Abstract
Infections due to methicillin-resistant Staphylococcus aureus (MRSA) are an important cause of morbidity and mortality in hospital patients. Moreover, increased incidences of outpatient MRSA have been recently reported. This study investigated the bactericidal activity of dalbavancin, a novel, semisynthetic glycopeptide antibiotic, against methicillin-sensitive S. aureus (MSSA) and MRSA in the rat granuloma pouch infection model. A single intravenous dose of 10 mg of dalbavancin/kg of body weight reduced the viable MRSA count in pouch exudates by more than 2 log CFU/ml, and regrowth was prevented for up to 120 h. Comparable results with vancomycin required four 100-mg/kg intramuscular doses. With one or two doses of vancomycin, the bacterial load declined over proportionately shorter periods of time, followed by regrowth. Reduction of the bacterial load obtained with 100- and 200-mg/kg oral doses of linezolid was relatively transient, with regrowth starting at 48 h. A single 10-mg/kg dose of dalbavancin reduced the MSSA count at 24 h to below the limit of detection, with no regrowth for at least 96 h. Dalbavancin demonstrated good exudate penetration; the ratio of the area under the curve (AUC) in plasma to the AUC in pouch exudate was 1.01. The in vivo activity of dalbavancin in this model is consistent with the antibiotic concentrations that are reached and maintained for extended periods of time after a single 10-mg/kg dose and with in vitro data showing that these concentrations are bactericidal for staphylococci. The pharmacokinetic and efficacy data seen in this relevant model of infection suggest that dalbavancin may be administered less frequently than vancomycin and linezolid.
The incidence of bacteremia, an important cause of morbidity and mortality in hospital patients, has increased significantly in recent years. The majority of these infections are nosocomial and are caused by gram-positive bacteria such as Staphylococcus aureus and coagulase-negative staphylococci (6). Antibiotic resistance is now a global problem, with increasing numbers of nosocomial infections caused by methicillin-resistant staphylococci (5). In many U.S. and European hospitals, methicillin-resistant S. aureus (MRSA) strains are now endemic (10) and are becoming increasingly resistant to multiple antibiotics (2, 23). Nosocomial infections due to MRSA significantly increase the lengths of hospital stays for patients and the costs of hospitalization (1). Furthermore, community-acquired MRSA infection is becoming more prevalent (4, 9).
The most widely used treatments for MRSA are the glycopeptide antibiotics vancomycin and, outside the United States, teicoplanin (11). However, glycopeptide-intermediate S. aureus strains, with reduced susceptibility to vancomycin and teicoplanin, are emerging (8, 12, 13), signaling the need for novel compounds with activity against this organism. Clinical isolates of MRSA that are resistant to linezolid, a recently introduced oxazolidinone antibiotic with activity against gram-positive bacteria, have also appeared (25).
As a result of a rational chemical approach to find new potent antibiotics with gram-positive activity, the semisynthetic glycopeptide dalbavancin (formerly BI 397) was synthesized (17, 24). This study compares the activities of dalbavancin, linezolid, and vancomycin against experimental bacterial infections caused by methicillin-sensitive S. aureus (MSSA) and MRSA in the rat granuloma pouch model. The pharmacokinetics of dalbavancin were also investigated.
(Part of this work was presented previously [G. Candiani, G. Romanó, C. Brunati, M. Cavaleri, S. Riva, G. Mosconi, and D. Jabés, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-1654, 2001].)
MATERIALS AND METHODS
Bacterial strains.
An MSSA type culture (ATCC 19636) and a clinical isolate of MRSA (SA 1400; Hôpital Pitié Salpêtrière, Paris, France) were used. The isolates were stored at −80°C in nutrient no. 2 broth (Oxoid S.p.A, Garbagnate, Italy) with 20% (vol/vol) glycerol (Baker Malinckrodt B. V., Denverter, The Netherlands).
Antimicrobial agents.
Dalbavancin was produced by Vicuron Pharmaceuticals (formerly Biosearch Italia SpA), and vancomycin was obtained as a powder (Sigma, Milan, Italy) and in lyophilized vials for clinical use (Eli Lilly). The commercial preparation of linezolid (Zyvox; Pharmacia) was used.
Animals.
Sprague-Dawley rats (Harlan Italy, S. Pietro al Natisone, Italy) of both sexes, weighing 100 to 150 g, were used. Procedures were approved by the Animal Care and Use Committee at our institution.
