Abstract
The etiology behind and physiological significance of spontaneous oscillations in the low-frequency spectrum in both systemic and cerebral vessels remain unknown. Experimental studies have proposed that spontaneous oscillations in cerebral blood flow reflect impaired cerebral autoregulation (CA). Analysis of CA by measurement of spontaneous oscillations in the low-frequency spectrum in cerebral vessels might be a useful tool for assessing risk and investigating different treatment strategies in carotid artery disease (CAD) and stroke. We reviewed studies exploring spontaneous oscillations in the low-frequency spectrum in patients with CAD and ischemic stroke, conditions known to involve impaired CA. Several studies have reported changes in oscillations after CAD and stroke after surgery and over time compared with healthy controls. Phase shift in the frequency domain and correlation coefficients in the time domain are the most frequently used parameters for analyzing spontaneous oscillations in systemic and cerebral vessels. At present, there is no gold standard for analyzing spontaneous oscillations in the low-frequency spectrum, and simplistic models of CA have failed to predict or explain the spontaneous oscillation changes found in CAD and stroke studies. Near-infrared spectroscopy is suggested as a future complementary tool for assessing changes affecting the cortical arterial system.
Keywords: Cerebral autoregulation, low frequency oscillations, stroke, carotid artery disease, transcranial Doppler, near-infrared spectroscopy
Cerebral autoregulation (CA)1 is controlled and affected by neurogenic,2,3 metabolic,4,5 and myogenic6,7 mechanisms, making it complex to study and interpret. Various neurologic disorders exhibit changes in CA.8–11 CA can be studied by static12 and dynamic5 interventional methods, but the use of these methods requires a certain level of compliance and can be stressful or even harmful to the patient. Over the last 10 years, an increasing number of studies have assessed CA by recording spontaneous oscillations in mean arterial blood pressure (MAP) and cerebral blood flow (CBF). This approach to assessing CA can be termed spontaneous dynamic CA. Spontaneous changes in MAP and CBF can be oscillations at respiratory and cardiac frequencies. Numerous studies13–28 have focused on changes in low-frequency oscillations (LFOs, known as Mayer waves,29 at 0.1 Hz) and very low-frequency oscillations (VLFOs, known as B-waves,30 at 0.04 Hz). These studies have reported interesting findings indicating impaired CA in neurologic pathologies. Investigating the level of CA impairment assessed through the analysis of spontaneous oscillation is of clinical interest, because this analysis might provide a useful tool for assessing risk and evaluating treatment strategies in cerebrovascular disease. In this article, we discuss the etiology behind spontaneous oscillations in the low-frequency spectrum and review studies that have investigated spontaneous oscillations in MAP and CBF in carotid artery disease (CAD) and ischemic stroke.
Etiology of Oscillations in the Low-Frequency Spectrum
Although spontaneous oscillations in the low-frequency spectrum in systemic blood pressure were discovered more than 130 years ago,29 the driving mechanisms responsible for these LFOs is unclear. Interventional studies have shown that neurogenic,31 metabolic32 and myogenic stimuli33 all affect LFOs. LFOs are believed to reflect changes in sympathetic tone34 based on human35 and animal36 studies, in which oscillations were found to be strongly attenuated by alpha-adrenoceptor blockade.
The pacemaker hypothesis is derived from observations in experimental animal studies of efferent sympathetic nervous activity and MAP oscillations in the absence of sensory input from the periphery.37–39 The exact location and function of a possible central pacemaker is unknown. It is known that CBF can be altered by electrical stimulation of brainstem centers.40 Serotonergic nerves from the brainstem’s median and dorsal raphe nuclei project to major resistance vessels,41 and electrical stimulation of the dorsal raphe nucleus leads to serotonergic activity in major cerebral arteries.42 These findings suggest that a central pacemaker may be located in the brain stem.
Guyton and Harris43 originally proposed a baroreceptor reflex effect on LFOs. This was later confirmed in animal studies showing that sinoaortic deafferentation attenuates Mayer waves.44,45 Interestingly, a study evaluating both sinoaortic baroreceptor denervation and sympathetic blockade in rats found that ~80% of the LFO power of MAP depended on the sympathetic nervous system activity, whereas the baroreflex accounted for ~50% of this power.46 This suggests that both a central pacemaker and the baroreflex might modulate LFO.
VLFOs, often referred to as B-waves, were originally observed by Lundberg30 as spontaneous oscillations in intracranial pressure (ICP) at frequencies below 0.04 Hz in patients with various intracranial diseases. VLFOs also have been observed as a normal physiological phenomenon in healthy persons measuring ICP,47 intraventricular cerebrospinal fluid flow,48 and flow velocity in the middle cerebral artery,49 suggesting a vascular myogenic mechanism based on the basic properties of the type of smooth muscle cells in cerebral vessels. Other studies have suggested that VLFOs, like LFOs, are caused by a central pacemaker.47,50 Given that VLFOs in CBF are mostly independent of MAP,51 a myogenic mechanism should be considered. Conversely, bilateral assessment of VLFOs by transcranial Doppler ultrasonography (TCD) showed that the changes are synchronized, suggesting a central pacemaker mechanism.47 Thus, the main difference between the different oscillation frequencies lies in the fact that LFOs are driven by spontaneous MAP changes, whereas VLFOs seem to be generated by mechanisms independent of MAP.
Functional Purpose of Oscillations in the Low-Frequency Spectrum
Very little data are available addressing the question of a physiological functional purpose of LFOs and VLFOs. Roche-Labarbe et al52 found associations between oscillations in the LFO range and spontaneous electroencephalogram bursts in neonates, suggesting that changes in oscillations are coupled to neuronal activity. In terms of blood flow and oxygen delivery, a mathematical model has shown greater oxygen conductance in vessels with an oscillating diameter compared with vessels with a constant diameter.53 A rat in vivo model examining the femoral artery found that during critical perfusion conditions, oscillations of arterioles at 0.034 Hz preserved nutritive perfusion.54 Whether these findings apply to the synchronized LFOs and VLFOs in systemic and cerebral vessels remains to be investigated. The finding that LFOs play a role in oxygen delivery could mean that impaired oscillations in a neurologic pathology is a direct causal factor in the outcome of the pathology, and thus would be important to monitor.
