Abstract
A diversity-oriented synthesis (DOS) strategy was developed for the synthesis of stereochemically diverse fused-ring systems containing a pyran moiety. Each scaffold contains an amine and methyl ester for future diversification via amine capping and amide coupling. Scaffold diversity was evaluated in comparison to previously prepared scaffolds via a shape-based principal moments of inertia (PMI) analysis.
INTRODUCTION
As part of ongoing efforts to produce a stereochemically and skeletally diverse collection of small molecules, we sought to develop methods for the synthesis of a set of pyran-containing fused ring systems.1 Pyrans are a common subunit in numerous natural products and biologically relevant small molecules.2 To access pyran-containing fused ring systems, we envisioned utilizing a 2,3-unsaturated C-glycoside scaffold (1, Figure 1) previously reported by our group.3 Having access to all eight stereoisomers of C-glycoside 1 and the corresponding allylic amine 2, we aimed to develop synthetic pathways that would yield fused bi- and tricyclic ring systems. This paper descibes the synthesis of tricyclic compounds 3 and 4 via radical cyclization and nucleophilic aromatic substitution (SNAr) reactions, and bicyclic compounds 5 and 6 through intramolecular Mitsunobu and epoxide ring opening reactions. All skeletons resulting from these pathways retain functional handles that can be utilized for solid-phase library synthesis and future analog development.
Figure 1.
Synthesis of fused bi-and tricyclic ring systems from a C-glycoside template.
RESULTS AND DISCUSSION
The benzofuran motif is present in a wide range of natural products.4 We aimed to access a [6,5,6] benzofuran scaffold (3) starting from C-glycoside 1 via a 5-exo-trig radical cyclization.5 This type of radical cyclization has been successfully employed in the construction of current drugs and numerous natural products including pregabalin 6 and morphine.7 We anticipated that the radical cyclization step would occur by a regio- and stereoselective mode of addition onto the alkene, providing a cis relationship between C-4 and the newly formed stereogenic center at C-3.
The synthesis of benzofuran scaffold 3 began with an intermolecular SNAr reaction between C-glycoside 1 and commercially available 2-bromo-1-fluoro-4-nitrobenzene 7 (Scheme 1). This reaction proceeded in the presence of sodium hydride in DMF with varying degrees of success (52-78% yield) across the diastereomers to afford 8a-d. Selective reduction of the aryl nitro group using Zn metal8 afforded the desired aniline 9a-d in good yield. Initial attempts to affect the 5-exo-trig radical cyclization of 9a by treatment with excess amounts of n-Bu3SnH and catalytic AIBN in refluxing benzene were successful, affording the desired benzofuran 10a in 53% yield. Concerns about toxicity and contamination by organo-tin reagents led us to explore the possibility of utilizing a catalytic amount of tin for the radical cyclization.9 Using catalytic amounts of n-Bu3SnCl and AIBN in the presence of NaBH3CN in i-PrOH 10 resulted in the formation of the cyclized product with similar efficiency as when using excess n-Bu3SnH. Notably, we were able to perform this reaction on multi-gram scale yielding >20 grams of product. Finally, removal of the TBDPS group using HF-pyridine followed by Fmoc protection of the aniline yielded the desired benzofuran scaffolds 3a-d in high yield.11
Scheme 1.
Benzofuran formation via a 5-exo-trig cyclization.
The intramolecular SNAr reaction has been widely used in the context of diversity-oriented synthesis (DOS)12 and small molecule synthesis in general.13 We envisioned employing a SNAr cyclization to produce a [6,8,6] tricyclic scaffold containing an 8-membered lactam starting from allylic amine 2. Thus, amine 2 was first acylated with 2-fluoro-5-nitrobenzoyl chloride 11 to afford amide 12 (Scheme 2). Initially we attempted the SNAr cyclization of 12 with a “one pot” TBDPS deprotection/cyclization sequence using either TBAF or CsF, however, only dimerization and decomposition was observed. We hypothesized that the success of the intramolecular cyclization may be affected by the conformation of the amide bond. Based on observations by Smith and coworkers,14 we decided to investigate the impact of an N-alkylated amide bond on intramolecular ring cyclization. Thus, amides 12a-d were treated with methyl iodide in the presence of sodium hydride in DMF to afford N-methyl amide 13a-d. Subsequent conversion of the alkylated amides to the cyclized products was successful, however, the SNAr reaction was found to have moderate stereochemical dependency. On treatment with cesium fluoride in DMF at 35 °C, TBDPS ethers 13c-d underwent a smooth deprotection/cyclization sequence to produce lactams 14c-d. Meanwhile, amides 13a-b required a two-step sequence involving TBDPS removal with HF/Pyridine followed by treatment with cesium fluoride to promote the SNAr-mediated ring closure.1a Finally, hydrogenation of the cyclized products 14a-d15 reduced both the double bond and aryl nitro group which afforded the desired tricyclic scaffolds 4a-d.
Scheme 2.
Tricyclic lactam formation via intramolecular SNAr.
We next investigated the possibility of azetidine ring formation16 to yield [6,4] ring system 5 through an intramolecular Mitsunobu reaction. Starting from amines 2a-b, the Mitsunobu precursor 16a-b was obtained in three steps including hydrogenation, N-nosylation and TBDPS deprotection (Scheme 3). This material was then treated with PPh3 and DIAD leading to the formation of the desired bicyclic azetidine 5a-b in high yield. The product was easily isolated from the Mitsunobu byproducts and the reaction could be carried out on multigram scale.
Scheme 3.

Azetidine formation via intramolecular Mitsunobu.
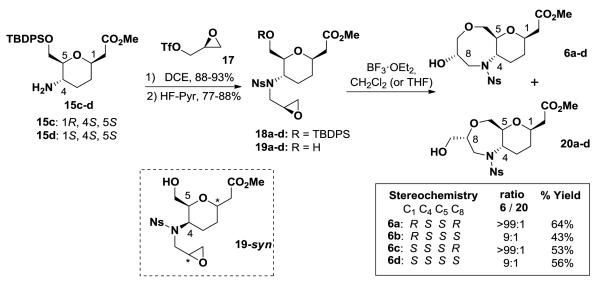
Finally, a number of interesting oxazapane and/or oxazacane ring systems were envisioned to be readily accessed through the use of chiral intermediate 15 (Scheme 4). We chose to explore the use of an epoxide-opening/ring closing reaction which would allow for the formation of a single diastereomeric product upon cyclization. A number of examples utilizing chiral epoxide as synthons for the assembly of complex small molecules, including both natural products17 and library scaffolds,18 have been well documented. Execution of this approach first required the incorporation of an epoxide into the C-glycoside template.
Scheme 4.
Oxazacane formation via epoxide opening/ring-closing reaction.
Initially, alkylation of sulfonamide 15a-d with epichlorohydrin proved difficult when using the all syn stereoisomer, presumably due to steric hindrance. Ultimately, this was overcome by using the triflate derived from (R)-or (S)-glycidol (17)19 (Scheme 4). Liberation of the primary alcohol by TBDPS deprotection gave monocyclic compound 19, the precursor to the epoxide-opening/ring closing reaction. Previous reports of epoxide-opening/ring closing reactions15c have mainly focused on the formation of smaller ring systems with varying degrees of endo/exo selectivity. Although the 7-exo-tet cyclization20 is favored based on Baldwin’s rules, there is precedent for the 8-endo-tet cyclization to occur under basic conditions.21 After running a series of trial experiments, we found that the endo/exo selectivity of the Lewis-acid mediated epoxide opening/ring closing reaction had a strong dependence on the stereochemical relationship between C-4 and C-5. With the syn configuration (19-syn), mixtures of both the 7-(20) and 8-membered ring (6) systems were observed with selectivity ranging from 1:1 to 9:1 favoring the oxazapane depending on the stereoisomer used. Interestingly, with the anti-configuration (19a-d), the cyclization occurs smoothly via 8-endo-tet in the presence of BF3·OEt2 in either CH2Cl2 or THF to give primarily the endo product, 8-membered oxazocane 6a-d.22 The stereochemistry at both C-1 of the pyran and of the epoxide has little impact on the regioselectivity of the reaction. The epoxide-opening/ring closing reaction was run on a multigram scale with 19a-d, obtaining acceptable yields of 6a-d while not effecting selectivity.
In order to visualize the chemical space represented by the pyran-containing fused-ring systems as compared to our previously reported aldol-1a-c and azetidine-based1e pathways we undertook a principal moments of inertia (PMI) analysis (Figure 2).23 Through this shape-based analysis, we are able to visualize the differences between the three collections and observed that the fused ring systems access different chemical space than occupied by the previously described scaffolds, especially compared to the aldol-based pathways, which included a variety of macrocycles.
Figure 2.
PMI analysis of pyran-containing scaffolds, along with previously reported scaffolds from aldol-and azetidine-based pathways.
CONCLUSIONS
In conclusion, we have reported the synthesis of a diverse set of fused-ring systems containing a pyran moiety. Utilizing all stereoisomers of a common C-glycoside intermediate, we are able to access all possible stereoisomers of each scaffold efficiently on multi-gram scale. Elaboration of these scaffolds to libraries suitable for high-throughput screening has been completed and will be the basis of future publications.
EXPERIMENTAL SECTION
General Methods
All oxygen and/or moisture-sensitive reactions were carried out under N2 atmosphere in glassware that had been flame-dried under vacuum (~0.5 mmHg) and purged with N2 prior to use. All reagents and solvents were purchased from commercial vendors and used as received or synthesized according to the footnoted references. 1H and 13C NMR spectra were recorded on 300 MHz and/or 500 MHz spectrometers. All chemical shifts are reported in parts per million (δ) referenced to residual nondeuterated solvent. Data are reported as follows: chemical shifts, multiplicity (br = broad, s = singlet, d = doublet, t = triplet, q = quartet, p = pentet, m = multiplet; coupling constant(s) in Hz; integration). Unless otherwise indicated, NMR data were collected at 25 °C. IR spectra were obtained with an FTIR spectrometer and are reported in cm−1. Flash chromatography was performed using 40–60 μm silica gel (60 Å mesh) with the indicated solvent. Analytical thin layer chromatography (TLC) was performed on 0.25 mm silica gel 60-F plates. Visualization was accomplished with UV light and aqueous potassium permanganate or ceric ammonium molybdate stain followed by heating. High-resolution mass spectra were obtained using a LCMS coupled with a quadrupole.
Methyl 2-((2S,5S,6R)-5-(2-bromo-4-nitrophenoxy)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5,6-dihydro-2H-pyran-2-yl)acetate 8a
To a solution of 1a (25.0 g, 57.2 mmol, 1.0 equiv) in DMF (570 ml) was added 2-bromo-1-fluoro-4-nitrobenzene 7 (12.6 g, 57.2 mmol, 1.0 equiv). The reaction mixture was cooled to 0 °C and sodium hydride (2.5 g, 62.9 mmol, 1.3 equiv) was added portion-wise over a period of 10 min. The reaction mixture was slowly warmed to rt and allowed to stir for 4 h. The reaction was then quenched with a saturated solution of aqueous ammonium chloride. DMF was removed in vacuo and the aqueous layer was extracted with EtOAc (3 × 200 mL). The combined organic layers were washed with brine, dried over MgSO4 and filtered. The organic layer was concentrated under reduced pressure and the crude residue was purified by chromatography on silica gel (gradient: 0% to 15% EtOAc in hexanes), which provided 28.6 g (78%) of 8a as a yellow oil. [α]D20 +80.2 (c 1.1, CHCl3). IR νmax (cm−1, film): 2930, 2856, 1740, 1582, 1519, 1478, 1343, 1272, 1113. 1H NMR (300 MHz, CDCl3) δ 8.50 (d, J = 2.7 Hz, 1H), 8.16 (dd, J = 9.1, 2.7 Hz, 1H), 7.76 – 7.63 (m, 2H), 7.64 – 7.52 (m, 2H), 7.50 – 7.23 (m, 6H), 7.14 (d, J = 9.2 Hz, 1H), 6.05 – 5.97 (m, 2H), 5.31 (d, J = 8.3 Hz, 1H), 4.76 (t, J = 6.1 Hz, 1H), 4.05 – 3.92 (m, 2H), 3.86 (d, J = 8.3 Hz, 1H), 3.74 (s, 3H), 2.74 – 2.59 (m, 2H), 1.05 (s, 9H). 13C NMR (125 MHz, CDCl3) δ 170.9, 159.7, 141.9, 135.5, 133.2, 133.8, 127.8, 124.9, 124.7, 113.3, 113.0, 77.6, 72.0, 70.7, 62.9, 51.9, 40.2, 26.0, 19.4. HRMS (ESI+) calcd for C31H34BrNNaO7Si [M+Na]+ : 662.1186. Found: 662.1181.
Methyl 2-((2R,5S,6R)-5-(2-bromo-4-nitrophenoxy)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5,6-dihydro-2H-pyran-2-yl)acetate 8b
Following the above protocol, 1b (29.5 g, 67 mmol, 1.0 equiv) was treated with 2-bromo-1-fluoro-4-nitrobenzene 7 (14.7 g, 67 mmol, 1.0 equiv) and sodium hydride (3.5 g, 87 mmol, 1.3 equiv) in DMF (890 mL). The reaction provided, after purification, 24.0 g (56%) of 8b as a yellow oil. [α]D20 +49.7 (c 1.0, CHCl3). IR νmax (cm−1, film): 2930, 2856, 1740, 1582, 1519, 1478, 1343, 1272, 1113. 1H NMR (300 MHz, CDCl3) δ 8.45 (d, J = 2.7 Hz, 1H), 8.09 (dd, J = 9.1, 2.7 Hz, 1H), 7.64 (d, J = 7.8 Hz, 2H), 7.55 (d, J = 7.7 Hz, 2H), 7.43 – 7.26 (m, 6H), 7.06 (d, J = 9.1 Hz, 1H), 5.99 (q, J = 10.4 Hz, 2H), 5.10 (d, J = 5.7 Hz, 1H), 4.79 (t, J = 6.0 Hz, 1H), 3.92 (dd, J = 7.1, 10.3 Hz, 3H), 3.72 (s, 3H), 2.78 (dd, J = 15.3, 8.6 Hz, 1H), 2.57 (dd, J = 15.3, 5.6 Hz, 1H), 1.15 – 0.82 (m, 9H). 13C NMR (75 MHz, CDCl3) δ 170.9, 159.4, 141.9, 135.7, 135.6, 133.2, 133.0, 132.9, 129.9, 129.6, 127.9, 124.7, 123.9, 113.1, 72.2, 70.2, 69.5, 52.1, 38.5, 26.9, 19.4. HRMS (ESI+) calcd for C31H34BrNNaO7Si [M+Na]+ : 662.1186. Found: 662.1180.
Methyl 2-((2S,5R,6R)-5-(2-bromo-4-nitrophenoxy)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5,6-dihydro-2H-pyran-2-yl)acetate 8c
Following the above protocol, 1c (10.0 g, 22.7 mmol, 1.0 equiv) was treated with 2-bromo-1-fluoro-4-nitrobenzene 7 (5.0 g, 22.7 mmol, 1.0 equiv) and sodium hydride (1.3 g, 31.8 mmol, 1.3 equiv) in DMF (280 mL). The reaction provided, after purification, 9.0 g (61%) of 8c as a yellow oil. [α]D20 −137.0 (c 0.9, CHCl3). IR νmax (cm−1, film): 2930, 2856, 1740, 1582, 1519, 1478, 1343, 1272, 1113. 1H NMR (300 MHz, CDCl3) δ 8.48 (d, J = 2.6 Hz, 1H), 8.18 (d, J = 9.1 Hz, 1H), 7.57 (dd, J = 18.8, 7.7 Hz, 4H), 7.47 – 7.26 (m, 6H), 7.06 (d, J = 9.1 Hz, 1H), 6.52 – 6.33 (m, 1H), 6.18 (d, J = 10.2 Hz, 1H), 4.90 (d, J = 4.5 Hz, 1H), 4.65 (t, J = 6.5 Hz, 1H), 4.13 – 4.03 (m, 1H), 4.02 – 3.89 (m, 2H), 3.70 (s, 3H), 2.71 (dd, J = 15.9, 7.2 Hz, 1H), 2.57 (dd, J = 16.0, 6.5 Hz, 1H), 0.95 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 170.9, 159.8, 141.4, 136.7, 135.4, 133.2, 133.1, 129.7, 129.4, 127.7, 127.6, 125.1, 124.5, 121.6, 116.0, 113.0, 112.4, 71.6, 68.8, 62.3, 51.8, 39.6, 26.7, 19.1. HRMS (ESI+) calcd for C31H34BrNNaO7Si [M+Na]+ : 662.1186. Found: 662.1194.