MIC and MBC determinations.
MICs of dalbavancin, vancomycin, and linezolid were determined by broth macrodilution as recommended by the National Committee for Clinical Laboratory Standards (19) in Mueller-Hinton broth (MHB) (Difco Laboratories, Detroit, Mich.) with and without addition of 50% rat granuloma pouch exudate from uninfected rats. Previous experiments demonstrated that the addition of 50% rat exudate alone does not affect bacterial growth in vitro (G. Candiani, G. Romanó, C. Brunati, M. Cavaleri, S. Riva, G. Mosconi, and D. Jabés, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-1654, 2001). Cultures were incubated at 35°C for 24 h, and the MIC was defined as the lowest concentration of antibiotic at which there was no visible growth. Minimum bactericidal concentrations (MBCs) were determined by plating duplicate 0.025-ml aliquots from tubes with no visible growth on Todd-Hewitt agar (THA) (Difco Laboratories) and incubating overnight at 35°C. The MBC was defined as the lowest concentration of antibiotic that reduced the viable count by 99.9% of the initial bacterial inoculum. Total counts were determined on at least two consistent serial dilutions. The limit of detection for the MBC determinations was 40 CFU/ml.
Time-kill experiments.
Colonies of MRSA grown on THA were inoculated into MHB supplemented with 50% rat granuloma pouch exudate (MHBRE) and incubated in a shaking water bath at 35°C. Log-phase cultures were diluted to 105 to 106 CFU/ml with MHBRE and exposed to different concentrations of antibiotics. At intervals, duplicate 0.1-ml aliquots (at least 10-fold in 0.1% peptone-0.9% NaCl) were plated on THA by the inclusion method in 2.5 ml of soft THA (0.7% agar in Todd-Hewitt broth). Plates were incubated at 35°C for at least 48 h for counting. Bactericidal activity was defined as a decrease of 3 log units in CFU per milliliter in 24 h (20). The bacterial titer was determined with at least two serial 10-fold dilutions. Five colonies per plate were the minimum number used for determining viable counts.
Granuloma pouch model.
The granuloma pouch model used in these experiments was based on the model described by Dalhoff in 1986 (7). Granuloma pouches were induced in rats by injecting 30 ml of sterile filtered air followed by 0.5 ml of 0.5% croton oil (Sigma) in sesame seed oil deep into loose connective dorsal tissue. Two to three days later, approximately 10 ml of air was withdrawn, and after 2 to 3 more days, 2 ml of saline containing 1% peptone (Difco Laboratories) and 0.35% agar (Becton Dickinson and Company, Sparks, Md.) was injected. Bacterial suspensions of MSSA were prepared by diluting frozen cultures in Todd-Hewitt broth containing 0.25% agar (Difco Laboratories). Bacterial suspensions of MRSA were prepared by diluting frozen cultures in saline containing 2.5% bacteriological gastric mucin (Becton Dickinson and Company), 0.5% peptone, and 0.35% agar. Gastric mucin and a higher concentration of agar were required for infection with SA 1400, which, like many MRSA strains, is generally less virulent than MSSA in this model and requires protection from host defenses (18). Bacterial suspensions (1 to 1.5 ml) containing approximately 106 CFU/ml were inoculated into rat granuloma pouches 7 to 10 days after the start of induction.
Antibiotic treatment.
Five to six animals per group were used. In a preliminary experiment, dalbavancin was administered as a single 2.5-, 5-, or 10-mg/kg intravenous (i.v.) bolus to rats infected with MSSA or MRSA in order to determine the optimal dose. In a second series of experiments, dalbavancin (10 mg/kg) was administered as a single i.v. injection to rats infected with MRSA.
Doses of comparator treatments, linezolid and vancomycin, were selected to mimic therapeutic doses given to patients and were also based on dose regimens used in other animal models of infection. A 25-mg/kg oral dose of linezolid in rats produces plasma exposures comparable to those obtained in humans following a 600-mg oral dose (D. J. Stalker, C. P. Wajszczuk, and D. H. Batts, 2nd Int. Conf. Macrolides, Azalides, Streptogramins, Ketolides, Oxazolidinones 5, poster 08.21, 2000; K. Chiba, K. L. Feenstra, J. G. Slatter, P. T. Daley-Yates, J. N. Duncan, P. E. Fagernes, M. R. Howard, I. J. Martin, N. Ozawa, B. J. Passingham, H. G. Parks, G. W. Peng. R. J. Simmonds, W. Speed, D. Yallop, and S. Yamazaki, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-123, 1998). Linezolid was given once orally at 25-, 50-, and 100-mg/kg doses in rats infected with MSSA and at 50-, 100-, and 200-mg/kg doses in rats infected with MRSA. Higher doses were used against the MRSA strain because it was less susceptible in vitro.