Measurement of Systemic and Cerebral Vessel Oscillations
Oscillations in the low-frequency spectrum in systemic MAP14,21,51 are commonly measured with a finger cuff system, using the volume clamp method of Penaz et al.55 TCD can be performed at the patient’s bedside and provides high-resolution measurement of velocity of the middle cerebral artery (VMCA), which supplies each hemisphere with up to 80% of the flow volume to the brain.56 Because VMCA is easy to insonate, and the diameter of the MCA does not change even under strong blood pressure manipulations,57 spontaneous changes in VMCA are believed to be caused by changes in CBF. VMCA may also be a reliable surrogate for blood flow changes in the internal carotid artery (ICA).58
A different approach to analyzing CA is through near-infrared spectroscopy (NIRS), which detects relative changes in cerebral oxygenation and blood volume over the cerebral cortex.59 Because the most comprehensive research on spontaneous dynamic CA to date has been done through analysis of changes seen on TCD, we focus on those studies in the following sections. We then address investigations of spontaneous dynamic CA performed with NIRS.
Data Analysis of Spontaneous Oscillations
The most common approach to studying spontaneous oscillations is in the frequency domain, but oscillations can be analyzed in the time domain as well. A power spectrum can be calculated using a fast Fourier transform algorithm,60 where power reflects the amplitude of the oscillation at a given frequency. Data on coherence, phase shift, and gain between physiological oscillation signals can be obtained through computerized cross-spectral analysis.51 This analytical model investigates the linear coupling between physiological oscillations, which are believed to reflect CA.
Comparing MAP and VMCA in healthy subjects shows a phase shift in the LFO spectrum, with VMCA oscillations preceding MAP oscillations by ~70°–45° (Table 1). Phase shift analysis requires stable frequencies over time, and because slow oscillations are transitional phenomena, paced breathing at slow frequencies is often used to induce stable frequencies,18,26,61,62 even though respiratory-induced oscillations are generated by different mechanisms than those of Mayer waves. The relationships between physiological parameters also can be expressed by gain, the ratio between corresponding amplitudes of physiological parameters.
Table 1.
Frequency domain analysis in CAD
| Study | Mean age, years (n) | Frequency (Hz) | Conclusions |
|---|---|---|---|
| Diehl et al (1995)61 | CO: 45 ± 15 (50) CAD: 49 ± 18 (20) |
Paced breathing: 0.1 | Paced LFO phase shift decreased on aS. |
| Diehl et al (1997)14 | CO: 44.7 ± 15.0 (50) CAD: 62.3 ± 9.7 (10) |
Spontaneous breathing: 0.05–0.15 | LFO phase shift decreased on aS. |
| Hu et al (1999)17 | CO: 61 ± 9 (37) CAD: 70 ± 7 (83) |
Spontaneous breathing: 0.04–0.15 | LFO phase shift and gain negatively correlated with degree of stenosis. |
| Reinhard et al (2003)18 | Collateral flow group I (best flow): (65) II: (24) III (worst flow): (12) |
Paced breathing: 0.1 | Paced LFO phase shift decreased on aS in groups II and III compared with group I and nS. |
| Reinhard et al (2003)26 | Unilateral CAD: 66 ± 8 (30) Bilateral CAD: 68 ± 7 (30) |
Paced breathing: 0.1 | No difference in paced LFO phase shift between most aS in unilateral and bilateral stenosis. |
| Haubrich et al (2004)72 | CO: 55 ± 12 (14) CAD: 65 ± 10 (102) |
Spontaneous breathing: 0.05–0.15 | LFO phase shift decreased in groups with > 70% stenosis. LFO was negatively correlated with degree of stenosis in CAD. |
| Reinhard et al (200)20 | CEA (41) SPAC (17) |
Spontaneous breathing: 0.06–0.12 | Both LFO phase shift and gain increased after CEA and SPAC operations. |
This table summarizes studies investigating oscillations in CAD through frequency domain analysis in the low-frequency spectrum.
Abbreviations: CO, healthy controls; aS, affected side; nS, nonaffected side; CEA, carotid endarterectomy; SPAC, stent-protected angioplasty of the carotid artery.
Diehl et al13 proposed that systemic and cerebral oscillations function as a biological system, and that spontaneous oscillations are regulated as a high-pass filter model. The Diehl model predicts positive phase shifts of up to 90° between MAP and VMCA oscillations,61 smaller for higher frequencies than for lower frequencies. In contrast, the gain between corresponding amplitudes of MAP and VMCA decreases with larger gains for higher frequencies (ie, less autoregulation) compared with lower frequencies (ie, more autoregulation). This model suggests that CA can be assessed by cerebral vessel resistance modulation of VMCA in response to changes in MAP in the low frequencies. These predictions were later confirmed in healthy subjects by different groups.63 The Diehl model implies that in impaired CA, phase shift decreases and gain increases between MAP and VMCA. This model must be interpreted with caution when analyzing phase and gain in pathological states, given that CA is clearly a nonlinear phenomenon64 with multiple variables. We address this issue later in this review by confronting the model with experimental data.
Analysis in the time domain also can describe the CA of spontaneous fluctuations by measuring a correlation coefficient between MAP and VMCA (Table 2). The concept of a correlation coefficient19,65 in assessing CA is based on the well-known autoregulatory plateau at which CBF is independent within a wide range of MAP and cerebral perfusion pressure changes.66 Within a normal MAP range, a correlation coefficient of <0.3 reflects functioning CA, whereas a higher correlation coefficient approximating 1 reflects impaired autoregulation.19,67 The correlation coefficient method in the time domain does not account for a specific frequency band and is not designed exclusively for analyzing LFOs.
Table 2.
Time domain analysis in patients with CAD and ischemic stroke
| Study | Pathology | Age (n) | Conclusions |
|---|---|---|---|
| Reinhard et al (2003)19 | Unilateral CAD | 67 ± 8 (150) | Increased CC between sides and groups (<80%) with stenosis ≥90%. |
| Gooskens et al (2003)73 | Unilateral CAD (89% stenosis); bilateral CAD (77% stenosis) | 64; range, 47–82 (38) | Increased CC in bilateral stenosis compared with unilateral stenosis. |
| Reinhard et al (2004)20 | CAD before and after SPAC or CEA | CEA (41); SPAC (17) | Decreased CC on aS after operation. |
| Reinhard et al (2005)24 | Acute stroke: MCA infarct size <35% Study 1: 22 ± 11 hours of ictus Study 2: 134 ± 25 hours of ictus |
CO: 61 ± 13 (25) Study 1: 61 ± 12 (33) Study 2: 59 ± 12 (29) |
Increased CC on both sides in study 2 compared with study 1. |
| Reinhard et al (2008)23 | Acute stroke: Study 1: 20 ± 9 hours of ictus Study 2: 64 ± 10 hours of ictus Study 3: 112 ± 7 hours of ictus |
CO: 64 ± 9 (71) Good outcome: 64 ± 14 (9) Poor outcome: 72 ± 6 (7) |
Increased CC on aS in poor outcome group compared with good outcome group and CO in study 2 and 3. Increased CC on aS compared with nS in poor outcome group in studies 1 and 3. |
This table summarizes studies measuring spontaneous oscillations analyzed in the time domain in patients with ischemic stroke and CAD.