Methyl 2-((2R,5R,6R)-5-(2-bromo-4-nitrophenoxy)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5,6-dihydro-2H-pyran-2-yl)acetate 8d
Following the above protocol, 1d (15.0 g, 34.0 mmol, 1.0 equiv) was treated with 2-bromo-1-fluoro-4-nitrobenzene 7 (7.49 g, 34.0 mmol, 1.0 equiv) and sodium hydride (1.12 g, 28.0 mmol, 1.3 equiv) in DMF (400 mL). The reaction provided, after purification, 11.4 g (52%) of 8d as a yellow oil. [α]D20 −116.2 (c 1.1, CHCl3). IR νmax (cm−1, film): 2930, 2856, 1740, 1582, 1519, 1478, 1343, 1272, 1113. 1H NMR (300 MHz, CDCl3) δ 8.36 (d, J = 2.7 Hz, 1H), 8.08 (dd, J = 9.1, 2.7 Hz, 1H), 7.48 (dd, J = 13.7, 7.9 Hz, 4H), 7.50 – 7.20 (m, 6H), 6.94 (d, J = 9.1 Hz, 1H), 6.35 – 6.09 (m, 2H), 4.77 (s, 2H), 3.98 (d, J = 4.6 Hz, 2H), 3.81 (d, J = 9.7 Hz, 1H), 3.59 (s, 3H), 2.64 (dd, J = 15.1, 8.9 Hz, 1H), 2.45 (dd, J = 15.1, 5.5 Hz, 1H), 0.86 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 170.9, 159.8, 141.7, 135.7, 135.6, 135.6, 133.4, 133.2, 130.0, 129.6, 128.7, 127.9 (2), 124.7, 121.6, 113.2, 112.6, 71.5, 69.6, 68.8, 62.1, 52.1, 37.5, 26.9, 19.3. HRMS (ESI+) calcd for C31H34BrNNaO7Si [M+Na]+: 662.1186. Found: 662.1195.
Methyl 2-((2S,5S,6R)-5-(4-amino-2-bromophenoxy)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5,6-dihydro-2H-pyran-2-yl)acetate 9a
To a solution of 8a (27.3 g, 42.6 mmol) in THF (426 mL) and acetic acid (426 mL) was added zinc powder (41.8 g, 639 mmol, 15 equiv) in small portions at rt. The reaction mixture was stirred at rt for 4 h. The reaction mixture was diluted with CH2Cl2, filtered over Celite and washed with CH2Cl2. After filtration, the solvent was removed in vacuo. The organic residue was dissolved in CH2Cl2 (300 mL) and then washed with water (2 × 200 mL), brine (100 mL) and dried over Na2SO4. After filtration, excess solvent was removed in vacuo to afford a crude residue, which was purified by chromatography on silica gel (gradient: 0% to 60% EtOAc in hexanes) to provide 19.5 g (73%) of 9a as a white foamy solid. [α]D20 +59.3 (c 1.3, CHCl3). IR νmax (cm−1, film): 2929, 2856, 1735, 1492, 1427, 1224, 1112. 1H NMR (300 MHz, CDCl3) δ 7.70 (d, J = 7.8 Hz, 2H), 7.60 (d, J = 7.8 Hz, 2H), 7.44 – 7.26 (m, 6H), 6.90 – 6.79 (m, 2H), 6.53 (dd, J = 8.7, 2.7 Hz, 1H), 6.01 (d, J = 10.3 Hz, 1H), 5.85 (d, J = 10.3 Hz, 1H), 4.92 (d, J = 7.5 Hz, 1H), 4.67 (br s, 1H), 3.97 (s, 2H), 3.76 (d, J = 8.5 Hz, 1H), 3.69 (s, 3H), 3.50 – 3.13 (m, 2H), 2.71 – 2.40 (m, 2H), 0.99 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 171.2, 147.6, 142.0, 136.0, 135.7, 134.0, 133.6, 131.0, 129.7, 129.6, 127.7 (2), 127.1, 120.1, 118.0, 115.3, 114.3, 78.0, 71.8, 71.5, 63.4, 51.9, 40.5, 26.9, 19.5. HRMS (ESI+) calcd for C31H36BrNNaO5Si [M+Na]+ : 632.1444. Found: 632.1447.
Methyl 2-((2R,5S,6R)-5-(4-amino-2-bromophenoxy)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5,6-dihydro-2H-pyran-2-yl)acetate 9b
Following the above protocol, 8b (24.0 g, 37.5 mmol, 1 equiv) was treated with zinc powder (36.8 g, 562 mmol, 15 equiv) in THF (375 mL) and acetic acid (375 mL). The reaction provided, after purification, 14.3 g (63%) of 9b as a white foamy solid. [α]D20 +38.6 (c 1.1, CHCl3). IR νmax (cm−1, film): 2929, 2856, 1735, 1492, 1427, 1224, 1112. 1H NMR (300 MHz, CDCl3) δ 7.64 (dd, J = 6.1, 2.0 Hz, 4H), 7.47 – 7.26 (m, 6H), 6.87 (dd, J = 15.2, 5.7 Hz, 2H), 6.52 (dd, J = 8.7, 2.7 Hz, 1H), 6.01 (d, J = 10.4 Hz, 1H), 5.88 (d, J = 10.3 Hz, 1H), 4.75 (br s, 2H), 3.91 (br s, 3H), 3.67 (s, 3H), 3.51 (br s, 1H), 2.78 (dd, J = 15.3, 8.8 Hz, 1H), 2.54 (dd, J = 15.3, 5.4 Hz, 1H), 0.99 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 171.4, 147.3, 142.1, 135.9, 135.8, 133.7, 133.5, 131.1, 129.7, 127.8, 126.1, 120.1, 118.3, 115.2, 114.6, 72.8, 71.2, 69.4, 63.3, 51.9, 38.6, 26.9, 19.4. HRMS (ESI+) calcd for C31H36BrNNaO5Si [M+Na]+ : 632.1444. Found: 632.1439.
Methyl 2-((2S,5R,6R)-5-(4-amino-2-bromophenoxy)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5,6-dihydro-2H-pyran-2-yl)acetate 9c
Following the general reaction protocol, 8c (30.0 g, 46.8 mmol, 1 equiv) was treated with zinc powder (45.9 g, 702 mmol, 15 equiv) in THF (468 mL) and acetic acid (468 mL). The reaction provided, after purification, 21.0 g (73%) of 9c as a white foamy solid. [α]D20 −137.0 (c 1.0, CHCl3). IR νmax (cm−1, film): 2929, 2856, 1735, 1492, 1427, 1224, 1112. 1H NMR (300 MHz, CDCl3) δ 7.73 – 7.57 (m, 4H), 7.45 – 7.22 (m, 6H), 6.83 (dd, J = 8.5, 5.7 Hz, 2H), 6.50 (dd, J = 8.6, 2.7 Hz, 1H), 6.00 (dd, J = 16.0, 6.8 Hz, 2H), 4.62 – 4.43 (m, 2H), 4.14 (dd, J = 10.2, 6.8 Hz, 1H), 3.95 (dd, J = 10.2, 6.2 Hz, 1H), 3.84 (d, J = 4.8 Hz, 1H), 3.66 (s, 3H), 3.47 (s, 2H), 2.69 (dd, J = 15.7, 7.3 Hz, 1H), 2.52 (dd, J = 15.7, 6.5 Hz, 1H), 1.03 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 171.3, 147.3, 142.1, 135.8, 134.7, 133.8, 129.7, 127.8, 124.2, 120.0, 119.3, 115.3, 115.0, 78.0, 71.8, 69.7, 63.4, 51.9, 39.9, 27.0, 19.4. HRMS (ESI+) calcd for C31H36BrNNaO5Si [M+Na]+: 632.1444. Found: 632.1441.
Methyl 2-((2R,5R,6R)-5-(4-amino-2-bromophenoxy)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5,6-dihydro-2H-pyran-2-yl)acetate 9d
Following the above protocol, 8d (15.0 g, 23.4 mmol, 1 equiv) was treated with zinc powder (23.0 g, 351 mmol, 15 equiv) in THF (234 mL) and acetic acid (234 mL). The reaction provided, after purification, 10.9 g (76%) of 9d as a white foamy solid. [α]D20 −23.9 (c, 1.1 CHCl3). IR νmax (cm−1, film): 2929, 2856, 1735, 1492, 1427, 1224, 1112. 1H NMR (300 MHz, CDCl3) δ 7.72 – 7.53 (m, 4H), 7.46 – 7.23 (m, 6H), 6.91 – 6.69 (m, 2H), 6.62 – 6.42 (m, 1H), 6.12 – 5.88 (m, 2H), 4.90 – 4.68 (m, 1H), 4.56 – 4.45 (m, 1H), 4.12 (dt, J = 18.7, 7.0 Hz, 2H), 4.03 – 3.85 (m, 3H), 3.63 (s, 3H), 2.66 (dd, J = 15.0, 8.6 Hz, 1H), 2.47 (dd, J = 15.0, 5.6 Hz, 1H), 1.10 – 0.86 (m, 9H). 13C NMR (75 MHz, CDCl3) δ 171.2, 147.4, 142.1, 135.8, 133.8 (2), 133.3, 129.8, 127.8 (2), 124.2, 120.1, 118.8, 115.1, 72.8, 69.9, 69.2, 62.5, 60.6, 52.0, 38.0, 27.0, 19.3. HRMS (ESI+) calcd for C31H36BrNNaO5Si [M+Na]+ : 632.1444. Found: 632.1441.
Methyl 2-((1R,3R,4aR,9aS)-6-amino-1-(((tert-butyldiphenylsilyl)oxy)methyl)-3,4,4a,9a-tetrahydro-1H-pyrano[3,4-b]benzofuran-3-yl)acetate 10a
To a solution of 9a (32.6 g, 53.4 mmol) in i-PrOH (530 mL) in a jacketed, 3-necked round bottom flask (equipped with a reflux condenser and recirculating chiller) was added AIBN (1.7 g, 10.7 mmol) and tributyltin chloride (2.2 mL, 8.0 mmol). The reaction mixture was carefully degassed with Ar for 10 min prior to heating. The reaction mixture was then heated at 85 °C and was allowed to stir for 5 min. Simultaneously, a solution of NaBH3CN (5.0 g, 80.0 mmol) in i-PrOH (120 mL) and a solution of AIBN (1.7g, 10.7 mmol) in benzene (120 mL) were added slowly over 2 h. After 2 h, the reaction was cooled to rt. i-PrOH was removed in vacuo and the residue was co-evaporated with benzene (3 × 20 mL). The crude purple solid was diluted in EtOAc (200 mL) and extracted with a saturated solution of aqueous ammonium chloride (2 × 100 mL). The organic layer was washed with brine, dried with MgSO4, filtered and concentrated. The resulting residue was diluted with MeCN (100 mL) and washed with hexanes (2 × 60 mL). The MeCN phase was then dried in vacuo to afford a pink residue, which was purified by chromatography on silica gel (gradient: 0% to 70% EtOAc in hexanes), which provided 28.5 g (57%) of 10a as a white/pink foamy solid. [α]D20 +36.4 (c 1.1, CHCl3). IR νmax (cm−1, film): 2930, 2856, 1736, 1488, 1428, 1217, 1112. 1H NMR (300 MHz, CDCl3) δ 7.63 – 7.60 (m, 4H), 7.29 – 7.27 (m, 6H), 6.50 – 6.37 (m, 3H), 4.46 (t, J = 8.7 Hz, 1H), 3.80 – 3.77 (m, 3H), 3.69 (m, 1H), 3.64 (s, 3H), 3.40 (m, 3H), 2.59 (dd, J = 15.4, 7.8 Hz, 1H), 2.45 (dd, J = 15.5, 5.2 Hz, 1H), 2.21 (d, J = 13.9 Hz, 1H), 1.78 (m, 1H), 0.95 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 171.5, 152.5, 140.8, 135.8 (2), 133.9, 133.7, 129.59, 129.54, 129.5, 127.6, 115.0, 111.3, 110.6, 77.6, 77.0, 70.0, 64.9, 60.5, 51.8, 40.8, 39.5, 30.3, 26.9, 19.4. HRMS (ESI+) calcd for C31H38NO5Si [M+H]+ : 532.2519. Found: 532.2516.
Methyl 2-((1R,3S,4aR,9aS)-6-amino-1-(((tert-butyldiphenylsilyl)oxy)methyl)-3,4,4a,9a-tetrahydro-1H-pyrano[3,4-b]benzofuran-3-yl)acetate 10b
Following the above protocol, 9b (15.0 g, 24.6 mmol, 1 equiv) was treated with AIBN (1.6 g, 8.9 mmol) and tributyltin chloride (1.0 mL, 3.6 mmol) followed by NaBH3CN (2.3 g, 36.8 mmol) in i-PrOH (250 mL). The reaction provided, after purification, 8.8 g (66%) of 10b as a white/pink foamy solid. [α]D20 +54.9 (c 1.1, CHCl3). IR νmax (cm−1, film): 2930, 2856, 1736, 1488, 1428, 1217, 1112. 1H NMR (300 MHz, CDCl3) δ 7.87 – 7.55 (m, 4H), 7.40 (m, 5H), 6.57 (dd, J = 15.0, 5.2 Hz, 2H), 6.46 (dd, J = 8.3, 2.4 Hz, 1H), 4.69 – 4.48 (m, 1H), 4.35 (td, J = 11.4, 5.0 Hz, 1H), 4.07 – 3.89 (m, 2H), 3.82 (dd, J = 10.9, 5.4 Hz, 1H), 3.63 (s, 3H), 3.49 – 3.29 (m, 2H), 2.61 (dd, J = 15.4, 7.5 Hz, 1H), 2.38 (dd, J = 15.4, 5.6 Hz, 1H), 2.00 (ddd, J = 13.7, 5.7, 3.7 Hz, 1H), 1.49 (m 1H), 1.05 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 171.6, 152.3, 140.4, 135.9, 135.8, 133.4, 133.4, 132.2, 129.8, 129.8, 127.9, 127.9, 115.2, 112.0, 110.2, 79.6, 77.5, 77.2, 77.0, 72.7, 68.2, 65.3, 51.8, 40.8, 38.9, 32.9, 27.0, 19.4. HRMS (ESI+) calcd for C31H37NNaO5Si [M+Na]+: 554.2339. Found: 554.2345.