Vancomycin (100 mg/kg) was administered by intramuscular (i.m.) injection either once, twice, or four times, at 12-h intervals, to rats infected with MRSA. This dose regimen has been shown to achieve plasma peak and trough vancomycin concentrations in rats comparable to those achieved in humans following i.v. administration of 1,000 mg (26).
For all three antibiotics studied, treatment commenced 1 h after infection with MSSA and 3 h after infection with MRSA. The difference in timing is due to the observation that MRSA strains have a longer lag phase in vivo than MSSA strains (18).
In all experiments, 0.2-ml samples of infected pouch exudates were collected just before treatment and at various time intervals after the first treatment. Samples were serially diluted 10-fold in 1% peptone in saline, and duplicate 0.025-ml aliquots of suitable dilutions were plated by inclusion in 3 ml of soft THA. Colonies were counted after incubation at 35°C for 48 to 72 h, and bacterial titers were expressed as the average log10 CFU per milliliter of exudates from five or six rats per treatment group.
Pharmacokinetics.
Exudate samples (0.5 ml) were collected at appropriate intervals, using heparinized syringes, for determination of dalbavancin and vancomycin concentrations. Blood samples were collected simultaneously in heparinized tubes from the retro-orbital sinuses of lightly anesthetized rats. Concentrations of linezolid were not determined.
Blood and exudate samples were centrifuged at 5,000 × g for 5 min to obtain plasma and cell-free exudate. Antibiotic concentrations were determined in both biologic matrices by a microbiological agar diffusion assay with Bacillus subtilis ATCC 6633 as the indicator organism. The limits of quantification were 0.4 mg/liter for dalbavancin and 0.8 mg/liter for vancomycin.
The total protein contents of exudates and plasma samples were measured by the method of Lowry et al. (16). WinNonLin Professional, version 3 (Pharsight, Mountain View, Calif.), was used to calculate pharmacokinetic parameters. A noncompartmental and a three-compartmental model with first-order output and macro constants as primary parameters were used to fit the temporal profile of dalbavancin concentrations.
RESULTS
MICs, MBCs, and protein determinations.
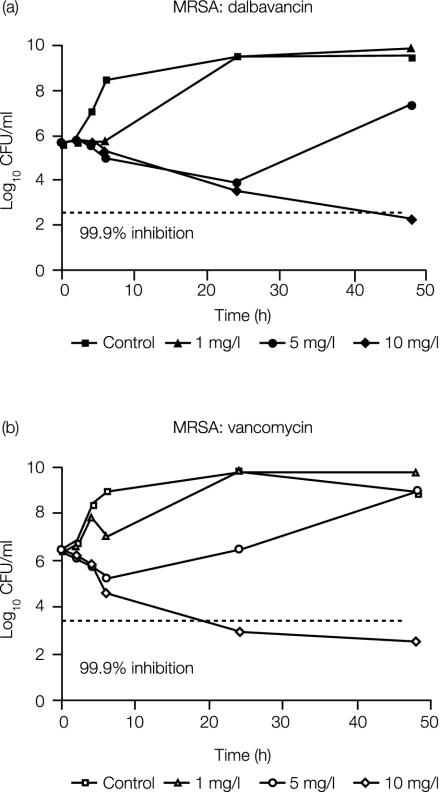
The MICs and MBCs of dalbavancin, vancomycin, and linezolid for MRSA and MSSA grown in MHB and MHBRE are shown in Table 1. The total protein concentrations were 32 mg/ml in rat plasma and 29.7 mg/ml in pouch exudates (averages of two determinations). Figure 1 shows the time-kill curves for dalbavancin and vancomycin versus the MRSA strain. At 10 mg/liter, both compounds reduced the viable count by at least 99.9% by 24 to 48 h of exposure.
TABLE 1.