Abbreviations: CO, healthy controls; aS, affected side; nS, nonaffected side; CC, Pearson’s correlation coefficient between mean MAP and VMCA through 1 minute; CEA, carotid endarterectomy; SPAC, stent-protected angioplasty of the carotid artery.
Oscillations in Carotid Stenosis Disease
Impairment of both static68 and dynamic68–71 autoregulation has been found in CAD. However, manipulation-based studies require a certain level of compliance and can be stressful or even harmful to the patient. Therefore, several studies have investigated spontaneous dynamic autoregulation through transfer function and time domain analyses (Tables 1 and 2) in resting subjects without the need for any stressful manipulations.14,17–20,26,61,72
The majority of studies investigating spontaneous oscillations in the low-frequency spectrum in CAD have come from 3 research groups.17,19,61 In these groups, data collection was similar, but the designs varied between paced and spontaneous oscillations. In addition, the groups applied different extraction rules, given the lack of a gold standard for analyzing data in the frequency domain. Despite these discrepancies, the studies using spontaneous dynamic CA on CAD patients reported findings that largely agree (Tables 1 and 2). The LFO phase shift was decreased on the affected side or negatively correlated with the degree of stenosis in all studies. However, the correlation coefficients were moderate,17,72 and stenosis must be at least 70% to demonstrate changes.72 This might arise from confounding factors, such as the level of collateral circulation and recent stroke, which will affect CA. In support of this, Reinhard et al18 reported decreased paced LFO phase shifts with the recruitment of secondary collateral pathways, that is, a retrograde flow via the external carotid and ophthalmic artery and leptomeningeal collateral flow via the posterior cerebral artery. Interestingly, the LFO phase shift approach has showed better sensitivity and specificity for detecting high-grade carotid stenosis compared with conventional standardized tests, such as CO2 vasoreactivity17 and the tilt test,72 making it a promising tool for CA assessment.
Hu et al17 analyzed the phase shift in VLFOs and found no change in phase shift in CAD. Because coherence between MAP and CBF61 in VLFOs is often very low, analysis of phase shift is difficult and uncertain. This is probably why most studies have not analyzed phase shift and gain at the VLFO spectrum.
An analysis of correlation coefficients between MAP and VMCA in the time domain in CAD studies appears to give the same information as an analysis in the frequency domain (Table 2). Studies have reported a decreased correlation coefficient on the affected side and with an increasing degree of stenosis.19,20 Gooskens et al73 found larger correlation coefficients in bilateral stenosis compared with unilateral stenosis, in contrast to Reinhard et al,26 who found no difference in phase shift between unilateral and bilateral stenosis. These differences could be caused by differences in patient factors, such as collateral flow. Although the correlation coefficient method is not confined to LFO and VLFO frequencies, the method has been shown to have moderate agreement with LFO phase shift in patients with severe unilateral carotid stenosis.19
This interpretation of increased carotid artery stenosis does not follow the Diehl model,13 because the gains in both LFO and VFLO frequencies decrease with increasing levels of stenosis.17 According to the Diehl model, this would imply increasing CA with increasing stenosis, which is meaningless. During hypercapnia, the blood flow velocity distal from an ICA occlusion was affected.74 Thus in carotid stenosis, the arterioles might be compensatively dilated by the demand of adequate perfusion and oxygen delivery distal to the stenosis. This could affect the capacity for LFO and thereby decrease gain. Thus, analyzing changes in gain as a single marker of CA might lead to misinterpretation. Hu et al17 found that gain, like phase shift, is negatively related to the degree of stenosis in both the VLFO and LFO spectrum, and Reinhard et al20 also reported increased gain after surgery in patients with CAD. However, most other studies did not analyze gain in CAD, and thus the use of gain analysis merits further exploration in future studies.
The studies investigating spontaneous dynamic autoregulation did not explore the etiology behind LFO and VLFO. In some studies, a disagreement between gain data and the Diehl model was explained by vasodilatation downstream of a stenosis.15,17,20 However, no study discussed the limitations of the model or the etiology behind the oscillations any further. Using a simplistic model to interpret results does not seem to provide any additional information on why oscillations differ in patients with CAD. There is a need for carefully designed clinical studies to explore the etiology behind LFOs and VLFOs in pathological states.
Reinhard et al20 have suggested that impairment of spontaneous dynamic CA might serve as a prognostic tool for stroke in asymptomatic severe carotid artery stenosis. In a recent study, the Reinhard group found a significant predictive effect on ipsilateral ischemic events for impaired spontaneous correlation coefficients, respiratory-induced LFO phase shift, and conventional CO2 vasoreactivitity in patients with unilateral ICP stenosis.25 When the analysis was restricted to severe stenosis alone, only respiratory-induced LFO phase shift remained a significant predictor of ischemic events. Thus, the authors concluded that analyzing CA by measuring spontaneous oscillations can detect an increased risk of subsequent ischemic events in patients with severe obstructive ICA disease, and might be a helpful tool in identifying patients at higher risk. That study yielded promising results, but the need remains for a standardized investigation of the use of spontaneous and respiratory-induced dynamic CA as a clinical tool for risk assessment in a large patient population.
Oscillations in Ischemic Stroke
The presence or absence of CA in ischemic stroke seems to be critical to the maintenance of stable blood flow in the ischemic penumbra and to avoid excessive hyperperfusion.24 Olsen et al9 reported impaired CA in areas of the brain perfused by collateral circulation, believed to be the ischemic penumbra, compared with surrounding brain areas with normal CA. Global impairment of both conventional static75 and dynamic76 autoregulation also has been reported in stroke patients. Dawson et al76 showed that dynamic CA was globally impaired for 4–14 days after stroke, whereas static autoregulation was unchanged compared with controls. This suggests that different mechanisms might influence static and dynamic CA. However, spontaneous dynamic CA might be more clinically relevant, because it mimics transient blood pressure changes occurring in stroke patients.77 To date, only 4 published studies have investigated spontaneous dynamic CA in acute stroke (Tables 2 and 3).23,24,27,28
Table 3.