Methyl 2-((1R,3R,4aS,9aR)-6-amino-1-(((tert-butyldiphenylsilyl)oxy)methyl)-3,4,4a,9a-tetrahydro-1H-pyrano[3,4-b]benzofuran-3-yl)acetate 10c
Following the above protocol, 9c (20.0 g, 32.8 mmol, 1 equiv) was treated with AIBN (2.1 g, 13.1 mmol) and tributyltin chloride (1.3 mL, 4.9 mmol) followed by NaBH3CN (3.1 g, 49.1 mmol) in i-PrOH (430 mL). The reaction provided, after purification, 13.8 g (79%) of 10c as a white/pink foamy solid. [α]D20 −53.0 (c 0.9, CHCl3). IR νmax (cm−1, film): 2930, 2856, 1736, 1488, 1428, 1217, 1112. 1H NMR (300 MHz, CDCl3) δ 7.71 (t, J = 8.0 Hz, 4H), 7.39 – 7.37 (m, 6H), 6.63 – 6.37 (m, 3H), 4.38 (d, J = 9.9 Hz, 1H), 4.03 (dt, J = 10.9, 5.6 Hz, 1H), 3.91 (m, 2H), 3.85 – 3.67 (m, 2H), 3.64 (s, 3H), 3.39 (s, 2H), 3.15 – 3.09 (m, 1H), 2.58 – 2.53 (dd, J = 14.9, 7.1 Hz, 1H), 2.30 – 2.27 (dd, J = 14.9, 5.6, 1H), 2.07 – 1.89 (m, 1H), 1.27 – 1.18 (m, 1H), 1.05 (s, 9H). 13C NMR (125 MHz, CDCl3) δ 171.7, 152.1, 140.2, 135.9, 135.8, 134.2, 133.8, 129.8, 129.7, 127.8, 115.0, 111.5, 110.8, 78.9, 78.0, 72.1, 63.9, 51.9, 40.9, 39.5, 35.4, 27.0, 19.4. HRMS (ESI+) calcd for C31H37NNaO5Si [M+Na]+ : 554.2339. Found: 554.2341.
Methyl 2-((1R,3S,4aS,9aR)-6-amino-1-(((tert-butyldiphenylsilyl)oxy)methyl)-3,4,4a,9a-tetrahydro-1H-pyrano[3,4-b]benzofuran-3-yl)acetate 10d
Following the above protocol, 9d (6.0 g, 9.8 mmol, 1 equiv) was treated with AIBN (0.6 g, 3.9 mmol) and tributyltin chloride (0.6 mL, 2.4 mmol) followed by NaBH3CN (0.9 g, 14.7 mmol) in i-PrOH (130 mL). The reaction provided, after purification, 3.2 g (61%) of 10d as a white/pink amorphous solid. [α]D20 −92.5 (c, 0.5 CHCl3). IR νmax (cm−1, film): 2930, 2856, 1736, 1488, 1428, 1217, 1112. 1H NMR (300 MHz, CDCl3) δ 7.76 – 7.71 (m, 4H), 7.43 – 7.41 (m, 6H), 6.61 – 6.50 (m, 3H), 6.46 (dd, J = 8.3, 1.7 Hz, 1H), 5.00 (d, J = 5.9 Hz, 1H), 4.10 – 4.04 (m, 1H), 3.90 – 3.80 (m, 2H), 3.77 – 3.70 (m, 2H), 3.61 (s, 3H), 3.50 (s, 2H), 2.57 – 2.49 (dd, J = 15.7, 7.3 Hz, 1H), 2.43 – 2.38 (dd, J = 15.7, 5.8 Hz, 1H), 2.10 – 1.95 (m, 1H), 1.90 – 1.80 (m, 1H), 1.09 (s, 9H). 13C NMR (125 MHz, CDCl3) δ 171.4, 153.3, 140.0, 135.9, 135.8, 133.8, 133.7, 130.1, 129.8, 129.8, 127.8, 127.8, 115.8, 112.0, 109.7, 79.7, 77.5, 77.2, 77.0, 71.1, 67.4, 63.3, 51.8, 40.9, 37.9, 30.4, 27.0, 19.4. HRMS (ESI+) calcd for C31H37NNaO5Si [M+Na]+ : 554.2339. Found: 554.2335.
Methyl 2-((1R,3R,4aR,9aS)-6-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-1-(hydroxymethyl)-3,4,4a,9a-tetrahydro-1H-pyrano[3,4-b]benzofuran-3-yl)acetate 3a
To a solution of 10a (6.70 g, 12.6 mmol, 1 equiv) in THF (130 mL) was added HF pyridine (70 wt%, 6.3 mL, 50 mmol, 4 equiv) at rt. The reaction was monitored by LC/MS until complete conversion of the starting material was observed. After stirring overnight, the reaction was quenched with TMSOMe (17.3 mL, 126 mmol) and excess of solvent was removed in vacuo to afford a crude oil, which was carried on to the next step without purification. To a solution of crude deprotected alcohol (3.70 g, 12.6 mmol) in dioxane (160 mL) was added a 10% aqueous NaHCO3 solution (100 mL) until pH 6~7 was reached. The reaction mixture was cooled to 0 °C and a solution of FmocCl (3.59 g, 13.9 mmol) in dioxane (20 mL) was added. The reaction was quenched with a saturated solution of aqueous ammonium chloride, and 1,4-dioxane was removed in vacuo. The aqueous layer was extracted with CH2Cl2 (3 × 100 mL) and the combined organic layers were then washed with brine, separated and dried over MgSO4. After filtration, the solvent was removed and a crude pink solid was obtained. The solid was triturated in cold CH2Cl2 then filtered and washed carefully with cold CH2Cl2 to give 4.7 g (72%) of the desired product 3a as a white-grey amorphous solid. [α]D20 +94.1 (c 0.8, CHCl3). IR νmax (cm−1, film): 2950, 1723, 1615, 1548, 1490, 1449, 1439, 1219, 1150, 1054. 1H NMR (500 MHz, DMSO-d6, 100 °C) δ 9.10 (s, 1H), 7.88 (d, J = 7.5 Hz, 2H), 7.73 (d, J = 6.6 Hz, 2H), 7.43 (t, J = 7.3 Hz, 2H), 7.40 – 7.31 (m, 3H), 7.16 (d, J = 8.3 Hz, 1H), 6.70 (d, J = 8.5 Hz, 1H), 4.61 (t, J = 8.7 Hz, 1H), 4.54 – 4.46 (m, 2H), 4.31 – 4.24 (m, 2H), 3.87 – 3.76 (m, 1H), 3.69 – 3.56 (m, 4H), 3.55 – 3.50 (m, 1H), 3.22 – 3.18 (m, 1H), 2.52 – 2.49 (m, 1H, obscured by solvent peak), 2.19 (d, J = 14.1, 1H), 1.99 - 1.89 (m, 1H), 1.38 - 1.32 (m, 1H). 13C NMR (125 MHz, DMSO-d6, 100 °C, as a mixture of rotamers) δ 170.1, 154.4, 153.3, 143.4, 140.3, 132.2, 128.8, 128.3, 127.0, 126.6, 126.4, 124.5, 120.6, 119.4, 119.3, 115.1, 108.8, 108.4, 108.1, 77.0, 69.3, 65.2, 61.7, 50.5, 46.5, 38.0, 29.5, 29.4. HRMS (ESI+) calcd for C30H30NO7 [M+H]+ : 516.2022. Found: 516.2027.
Methyl 2-((1R,3S,4aR,9aS)-6-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-1-(hydroxymethyl)-3,4,4a,9a-tetrahydro-1H-pyrano[3,4-b]benzofuran-3-yl)acetate 3b
Following the above protocol, 10b (6.8 g, 12.7 mmol, 1 equiv) was treated with HF pyridine (70 wt%, 7.9 mL, 64.0 mmol) in THF (130 mL). The crude alcohol was dissolved in 1,4-dioxane (160 mL) and 10% aqueous NaHCO3 solution (100 mL) was added followed by a solution of FmocCl (6.6 g, 25.6 mmol) in 1,4-dioxane (20 ml). The reaction provided, after filtration, 5.84 g (89%) of 3b as a white foamy solid. [α]D20 +89.3 (c 1.0, CHCl3). IR νmax (cm−1, film): 2950, 1723, 1615, 1548, 1490, 1449, 1439, 1219, 1150, 1054. 1H NMR (500 MHz, DMSO-d6, 100 °C) δ 9.07 (s, 1H), 7.88 (d, J = 7.5 Hz, 2H), 7.72 (d, J = 7.4 Hz, 2H), 7.43 (t, J = 7.4 Hz, 2H), 7.37 – 7.34 (m, 3H), 7.11 (d, J = 8.5 Hz, 1H), 6.68 (d, J = 8.5 Hz, 1H), 4.62 – 4.53 (m, 1H), 4.48 (d, J = 6.6 Hz, 2H), 4.30 (t, J = 6.5 Hz, 1H), 4.16 (dt, J = 16.5, 8.3 Hz, 1H), 3.87 (dd, J = 10.1, 5.5 Hz, 1H), 3.73 – 3.57 (m, 5H), 3.56 – 3.40 (m, 1H), 2.54 – 2.36 (m, 2H), 2.08 – 1.91 (m, 1H), 1.40 (m, 1H). 13C NMR (125 MHz, DMSO-d6, 100 °C) δ 170.2, 154.1, 153.3, 143.4, 140.3, 131.7, 131.3, 127.0, 126.5, 124.5, 119.4, 119.1, 115.8, 108.4, 79.0, 71.7, 66.6, 65.1, 61.3, 50.5, 46.5, 37.3, 31.8. HRMS (ESI+) calcd for C30H30NO7 [M+H]+ : 516.2022. Found: 516.2021.
Methyl 2-((1R,3R,4aR,9aR)-6-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-1-(hydroxymethyl)-3,4,4a,9a-tetrahydro-1H-pyrano[3,4-b]benzofuran-3-yl)acetate 3c
Following the above protocol, 10c (7.5 g, 14.1 mmol, 1 equiv) was treated with HF pyridine (70 wt%, 8.7 mL, 70.5 mmol) in THF (140 mL). The crude alcohol was dissolved in 1,4-dioxane (180 mL) and 10% aqueous NaHCO3 solution (120 mL) was added, followed by a solution of FmocCl (7.3 g, 28.2 mmol) in 1,4-dioxane (20 ml). The reaction provided, after filtration, 6.2 g (85%) of 3c as a white foamy solid. [α] D20 −121.9 (c 0.8, CHCl3). IR νmax (cm−1, film): 2950, 1723, 1615, 1548, 1490, 1449, 1439, 1219, 1150, 1054. 1H NMR (500 MHz, DMSO-d6, 100 °C) δ 9.06 (s, 1H), 8.20 (s, 1H), 7.88 (d, J = 7.6 Hz, 2H), 7.72 (d, J = 7.4 Hz, 2H), 7.43 (t, J = 7.4 Hz, 2H), 7.34 (m, 2H), 7.10 (d, J = 8.5 Hz, 1H), 6.70 (d, J = 8.5 Hz, 1H), 4.48 (d, J = 6.6 Hz, 2H), 4.39 (d, J = 6.2 Hz, 1H), 4.35 – 4.22 (m, 2H), 3.79 – 3.70 (m, 3H), 3.69 – 3.48 (m, 4H), 3.40 – 3.23 (m, 1H), 2.56 – 2.32 (m, 2H), 1.98 (dd, J = 13.5, 7.0 Hz, 1H), 1.03 – 0.98 (m, 1H). 13C NMR (125 MHz, DMSO-d6, 100 °C) δ 170.0, 153.8, 153.3, 143.5, 140.4, 133.2, 131.7, 127.1, 126.4, 124.5, 119.4, 118.9, 115.2, 108.7, 78.7, 77.1, 71.0, 65.1, 60.8, 50.5, 46.5, 38.0, 34.4. HRMS (ESI+) calcd for C30H30NO7 [M+H]+ : 516.2022. Found: 516.2027.
Methyl 2-((1R,3S,4aR,9aR)-6-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-1-(hydroxymethyl)-3,4,4a,9a-tetrahydro-1H-pyrano[3,4-b]benzofuran-3-yl)acetate 3d
Following the above protocol, 10d (3.2 g, 6.0 mmol, 1 equiv) was treated with HFpyridine (70 wt%, 3.0 mL, 24.0 mmol) in THF (60 mL). The crude alcohol was dissolved in dioxane (70 mL) and 10% aqueous NaHCO3 solution (80 mL) was added, followed by a solution of FmocCl (1.6 g, 6.2 mmol) in 1,4-dioxane (10 ml). The reaction provided, after filtration, 2.4 g (82%) of 3d as a white foamy solid. [α]D20 −65.8 (c 1.0, CHCl3). IR νmax (cm−1, film): 2950, 1723, 1615, 1548, 1490, 1449, 1439, 1219, 1150, 1054. 1H NMR (500 MHz, DMSO-d6, 100 °C) δ 9.07 (s, 1H), 7.88 (d, J = 7.5 Hz, 2H), 7.73 (d, J = 7.3 Hz, 2H), 7.43 (t, J = 7.4 Hz, 2H), 7.36 – 7.31 (m, 3H), 7.11 (d, J = 8.3 Hz, 1H), 6.64 (d, J = 8.5 Hz, 1H), 4.91 (d, J = 9.8 Hz, 1H), 4.47 (d, J = 6.7 Hz, 2H), 4.32 – 4.24 (m, 2H), 3.85 – 3.75 (m, 3H), 3.65 – 3.60 (m, 4H), 3.51 – 3.47 (m, 1H), 2.56 – 2.42 (m, 4H), 2.03 (s, 1H), 1.83 – 1.78 (m, 1H). 13C NMR (125 MHz, DMSO-d6) δ 170.1, 155.0, 153.3, 143.4, 140.3, 131.4, 129.5, 127.0, 126.4, 124.5, 119.4, 119.3, 115.8, 107.6, 93.7, 70.2, 66.4, 65.1, 60.2, 50.4, 46.5, 36.5, 29.0. HRMS (ESI+) calcd for C30H30NO7 [M+H]+ : 516.2022. Found: 516.2030.
Methyl 2-((2S,5R,6S)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5-(2-fluoro-N-methyl-5-nitrobenzamido)-5,6-dihydro-2H-pyran-2-yl)acetate 13a
To a solution of 2a (21.0 g, 47.8 mmol, 1.0 equiv) in CH2Cl2 (480 mL) at 0 °C was added 2,6-lutidine (11.1 mL, 96.0 mmol, 2.0 equiv) followed by 2-fluoro-5-nitrobenzoyl chloride 11 (11.7 g, 57.3 mmol, 1.2 equiv). The reaction mixture was stirred at 0 °C for 2 h. After complete conversion (determined by LC/MS) the reaction mixture was quenched with a saturated aqueous solution of aqueous ammonium chloride. The organic layer was washed with water and the combined aqueous phases were extracted twice with CH2Cl2, dried over MgSO4, filtered and concentrated. The crude material was purified by chromatography on silica gel, which provided 25.2 g of the amide (87%). The purified amide (21.0 g, 34.6 mmol, 1.0 equiv) was dissolved in dry DMF (700 mL) and neat methyl iodide (4.3 mL, 69.2 mmol, 2.0 equiv) was added to the solution. At 0 °C, sodium hydride (60% dispersion in mineral oil, 2.1 g, 51.9 mmol, 1.5 equiv) was added portion-wise to the mixture, over a period of 15 min. After 2 h at 0 °C, the reaction mixture was quenched with a saturated solution of aqueous ammonium chloride and the layers were separated. The aqueous phase was extracted with ether and the combined organic phases were washed with brine, dried with MgSO4, filtered and concentrated to afford a crude material that was purified by silica gel chromatography (gradient: 0% to 30% EtOAc in hexanes) to afford 13a as a pale yellow oil (18.0 g, 84% yield). [α]D20 −42.7 (c 1.0, CHCl3). IR νmax (cm−1, film): 2931, 2857, 1739, 1641, 1533, 1350, 1112, 1097. 1H NMR (300 MHz, CDCl3, mixture of rotamers, ratio 4:1) δ 8.26 (ddd, J = 9.0, 4.4, 2.9 Hz, 1H), 8.11 (ddd, J = 12.3, 5.3, 2.7 Hz, 1H), 7.75 – 7.67 (m, 2H), 7.65 – 7.57 (m, 1H), 7.45 – 7.33 (m, 6H), 7.24 – 7.12 (m, 2H), 6.17 (d, J = 10.0 Hz, 1H), 5.76 (ddd, J = 10.1, 5.5, 1.9 Hz, 1H), 5.25 (s, 1H), 4.62 – 4.41 (m, 1H), 4.01 – 3.86 (m, 2H), 3.78 – 3.70 (dd, J = 10.7, 7.4 Hz, 1H), 3.70 (s, 3H × 0.2), 3.66 (s, 3H × 0.8), 2.95 (s, 3H × 0.2), 2.69 (br s, 3H × 0.8), 2.63 – 2.43 (m, 2H), 1.06 (s, 9H × 0.8), 1.00 (s, 9H × 0.2). 13C NMR (75 MHz, CDCl3) δ 170.6, 164.8, 144.5, 137.9, 135.7, 135.6, 135.4, 135.3, 133.6, 133.5, 129.7, 129.5, 129.0, 128.2, 127.7, 127.6, 127.5, 126.7, 126.6, 126.3, 126.0, 125.3, 125.2, 125.1, 123.6, 117.1, 116.8, 78.4, 71.9, 63.9, 51.8, 46.6, 39.6, 39.5, 33.6, 26.8, 26.7, 21.4, 19.3, 19.1. HRMS (ESI) calcd for C33H37FN2NaO7Si [M+Na]+ : 643.2252. Found: 643.2250.