In vitro activities of dalbavancin, vancomycin, and linezolid against ATCC 19636 (MSSA) and SA 1400 (MRSA) in the presence and absence of rat granuloma exudates
| Strain | Antibiotic | MIC (mg/liter)a in:
|
MBC (mg/liter)a in:
|
||
|---|---|---|---|---|---|
| MHB | MHBRE | MHB | MHBRE | ||
| ATCC 19636 | Dalbavancin | ≤0.13-0.25 | 4 | 2 | 32 |
| Vancomycin | 0.5-2 | 2 | 128 | 32 | |
| Linezolid | 4 | 2 | 4 | 2 | |
| SA 1400 | Dalbavancin | 0.25-0.5 | 2-4 | 16-32 | 8-32 |
| Vancomycin | 1-2 | 2 | 16-128 | 4-16 | |
| Linezolid | 2-4 | 2-8 | 8 | 4-8 | |
Ranges are from three experiments.
FIG. 1.
In vitro bactericidal activities of dalbavancin (a) and vancomycin (b) against MRSA SA 1400 in the presence of 50% rat exudate.
Granuloma pouch model.
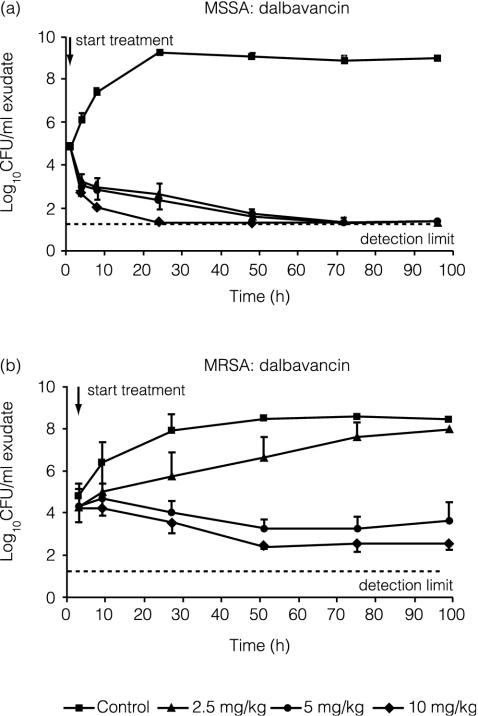
The effect of different doses of dalbavancin on S. aureus in granuloma pouches is shown in Fig. 2. Reduction of the bacterial load by dalbavancin was seen at all doses with the MSSA strain. A dose-dependent reduction in bacterial load was evident with the MRSA strain. A single i.v. 2.5-mg/kg dose of dalbavancin slowed the growth compared with the untreated control group, whereas a reduction in the number of viable bacteria was seen at 5 and 10 mg/kg and was more marked at the higher dose. Additionally, the single i.v. 10-mg/kg dose of dalbavancin prevented regrowth of MRSA for at least 96 h after treatment.
FIG. 2.
Efficacies of single doses of 2.5, 5, and 10 mg of dalbavancin per kg against MSSA (a) and MRSA (b) strains in the rat granuloma pouch. Error bars indicate standard deviations.
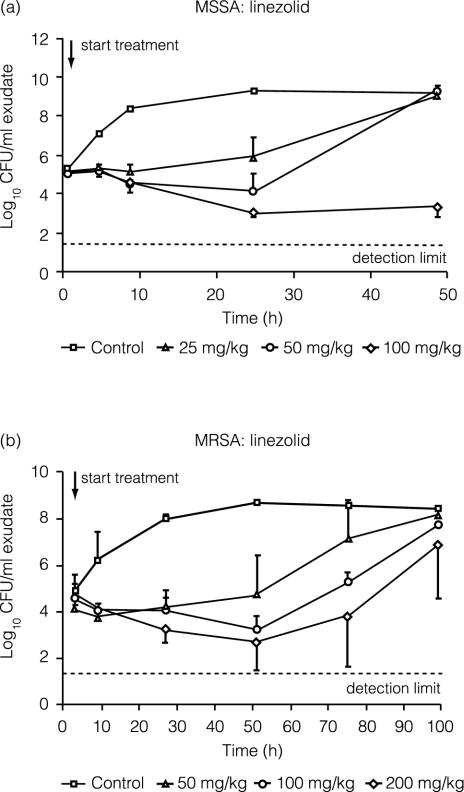
Figure 3 shows the effect of linezolid treatment on the bacterial load. Treatment with a single oral 100-mg/kg dose of linezolid reduced MSSA counts by approximately 2 log units by 24 h and suppressed regrowth for up to 48 h. Regrowth began by 24 h with the lower doses. Against the MRSA strain, a 2-log-unit reduction in CFU was seen by 48 h after linezolid doses of 100 and 200 mg/kg, but regrowth was seen at all linezolid doses. The CFU increased above the zero-time concentration and approached untreated control values by 96 h.