Frequency domain analysis in ischemic stroke
| Study | Pathology | Mean age, years (n) | Frequency, Hz | Conclusions |
|---|---|---|---|---|
| Kwan et al (2004)28 | MCA infarcts investigated <7 days, 6 weeks, and 3 months after stroke | 73 ± 11 (10) | Oscillation induced by handgrip, 0.025 | No changes between sides; induced phase shift increases over time; no change in gain. |
| Reinhard et al (2005)24 | Acute stroke: MCA infarct size <35% Study 1: 22 ± 11 hours of ictus Study 2: 134 ± 25 hours of ictus |
CO: 61 ± 13 (25) Study 1: 61 ± 12 (33) Study 2: 59 ± 12 (29) |
Spontaneous breathing: 0.06–0.12 | No changes in LFO phase shift or gain. |
| Immink et al (2005)27 | MCA and lacunar infarcts | CO: 57 ± 2 (10) MCA infarct: 59 ± 5 (10) Lacunar infarct: 63 ± 3 (10) |
Spontaneous breathing: 0.07–0.15 | LFO phase shift decreased on aS in MCA infarcts but bilaterally decreased in lacunar infarcts. |
| Reinhard et al (2008)23 | Acute stroke: Good outcome and bad outcome groups Study 1: 20 ± 9 hours of ictus Study 2: 64 ± 10 hours of ictus Study 3: 112 ± 7 hours of ictus |
CO: 64 ± 9 (71) Good outcome group: 64 ± 14 (9) Poor outcome group: 72 ± 6 (7) |
Spontaneous breathing: 0.06–0.12 | Good outcome group: LFO phase shift decreased on aS in study 2. Poor outcome: LFO phase shift decreased on aS in studies 1 and 3. LFO phase shift decreased on aS compared with good outcome group and CO in all 3 studies. LFO phase shift decreased on nS between studies 1 and 3. |
This table summarizes studies measuring spontaneous LFOs in patients with ischemic stroke.
Abbreviations: CO, healthy controls; aS, affected side; nS, nonaffected side; lS, left side; rS, right side.
Two studies by Reinhard et al examined patients with ischemic stroke.23,24 A study published in 2005 reported no change in transfer function analysis (phase and gain) in 33 stroke patients compared with healthy controls.24 Most patients (32/33) had an infarct of <35% in the MCA territory. However, the authors reported an increase in correlation coefficients on both sides after ictus, which reflected a decline in CA over time. In 2008, the same research group analyzed 9 patients with good outcome and a MCA infarct volume of 14% ± 6% at 20, 64, and 112 hours after receiving thrombolysis, and found no change after ictus or over time by the correlation coefficient method.23 Phase shift analysis revealed a decrease in phase shift on the affected side compared with the healthy side, but only at 64 hours after ictus; however, 7 patients with bad outcome and an MCA infarct volume of 62% ± 21% exhibited a decrease in phase shift and an increase in correlation coefficient on the affected side compared with healthy controls. In addition, a decrease in phase shift was observed on the contralateral side at 20–112 hours after ictus. This suggests that a significant part of the MCA volume must be affected before statistically significant changes in autoregulation between groups can be detected. Moreover, minor focal autoregulatory changes in the ischemic territory might not be sufficiently powerful to significantly affect oscillations in MCA velocity. Both studies indicated a decline in CA over time. In contrast, a study using a 0.025-Hz rhythmic handgrip technique demonstrated a significant increase in phase shift over time from 1 week to 3 months after is-chemic stroke.28 This finding might be interpreted as indicating improved CA over time. However, the difference in methods (ie, the handgrip induction of 0.025-Hz oscillations) and patient selection make a comparison between that study and the studies of the Reinhard group difficult. A comparison of these studies is also hindered by the different periods analyzed (1 week to 3 months vs the first 5 days), which could be lead to an interpretation of the results as an initial decline in CA with secondary recovery. Given that loss of CA might promote further ischemia and edema after a large stroke,66,78 Reinhard et al25 proposed a vicious cycle model of reperfusion and cerebral dysautoregulation. This model predicts that re-perfusion leads to secondary vasculopathy through increased local production of free radicals, which impairs CA. The resulting autoregulatory failure leads to further increased reperfusion, followed by further increased local production of radicals.
Immink et al27 reported decreased LFO phase in MCA infarcts (size not stated) on the affected side, in agreement with Reinhard et al.25 Interestingly, Immink et al27 also found a decreased phase shift in both cerebral hemispheres in unilateral lacunar infarcts. This finding indicates that the ischemic event it is not necessarily causative of the change in CA. Lacunar infarcts are usually caused by small-vessel disease, and a disturbance in CA can be seen in other diseases affecting these vessels, such as diabetes.79 Thus, impaired CA might serve as a marker for vessel disease and prognostic for ischemic events, rather than a marker of the particular ischemic event per se.
All of the stroke studies included patients with stenosis of <70%,23,24,27 except for the study of Kwan et al,28 which included patients with stenosis of <80%. The impaired CA in the stroke studies seems to be independent of CAD status. The stroke studies indicate that changes in dynamic CA might be related to the size of the infarction. CA impairment also appears to be independent of CAD status. Thus, CA impairment in acute ischemic events measured by spontaneous LFOs might be a useful tool for predicting clinical outcome in stroke patients. CA impairment in lacunar infarcts might be due to a more general pathological state of all resistance vessels, however. Thus, methods for measuring both the focal and global state of the spontaneous dynamic CA are needed.
Investigating LFOs by NIRS
Measuring changes in MCA blood flow velocity is only an indirect method of investigating oscillations, because fluctuations in CBF are caused by changes in small resistance vessels downstream from the MCA. NIRS is a noninvasive technique with a high time resolution that can measure relative changes in the concentrations of oxygenated (oxyHb) and deoxygenated hemoglobin (deoxyHb) at the cerebral cortex level.48,80 Thus, NIRS measures local changes in cortical resistance vessels. The NIRS signal is a mixture of arterial and venous compartments, and measurements can vary among subjects due to anatomical variations. Nonetheless, several studies have demonstrated detection of LFOs in humans by NIRS,22,81–85 and the method can be performed simultaneously with VMCA and MAP analysis (Fig 1). Franceschini et al86 investigated phase differences between systemic blood pressure and NIRS signals using a 50-channel system covering the entire skull. Interestingly, they found that phase differences between the optical signals and MAP demonstrated spatial differences that were consistent among all subjects. Such intricate regional differences between the posterior and middle cerebral arteries have also been demonstrated by TCD.21 Cerebrovascular changes measured by NIRS have already been applied in some pathologies; for instance, LFO amplitude measured by NIRS was shown to be impaired secondary to hypertension during neuronal activation in patients with cerebral microangiopathy compared with healthy controls.84 This suggests that changes in the structure and function of the cerebral vasculature might be detected by measuring oscillations with NIRS. Likewise, Schroeter et al83 found a significant decline in the LFO amplitude of both oxyHb and deoxyHb with age during both rest and visual stimulation. A study of NIRS oscillations has shown that hypercapnia attenuates LFOs,81 as has been reported in animal studies. In healthy subjects, the amplitude of oxyHb was found to decrease during neuronal stimulation.81 In contrast, Vetri et al87 found an increase in amplitude during neural activation in cats. However, during neuronal activation an increase in blood flow is induced through vasodilatation of arteriolar resistance vessels.88,89 If it is assumed that vasodilatation attenuates oscillations, then the finding of decreased amplitude is reasonable.