Methyl 2-((2R,5R,6S)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5-(2-fluoro-N-methyl-5-nitrobenzamido)-5,6-dihydro-2H-pyran-2-yl)acetate 13b
Compound 13b was prepared using the above protocol starting from 2b (24.2 g, 55.0 mmol, 1.0 equiv) via treatment with 2,6-lutidine (12.8 mL, 110.0 mmol, 2.0 equiv) and 2-fluoro-5-nitrobenzoyl chloride 11 (13.5 g, 66.1 mmol, 1.2 equiv) in CH2Cl2 (550 mL) to provid 29.2 g (87%) of the amide. The methylation was performed on 23.6 g (38.8 mmol, 1.0 equiv) of the amide intermediate with sodium hydride (60% dispersion in mineral oil, 2.2 g, 54.4 mmol, 1.4 equiv) and methyl iodide (4.8 mL, 78.0 mmol, 2.0 equiv) in DMF (650 mL). The reaction provided, after purification, 19.1 g (79%) of 13b as a pale yellow oil. [α]D20 −96.8 (c 1.1, CHCl3). IR νmax (cm−1, film) 2926, 2852, 1736, 1640, 1532, 1349, 1093. 1H NMR (300 MHz, CDCl3, mixture of rotamers, ratio 9:2) δ 8.29 – 8.20 (m, 1H), 8.17 – 8.04 (m, 1H), 7.69 (br t, J = 6.0 Hz, 3H), 7.55 (br dd, J = 14.1, 7.2 Hz, 1H), 7.47 – 7.29 (m, 6H), 7.20 (t, J = 8.6 Hz, 1H), 6.18 (br d, J = 9.9 Hz, 1H), 5.90 – 5.73 (m, 1H), 5.21 (br s, 1H), 4.85 (br s, 1H), 3.98 (br s, 1H), 3.87 (br dd, J = 11.0, 4.0 Hz, 1H), 3.76 – 3.65 (m, 4H), 2.99 (s, 3H × 0.2), 2.82 – 2.69 (m, 1H), 2.74 (s, 3H × 0.8), 2.64 – 2.35 (m, 1H), 1.05 (s, 9H × 0.8), 0.97 (s, 9H × 0.2). 13C NMR (75 MHz, CDCl3) δ 170.9, 170.6, 164.8, 164.3, 162.8, 159.4, 144.5, 135.7, 135.6, 135.5, 135.3, 134.6, 133.5, 129.8, 129.7, 129.6, 127.7, 127.6, 126.7, 126.6, 126.3, 126.0, 125.2, 125.1, 123.1, 117.2, 116.8, 72.8, 72.5, 69.9, 69.7, 63.9, 51.9, 51.7, 46.1, 36.9, 36.7, 33.5, 33.4, 26.8, 19.2, 19.1. HRMS (ESI) calcd for C33H37FN2NaO7Si [M+Na]+: 643.2252. Found: 643.2250.
Methyl 2-((2S,5S,6S)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5-(2-fluoro-N-methyl-5-nitrobenzamido)-5,6-dihydro-2H-pyran-2-yl)acetate 13c
Compound 13c was prepared using starting from 2a (20.3 g, 46.2 mmol, 1.0 equiv) via treatment with 2,6-lutidine (10.8 mL, 92.4 mmol, 2.0 equiv) and 2-fluoro-5-nitrobenzoyl chloride 11 (11.3 g, 55.5 mmol, 1.2 equiv) in CH2Cl2 (460 mL). This stereoisomer was not purified, and the crude mixture was used directly in the next step. The methylation was performed when the crude amide intermediate was reacted with sodium hydride (60% dispersion in mineral oil, 3.7 g, 92.0 mmol, 2.0 equiv) and methyl iodide (28.9 mL, 462 mmol, 10.0 equiv) in THF (750 mL). The reaction provided, after purification, 15.2 g of 13c as a pale yellow oil (53% yield over two steps). [α]D20 +13.6 (c 1.0, CHCl3). IR νmax (cm−1, film): 2926, 2852, 1737, 1643, 1532, 1348, 1111. 1H NMR (300 MHz, CDCl3, mixture of rotamers, ratio 1:1) δ 8.29 (ddd, J = 9.0, 4.4, 2.9 Hz, 1H × 0.5), 8.15 (m, 3H × 0.5), 7.76 – 7.67 (m, 2H), 7.57 (d, J = 7.7 Hz, 1H), 7.50 (d, J = 6.9 Hz, 1H), 7.43 – 7.29 (m, 7H), 6.00 (d, J = 9.5 Hz, 1H), 5.75 (br d, J = 9.5 Hz, 1H × 0.5), 5.65 (d, J = 10.2 Hz, 1H × 0.5), 5.25 (br d, J = 8.2 Hz, 1H × 0.5), 4.66 – 4.50 (m, 1H), 4.16 (br s, 1H × 0.5), 3.88 – 3.72 (m, 3H), 3.70 (s, 3H × 0.5), 3.66 (s, 3H × 0.5), 2.89 (s, 3H × 0.5), 2.64 (s, 3H × 0.5), 2.70 – 2.51 (m, 1H), 2.51 – 2.36 (m, 1H), 1.04 (s, 9H × 0.5), 0.91 (s, 9H × 0.5). 13C NMR (75 MHz, CDCl3, mixture of rotamers) δ 171.0, 170.7, 164.8, 159.5, 144.5, 135.8, 135.7, 135.3, 133.7, 133.5, 133.0, 129.8, 129.7, 129.6, 128.3, 127.7, 127.6, 126.9, 126.8, 126.5, 125.9, 125.3, 125.3, 125.2, 117.2, 116.9, 75.7, 75.4, 71.2, 64.0, 63.3, 51.8, 49.2, 40.1, 39.8, 32.0, 26.7, 19.3, 19.2. HRMS (ESI) calcd for C33H37FN2NaO7Si [M+Na]+ : 643.2252. Found: 643.2245.
Methyl 2-((2R,5S,6S)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5-(2-fluoro-N-methyl-5-nitrobenzamido)-5,6-dihydro-2H-pyran-2-yl)acetate 13d
Compound 13d was prepared using the above protocol starting from 2d (41.6 g, 95.0 mmol, 1.0 equiv) via treatment with 2,6-lutidine (22.0 mL, 189.0 mmol, 2.0 equiv) and 2-fluoro-5-nitrobenzoyl chloride 11 (23.1 g, 113.0 mmol, 1.2 equiv) in CH2Cl2 (1000 mL), to provide 52.0 g (91%) of the amide. The methylation was performed on 52.0 g (86.0 mmol, 1.0 equiv) of the amide intermediate with sodium hydride (60% dispersion in mineral oil, 4.8 g, 120.0 mmol, 1.4 equiv) and methyl iodide (10.7 mL, 171.0 mmol, 2.0 equiv) in DMF (1750 mL). The reaction provided, after purification, 40.1 g (75%) of 13d as a yellow oil. [α]D20 +40.3 (c 1.0, CHCl3). IR νmax (cm−1, film): 2931, 2857, 1737, 1643, 1533, 1349, 1111. 1H NMR (300 MHz, CDCl3, mixture of rotamers 3:2) δ 8.35 – 8.02 (m, 2H), 7.75 – 7.65 (m, 2H), 7.52 (dd, J = 18.1, 7.2 Hz, 2H), 7.45 – 7.31 (m, 7H), 6.07 (br d, J = 10.3 Hz, 1H), 5.72 (br d, J = 10.2 Hz, 1H), 5.18 (s, 1H), 4.66 (br s, 1H), 3.97 (dd, J = 8.9, 4.3 Hz, 3H × 0.4), 3.90 – 3.78 (m, 3H × 0.6), 3.67 (s, 3H), 2.99 (br s, 3H × 0.4), 2.79 (s, 3H × 0.6), 2.66 (dd, J = 14.4, 8.4 Hz, 1H), 2.54 (br dd, J = 14.9, 6.0 Hz, 1H), 1.07 (s, 9H × 0.6), 0.93 (s, 9H × 0.4). 13C NMR (75 MHz, CDCl3) δ 170.8, 170.6, 164.4, 164.2, 163.0, 159.6, 144.5, 135.7, 135.6, 135.3, 133.3, 133.2, 129.9, 129.8, 129.7, 127.7, 126.8, 126.7, 125.3, 125.2, 123.9, 117.2, 116.9, 74.1, 67.2, 63.4, 51.9, 51.8, 47.6, 39.2, 32.5, 32.5, 29.1, 26.8, 26.7, 19.2, 19.1. HRMS (ESI) calcd for C33H37FN2NaO7Si [M+Na]+ : 643.2252. Found: 643.2245.
Methyl 2-((2S,4aR,12aS)-5-methyl-8-nitro-6-oxo-2,4a,5,6,12,12a-hexahydrobenzo[b]pyrano[3,2-f][1,5]oxazocin-2-yl)acetate 14a
A solution of HF pyridine (70% by wt. in pyridine, 11.2 mL, 90.0 mmol, 4.0 equiv) was added via syringe at 0 °C to a solution of 13a (14.0 g, 22.6 mmol, 1.0 equiv) in 250 mL THF. Once the addition was complete, the cold bath was removed allowing the reaction mixture to reach rt and the mixture was stirred until complete conversion of the starting material was observed (4h, LC/MS). The reaction was quenched at 0 °C with TMSOMe (24.9 mL, 180.4 mmol, 8.0 equiv) and solvent was removed in vacuo to afford a crude material (7.87 g, 20.6 mmoles, 91% yield), which was used in the next step without further purification. A solution of the intermediate alcohol (7.87 g, 20.6 mmol, 1.0 equiv) in DMF (700 mL) was added via cannula at 0 °C to a flame-dried round bottom flask containing dry CsF (31.3 g, 206.0 mmol, 10.0 equiv). The mixture was warmed to 35 °C overnight. The reaction was carefully quenched at 0 °C with brine (350 mL) and the compound was partitioned between brine and ether. The aqueous layer was extracted with ether (3x) and the combined organic phases were dried over Na2SO4, filtered and concentrated to afford a crude material that was purified on silica gel (gradient: 0% to 60% EtOAc in hexanes) to afford 14a (3.7 g, 10.2 mmol, 50% yield) as a yellow foamy solid. [α]D20 −109.8 (c 1.1, CHCl3). IR νmax (cm−1, film) 2995, 2947, 2856, 1736, 1632, 1518, 1436, 1343, 1256, 1085. 1H NMR (300 MHz, CDCl3) δ 8.39 (d, J = 2.8 Hz, 1H), 8.10 (dd, J = 9.2, 2.8 Hz, 1H), 6.93 (d, J = 9.2 Hz, 1H), 6.18 (d, J = 10.1 Hz, 1H), 6.00 – 5.85 (m, 1H), 4.67 (s, 1H), 4.36 (dd, J = 12.6, 4.0 Hz, 1H), 4.26 (dd, J = 12.5, 9.5 Hz, 1H), 4.06 – 4.00 (m, 1H), 3.96 (ddd, J = 9.4, 3.9, 2.4 Hz, 1H), 3.72 (s, 3H), 3.08 (s, 3H), 2.64 (dd, J = 15.7, 7.6 Hz, 1H), 2.55 (dd, J = 15.7, 6.1 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 170.3, 168.3, 159.0, 141.6, 135.4, 128.7, 126.3, 122.3, 121.1, 120.1, 74.8, 72.2, 65.9, 52.0, 51.9, 38.8, 32.3. HRMS (ESI) calcd for C17H19N2O7 [M+H]+: 363.1187. Found: 363.1193.
Methyl 2-((2R,4aR,12aS)-5-methyl-8-nitro-6-oxo-2,4a,5,6,12,12a-hexahydrobenzo[b]pyrano[3,2-f][1,5]oxazocin-2-yl)acetate 14b
Compound 14b was prepared using above protocol starting from 13b (8.5 g, 13.7 mmol, 1.0 equiv). TBDPS deprotection was accomplished via treatment with HF pyridine (70% by wt. in pyridine, 6.8 mL, 54.8 mmol, 4.0 equiv) in THF (150 mL) and the reaction was quenched with TMSOMe (18.8 mL, 137.0 mmoles, 10.0 equiv) to afford the intermediate alcohol (4.8 g, 12.5 mmol) in 91% yield. Cyclization of the crude material (4.8 g, 12.5 mmol, 1.0 equiv) was conducted in DMF (420 mL) with CsF (19.0 g, 125.0 mmol, 10.0 equiv) at 40 °C for 5 h to afford 14b (2.8 g, 7.8 mmol, 63% yield) a yellow foamy solid. [α]D20 −91.0 (c 1.1, CHCl3). IR νmax (cm−1, film) 2952, 2900, 1736, 1634, 1518, 1437, 1343, 1306, 1257, 1104. 1H NMR (300 MHz, CDCl3) δ 8.39 (d, J = 2.8 Hz, 1H), 8.10 (dd, J = 9.2, 2.9 Hz, 1H), 6.93 (d, J = 9.2 Hz, 1H), 6.21 (dd, J = 10.0, 3.0 Hz, 1H), 5.98 (ddd, J = 8.8, 6.2, 2.1 Hz, 1H), 4.81 (ddt, J = 7.7, 5.1, 2.6 Hz, 1H), 4.34 – 4.20 (m, 2H), 4.06 – 3.96 (m, 2H), 3.74 (s, 3H), 3.07 (s, 3H), 2.74 (dd, J = 15.3, 9.4 Hz, 1H), 2.56 (dd, J = 15.3, 5.0 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 170.4, 168.3, 156.0, 141.7, 134.9, 128.9, 126.4, 122.1, 121.2, 120.1, 70.3, 69.2, 66.3, 52.1, 51.5, 37.2, 32.1. HRMS (ESI) calcd for C17H19N2O7 [M+H]+: 363.1187. Found: 363.1194.