FIG. 3.
Efficacies of single doses of 25, 50, and 100 mg of linezolid per kg against MSSA (a) and of 50, 100, and 200 mg of linezolid per kg against MRSA (b) in the rat granuloma pouch. Error bars indicate standard deviations.
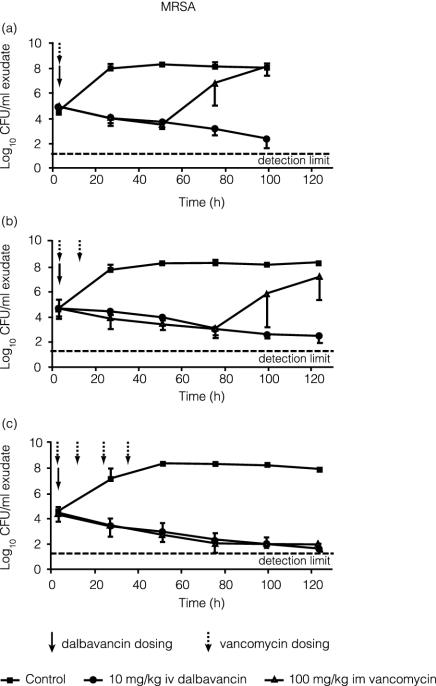
Figure 4 compares the activities of dalbavancin and vancomycin against MRSA in rat granuloma pouches. A single 10-mg/kg i.v. dose of dalbavancin progressively reduced the viable count of MRSA in pouch exudates by 2 or 3 log units over 96 to 120 h. A single 100-mg/kg i.m. dose of vancomycin produced a similar rate of reduction in bacterial load, but regrowth began by 48 h after treatment. With two vancomycin doses, at 0 and 12 h after infection, regrowth was delayed until about 72 h. Four 100-mg/kg i.m. doses of vancomycin at 0 to 36 h postinfection were required to prevent regrowth for at least 120 h, a result similar to that seen with a single 10-mg/kg i.v. dose of dalbavancin.
FIG. 4.
Efficacy of a single 10-mg/kg dose of dalbavancin compared with one (a), two (b), or four (c) 100-mg/kg i.m. doses of vancomycin against MRSA in the rat granuloma pouch. Error bars indicate standard deviations.
Pharmacokinetics.
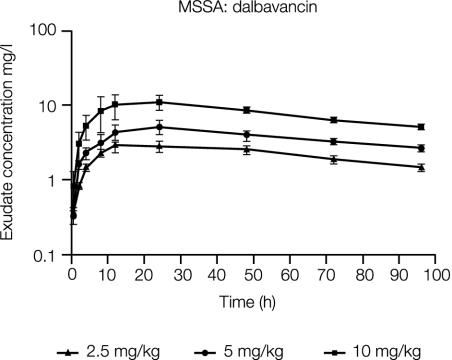
The concentrations of dalbavancin in rat granuloma pouches infected with MSSA through 96 h postdose are shown in Fig. 5. Concentrations of dalbavancin in exudate peaked by about 12 to 24 h postdose. Table 2 illustrates the dose proportionality of the maximum observed concentration in exudate (Cmax) and the concentration in exudate at 96 h (C96) following 2.5-, 5-, and 10-mg/kg i.v. doses.
FIG. 5.
Concentration of dalbavancin in granuloma pouch exudates over 96 h following i.v. administration of 2.5 (n = 4), 5 (n = 4), or 10 (n = 3) mg/kg to MSSA-infected rats. Error bars indicate standard deviations.
TABLE 2.