Figure 1.
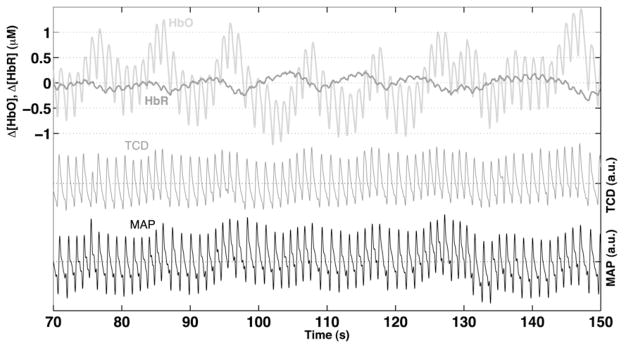
Example of an 80-second recording by NIRS in a healthy subject doing paced breathing at 0.1 Hz. The upper part shows a recording of oxygenated (HbO) and dexoxygenated (HbR) hemoglobin from the right frontal lobe. The middle part shows a TCD recording of the right middle cerebral artery. The lower part shows systemic blood pressure measurement by finger cuff.
NIRS has already been used in a study of CAD in which oxyHb LFOs were found to follow the LFOs of blood flow velocity in the MCA.51 Thus, the application of NIRS in studies of LFOs in cerebral vessels has already demonstrated interesting findings, and this technique merits further exploration in future studies.
Conclusion
The studies reviewed herein provide no clear link to the underlying etiology behind oscillations in the low-frequency spectrum. So far, no valid model can explain or predict the changes occurring in CAD and ischemic stroke, and there remains no gold standard for analyzing oscillations in the low-frequency spectrum. Analysis of spontaneous oscillations has demonstrated changes in patients with CAD and ischemic stroke compared with healthy controls both over time and after interventions in several studies. LFO phase shift changes in the frequency domain and correlation coefficients in the time domain are the most frequently used parameters for investigating spontaneous changes after CAD and stroke. In acute ischemic stroke, the impaired CA revealed by these techniques seems to be independent of underlying CAD status. The findings reported to date suggest that analysis of oscillations in the low-frequency spectrum might be a useful clinical tool for identifying risk and evaluating treatment strategies in patients with CAD and ischemic stroke.
Future Perspectives
Modeling of the complex interplay among modulations of vascular tone in different vascular compartments, blood flow, and delivery of oxygenated hemoglobin is likely required to guide the interpretation of data obtained from analysis of LFOs and VLFOs in cerebral vessels. This might lead to a standardized test for assessing oscillations in the low-frequency spectrum. Studies using both TCD and NIRS are warranted, because the complementary information regarding blood flow on the one hand and blood volume and oxygenation on the other hand might provide important information to identify the affected vascular compartments.
References
- 1.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39:183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- 2.Seylaz J, Hara H, Pinard E, et al. Effect of stimulation of the sphenopalatine ganglion on cortical blood flow in the rat. J Cereb Blood Flow Metab. 1988;8:875–878. doi: 10.1038/jcbfm.1988.145. [DOI] [PubMed] [Google Scholar]
- 3.Kawamura Y, Meyer JS, Hiromoto H, et al. Neurogenic control of cerebral blood flow in the baboon. J Neurosurg. 1975;43:676–688. doi: 10.3171/jns.1975.43.6.0676. [DOI] [PubMed] [Google Scholar]
- 4.Kontos HA, Wei EP. Oxygen-dependent mechanisms in cerebral autoregulation. Ann Biomed Eng. 1985;13:329–334. doi: 10.1007/BF02584251. [DOI] [PubMed] [Google Scholar]
- 5.Aaslid R, Lindegaard KF, Sorteberg W, et al. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- 6.Folkow B. Description of the myogenic hypothesis. Circ Res. 1964;15(Suppl):279–287. [PubMed] [Google Scholar]
- 7.Kontos HA, Wei EP, Navari RM, et al. Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol. 1978;234:H371–H383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- 8.Lang EW, Diehl RR, Mehdorn HM. Cerebral autoregulation testing after aneurysmal subarachnoid hemorrhage: The phase relationship between arterial blood pressure and cerebral blood flow velocity. Crit Care Med. 2001;29:158–163. doi: 10.1097/00003246-200101000-00031. [DOI] [PubMed] [Google Scholar]
- 9.Olsen TS. Regional cerebral blood flow after occlusion of the middle cerebral artery. Acta Neurol Scand. 1986;73:321–337. doi: 10.1111/j.1600-0404.1986.tb03286.x. [DOI] [PubMed] [Google Scholar]
- 10.Tang SC, Huang SJ, Chiu MJ, et al. Impaired cerebral autoregulation in a case of severe acute encephalitis. J Formos Med Assoc. 2007;106:S7–S12. doi: 10.1016/s0929-6646(09)60345-4. [DOI] [PubMed] [Google Scholar]
- 11.Lundar T, Lindegaard KF, Nornes H. Continuous recording of middle cerebral artery blood velocity in clinical neurosurgery. Acta Neurochir (Wien) 1990;102:85–90. doi: 10.1007/BF01405419. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt JF, Waldemar G, Vorstrup S, et al. Computerized analysis of cerebral blood flow autoregulation in humans: Validation of a method for pharmacologic studies. J Cardiovasc Pharmacol. 1990;15:983–988. doi: 10.1097/00005344-199006000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Diehl RR, Linden D, Lucke D, et al. Spontaneous blood pressure oscillations and cerebral autoregulation. Clin Auton Res. 1998;8:7–12. doi: 10.1007/BF02267598. [DOI] [PubMed] [Google Scholar]
- 14.Diehl RR, Kretzschmacher A, Linden D, et al. Autoregulation monitoring by analysis of spontaneuos TCD and blood pressure waves. New Trends Cereb Hemodyn Neurosonol. 1997:605–610. [Google Scholar]
- 15.Haubrich C, Kruska W, Diehl RR, et al. Dynamic autoregulation testing in patients with middle cerebral artery stenosis. Stroke. 2003;34:1881–1885. doi: 10.1161/01.STR.0000080936.36601.34. [DOI] [PubMed] [Google Scholar]