Methyl 2-((2S,4aS,12aS)-5-methyl-8-nitro-6-oxo-2,4a,5,6,12,12a-hexahydrobenzo[b]pyrano[3,2-f][1,5]oxazocin-2-yl)acetate 14c
Cesium fluoride (CsF, 25.0 g, 165.0 mmol, 13.1 equiv) was added in one portion at 0 °C to a solution of 13c (7.8 g, 12.6 mmol, 1.0 equiv) in DMF (1 L) under argon. The temperature was slowly raised to 35 °C and the mixture was stirred at this temperature until complete conversion of the starting material was observed (LC/MS). The reaction was carefully quenched with brine (500 mL) at 0 °C and then partially concentrated. The crude mixture was partitioned between Et2O and brine. The organic layers were combined, dried over Na2SO4, filtered and concentrated. The crude material was then purified on silica gel (gradient: 0% to 50% EtOAc in hexanes) to afford 14c (3.5 g, 9.7 mmol, 77% yield) as a yellow powder. [α]D20 −42.5 (c 1.0, CHCl3). IR νmax (cm−1, film) 2943, 2847, 1735, 1636, 1518, 1437, 1345, 1327, 1255, 1092. 1H NMR (300 MHz, CDCl3) δ 8.52 (d, J = 2.8 Hz, 1H), 8.17 (dd, J = 9.1, 2.9 Hz, 1H), 7.09 (d, J = 9.1 Hz, 1H), 5.97 (dd, J = 10.3, 2.5 Hz, 1H), 5.84 (br d, J = 10.3 Hz, 1H), 4.76 – 4.66 (m, 1H), 4.45 (dd, J = 13.9, 2.6 Hz, 1H), 4.43 – 4.37 (m, 1H), 4.34 (dd, J = 13.8, 2.3 Hz, 1H), 3.80 (ddd, J = 10.0, 2.5, 2.5 Hz, 1H), 3.71 (s, 3H), 3.01 (s, 3H), 2.67 (dd, J = 16.0, 7.3 Hz, 1H), 2.52 (dd, J = 16.0, 6.4 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 170.6, 168.3, 161.2, 142.4, 132.6, 129.3, 126.7, 124.6, 122.8, 121.1, 73.3, 71.8, 69.7, 52.0, 51.8, 39.5, 29.5. HRMS (ESI) calcd for C17H19N2O7 [M+H]+ : 363.1187. Found: 363.1190.
Methyl 2-((2R,4aS,12aS)-5-methyl-8-nitro-6-oxo-2,4a,5,6,12,12a-hexahydrobenzo[b]pyrano[3,2-f][1,5]oxazocin-2-yl)acetate 14d
Treatment of 13d (6.3 g, 10.2 mmol, 1.0 equiv) with CsF (20.0 g, 132.0 mmoles, 13.0 equiv) in DMF (800 mL) afforded 14d (2.7 g, 7.5 mmol, 73% yield) as a yellow foamy solid. [α]D20 −63.9 (c 1.2, CHCl3). IR νmax (cm−1, film) 3008, 2947, 1735, 1635, 1518, 1438, 1345, 1324, 1255, 1093. 1H NMR (300 MHz, CDCl3) δ 8.50 (s, 1H), 8.14 (dd, J = 9.1, 2.8 Hz, 1H), 7.06 (d, J = 9.1 Hz, 1H), 5.99 (d, J = 10.4 Hz, 1H), 5.89 (d, J = 10.5 Hz, 1H), 4.82 (br s, 1H), 4.51 – 4.19 (m, 3H), 3.80 (d, J = 9.8 Hz, 1H), 3.72 (s, 3H), 2.99 (s, 3H), 2.73 (dd, J = 15.1, 9.1 Hz, 1H), 2.59 (dd, J = 15.0, 5.1 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 170.3, 168.4, 161.1, 142.2, 131.7, 129.6, 126.7, 124.7, 122.0, 120.9, 70.6, 69.2, 67.8, 52.0, 51.8, 38.4, 29.7. HRMS (ESI) calcd for C17H19N2O7 [M+H]+: 363.1187. Found: 363.1193.
Methyl 2-((2R,4aR,12aS)-8-amino-5-methyl-6-oxo-2,3,4,4a,5,6,12,12a-octahydrobenzo[b]pyrano[3,2-f][1,5]oxazocin-2-yl)acetate 4a
To a solution of 14a (6.3 g, 17.5 mmol, 1.0 equiv) in THF:MeOH (3:1, 175 mL) under N2 was added palladium on carbon (10 wt%, 0.5 g, 4.4 mmol, 0.3 equiv). The solution was purged with H2 for 30 min and the mixture was stirred overnight at rt under an atmosphere of H2 (balloon). Celite was added to the reaction mixture and after 30 min, the crude material was filtered through a plug of Celite and rinsed with a solution of 10% MeOH in CH2Cl2 (50 mL). The solvents were removed in vacuo and the residue was purified by silica gel chromatography (gradient: 0% to 7% MeOH in CH2Cl2). Aniline 4a was isolated as a red-brown powder (5.8 g, 17.4 mmoles, 99% yield). [α]D20 +21.6 (c 1.0, CHCl3). IR νmax (cm−1, film) 3434, 3347, 2950, 2895, 1732, 1612, 1494, 1436, 1201, 1045. 1H NMR (300 MHz, CDCl3) δ 6.72 – 6.53 (m, 3H), 4.07 (d, J = 3.0 Hz, 1H), 4.05 (s, 1H), 3.84 – 3.99 (m, 1H), 3.84 – 3.72 (m, 2H), 3.68 (s, 3H), 3.58 (br s, 2H), 3.24 (s, 3H), 2.57 (dd, J = 15.5, 7.4 Hz, 1H), 2.44 (dd, J = 15.5, 5.3 Hz, 1H), 2.18 – 1.96 (m, 2H), 1.96 – 1.76 (m, 1H), 1.77 – 1.61 (m, 1H). 13C NMR (75 MHz, CDCl3) δ 171.2, 170.8, 147.6, 139.8, 121.1, 119.8, 119.5, 117.2, 78.8, 74.6, 65.2, 51.9, 50.6, 41.3, 32.1, 27.3, 24.2. HRMS (ESI) calcd for C17H22N2NaO5 [M+Na]+: 357.1421. Found: 357.1427.
Methyl 2-((2S,4aR,12aS)-8-amino-5-methyl-6-oxo-2,3,4,4a,5,6,12,12a-octahydrobenzo[b]pyrano[3,2-f][1,5]oxazocin-2-yl)acetate 4b
Compound 4b was prepared using the above protocol starting from 14b (6.0 g, 16.4 mmol, 1.0 equiv) and Pd/C (10 wt%, 0.4 g, 4.1 mmol, 0.3 equiv) in THF:MeOH (3:1, 165 mL). The reaction provided, after purification, 5.2 g (94%) of 4b as a red-brown powder. [α]D20 +41.2 (c 1.0, CHCl3). IR νmax (cm−1, film) 3347, 2950, 1731, 1610, 1493, 1396, 1205, 1022. 1H NMR (500 MHz, DMSO-d6, 100 °C) δ 6.70 (d, J = 8.6 Hz, 1H), 6.65 (dd, J = 8.6, 2.5 Hz, 1H), 6.50 (d, J = 2.4 Hz, 1H), 4.66 (br s, 2H), 4.17 – 4.04 (m, 3H), 3.98 (dd, J = 12.9, 11.0 Hz, 1H), 3.71 – 3.66 (m, 1H), 3.61 (s, 3H), 3.14 (s, 3H), 2.58 (dd, J = 14.9, 7.8 Hz, 1H), 2.47 (dd, J = 15.1, 5.5 Hz, 1H), 1.85 (br app t, J = 15.0 Hz, 2H), 1.58 (br d, J = 8.1 Hz, 1H), 1.46 – 1.37 (m, 1H). 13C NMR (125 MHz, DMSO-d6, 100 °C) δ 171.1, 169.4, 146.5, 143.7, 120.8, 118.5 (2), 114.7, 69.6, 68.8, 68.1, 57.1, 51.5, 39.4, 34.8, 28.3, 22.5. HRMS (ESI) calcd for C17H22N2NaO5 [M+Na]+: 357.1421. Found: 357.1426.
Methyl 2-((2R,4aS,12aS)-8-amino-5-methyl-6-oxo-2,3,4,4a,5,6,12,12a-octahydrobenzo[b]pyrano[3,2-f][1,5]oxazocin-2-yl)acetate 4c
Compound 4c was prepared as described above starting from 14c (6.3 g, 17.5 mmol, 1.0 equiv) and Pd/C (10 wt%, 0.5 g, 4.4 mmol, 0.3 equiv) in THF:MeOH (3:1, 180 mL). The reaction provided, after purification, 5.8 g (99%) of 4c as a red-brown powder. [α]D20 −32.0 (c 1.0, CHCl3). IR νmax (cm−1, film) 3427, 3349, 2951, 2873, 1733, 1616, 1493, 1436, 1202, 1091. 1H NMR (300 MHz, CDCl3) δ 6.82 (d, J = 2.7 Hz, 1H), 6.79 (dd, J = 9.0 Hz, 1H), 6.69 (dd, J = 8.7, 2.8 Hz, 1H), 4.18 (dd, J = 13.8, 1.6 Hz, 1H), 4.06 (dd, J = 13.8, 1.9 Hz, 1H), 3.93 – 3.70 (m, 4H), 3.67 (s, 3H), 3.60 (d, J = 10.6 Hz, 1H), 3.01 (s, 3H), 2.65 (dd, J = 16.0, 7.1 Hz, 1H), 2.42 (dd, J = 16.0, 5.8 Hz, 1H), 2.02 – 1.75 (m, 3H), 1.39 (ddd, J = 24.3, 11.3, 5.4 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 171.5, 170.9, 149.8, 140.1, 121.7, 120.5, 120.1, 117.9, 76.4, 74.1, 68.5, 53.2, 51.8, 40.5, 30.6, 28.7, 27.0. HRMS (ESI) calcd for C17H22N2NaO5 [M+Na]+: 357.1421. Found: 357.1419.
Methyl 2-((2S,4aS,12aS)-8-amino-5-methyl-6-oxo-2,3,4,4a,5,6,12,12a-octahydrobenzo[b]pyrano[3,2-f][1,5]oxazocin-2-yl)acetate 4d
Compound 4d was prepared using the above protocol starting from 14d (6.1 g, 17.0 mmol, 1.0 equiv) and Pd/C (10 wt%, 0.5 g, 4.2 mmol, 0.3 equiv) in THF:MeOH (3:1, 170 mL). The reaction provided, after purification, 5.5 g (98%) of 4d as a red-brown powder. [α]D20 +10.4 (c 1.0, CHCl3). IR νmax (cm−1, film) 3437, 3350, 2950, 1733, 1623, 1493, 1437, 1206, 1095. 1H NMR (300 MHz, CDCl3) δ 6.72 (dd, J = 5.7, 2.9 Hz, 2H), 6.61 (dd, J = 8.8, 2.7 Hz, 1H), 4.42 – 4.30 m, 1H), 4.11 (d, J = 13.6 Hz, 1H), 3.89 (d, J = 13.5 Hz, 1H), 3.72 (d, J = 3.2 Hz, 2H), 3.60 (s, 3H), 3.48 (br s, 2H), 2.95 (s, 3H), 2.76 (dd, J = 14.4, 9.1 Hz, 1H), 2.41 (dd, J = 14.5, 6.0 Hz, 1H), 1.99 – 1.50 (m, 4H). 13C NMR (75 MHz, CDCl3) δ 171.1, 170.6, 149.6, 140.7, 128.4, 122.5, 120.7, 119.9, 117.4, 69.6, 69.2, 68.8, 53.8, 52.0, 36.5, 28.6, 27.6, 22.0. HRMS (ESI) calcd for C17H22N2NaO5 [M+Na]+: 357.1421. Found: 357.1419.
Methyl 2-((2R,5R,6S)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5-(2-nitrophenylsulfonamido)tetrahydro-2H-pyran-2-yl)acetate 15a
A solution of 2a (17.1 g, 38.9 mmol, 1.0 equiv) in MeOH (389 ml) was degassed for 20 min by sparging with dry N2. To the solution was added Pd(OH)2/C (20% by weight, 2.73 g, 3.89 mmol, 0.1 equiv), and the suspension was sparged with H2 for 20 min before being placed under a static an atmosphere of H2 (balloon) at rt while stirring for 20 h. Upon completion of the reaction (LC/MS), the mixture was filtered through Celite. The filter cake was washed with CH2Cl2 and the filtrate was concentrated under reduced pressure to provide 16.45 g of crude product (96%), which was used in the next step without further purification. The crude material from above 16.45 g (37.2 mmol, 1.0 equiv) was dissolved in CH2Cl2 (372 mL) at 0 °C (ice/water bath), and to this solution was added sequentially Et3N (15.7 mL, 112 mmol, 3.0 equiv), DMAP (0.46 g, 3.72 mmol, 0.1 equiv) and 2-nitrobenzenesulfonyl chloride (12.38 g, 55.9 mmol, 1.5 equiv). The reaction was stirred at 0 °C for 15 min before removing the ice bath and stirring for an additional 100 min. When the reaction was deemed complete by LC/MS, the reaction was concentrated under reduced pressure and the crude residue was purified by chromatography on silica gel (gradient: 0% to 40% EtOAc in hexanes), which provided 19.0 g (82%) of 15a as a yellow foamy solid. [α]D20 −18.9 (c 1.0, CHCl3). IR νmax (cm−1, film): 2931 (w), 2857 (w), 1737 (m), 1540 (s), 1427 (m), 1352 (s), 1165 (s), 1111 (s). 1H NMR (300 MHz, CDCl3) δ 8.12 – 8.00 (m, 1H), 7.73 – 7.65 (m, 1H), 7.66 – 7.55 (m, 4H), 7.50 (dd, J = 3.4, 5.9 Hz, 2H), 7.48 – 7.32 (m, 6H), 5.81 (d, J = 8.7 Hz, 1H), 3.95 – 3.77 (m, 2H), 3.63 (s, 3H), 3.59 (d, J = 5.6 Hz, 1H), 3.48 – 3.35 (m, 1H), 3.38 (dd, J = 5.9, 10.5 Hz, 1H), 2.55 (dd, J = 7.2, 15.6 Hz, 1H), 2.39 (dd, J = 5.5, 15.6 Hz, 1H), 1.88 (d, J = 13.6 Hz, 1H), 1.71 (brs, 1H), 1.63 – 1.46 (m, 2H), 0.99 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 171.1, 147.4, 135.4, 135.4, 135.1, 133.1, 133.1, 133.0, 132.7, 129.9, 129.6, 129.6, 127.6, 127.6, 125.1, 80.1, 74.6, 63.4, 51.6, 49.2, 40.7, 29.3, 26.6, 25.3, 19.0. HRMS (ESI) calcd for C31H38N2NaO8SSi [M+Na]+ : 649.2016. Found: 649.2012.
Methyl 2-((2S,5R,6S)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5-(2-nitrophenylsulfonamido)tetrahydro-2H-pyran-2-yl)acetate 15b
Compound 15b was prepared using the above protocol from 2b (21 g, 47.7 mmol, 1.0 equiv) in MeOH (477 ml) and Pd(OH)2/C (20% by weight, 3.35 g, 4.77 mmol, 0.1 equiv), which provide 20.1 g of crude saturated amine (96%), which was used in the next step without further purification. The crude material from above (20.13 g, 45.6 mmol, 1.0 equiv) was subjected to a nosylation using CH2Cl2 (456 mL), Et3N (19.22 mL, 137 mmol, 3.0 equiv), DMAP (0.55 g, 4.56 mmol, 0.1 equiv) and 2-nitrobenzenesulfonyl chloride (15.15 g, 68.4 mmol, 1.5 equiv) which provided, after purification, 23.6 g (83%) of 15b as a yellow foamy solid. [α]D20 +22.6 (c 1.0, CHCl3). IR νmax (cm−1, film): 2932 (w), 2858 (w), 1736 (s), 1541 (s), 1427 (m), 1360 (s), 1168 (s), 1111 (s). 1H NMR (300 MHz, CDCl3) δ 8.11 – 8.00 (m, 1H), 7.71 – 7.63 (m, 1H), 7.63 – 7.49 (m, 6H), 7.40 (dt, J = 13.7, 6.7 Hz, 6H), 5.86 (d, J = 7.8 Hz, 1H), 4.33 (t, J = 6.9 Hz, 1H), 3.85 – 3.72 (m, 2H), 3.61 (obscured s, 2H), 3.59 (s, 3H), 2.64 (dd, J = 14.9, 7.9 Hz, 1H), 2.41 (dd, J = 14.8, 6.5 Hz, 1H), 2.03 – 1.69 (m, 3H), 1.43 – 1.30 (m, 1H), 1.01 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 171.2, 147.8, 135.7, 135.0, 133.4, 133.1, 133.0, 132.9, 130.4, 130.0, 129.9, 127.9, 125.3, 73.0, 69.2, 63.4, 51.8, 50.5, 37.6, 26.9, 25.8, 25.2, 19.2. HRMS (ESI) calcd for C31H38N2NaO8SSi [M+Na]+: 649.2016. Found: 649.2012.