Mean Cmax, C96h, and Tmax of dalbavancin in granuloma pouch exudates following i.v. administration of 2.5, 5, or 10 mg of dalbavancin per kg to rats infected with the MSSA strain
| Dalbavancin dose (mg/kg)a | Mean Cmax (mg/liter)b | Mean C96 (mg/liter)b | Tmax (h) |
|---|---|---|---|
| 2.5 (4) | 2.85 (0.48) | 1.49 (0.19) | 12 |
| 5 (4) | 5.14 (1.06) | 2.68 (0.32) | 24 |
| 10 (3) | 11.06 (2.47) | 5.13 (0.06) | 24 |
The number of rats is given in parentheses.
The standard deviation is given in parentheses.
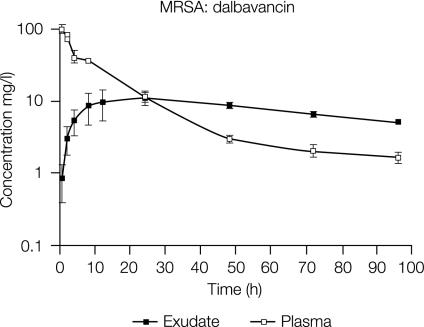
Figure 6 compares the concentrations of dalbavancin in rat granuloma pouch exudate and plasma after i.v. administration of a 10-mg/kg dose to MRSA-infected animals. The ratio of the area under the concentration-time curve in plasma to the area under the concentration-time curve in exudate was 1.01, indicating good penetration of dalbavancin into the granuloma compartment.
FIG. 6.
Mean concentrations of dalbavancin in plasma and granuloma pouch exudates over 96 h following i.v. administration of a single 10-mg/kg dose to MRSA-infected rats. Error bars indicate standard deviations.
For MRSA-infected animals receiving a single 100-mg/kg i.m. dose of vancomycin, the Cmax and time to maximum concentration (Tmax) of vancomycin in rat granuloma pouch exudates were 13.7 mg/liter and 8 h, respectively. At 24 h the concentration of vancomycin in rat granuloma pouch exudates was 3.6 mg/liter.
DISCUSSION
Previous studies have demonstrated that dalbavancin is active in vitro against a wide range of antimicrobial-resistant gram-positive bacteria and in different animal models of gram-positive infection (3, 14). In this study, we compared the bactericidal activities of dalbavancin, vancomycin, and linezolid, in vitro and in vivo, against MSSA and MRSA strains. The granuloma pouch model of infection permits concurrent determination of drug concentration at the infected site and of the number of viable bacteria and also allows for repeated sampling of the same animals. Thus, correlations between antibiotic concentration and antibacterial activity are possible.
Dalbavancin is highly protein bound, and previous studies demonstrated an effect of serum on its in vitro activity (3, 14). The extents of binding to albumin are similar in rat and human sera and exceed 98%. In this study, we determined that the rat granuloma pouch exudates contained approximately the same concentration of protein as that measured in rat plasma (∼30 mg/liter). These values are consistent with literature values and are representative of values reported for human plasma (35 to 50 mg/liter). Despite the high degree of dalbavancin protein binding, bactericidal activity of dalbavancin was observed in vitro in the presence of 50% granuloma pouch exudate, and the value obtained is similar to what has been observed in the presence of 50% human plasma (G. Candiani, G. Romano, C. Brunati, M. Cavaleri, S. Riva, G. Mosconi, D. Jabes, S. Lopez, and C. Hackbarth, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F5, 2001). Neither vancomycin nor linezolid is highly protein bound; the extent of binding for both drugs is approximately 30%.
The in vitro and in vivo results for vancomycin observed in this study are consistent with those which have been reported in the literature (26). While dalbavancin and vancomycin had similar bactericidal activities in vitro in the presence of protein-containing rat exudates (Fig. 1), a similar extent of in vivo activity, as demonstrated with the granuloma pouch model, required four 100-mg/kg i.m. doses of vancomycin, compared to a single 10-mg/kg i.v. dose of dalbavancin (Fig. 4). A single 10-mg/kg i.v. dose of dalbavancin and four 100-mg/kg i.m. doses of vancomycin maintained a low bacterial load for at least 96 to 120 h after treatment.
The efficacy in the granuloma pouch model of a single 10-mg/kg i.v. dose of dalbavancin is consistent with previous studies in which this dosage regimen was more effective than multiple 100-mg/kg doses of vancomycin in reducing S. aureus loads in a rat endocarditis model (3).