- 16.Haubrich C, Wendt A, Diehl RR, et al. Dynamic autoregulation testing in the posterior cerebral artery. Stroke. 2004;35:848–852. doi: 10.1161/01.STR.0000120729.99039.B6. [DOI] [PubMed] [Google Scholar]
- 17.Hu HH, Kuo TB, Wong WJ, et al. Transfer function analysis of cerebral hemodynamics in patients with carotid stenosis. J Cereb Blood Flow Metab. 1999;19:460–465. doi: 10.1097/00004647-199904000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Reinhard M, Muller T, Guschlbauer B, et al. Dynamic cerebral autoregulation and collateral flow patterns in patients with severe carotid stenosis or occlusion. Ultrasound Med Biol. 2003;29:1105–1113. doi: 10.1016/s0301-5629(03)00954-2. [DOI] [PubMed] [Google Scholar]
- 19.Reinhard M, Roth M, Muller T, et al. Cerebral autoregulation in carotid artery occlusive disease assessed from spontaneous blood pressure fluctuations by the correlation coefficient index. Stroke. 2003;34:2138–2144. doi: 10.1161/01.STR.0000087788.65566.AC. [DOI] [PubMed] [Google Scholar]
- 20.Reinhard M, Roth M, Muller T, et al. Effect of carotid endarterectomy or stenting on impairment of dynamic cerebral autoregulation. Stroke. 2004;35:1381–1387. doi: 10.1161/01.STR.0000127533.46914.31. [DOI] [PubMed] [Google Scholar]
- 21.Reinhard M, Waldkircher Z, Timmer J, et al. Cerebellar autoregulation dynamics in humans. J Cereb Blood Flow Metab. 2008;28:1605–1612. doi: 10.1038/jcbfm.2008.48. [DOI] [PubMed] [Google Scholar]
- 22.Reinhard M, Wehrle-Wieland E, Grabiak D, et al. Oscillatory cerebral hemodynamics—the macro- vs. microvascular level. J Neurol Sci. 2006;250:103–109. doi: 10.1016/j.jns.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Reinhard M, Wihler C, Roth M, et al. Cerebral autoregulation dynamics in acute ischemic stroke after rt-PA thrombolysis. Cerebrovasc Dis. 2008;26:147–155. doi: 10.1159/000139662. [DOI] [PubMed] [Google Scholar]
- 24.Reinhard M, Roth M, Guschlbauer B, et al. Dynamic cerebral autoregulation in acute ischemic stroke assessed from spontaneous blood pressure fluctuations. Stroke. 2005;36:1684–1689. doi: 10.1161/01.STR.0000173183.36331.ee. [DOI] [PubMed] [Google Scholar]
- 25.Reinhard M, Gerds TA, Grabiak D, et al. Cerebral dysautoregulation and the risk of ischemic events in occlusive carotid artery disease. J Neurol. 2008;255:1182–1189. doi: 10.1007/s00415-008-0865-z. [DOI] [PubMed] [Google Scholar]
- 26.Reinhard M, Muller T, Roth M, et al. Bilateral severe carotid artery stenosis or occlusion: Cerebral autoregulation dynamics and collateral flow patterns. Acta Neurochir (Wien) 2003;145:1053–1059. doi: 10.1007/s00701-003-0137-8. [DOI] [PubMed] [Google Scholar]
- 27.Immink RV, van Montfrans GA, Stam J, et al. Dynamic cerebral autoregulation in acute lacunar and middle cerebral artery territory ischemic stroke. Stroke. 2005;36:2595–2600. doi: 10.1161/01.STR.0000189624.06836.03. [DOI] [PubMed] [Google Scholar]
- 28.Kwan J, Lunt M, Jenkinson D. Assessing dynamic cerebral autoregulation after stroke using a novel technique of combining transcranial Doppler ultrasonography and rhythmic handgrip. Blood Press Monit. 2004;9:3–8. doi: 10.1097/00126097-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Mayer S. Studien zur physiologie des herzens und der blutgefässe. Sitz Kaiser Akad Wiss. 1876;74:281–307. [Google Scholar]
- 30.Lundberg N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr Scand Suppl. 1960;36:1–193. [PubMed] [Google Scholar]
- 31.Mayhew JE, Askew S, Zheng Y, et al. Cerebral vasomotion: A 0.1-Hz oscillation in reflected light imaging of neural activity. Neuroimage. 1996;4:183–193. doi: 10.1006/nimg.1996.0069. [DOI] [PubMed] [Google Scholar]
- 32.Hudetz AG, Smith JJ, Lee JG, et al. Modification of cerebral laser-Doppler flow oscillations by halothane, PCO2, and nitric oxide synthase blockade. Am J Physiol. 1995;269:H114–H120. doi: 10.1152/ajpheart.1995.269.1.H114. [DOI] [PubMed] [Google Scholar]
- 33.Auer LM, Gallhofer B. Rhythmic activity of cat pial vessels in vivo. Eur Neurol. 1981;20:448–468. doi: 10.1159/000115278. [DOI] [PubMed] [Google Scholar]
- 34.Tachtsidis I, Elwell CE, Leung TS, et al. Investigation of cerebral haemodynamics by near-infrared spectroscopy in young healthy volunteers reveals posture-dependent spontaneous oscillations. Physiol Meas. 2004;25:437–445. doi: 10.1088/0967-3334/25/2/003. [DOI] [PubMed] [Google Scholar]
- 35.Cevese A, Gulli G, Polati E, et al. Baroreflex and oscillation of heart period at 0.1 Hz studied by alpha-blockade and cross-spectral analysis in healthy humans. J Physiol. 2001;531:235–244. doi: 10.1111/j.1469-7793.2001.0235j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Japundzic N, Grichois ML, Zitoun P, et al. Spectral analysis of blood pressure and heart rate in conscious rats: Effects of autonomic blockers. J Auton Nerv Syst. 1990;30:91–100. doi: 10.1016/0165-1838(90)90132-3. [DOI] [PubMed] [Google Scholar]
- 37.Montano N, Gnecchi-Ruscone T, Porta A, et al. Presence of vasomotor and respiratory rhythms in the discharge of single medullary neurons involved in the regulation of cardiovascular system. J Auton Nerv Syst. 1996;57:116–122. doi: 10.1016/0165-1838(95)00113-1. [DOI] [PubMed] [Google Scholar]
- 38.Montano N, Cogliati C, da Silva VJ, et al. Effects of spinal section and of positive-feedback excitatory reflex on sympathetic and heart rate variability. Hypertension. 2000;36:1029–1034. doi: 10.1161/01.hyp.36.6.1029. [DOI] [PubMed] [Google Scholar]
- 39.Preiss G, Polosa C. Patterns of sympathetic neuron activity associated with Mayer waves. Am J Physiol. 1974;226:724–730. doi: 10.1152/ajplegacy.1974.226.3.724. [DOI] [PubMed] [Google Scholar]