Methyl 2-((2R,5R,6S)-6-(hydroxymethyl)-5-(2-nitrophenylsulfonamido)tetrahydro-2H-pyran-2-yl)acetate 16a
To a solution of 15a (21.7 g, 34.6 mmol, 1.0 equiv) in THF (173 mL) at 0 °C (ice/water bath) in a plastic bottle was added HF pyridine (70 wt%, 8.60 mL, 69.2 mmol, 2.0 equiv). The reaction was stirred, slowly warming to rt overnight for 20 h. The reaction was deemed complete by TLC, and subsequently quenched with TMSOMe (38.2 mL, 277 mmol, 8.0 equiv). The mixture was stirred for an additional 30 min and then diluted with EtOAc and washed with aqueous saturated copper sulfate solution (2 × 100 mL). The organic layer was dried over MgSO4, filtered and concentrated under reduced pressure. The crude residue was purified by chromatography on silica gel (gradient: 0% to 100% EtOAc in hexanes) to provide 12.1 g (90%) of 16a as a pale yellow foamy solid. [α]D20 −107.9 (c 1.0, CHCl3). IR νmax (cm−1, film): 3278 (w), 2953 (w), 1728 (m), 1541 (s), 1442 (m), 1344 (m), 1164 (s). 1H NMR (300 MHz, CDCl3) δ 8.12 (dd, J = 3.4, 5.8 Hz, 1H), 7.86 (dd, J = 3.5, 5.8 Hz, 1H), 7.80 – 7.73 (m, 2H), 5.83 (d, J = 9.0 Hz, 1H), 3.90 – 3.79 (m, 1H), 3.69 (s, 4H), 3.67 – 3.59 (m, 2H), 3.58 – 3.40 (m, 1H), 2.59 (dd, J = 7.3, 15.7 Hz, 1H), 2.43 (dd, J = 5.5, 15.8 Hz, 1H), 2.23 – 2.13 (m, 1H), 1.79 – 1.63 (m, 2H), 1.62 – 1.41 (m, 2H). 13C NMR (75 MHz, CDCl3) δ 171.1, 147.7, 134.5, 133.6, 132.9, 130.4, 125.3, 79.3, 74.4, 62.0, 51.7, 48.8, 40.6, 28.7, 25.4. HRMS (ESI) calcd for C15H20N2NaO8S [M+Na]+ : 411.0838. Found: 411.0844.
Methyl 2-((2S,5R,6S)-6-(hydroxymethyl)-5-(2-nitrophenylsulfonamido)tetrahydro-2H-pyran-2-yl)acetate 16b
Compound 16b was prepared following the above protocol using a solution of 15b (23.59 g, 37.6 mmol, 1.0 equiv), THF (188 mL), HF pyridine (70 wt%, 7.0 mL, 56.5 mmol, 1.5 equiv), TMSOMe (41.5 mL, 300 mmol, 8.0 equiv) which provided, after purification, 13.59 g (93%) of 16b as a pale yellow foamy solid. [α]D20 +23.3 (c 1.0, CHCl3). IR νmax (cm−1, film): 3395 (w), 2932 (w), 2857 (w), 1737 (m), 1541 (s), 1427 (m), 1360 (s), 1168 (s), 1112 (s). 1H NMR (300 MHz, CDCl3) δ 8.12 (dd, J = 5.9, 3.3 Hz, 1H), 7.84 (dd, J = 7.5, 3.8 Hz, 1H), 7.73 (dd, J = 5.7, 3.3 Hz, 2H), 5.76 (br s, 1H), 4.23 – 4.11 (m, 1H), 3.95 – 3.76 (m, 2H), 3.76 – 3.50 (m, 2H), 3.66 (s, 3H), 2.72 (br d, J = 6.1 Hz, 1H), 2.61 (dd, J = 15.9, 9.4 Hz, 1H), 2.42 (dd, J = 15.9, 4.4 Hz, 1H), 1.93 – 1.59 (m, 3H), 1.48 – 1.31 (m, 1H). 13C NMR (75 MHz, CDCl3) δ 171.9, 147.9, 134.3, 133.9, 133.1, 130.7, 125.6, 73.0, 67.1, 59.4, 52.1, 50.5, 37.9, 26.8, 25.7. HRMS (ESI) calcd for C15H21N2O8S [M+H]+ : 389.1019. Found: 389.1022.
Methyl 2-((1R,3R,6R)-7-((2-nitrophenyl)sulfonyl)-2-oxa-7-azabicyclo[4.2.0]octan-3-yl)acetate 5a
To a solution of alcohol 16a (12.1 g, 31.2 mmol, 1.0 equiv) and PPh3 (16.3 g, 62.3 mmol, 2.0 equiv) in THF (312 mL) at 0 °C (ice/water bath) was added DIAD (13.5 mL, 68.5 mmol, 2.2 equiv) dropwise over 5 min. The reaction was stirred, slowly warming to rt over 1 h until the reaction was deemed complete by LC/MS. The reaction mixture was concentrated under reduced pressure, and the crude residue was purified by chromatography on silica gel (gradient: 0% to 100% EtOAc in hexanes), to afford 9.84 g (85%) of 5a as a white foamy solid. [α]D20 −108.6 (c 1.0, CHCl3). IR νmax (cm−1, film): 2952 (w), 1736 (m), 1544 (s), 1371 (m), 1168 (s). 1H NMR (300 MHz, CDCl3) δ 8.02 (d, J = 8.0 Hz, 1H), 7.86 – 7.73 (m, 2H), 7.66 (d, J = 9.0 Hz, 1H), 4.28 (brs, 1H), 4.23 (t, J = 4.8 Hz, 1H), 3.93 (dd, J = 4.6, 8.5 Hz, 1H), 3.67 (s, 4H), 3.61 (d, J = 8.6 Hz, 1H), 2.58 (dd, J = 8.0, 15.8 Hz, 1H), 2.44 (dd, J = 4.8, 15.7 Hz, 1H), 2.18 (d, J = 11.6 Hz, 1H), 1.76 (dd, J = 8.5, 17.4 Hz, 2H), 1.55 (d, J = 9.4 Hz, 1H), 1.24 (t, J = 5.8 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 171.4, 148.7, 134.0, 131.6, 130.9, 129.7, 124.1, 70.6, 66.5, 60.7, 57.2, 51.6, 40.8, 25.3, 24.6. HRMS (ESI) calcd for C15H18N2NaO7S [M+Na]+: 393.0732. Found: 393.0730.
Methyl 2-((1R,3S,6R)-7-((2-nitrophenyl)sulfonyl)-2-oxa-7-azabicyclo[4.2.0]octan-3-yl)acetate 5b
Compound 5b was obtained following the above procedure using 16b (13.53 g, 34.8 mmol, 1.0 equiv) PPh3 (18.27 g, 69.7 mmol, 2.0 equiv), DIAD (15.1 mL, 77.0 mmol, 2.2 equiv) in THF (348 mL). Purification of the reaction mixture afforded 11.46 g (89%) of 5b as a white foamy solid. [α]D20 −86.2 (c 1.0, CHCl3). IR νmax (cm−1, film): 2952 (w), 1735 (m), 1544 (s), 1371 (m), 1168 (s). 1H NMR (300 MHz, CDCl3) δ 7.93 (dd, J = 7.2, 3.2 Hz, 1H), 7.75 – 7.64 (m, 2H), 7.64 – 7.56 (m, 1H), 4.56 – 4.39 (m, 2H), 4.31 (td, J = 6.3, 2.7 Hz, 1H), 4.05 (dd, J = 9.3, 6.2 Hz, 1H), 3.94 (dd, J = 9.3, 2.6 Hz, 1H), 3.61 (s, 3H), 2.53 (dd, J = 15.2, 8.8 Hz, 1H), 2.38 (dd, J = 15.2, 4.9 Hz, 1H), 2.08 – 1.81 (m, 3H), 1.44 – 1.28 (m, 1H). 13C NMR (75 MHz, CDCl3) δ 171.2, 148.5, 134.1, 131.9, 130.7, 130.5, 124.2, 67.1, 63.5, 61.9, 56.3, 51.7, 39.4, 23.4, 21.4. HRMS (ESI) calcd for C15H19N2O7S [M+H]+: 371.0913. Found: 371.0913.
Methyl 2-((2R,5S,6S)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5-(2-nitrophenylsulfonamido)tetrahydro-2H-pyran-2-yl)acetate 15c
To allylic amine 2c (4.5 g, 10.2 mmol) under Ar was added ethanol (100 mL) and Pd/C (10 wt%, 0.1 g, 1.0 mmol, 0.1 equiv). The solution was subsequently purged with H2 (balloon) for 10 min and left to stir under the H2 atmosphere for 16 h. The reaction mixture was then filtered through Celite and the filter cake was washed with CH2Cl2 (3 × 75 mL). The filtrate was then concentrated to give the primary amine, which was used in the next reaction without further purification. To a solution of the saturated primary amine (4.5 g, 10.2 mmol, 1.0 equiv) in CH2Cl2 (41 mL) was added Et3N (1.7 mL, 12.2 mmol, 1.2 equiv) followed by 2-nitrobenzenesulfonyl chloride (2.5 g, 11.2 mmol, 1.1 equiv). The reaction was stirred at rt until analysis of the reaction mixture by LC/MS showed that all starting material had been consumed (~1 h). The reaction was quenched with a saturated solution of aqueous ammonium chloride (100 mL). The organic layer was separated and the aqueous layer was then washed with CH2Cl2 (2 × 100 mL). The combined organic layers were washed with brine, dried over MgSO4 and filtered. The organic layer was concentrated under reduced pressure and the crude residue was purified by chromatography on silica gel (gradient: 5% to 50% EtOAc in hexanes), which provided 5.5 g (86%) of 15c as a yellow foamy solid. [α]D22 +8.3 (c 1.0, CHCl3). IR νmax (cm−1, film): 3327, 2932, 2857, 1734, 1542, 1437, 1362, 1188, 1168, 1112. 1H NMR (300 MHz, CDCl3) δ 8.10 (d, J = 6.9 Hz, 1H), 8.04 – 7.97 (m, 1H), 7.80 – 7.27 (m, 13H), 5.26 (d, J = 8.3 Hz, 1H), 4.33 (q, J = 7.1 Hz, 1H), 3.82 – 3.70 (m, 2H), 3.62 (s, 3H), 3.43 (s, 1H), 3.30 (s, 1H), 2.44 (ddd, J = 20.9, 15.5, 6.4 Hz, 2H), 1.96 (d, J = 12.1 Hz, 1H), 1.68 (d, J = 11.9 Hz, 1H), 1.48 (s, 1H), 1.41 – 1.34 (m, 2H), 0.99 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 171.5, 147.8, 135.9, 135.8, 134.89, 134.86, 133.9, 133.55, 133.48, 133.0, 132.4, 131.3, 130.6, 129.9, 129.67, 129.62, 127.9, 127.7, 127.7, 125.4, 124.9, 81.0, 76.8, 73.8, 69.1, 63.9, 51.8, 50.9, 40.8, 31.8, 30.6, 26.9, 26.7, 22.1, 19.4, 14.9. HRMS (ESI+) calcd for C31H38N2NaO8SSi [M+Na]+ : 649.2016. Found: 649.2020.
Methyl 2-((2S,5S,6S)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5-(2-nitrophenylsulfonamido)tetrahydro-2H-pyran-2-yl)acetate 15d
Compound 15d was prepared as described above using allylic amine (R,S,S)-2d (41.4 g, 94 mmol) and 10% Pd/C (10.0 g, 9.42 mmol, 0.1 equiv) in MeOH (942 mL). The resulting crude amine (42.0 g, 95.0 mmol) was treated with Et3N (15.9 ml, 114 mmol, 1.2 equiv), and 2-nitrobenzenesulfonyl chloride (23.2 g, 105 mmol, 1.1 equiv) in CH2Cl2 (476 mL) to afford 54.9 g (92%) of the desired amine 15d as a yellow foamy solid. [α]D22 −50.6 (c 1.0, CHCl3). IR νmax (cm−1, film) 3361, 2932, 2858, 1733, 1541, 1427, 1361, 1166, 1111. 1H NMR (300 MHz, CDCl3) δ 8.13 – 8.01 (m, 1H), 7.86 – 7.77 (m, 1H), 7.65 (dd, J = 11.2, 4.3 Hz, 1H), 7.60 – 7.48 (m, 5H), 7.46 – 7.30 (m, 6H), 5.97 (d, J = 8.2 Hz, 1H), 3.99 – 3.65 (m, 5H), 3.62 (s, 4H), 2.42 (ddd, J = 20.9, 15.5, 6.3 Hz, 2H), 1.73 (s, 2H), 1.53 (s, 2H), 1.01 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 171.2, 147.9, 135.6, 135.5, 134.7, 133.5, 133.0, 132.9, 132.9, 130.7, 130.0, 129.9, 127.9, 127.8, 125.4, 77.0, 68.0, 61.8, 51.7, 48.8, 40.4, 26.9, 25.2, 24.3, 19.2. HRMS (ESI) calcd for C3138N2NaO8SSi [M+Na]+ : 649.2016. Found: 649.2011.
Methyl 2-((2R,5S,6S)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5-(2-nitro-N-((R)-oxiran-2-ylmethyl)phenylsulfonamido)tetrahydro-2H-pyran-2-yl)acetate 18a
To a solution of sulfonamide 15c (6.5 g, 10.4 mmol, 1.0 equiv) in 1,2-dichloroethane (42 mL) was added cesium carbonate (13.5 g, 41.5 mmol, 4.0 equiv) followed by the (S)-glycidyl triflate [(S)-17] (4.3 g, 20.7 mmol, 2.0 eq). The reaction mixture was stirred at rt until deemed completion (~1 h) and then quenched with 100 mL of a saturated solution of aqueous ammonium chloride. The organic layer was separated and the aqueous layer was then washed with CH2Cl2 (2 × 100 mL). The combined organic layers were washed with brine, dried over MgSO4 and filtered. The organic layer was concentrated under reduced pressure and the crude residue was purified by chromatography on silica gel (gradient: 5% to 55% EtOAc in hexanes), which provided 6.4 g (90%) of pure 18a as a yellow oil. [α]D22 −10.3 (c 1.0, CHCl3). IR νmax (cm−1, film): 2932, 2857, 1740, 1545, 1437, 1373, 1265, 1113. 1H NMR (300 MHz, CDCl3) δ 7.94 (d, J = 9.0 Hz, 1H), 7.70 – 7.31 (m, 13H), 3.90 – 3.27 (m, 7H), 3.62 (s, 3H), 3.05 (dd, J = 6.1, 14.9 Hz, 1H), 2.94 (bs, 1H), 2.76 (t, J = 3.1 Hz, 1H), 2.58 – 2.47 (m, 2H), 2.38 (dd, J = 6.1, 17.8 Hz, 1H), 1.83 – 1.70 (m, 2H), 1.48 – 1.35 (m, 1H), 0.97 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 171.4, 147.6, 135.8, 135.7, 133.8, 131.7, 131.3, 129.6, 129.5, 127.6, 124.2, 79.6, 73.4, 63.9, 51.7, 51.3, 46.3, 40.7, 39.5, 31.5, 27.5, 26.8, 19.2. HRMS (ESI+) calcd for C34H42N2NaO9SSi [M+Na]+: 705.2278. Found: 705.2299.