The results for linezolid in this study were somewhat unexpected. Based on the pharmacokinetics and MBCs of linezolid against the strains tested, single doses of 25 and 50 mg/kg were expected to be effective against MSSA and MRSA, respectively, in this granuloma pouch model of infection. The oral bioavailability is 100%, and tissue penetration is excellent; therefore, concentrations at the site of infection should have been adequate. These doses were not effective in reducing bacterial loads of the MSSA or MRSA strains (Fig. 3). A single 100-mg/kg oral dose of linezolid was necessary to achieve the same reduction of bacterial titer in the granuloma pouches as was seen with a single 10-mg/kg i.v. dose of dalbavancin (Fig. 2 and 3). However, regrowth of bacteria began by 24 to 48 h in animals treated with linezolid regardless of the dose. After the present study was conducted, others reported that a 25-mg/kg i.v. dose of linezolid given three times a day for 5 days was not effective in the treatment of experimental staphylococcal endocarditis in a rabbit model (21). Efficacy in that model was not seen until the dose was 50 or 75 mg/kg with the same treatment schedule.
The main factor accounting for the in vivo efficacy of single doses of dalbavancin is its pharmacokinetics. Dalbavancin had excellent penetration into the granuloma pouch, and high concentrations were maintained for extended periods of time after a single 10-mg/kg i.v. dose. Levels similar to those that are bactericidal in vitro in the presence of protein were maintained for at least 96 h.
This study demonstrated greater efficacy of dalbavancin than of vancomycin or linezolid against MSSA and MRSA in the rat granuloma pouch infection model. Pharmacokinetic data for animals (unpublished data) and humans (15) suggest that dalbavancin may be administered less frequently than other treatments for nosocomial S. aureus infections. Infrequent dosing may reduce the need for indwelling catheters. Recent clinical experience suggests that a once-weekly dosage of dalbavancin may be used to treat skin and soft tissue infections (22).
REFERENCES
- 1.Abramson, M. A., and D. J. Sexton. 1999. Nosocomial methicillin-resistant and methicillin-susceptible Staphylococcus aureus primary bacteremia: at what costs? Infect. Control Hosp. Epidemiol. 20:408-411. [DOI] [PubMed] [Google Scholar]
- 2.Aucken, H. M., M. Ganner, S. Murchan, B. D. Cookson, and A. P. Johnson. 2002. A new UK strain of epidemic methicillin-resistant Staphylococcus aureus (EMRSA-17) resistant to multiple antibiotics. J. Antimicrob. Chemother. 50:171-175. [DOI] [PubMed] [Google Scholar]
- 3.Candiani, G., M. Abbondi, M. Borgonovi, G. Romano, and F. Parenti. 1999. In-vitro and in-vivo antibacterial activity of BI 397, a new semi-synthetic glycopeptide antibiotic. J. Antimicrob. Chemother. 44:179-192. [DOI] [PubMed] [Google Scholar]
- 4.Chambers, H. F. 2001. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 7:178-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collignon, P. J. 2002. Antibiotic resistance. Med. J. Aust. 177:325-329. [DOI] [PubMed] [Google Scholar]
- 6.Crowe, M., P. Ispahani, H. Humphreys, T. Kelley, and R. Winter. 1998. Bacteraemia in the adult intensive care unit of a teaching hospital in Nottingham, UK, 1985-1996. Eur. J. Clin. Microbiol. Infect. Dis. 17:377-384. [DOI] [PubMed] [Google Scholar]
- 7.Dalhoff, A. 1986. The granuloma pouch, p. 123-137. In O. Zak and M. A. Sande (ed.), Experimental models in antimicrobial chemotherapy. Academic Press, London, United Kingdom.