- 40.Molnar L. Role of brain stem in CBF regulation. Scand J Clin Lab Invest Suppl. 1968;102:VI:D. [PubMed] [Google Scholar]
- 41.Edvinsson L, Degueurce A, Duverger D, et al. Central serotonergic nerves project to the pial vessels of the brain. Nature. 1983;306:55–57. doi: 10.1038/306055a0. [DOI] [PubMed] [Google Scholar]
- 42.Marco EJ, Moreno MJ, de Pablo AL. Local treatments of dorsal raphe nucleus induce changes in serotonergic activity in rat major cerebral arteries. Stroke. 1999;30:1695–1701. doi: 10.1161/01.str.30.8.1695. [DOI] [PubMed] [Google Scholar]
- 43.Guyton AC, Harris JW. Pressoreceptor-autonomic oscillation: A probable cause of vasomotor waves. Am J Physiol. 1951;165:158–166. doi: 10.1152/ajplegacy.1951.165.1.158. [DOI] [PubMed] [Google Scholar]
- 44.Julien C, Zhang ZQ, Barres C. How sympathetic tone maintains or alters arterial pressure. Fundam Clin Pharmacol. 1995;9:343–349. doi: 10.1111/j.1472-8206.1995.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 45.Jacob HJ, Ramanthan A, Pan SG, et al. Spectral analysis of arterial pressure lability in rats with sinoaortic deafferentation. Am J Physiol. 1995;269:R1481–R1488. doi: 10.1152/ajpregu.1995.269.6.R1481. [DOI] [PubMed] [Google Scholar]
- 46.Cerutti C, Barres C, Paultre C. Baroreflex modulation of blood pressure and heart rate variabilities in rats: Assessment by spectral analysis. Am J Physiol. 1994;266:H1993–H2000. doi: 10.1152/ajpheart.1994.266.5.H1993. [DOI] [PubMed] [Google Scholar]
- 47.Newell DW, Aaslid R, Stooss R, et al. The relationship of blood flow velocity fluctuations to intracranial pressure B waves. J Neurosurg. 1992;76:415–421. doi: 10.3171/jns.1992.76.3.0415. [DOI] [PubMed] [Google Scholar]
- 48.Strik C, Klose U, Kiefer C, et al. Slow rhythmic oscillations in intracranial CSF and blood flow registered by MRI. Acta Neurochir Suppl. 2002;81:139–142. doi: 10.1007/978-3-7091-6738-0_36. [DOI] [PubMed] [Google Scholar]
- 49.Lang EW, Diehl RR, Timmermann L, et al. Spontaneous oscillations of arterial blood pressure, cerebral and peripheral blood flow in healthy and comatose subjects. Neurol Res. 1999;21:665–669. doi: 10.1080/01616412.1999.11740995. [DOI] [PubMed] [Google Scholar]
- 50.Einhäubel KM. Oscillations of ICP related to cardiovacular parameters. In: Miller JD, Teasdale GM, Rowan JO, et al., editors. Intracranial Pressure VI. Berlin: Springer-Verlag; 1986. [Google Scholar]
- 51.Kuo TB, Chern CM, Sheng WY, et al. Frequency domain analysis of cerebral blood flow velocity and its correlation with arterial blood pressure. J Cereb Blood Flow Metab. 1998;18:311–318. doi: 10.1097/00004647-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Roche-Labarbe N, Wallois F, Ponchel E, et al. Coupled oxygenation oscillation measured by NIRS and intermittent cerebral activation on EEG in premature infants. Neuroimage. 2007;36:718–727. doi: 10.1016/j.neuroimage.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Meyer C, de Vries G, Davidge ST, et al. Reassessing the mathematical modeling of the contribution of vasomotion to vascular resistance. J Appl Physiol. 2002;92:888–889. doi: 10.1152/jappl.2002.92.2.888. [DOI] [PubMed] [Google Scholar]
- 54.Rucker M, Strobel O, Vollmar B, et al. Vasomotion in critically perfused muscle protects adjacent tissues from capillary perfusion failure. Am J Physiol Heart Circ Physiol. 2000;279:H550–H558. doi: 10.1152/ajpheart.2000.279.2.H550. [DOI] [PubMed] [Google Scholar]
- 55.Penaz J, Voigt A, Teichmann W. Contribution to continuous indirect blood pressure measurement. Z Gesamte Inn Med. 1976;31:1030–1033. (in German) [PubMed] [Google Scholar]
- 56.Toole J. Cerebrovascular Disorders. New York: Raven Press; 1984. pp. 1–18. [Google Scholar]
- 57.Olesen J. The effect of intracarotid epinephrine, norepinephrine, and angiotensin on regional cerebral blood flow in man. Neurology. 1972;22:978–987. doi: 10.1212/wnl.22.9.978. [DOI] [PubMed] [Google Scholar]
- 58.Newell DW, Aaslid R, Lam A, et al. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke. 1994;25:793–797. doi: 10.1161/01.str.25.4.793. [DOI] [PubMed] [Google Scholar]
- 59.Boas DA, Gaudette T, Strangman G, et al. The accuracy of near-infrared spectroscopy and imaging during focal changes in cerebral hemodynamics. Neuroimage. 2001;13:76–90. doi: 10.1006/nimg.2000.0674. [DOI] [PubMed] [Google Scholar]
- 60.Welch PD. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroacoust. 1967;AU15:70–73. [Google Scholar]
- 61.Diehl RR, Linden D, Lucke D, et al. Phase relationship between cerebral blood flow velocity and blood pressure: A clinical test of autoregulation. Stroke. 1995;26:1801–1804. doi: 10.1161/01.str.26.10.1801. [DOI] [PubMed] [Google Scholar]
- 62.Reinhard M, Muller T, Guschlbauer B, et al. Transfer function analysis for clinical evaluation of dynamic cerebral autoregulation: A comparison between spontaneous and respiratory-induced oscillations. Physiol Meas. 2003;24:27–43. doi: 10.1088/0967-3334/24/1/303. [DOI] [PubMed] [Google Scholar]
- 63.Zhang R, Zuckerman JH, Levine BD. Spontaneous fluctuations in cerebral blood flow: Insights from extended-duration recordings in humans. Am J Physiol Heart Circ Physiol. 2000;278:H1848–H1855. doi: 10.1152/ajpheart.2000.278.6.H1848. [DOI] [PubMed] [Google Scholar]
- 64.Panerai RB. Cerebral autoregulation: From models to clinical applications. Cardiovasc Eng. 2008;8:42–59. doi: 10.1007/s10558-007-9044-6. [DOI] [PubMed] [Google Scholar]