Methyl 2-((2R,5S,6S)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5-(2-nitro-N-((S)-oxiran-2-ylmethyl)phenylsulfonamido)tetrahydro-2H-pyran-2-yl)acetate 18b
Following the above protocol, 15c (15.9 g, 50.8 mmol, 1.0 equiv) was treated with (R)-glycidyl triflate [(R)-17] (10.5 g, 50.8 mmol, 2.0 equiv), cesium carbonate (33.1 g, 102 mmol, 4.0 equiv) in 1,2-dichloroethane (100 mL). The reaction provided, after purification, 15.0 g (93%) of 18b as a yellow oil. [α]D22 −86.8 (c 1.0, CHCl33). IR νmax (cm−1, film): 2956, 2931, 2857, 1741, 1544, 1428, 1356, 1264, 1164, 1112. 1H NMR (300 MHz, CDCl3) δ 7.88 (d, J = 8.4 Hz, 1H), 7.51 (t, J = 8.4 Hz, 4H), 7.43-7.32 (m, 8H), 7.15 (d, J = 8.4 Hz, 1H), 4.41 (dd, J = 12.1, 3.2 Hz, 1H), 4.03 (dd, J = 12.1, 6.1 Hz, 1H), 3.91 – 3.69 (m, 2H), 3.58 (s, 3H), 3.41 (d, J = 11.2 Hz, 1H), 3.39 – 3.29 (m, 1H), 3.26 – 3.17 (m, 1H), 3.08 – 3.02 (m, 1H), 2.82 (dt, J = 9.0, 4.5 Hz, 2H), 2.66 (dd, J = 4.8, 2.6 Hz, 1H), 2.60 – 2.37 (m, 3H), 2.29 – 1.95 (m, 1H), 1.84 (d, J = 13.3 Hz, 1H), 1.48 (d, J = 12.4 Hz, 1H), 0.92 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 171.4, 155.0, 147.3, 135.9, 135.8, 133.7, 133.5, 132.9, 131.8, 131.4, 129.8, 129.7, 127.8, 127.7, 124.4, 80.0, 79.2, 74.1, 68.7, 63.7, 55.2, 51.9, 49.1, 47.9, 46.7, 44.73, 40.9, 31.8, 29.4, 26.8, 19.3. HRMS (ESI+) calcd for C34H43N2O9SSi [M+H]+ : 683.2459. Found: 683.2468.
Methyl 2-((2S,5S,6S)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5-(2-nitro-N-((R)-oxiran-2-ylmethyl)phenylsulfonamido)tetrahydro-2H-pyran-2-yl)acetate 18c
Following the above protocol, (S,S,S)-15d (10.0 g, 16.0 mmol, 1.0 equiv) was treated with (S)-glycidyl triflate [(S)-17] (6.6 g, 31.9 mmol, 2.0 equiv), cesium carbonate (20.8 g, 63.8 mmol, 4.0 equiv) in DCE (64 mL). The reaction provided, after purification, 10.1 g (93%) of 18c as a yellow oil. [α]D22 +3.2 (c 1.0, CHCl3). IR νmax (cm−1, film) 2931, 2857, 1734, 1543, 1360, 1165, 1110, 1006. 1H NMR (300 MHz, CDCl3) δ 7.97 (dd, J = 7.3, 1.5 Hz, 1H), 7.71 – 7.57 (m, 4H), 7.57 – 7.46 (m, 2H), 7.45 – 7.28 (m, 7H), 4.43 (s, 1H), 4.06 – 3.93 (m, 1H), 3.92 – 3.66 (m, 3H), 3.62 (s, 3H), 3.49 (dd, J = 11.1, 6.6 Hz, 1H), 3.01 (dd, J = 15.8, 7.0 Hz, 1H), 2.93 – 2.71 (m, 2H), 2.67 – 2.48 (m, 2H), 2.13 – 1.84 (m, 2H), 1.79 – 1.52 (m, 2H), 1.00 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 171.5, 147.8, 135.8, 135.8, 133.8, 133.7, 133.6, 133.2, 131.7, 131.3, 129.69, 129.61, 127.69, 127.65, 124.3, 72.8, 68.9, 64.3, 55.3, 51.8, 51.3, 47.9, 46.2, 36.4, 28.3, 26.9, 22.8, 19.3. HRMS (ESI) calcd for C34H42N2NaO9SSi [M+Na]+: 705.2278. Found: 705.2296.
Methyl 2-((2S,5S,6S)-6-(((tert-butyldiphenylsilyl)oxy)methyl)-5-(2-nitro-N-((S)-oxiran-2-ylmethyl)phenylsulfonamido)tetrahydro-2H-pyran-2-yl)acetate 18d
Following the above protocol, 15d (20.1 g, 32.0 mmol, 1.0 equiv) was treated with (R)-glycidyl triflate [(R)-17] (13.2 g, 64.0 mmol, 2.0 equiv), cesium carbonate (41.7 g, 128.0 mmol, 4.0 equiv) in DCE (128 mL). The reaction provided, after purification, 19.3 g (88%) 18d as a yellow oil. [α]D22 −74.5 (c 1.0, CHCl3). IR νmax (cm−1, film) 2931, 2857, 1736, 1542, 1428, 1360, 1165, 1111. 1H NMR (300 MHz, CDCl3) δ 7.96 – 7.86 (m, 1H), 7.53 (dd, J = 15.6, 6.7 Hz, 4H), 7.46 – 7.29 (m, 8H), 4.54 – 4.40 (m, 1H), 3.96 (d, J = 16.4 Hz, 1H), 3.79 (t, J = 7.9 Hz, 1H), 3.63 (s, 3H), 3.40 (d, J = 10.7 Hz, 2H), 3.17 – 3.02 (m, 1H), 3.02 – 2.77 (m, 3H), 2.62 (dd, J = 15.2, 6.6 Hz, 1H), 2.56 – 2.48 (m, 1H), 2.46 – 2.14 (m, 2H), 2.04 – 1.90 (m, 2H), 1.78 – 1.59 (m, 1H), 0.92 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 171.5, 147.4, 135.7, 133.65, 133.61, 133.4, 132.9, 131.6, 131.3, 129.8, 129.6, 127.76, 127.72, 124.3, 71.6, 69.2, 64.0, 55.0, 52.4, 51.8, 48.0, 46.4, 36.1, 28.5, 26.8, 24.8, 19.2. HRMS (ESI) calcd for C34H43N2O9SSi [M+H]+: 683.2459. Found: 683.2444.
Methyl 2-((2R,5S,6S)-6-(hydroxymethyl)-5-(2-nitro-N-((R)-oxiran-2-ylmethyl)phenylsulfonamido)tetrahydro-2H-pyran-2-yl)acetate 19a
To a solution of 18a (5.6 g, 8.2 mmol, 1.0 equiv) in THF (41 mL) was added HF pyridine (70 wt%, 6.1 mL, 49.2 mmol, 6.0 equiv) at rt. The reaction mixture was stirred at rt for 2 h. The mixture reaction was quenched with TMSOMe (17.0 mL, 123.0 mmol, 15.0 equiv) and stirring was continued for 1 h. The solvent was then removed under reduced pressure and the crude residue was purified by chromatography on silica gel (gradient: 0% to 5% MeOH in CH2Cl2), which provided 2.8 g (77%) of 19a as a foamy solid. [α]D22 −22.6 (c 1.0, CHCl3). IR νmax (cm−1, film): 3436, 3350, 2933, 1689, 1609, 1450, 1396, 1241, 1193, 1081. 1H NMR (300 MHz, CDCl3) δ 8.20 – 8.02 (m, 1H), 7.73 – 7.58 (m, 3H), 4.11 – 3.75 (m, 4H), 3.65 (s, 3H), 3.62 – 3.43 (m, 3H), 3.18 – 3.12 (m, 1H), 2.93 – 2.85 (m, 1H), 2.63 – 2.32 (m, 3H), 2.15 – 2.08 (m, 1H), 1.91 – 1.37 (m, 4H). 13C NMR (75 MHz, CDCl3) δ 171.3, 134.2, 132.0, 131.9, 130.9, 124.6, 124.3, 81.2, 78.3, 75.3, 74.6, 74.1, 70.3, 62.2, 51.9, 40.7, 31.7, 31.3, 28.7, 27.7. HRMS (ESI+) calcd for C18H25N2O9S [M+H]+: 445.1281. Found: 445.1288.
Methyl 2-((2R,5S,6S)-6-(hydroxymethyl)-5-(2-nitro-N-((S)-oxiran-2-ylmethyl)phenylsulfonamido)tetrahydro-2H-pyran-2-yl)acetate 19b
Following the above protocol, 18b (11.0 g, 16.1 mmol, 1.0 equiv) was treated with HF pyridine (70 wt%, 10.0 mL, 81.0 mmol, 5.0 equiv) in THF (81 mL). The reaction provided, after purification, 6.3 g (88%) of 19b as a foamy solid. [α]D22 −62.5 (c 1.0, CHCl3). IR νmax (cm−1, film): 3485, 2951, 2874, 1732, 1541, 1373, 1349, 1260, 1163. 1H NMR (300 MHz, CDCl3) δ 8.24 – 8.00 (m, 1H), 7.71 – 7.56 (m, 3H), 3.98 (dd, J = 16.3, 2.6 Hz, 1H), 3.88 – 3.76 (m, 1H), 3.64 (s, 4H), 3.61 – 3.39 (m, 3H), 3.19 – 3.11 (m, 2H), 2.96 – 2.81 (m, 2H), 2.60 (dd, J = 4.5, 2.6 Hz, 1H), 2.46 (ddd, J = 20.8, 15.5, 6.5 Hz, 2H), 2.18 – 1.98 (m, 2H), 1.87 – 1.79 (m, 2H), 1.59 – 1.36 (m, 1H). 13C NMR (75 MHz, CDCl3) δ 171.1, 147.7, 133.9, 133.1, 131.86, 131.83, 124.4, 78.1, 73.9, 62.0, 54.2, 51.8, 51.7, 47.6, 46.6, 40.4, 31.5, 28.4. HRMS (ESI+) calcd for C18H25N2O9S [M+H]+: 445.1281. Found: 445.1281.
Methyl 2-((2S,5S,6S)-6-(hydroxymethyl)-5-(2-nitro-N-((R)-oxiran-2-ylmethyl)phenylsulfonamido)tetrahydro-2H-pyran-2-yl)acetate 19c
Following the above protocol, 18c (10.0 g, 14.6 mmol, 1.0 equiv) was treated with HF pyridine (70 wt%, 5.5 mL, 43.9 mmol, 3.0 equiv) in THF (146 mL). The reaction provided, after purification, 5.7 g (88%) of 19c as a foamy solid. [α]D22 +77.1 (c 1.0, CHCl3). IR νmax (cm−1, film) 3537, 2951, 1732, 1543, 1372, 1165. 1H NMR (300 MHz, CDCl3) δ 8.11 (dd, J = 7.3, 1.7 Hz, 1H), 7.80 – 7.57 (m, 3H), 4.40 (s, 1H), 4.16 (d, J = 16.0 Hz, 1H), 4.03 – 3.91 (m, 1H), 3.89 – 3.70 (m, 2H), 3.67 (s, 3H), 3.24 – 3.06 (m, 1H), 3.00 – 2.72 (m, 3H), 2.71 – 2.41 (m, 3H), 2.08 – 1.80 (m, 2H), 1.76 – 1.55 (m, 2H), 1.53 – 1.35 (m, 1H). 13C NMR (75 MHz, CDCl3) δ 171.6, 148.0, 134.2, 132.0, 131.8, 124.5, 72.0, 69.0, 62.3, 54.3, 51.9, 51.8, 48.0, 46.1, 35.9, 28.3, 21.8. HRMS (ESI) calcd for C18H24N2NaO9S [M+Na]+: 467.1100. Found: 467.1108.
Methyl 2-((2S,5S,6S)-6-(hydroxymethyl)-5-(2-nitro-N-((S)-oxiran-2-ylmethyl)phenylsulfonamido)tetrahydro-2H-pyran-2-yl)acetate 19d
Following the above protocol, 18d (19.3 g, 28.3 mmol, 1.0 equiv) was treated with HF pyridine (70 wt%, 10.5 mL, 85.0 mmol, 3.0 equiv) in THF (283 mL). The reaction provided, after purification, 9.9 g (79%) of 19d as a foamy solid. [α]D22 −57.8 (c 1.0, CHCl3). IR νmax (cm−1, film) 3500, 2951, 1731, 1541, 1439, 1348, 1163. 1H NMR (300 MHz, CDCl3) δ 8.18 – 8.05 (m, 1H), 7.77 – 7.60 (m, 3H), 4.36 (s, 1H), 4.16 (d, J = 16.2 Hz, 1H), 3.92 – 3.75 (m, 1H), 3.62 (s, 4H), 3.47 – 3.31 (m, 1H), 3.25 – 3.07 (m, 2H), 3.03 – 2.78 (m, 3H), 2.72 – 2.61 (m, 1H), 2.54 (dd, J = 15.0, 5.7 Hz, 1H), 2.39 – 2.16 (m, 1H), 2.08 – 1.88 (m, 3H), 1.80 – 1.62 (m, 1H). 13C NMR (75 MHz, CDCl3) δ 171.6, 147.8, 134.1, 133.1, 132.0, 131.9, 124.5, 70.8, 69.2, 61.8, 53.9, 52.2, 51.9, 48.1, 46.6, 35.9, 28.4, 24.3. HRMS (ESI) calcd for C18H24N2NaO9S [M+Na]+ : 467.1100. Found: 467.1089.
Methyl 2-((3R,6aS,8R,10aS)-3-hydroxy-1-((2-nitrophenyl)sulfonyl)decahydropyrano[2,3-c][1,5]oxazocin-8-yl)acetate 6a
To 19a (5.0 g, 11.3 mmol) in CH2Cl2 (112 mL) was added BF3-Et2O (1.6 ml, 12.4 mmol, 1.1 equiv) at rt. After 2 h, the reaction was concentrated under reduced pressure to afford a light brown residue, which was purified by chromatography on silica gel (gradient: 40% to 90% EtOAc in hexanes) to provide 3.2 g (64%) of 6a as a white powder. [α]D22 +69.5 (c 1.0, CHCl3). IR νmax (cm−1, film): 3516, 2951, 2874, 1735, 1532, 1439, 1346, 1160, 1058. 1H NMR (300 MHz, CDCl3) δ 8.07 (d, J = 8.3 Hz, 1H), 7.83 – 7.47 (m, 3H), 3.99 – 3.68 (m, 6H), 3.63 (s, 3H), 3.58 – 3.47 (m, 3H), 3.41 – 3.21 (m, 2H), 2.41 (ddd, J = 20.9, 15.5, 6.5 Hz, 2H), 1.68 (d, J = 16.3 Hz, 2H), 1.52 – 1.21 (m, 2H). 13C NMR (75 MHz, CDCl3) δ 171.3, 148.2, 134.4, 132.0, 131.1, 128.5, 124.4, 81.2, 77.4, 75.2, 73.4, 69.6, 58.8, 51.9, 51.8, 40.6, 31.4, 27.1. HRMS (ESI+) calcd for C18H25N2O9S [M+H]+: 445.1281. Found: 445.1276.
