl Acad Sci U S A. 2006;103:14507–12. doi: 10.1073/pnas.0606586103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singer, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115:297–306. doi: 10.1016/s0016-5085(98)70196-9. [DOI] [PubMed] [Google Scholar]
- 41.Altorki NK, Port JL, Zhang F, Golijanin D, Thaler HT, Duffield-Lillico AJ, et al. Chemotherapy induces the expression of cyclooxygenase-2 in non-small cell lung cancer. Clin Cancer Res. 2005;11:4191–7. doi: 10.1158/1078-0432.CCR-05-0108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Additional patient characteristics
Supplemental Table 2. Patient enrollment by timing of study amendment
Supplemental Table 3. PGE-M levels in current vs. former vs. never smokers