- 65.Czosnyka M, Smielewski P, Kirkpatrick P, et al. Monitoring of cerebral autoregulation in head-injured patients. Stroke. 1996;27:1829–1834. doi: 10.1161/01.str.27.10.1829. [DOI] [PubMed] [Google Scholar]
- 66.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- 67.Lang EW, Lagopoulos J, Griffith J, et al. Noninvasive cerebrovascular autoregulation assessment in traumatic brain injury: Validation and utility. J Neurotrauma. 2003;20:69–75. doi: 10.1089/08977150360517191. [DOI] [PubMed] [Google Scholar]
- 68.Tiecks FP, Lam AM, Aaslid R, et al. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995;26:1014–1019. doi: 10.1161/01.str.26.6.1014. [DOI] [PubMed] [Google Scholar]
- 69.Panerai RB, White RP, Markus HS, et al. Grading of cerebral dynamic autoregulation from spontaneous fluctuations in arterial blood pressure. Stroke. 1998;29:2341–2346. doi: 10.1161/01.str.29.11.2341. [DOI] [PubMed] [Google Scholar]
- 70.Gong XP, Li Y, Jiang WJ, et al. Impaired dynamic cerebral autoregulation in middle cerebral artery stenosis. Neurol Res. 2006;28:76–81. doi: 10.1179/016164106X91915. [DOI] [PubMed] [Google Scholar]
- 71.Czosnyka M, Smielewski P, Czosnyka Z, et al. Continuous assessment of cerebral autoregulation: Clinical and laboratory experience. Acta Neurochir Suppl. 2003;86:581–585. doi: 10.1007/978-3-7091-0651-8_118. [DOI] [PubMed] [Google Scholar]
- 72.Haubrich C, Klemm A, Diehl RR, et al. M-wave analysis and passive tilt in patients with different degrees of carotid artery disease. Acta Neurol Scand. 2004;109:210–216. doi: 10.1034/j.1600-0404.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 73.Gooskens I, Schmidt EA, Czosnyka M, et al. Pressure-autoregulation, CO2 reactivity and asymmetry of haemodynamic parameters in patients with carotid artery stenotic disease: A clinical appraisal. Acta Neurochir (Wien) 2003;145:527–532. doi: 10.1007/s00701-003-0045-y. [DOI] [PubMed] [Google Scholar]
- 74.Bishop CC, Powell S, Insall M, et al. Effect of internal carotid artery occlusion on middle cerebral artery blood flow at rest and in response to hypercapnia. Lancet. 1986;1:710–712. doi: 10.1016/s0140-6736(86)91102-5. [DOI] [PubMed] [Google Scholar]
- 75.Olsen TS, Larsen B, Herning M, et al. Blood flow and vascular reactivity in collaterally perfused brain tissue: Evidence of an ischemic penumbra in patients with acute stroke. Stroke. 1983;14:332–341. doi: 10.1161/01.str.14.3.332. [DOI] [PubMed] [Google Scholar]
- 76.Dawson SL, Panerai RB, Potter JF. Serial changes in static and dynamic cerebral autoregulation after acute ischaemic stroke. Cerebrovasc Dis. 2003;16:69–75. doi: 10.1159/000070118. [DOI] [PubMed] [Google Scholar]
- 77.Panerai RB. Assessment of cerebral pressure autoregulation in humans: A review of measurement methods. Physiol Meas. 1998;19:305–338. doi: 10.1088/0967-3334/19/3/001. [DOI] [PubMed] [Google Scholar]
- 78.Iannotti F, Hoff J. Ischemic brain edema with and without reperfusion: An experimental study in gerbils. Stroke. 1983;14:562–567. doi: 10.1161/01.str.14.4.562. [DOI] [PubMed] [Google Scholar]
- 79.Mankovsky BN, Piolot R, Mankovsky OL, et al. Impairment of cerebral autoregulation in diabetic patients with cardiovascular autonomic neuropathy and orthostatic hypotension. Diabetes Med. 2003;20:119–126. doi: 10.1046/j.1464-5491.2003.00885.x. [DOI] [PubMed] [Google Scholar]
- 80.Mautner-Huppert D, Haberl RL, Dirnagl U, et al. B-waves in healthy persons. Neurol Res. 1989;11:194–196. doi: 10.1080/01616412.1989.11739891. [DOI] [PubMed] [Google Scholar]
- 81.Obrig H, Neufang M, Wenzel R, et al. Spontaneous low-frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage. 2000;12:623–639. doi: 10.1006/nimg.2000.0657. [DOI] [PubMed] [Google Scholar]
- 82.Tachtsidis I, Elwell CE, Lee CW, et al. Spectral characteristics of spontaneous oscillations in cerebral haemodynamics are posture-dependent. Adv Exp Med Biol. 2003;540:31–36. doi: 10.1007/978-1-4757-6125-2_6. [DOI] [PubMed] [Google Scholar]
- 83.Schroeter ML, Schmiedel O, von Cramon DY. Spontaneous low-frequency oscillations decline in the aging brain. J Cereb Blood Flow Metab. 2004;24:1183–1191. doi: 10.1097/01.WCB.0000135231.90164.40. [DOI] [PubMed] [Google Scholar]
- 84.Schroeter ML, Bucheler MM, Preul C, et al. Spontaneous slow hemodynamic oscillations are impaired in cerebral microangiopathy. J Cereb Blood Flow Metab. 2005;25:1675–1684. doi: 10.1038/sj.jcbfm.9600159. [DOI] [PubMed] [Google Scholar]
- 85.Elwell CE, Springett R, Hillman E, et al. Oscillations in cerebral haemodynamics: Implications for functional activation studies. Adv Exp Med Biol. 1999;471:57–65. [PubMed] [Google Scholar]
- 86.Franceschini MA, Joseph DK, Huppert TJ, et al. Diffuse optical imaging of the whole head. J Biomed Opt. 2006;11:054007. doi: 10.1117/1.2363365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vetri F, Menicucci D, Lapi D, et al. Pial arteriolar vasomotion changes during cortical activation in rats. Neuroimage. 2007;38:25–33. doi: 10.1016/j.neuroimage.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 88.Hillman EM, Devor A, Bouchard MB, et al. Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. Neuroimage. 2007;35:89–104. doi: 10.1016/j.neuroimage.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Devor A, Tian P, Nishimura N, et al. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level–dependent signal. J Neurosci. 2007;27:4452–4459. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