- 8.Denis, O., C. Nonhoff, B. Byl, C. Knoop, S. Bobin-Dubreux, and M. J. Struelens. 2002. Emergence of vancomycin-intermediate Staphylococcus aureus in a Belgian hospital: microbiological and clinical features. J. Antimicrob. Chemother. 50:383-391. [DOI] [PubMed] [Google Scholar]
- 9.Dufour, P., Y. Gillet, M. Bes, G. Lina, F. Vandenesch, D. Floret, J. Etienne, and H. Richet. 2002. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clin. Infect. Dis. 35:819-824. [DOI] [PubMed] [Google Scholar]
- 10.Haddadin, A. S., S. A. Fappiano, and P. A. Lipsett. 2002. Methicillin resistant Staphylococcus aureus (MRSA) in the intensive care unit. Postgrad. Med. J. 78:385-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton-Miller, J. M. 2002. Vancomycin-resistant Staphylococcus aureus: a real and present danger? Infection 30:118-124. [DOI] [PubMed] [Google Scholar]
- 12.Heym, B., M. Le Moal, L. Armand-Lefevre, and M. H. Nicolas-Chanoine. 2002. Multilocus sequence typing (MLST) shows that the ‘Iberian’ clone of methicillin-resistant Staphylococcus aureus has spread to France and acquired reduced susceptibility to teicoplanin. J. Antimicrob. Chemother. 50:323-329. [DOI] [PubMed] [Google Scholar]
- 13.Hussain, F. M., S. Boyle-Vavra, P. B. Shete, and R. S. Daum. 2002. Evidence for a continuum of decreased vancomycin susceptibility in unselected Staphylococcus aureus clinical isolates. J. Infect. Dis. 186:661-667. [DOI] [PubMed] [Google Scholar]
- 14.Jones, R. N., D. J. Biedenbach, D. M. Johnson, and M. A. Pfaller. 2001. In vitro evaluation of BI 397, a novel glycopeptide antimicrobial agent. J. Chemother. 13:244-254. [DOI] [PubMed] [Google Scholar]
- 15.Leighton, A., A. B. Gottlieb, M. B. Dorr, D. Jabes, G. Mosconi, C. VanSaders, E. J. Mroszczak, K. C. M. Campbell, and E. Kelly. Tolerability, pharmacokinetics, and pharmacodynamics of intravenous dalbavancin in healthy volunteers. Antimicrob. Agents Chemother. 48:940-945. [DOI] [PMC free article] [PubMed]
- 16.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 17.Malabarba, A., and R. Ciabatti. 2001. Glycopeptide derivatives. Curr. Med. Chem. 8:1759-1773. [DOI] [PubMed] [Google Scholar]
- 18.Mizobuchi, S., J. Minami, F. Jin, O. Matsushita, and A. Okabe. 1994. Comparison of the virulence of methicillin-resistant and methicillin-sensitive Staphylococcus aureus. Microbiol. Immunol. 38:599-605. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standards, 4th ed. M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.National Committee for Clinical Laboratory Standards. 1999. Methods for determining bactericidal activity of antimicrobial agents. Approved standards, 4th ed. M26-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 21.Oramas-Shirey, M. P., L. V. Buchanan, C. L. Dileto-Fang, C. F. Dailey, C. W. Ford, D. H. Batts, and J. K. Gibson. 2001. Efficacy of linezolid in a staphylococcal endocarditis rabbit model. J. Antimicrob. Chemother. 47:349-352. [DOI] [PubMed] [Google Scholar]
- 22.Seltzer, E., M. B. Dorr, B. P. Goldstein, M. Perry, J. A. Dowell, T. Henkel, et al.. 2003. Once-weekly dalbavancin versus standard of care antimicrobial regimens for treatment of skin and soft tissue infections. Clin. Infect. Dis. 37:1298-1303. [DOI] [PubMed] [Google Scholar]
- 23.Speller, D. C., A. P. Johnson, D. James, R. R. Marples, A. Charlett, and R. C. George. 1997. Resistance to methicillin and other antibiotics in isolates of Staphylococcus aureus from blood and cerebrospinal fluid, England and Wales, 1989-95. Lancet 350:323-325. [DOI] [PubMed] [Google Scholar]
- 24.Steiert, M., and F. J. Schmitz. 2002. Dalbavancin (Biosearch Italia/Versicor). Curr. Opin. Investig. Drugs 3:229-233. [PubMed] [Google Scholar]
- 25.Tsiodras, S., H. S. Gold, G. Sakoulas, G. M. Eliopoulos, C. Wennersten, L. Venkataraman, R. C. Moellering, and M. J. Ferraro. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207-208. [DOI] [PubMed] [Google Scholar]
- 26.Voorn, G. P., J Kuyvenhoven, W. H. Goessens, W. C. Schmal-Bauer, P. H. Broeders, J. Thompson, and M. F. Michel. 1994. Role of tolerance in treatment and prophylaxis of experimental Staphylococcus aureus endocarditis with vancomycin, teicoplanin, and daptomycin. Antimicrob. Agents Chemother. 38:487-493. [DOI] [PMC free article] [PubMed] [Google Scholar]