Methyl 2-((3S,6aS,8R,10aS)-3-hydroxy-1-((2-nitrophenyl)sulfonyl)decahydropyrano[2,3-c][1,5]oxazocin-8-yl)acetate 6b
Following the above protocol, 19b (6.0 g, 13.5 mmol, 1.0 equiv) was treated with BF3-Et2O (0.34 ml, 2.7 mmol, 0.2 equiv) in CH2Cl2 (270 mL). The reaction provided, after purification, 2.6 g (43%) of 6b as a 9:1 mixture with 20b. 6b (white powder): [α]D22 +122.5 (c 1.0, CHCl3). IR νmax (cm−1, film): 3436, 2951, 2871, 1734, 1536, 1439, 1372, 1351, 1185, 1065. 1H NMR (300 MHz, CDCl3) δ 8.04 (d, J = 8.2 Hz, 1H), 7.73 – 7.62 (m, 2H), 7.59 (d, J = 9.0 Hz, 1H), 4.10 - 4.02 (m, 1H), 3.90 - 3.75 (m, 5H), 3.64 (s, 3H), 3.62 – 3.40 (m, 3H), 2.95 (b, 1H), 2.50 (dd, J = 15.5, 7.6 Hz, 1H), 2.35 (dd, J = 15.5, 5.3 Hz, 2H), 1.72 – 1.26 (m, 4H). 13C NMR (75 MHz, CDCl3) δ 171.4, 148.1, 134.2, 134.1, 131.9, 130.9, 128.5, 124.30, 80.9, 75.2, 74.6, 70.1, 58.7, 51.9, 47.9, 40.7, 31.3, 27.7. HRMS (ESI+) calcd for C18H25N2O9S [M+H]+ : 445.1281. Found: 445.1271.
Methyl 2-((3R,6aS,8S,10aS)-3-hydroxy-1-((2-nitrophenyl)sulfonyl)decahydropyrano[2,3-c][1,5]oxazocin-8-yl)acetate 6c
Following the general reaction protocol, 19c (5.3 g, 11.8 mmol, 1.0 equiv) was treated with BF3-Et2O (0.30 ml, 2.4 mmol, 0.2 equiv) in CH2Cl2 (236 mL). The reaction provided, after purification, 2.8 g (53%) of 6c as as a white powder. [α]D22 +149.8 (c 1.0, CHCl3). IR νmax (cm−1, film) 3516, 2950, 1733, 1542, 1439, 1344, 1160. 1H NMR (300 MHz, CDCl3) δ 8.10 (d, J = 7.1 Hz, 1H), 7.80 – 7.56 (m, 3H), 4.47 – 4.23 (m, 1H), 4.00 – 3.71 (m, 4H), 3.67 (s, 3H), 3.63 – 3.43 (m, 3H), 2.80 (dd, J = 14.5, 8.2 Hz, 2H), 2.48 (dd, J = 14.5, 6.9 Hz, 2H), 2.05 – 1.80 (m, 2H), 1.69 (s, 1H), 1.59 – 1.45 (m, 1H), 1.42 – 1.15 (m, 1H). 13C NMR (75 MHz, CDCl3) δ 171.1, 148.2, 134.3, 131.9, 130.9, 128.4, 124.4, 77.3, 73.4, 73.2, 70.0, 69.5, 52.0, 35.9, 28.4, 23.1. HRMS (ESI) calcd for C18H24N2NaO9S [M+Na]+ : 467.1100. Found: 467.1107.
Methyl 2-((3S,6aS,8S,10aS)-3-hydroxy-1-((2-nitrophenyl)sulfonyl)decahydropyrano[2,3-c][1,5]oxazocin-8-yl)acetate 6d
Following the above protocol, 19d (4.2 g, 9.5 mmol, 1.0 equiv) was treated with BF3-Et2O (0.24 ml, 1.9 mmol, 0.2 equiv) in CH2Cl2 (189 mL). The reaction provided, after purification, 2.35 g (56%) of 6d as a 9:1 mixture with 20d as a white powder: [α]D22 +146.0 (c 1.0, CHCl3). IR νmax (cm−1, film) 3432, 2950, 1732, 1542, 1439, 1371, 1348, 1161. 1H NMR (300 MHz, CDCl3) δ 8.16 – 7.98 (m, 1H), 7.80 – 7.65 (m, 2H), 7.64 – 7.52 (m, 1H), 4.36 (dd, J = 13.7, 6.8 Hz, 1H), 4.17 – 3.98 (m, 1H), 3.97 – 3.70 (m, 4H), 3.66 (s, 3H), 3.63 – 3.39 (m, 3H), 3.03 (s, 1H), 2.83 (dd, J = 14.5, 8.4 Hz, 1H), 2.57 – 2.28 (m, 2H), 1.97 – 1.82 (m, 1H), 1.80 – 1.43 (m, 2H), 1.42 – 1.24 (m, 1H). 13C NMR (75 MHz, CDCl3) δ 171.3, 148.0, 134.1, 134.0, 131.9, 130.8, 124.2, 74.4, 72.8, 70.0, 69.9, 59.1, 52.0, 35.9, 28.2, 23.6. HRMS (ESI) calcd for C18H25N2O9S [M+H]+ : 445.1281. Found: 445.1293.
Supplementary Material
ACKNOWLEDGEMENT
This work was funded in part by the NIGMS-sponsored Center of Excellence in Chemical Methodology and Library Development (Broad Institute CMLD; P50 GM069721), as well as the NIH Genomics Based Drug Discovery U54 grants Discovery Pipeline RL1CA133834 (administratively linked to NIH grants RL1HG004671, RL1GM084437, and UL1DE019585). High-resolution mass spectra were obtained at the Boston University Chemical Instrumentation Center. x-ray crystallographic analysis was performed by Dr. Peter Muller at the MIT x-ray crystallographic laboratory.
Footnotes
Supporting Information 1H and 13C NMR spectra for all new compounds and x-ray crystallographic information for select compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1 (a).Marcaurelle LA, Comer E, Dandapani S, Duvall JR, Gerard B, Kesavan S, Lee MD, IV, Liu H, Lowe JT, Marié J-C, Mulrooney CA, Pandya BA, Rowley A, Ryba TD, Suh B-C, Wei J, Young DW, Akella LB, Ross NB, Zhang Y-L, Fass DM, Reis SA, Zhao W-N, Haggarty SJ, Palmer M, Foley MA. J. Am. Chem. Soc. 2010;132:16962–16976. doi: 10.1021/ja105119r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fitzgerald MF, Mulrooney CA, Duvall JR, Wei J, Suh B-C, Akella LB, Vrcic A, Marcaurelle LA. ACS Comb. Sci. 2012;14:89–96. doi: 10.1021/co200161z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gerard B, Duvall JR, Lowe JT, Murillo T, Wei J, Akella LB, Marcaurelle LA. ACS. Comb. Sci. 2011;13:365–374. doi: 10.1021/co2000218. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Comer E, Liu H, Joliton A, Clabaut A, Johnson C, Akella LB, Marcaurelle L. A. Proc Natl. Acad Sci USA. 2011, 108, 6751-6756. (e) Lowe, J. T., Lee MD, IV, Akella LB, Davoine E, Donckele EJ, Durak L, Duvall JR, Gerard B, Holson EB, Joliton A, Kesavan S, Lemercier BC, Liu H, Marié J-C, Mulrooney CA, Muncipinto G, Welzel-O’Shea M, Panko LM, Rowley A, Suh B-C, Thomas M, Wagner FF, Wei J, Foley MA, Marcaurelle LA. J. Org. Chem. 2012;77:7187–7211. [Google Scholar]
- 2 (a).Schreiber SL. Science. 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]; (b) Hale KJ, Hummersone MG, Manaviazar S, Frigerio M. Nat. Prod. Rev. 2002;19:413–453. doi: 10.1039/b009211h. [DOI] [PubMed] [Google Scholar]; (c) Pietruszka J. Angew. Chem. Int. Ed. 1998;37:2629–2636. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2629::AID-ANIE2629>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]; (d) Cereghetti DM, Carreira EM. Synthesis. 2006;6:914–942. [Google Scholar]; (e) Smith AB, Dong S, Brenneman JB, Fox RJ. J. Am. Chem. Soc. 2009;131:12109–12111. doi: 10.1021/ja906115a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Nicolaou KC, Ajito K, Patron AP, Khatuya H, Richter PK, Bertinato P. J. Am. Chem. Soc. 1996;118:3059–3060. [Google Scholar]; (g) Hoye TR, Danielson ME, May AE, Zhao H. J. Org. Chem. 2010;75:7052–7060. doi: 10.1021/jo101598y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Jackson KL, Henderson JA, Phillips AJ. Chem. Rev. 2009;109:3044–3079. doi: 10.1021/cr900016w. [DOI] [PubMed] [Google Scholar]
- 3.Gerard B, Marie J-M, Pandya B, Lee MD, IV, Liu H, Marcaurelle LA. J. Org. Chem. 2011;76:1898–1901. doi: 10.1021/jo1022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4 (a).McCalion G. Curr. Org. Chem. 1999;3:67–79. [Google Scholar]; (b) Welsch ME, Snyder SA, Stockwell BR. Curr. Opin. Chem. Biol. 2010;14:347–361. doi: 10.1016/j.cbpa.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sunden H, Osslon R. Org. Biomol. Chem. 2010;8:4831–4833. doi: 10.1039/c0ob00331j. [DOI] [PubMed] [Google Scholar]
- 5 (a).Rowlands GJ. Tetrahedron. 2010;66:1593–1636. [Google Scholar]; (b) Giese B, Kopping B, Gobel T, Dickhaut J, Thomas G, Kulicke KJ, Trach J. Org. React. 1996;48 [Google Scholar]
- 6.Rodriguez V, Quintero L, Sartillo-Piscil F. Tetrahedron Lett. 2007;48:4305–4308. [Google Scholar]
- 7 (a).Parker KA, Spero DM, Van Epp J. J. Org. Chem. 1988;53:4628–4630. [Google Scholar]; (b) Parker KA, Fokas D. J. Am. Chem. Soc. 1992;114:9689–9691. [Google Scholar]
- 8.For an example of nitro reduction in presence of zinc metal, see: Comer E, Rohan E, Deng L, Porco JA. Org. Lett. 2007;9:2123–2126. doi: 10.1021/ol070606t.
- 9 (a).Corey EJ, Suggs JW. J. Org. Chem. 1975;40:2554–2555. [Google Scholar]; (b) Stork G, Sher PM. J. Am. Chem. Soc. 1986;108:303–304. [Google Scholar]
- 10.Attrill RP, Blower MA, Mulholland KR, Roberts JK, Richardson JE, Teasdale MJ, Wanders A. Org. Proc. Res. Dev. 2000;4:98–101. [Google Scholar]
- 11.See Supporting Information for x-ray crystal structure of benzofuran 3c.
- 12.See ref. 1a and 1c. See also Loh JK, Yoon SY, Samarakoon TB, Rolfe A, Porubsky P, Neuenswander B, Lushinton GH, Hanson PR. Beilstein J. Org. Chem. 2012;8:1293–1302. doi: 10.3762/bjoc.8.147.
- 13 (a).For reviews on the synthesis of biaryl ethers via intramolecular SNAr, see: Rao AVR, Gurjar MK, Reddy L, Rao AS. Chem. Rev. 1995;95:2135–2167.. Burgess K, Lim D, Martinez CI. Angew. Chem., Int. Ed. 1996;35:1077–1078.. Zhu J. Synlett. 1997:133–144.. Nicolaou KC, Boddy CNC, Bräse S, Winssinger N. Angew. Chem., Int. Ed. 1999;38:2096–2152. doi: 10.1002/(sici)1521-3773(19990802)38:15<2096::aid-anie2096>3.0.co;2-f.. Sawyer JS. Tetrahedron. 2000;56:5045–5065.. Goldberg M, Smith L, II, Tamayo N, Kiselyov AS. Tetrahedron. 1999;55:13887–13898.. Jefferson EA, Swayze E. E. Tetrahedron Lett. 1999, 40, 7757-7760. (h) Temal-Laib, T., Chastanet J, Zhu J. J. Am. Chem. Soc. 2002;124:583–590. doi: 10.1021/ja0170807.. Tempest P, Ma V, Kelly MG, Jones W, Hulme C. Tetrahedron Lett. 2001;42:4963–4968.
- 14.Abrous L, Jokiel PA, Friedrich SR, Hynes J, Jr., Smith AB, III, Hirschman R. J. Org. Chem. 2004;69:280–302. doi: 10.1021/jo0352068. [DOI] [PubMed] [Google Scholar]
- 15.See Supporting Information for x-ray crystal structure of lactam 14b.
- 16 (a).Lowe JT, Lee IV MD, Akella LB, Davoine E, Donckele EJ, Durak L, Duvall JR, Gerard B, Holson EB, Joliton A, Kesavan S, Lemercier BC, Liu H, Marie J-C, Mulrooney CA, Muncipinto G, Welzel-O’Shea M, Panko LM, Rowley A, Suh B-C, Thomas M, Wagner FF, Wei J, Foley MA, Marcaurelle LA. J. Org. Chem. 2012;77:7187–7211. doi: 10.1021/jo300974j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Brandi A, Cicchi S, Cordero FM. Chem. Rev. 2008;108:3988–4035. doi: 10.1021/cr800325e. [DOI] [PubMed] [Google Scholar]; (c) Couty F, Evano G, Prim D. Mini-Rev. Org. Chem. 2004;1:133–148. [Google Scholar]
- 17 (a).For uses of chiral epoxide in natural product synthesis, see: Grove CI, Di Maso MJ, Jaipuri FA, Kim MB, Shaw JT. Org. Lett. 2012;14:4338–4341. doi: 10.1021/ol301743t.. Jeker OF, Carreira EM. Ang. Chem., Int. Ed. 2012;51:3474–3477. doi: 10.1002/anie.201109175.. Vilotijevic I, Jamison TF. Angew. Chem. Int. Ed. 2009;48:5250–5281. doi: 10.1002/anie.200900600.. xia QH, Ge HQ, Ye CP, Liu ZM, Su K. x. Chem. Rev. 2005;105:1603–1662. doi: 10.1021/cr0406458.
- 18 (a).Rolfe A, Samarakoon TB, Hanson PR. Org. Lett. 2010;12:1216–1219. doi: 10.1021/ol100035e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Organ MG, Hanson PR, Rolfe A, Samarakoon TB, Ullah F. J. Flow Chem. 2011;1:32–39. doi: 10.1556/jfchem.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19 (a).Baldwin JJ, McClure DE, Gross DM, Williams M. J. Med. Chem. 1982;25:931–936. doi: 10.1021/jm00350a009. [DOI] [PubMed] [Google Scholar]; (b) Du Y, Zheng J-F, Wang Z-G, Jiang L-J, Ruan Y-P, Haung P-Q. J. Org. Chem. 2010;75:4619–4622. doi: 10.1021/jo1007042. [DOI] [PubMed] [Google Scholar]
- 20.For an example of a 7-exo epoxide-opening/ring closing reaction, see: Matsumura R, Suzuki T, Sato K, Oku KI, Hagiwara H, Hoshi T, Ando M, Kamat VP. Tetrahedron Lett. 2000;41:7701–7704.
- 21.Sánchez I, Pujol MD, Guillaumet G, Massingham R, Monteil A. Sci. Pharma. 2001;69:11–19. [Google Scholar]
- 22.See Supporting Information for x-ray crystal structure of oxazocane 6a.
- 23.Sauer WHB, Schwarz MK. J. Chem. Inf. Comput. Sci. 2003;43:987–1003. doi: 10.1021/ci025599w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.