Abstract
Non-melanoma skin cancers (NMSCs) and psoriasis represent common hyperproliferative skin disorders, with approximately one million new NMSC diagnoses each year in the United States alone and a psoriasis prevalence of about 2% worldwide. We recently demonstrated that the glycerol channel, aquaporin-3 (AQP3) and the enzyme phospholipase D2 (PLD2) interact functionally in epidermal keratinocytes of the skin to inhibit their proliferation. However, others have suggested that AQP3 is pro-proliferative in keratinocytes and is upregulated in the NMSC, squamous cell carcinoma (SCC). To evaluate the AQP3/PLD2 signaling module in skin diseases, we determined their levels in SCC, basal cell carcinoma (BCC) and psoriasis compared to normal epidermis. Skin biopsies with the appropriate diagnoses (ten normal, five SCC, thirteen BCC and ten plaque psoriasis samples) were obtained from the pathology archives and examined by immunohistochemistry using antibodies recognizing AQP3 and PLD2. In normal epidermis AQP3, an integral membrane protein, was localized mainly to the plasma membrane and PLD2 to the cell periphery, particularly in suprabasal layers. In BCC, AQP3 and PLD2 levels were reduced compared to the normal-appearing overlying epidermis. In SCC, AQP3 staining was “patchy,” with areas of reduced AQP3 immunoreactivity exhibiting positivity for Ki67, a marker of proliferation. PLD2 staining was unchanged in SCC. In psoriasis, AQP3 staining was usually observed in the cytoplasm rather than in the membrane. Also, in the majority of psoriatic samples, PLD2 showed weak immunoreactivity or aberrant localization. These results suggest that abnormalities in the AQP3/PLD2 signaling module correlate with hyperproliferation in psoriasis and the NMSCs.
Keywords: (aquaporin-3), (basal cell carcinoma), epidermis, keratinocytes, (phospholipase D2), skin, (skin cancer), (squamous cell carcinoma)
INTRODUCTION
Non-melanoma skin cancer (NMSC) is one of the most common neoplasms in humans, with a million new cases diagnosed each year in the United States alone and an incidence that is on the rise [22]. NMSC can lead to major cosmetic deformities and sporadic mortality. In the two most common NMSCs, basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), the major cell type in the epidermis, keratinocytes fail to differentiate and exhibit hyperproliferation. Keratinocytes continually regenerate through regulated proliferation followed by differentiation into the suprabasal epidermal cell types. In the integumentary differentiation program, following cell division, early differentiation gives rise to the stratum spinosum, late differentiation generates the stratum granulosum, and terminal differentiation occurs in the stratum corneum, the outermost layer of the epidermis (reviewed in [3,1]). This pattern of proliferation and differentiation is essential for the protective barrier function of skin. Skin disorders, including NMSCs such as SCC and BCC, can result from dysregulation of the homeostasis of keratinocyte proliferation and differentiation (reviewed in [4,2]). Although environmental insults are known to predispose to skin carcinogenesis, the mechanisms underlying the development and progression of BCC and SCC remain unclear.
Similar to the keratinocyte-derived carcinomas BCC and SCC, psoriasis is a common disabling hyperproliferative skin disorder in which keratinocytes fail to differentiate and proliferate excessively. Although the autoimmune etiology of psoriasis is well-appreciated (reviewed in [9]), with the recent creation of genetically engineered mouse models, it has become clear that changes in the keratinocytes themselves can also trigger a psoriasis-like condition (reviewed in [12]). For example, mice in which genes for the transcription factors c-jun and JunB are deleted only in keratinocytes (i.e., an epidermal-targeted conditional c-jun/JunB knockout mouse model) exhibit a hyperproliferative epidermis and other characteristics of a psoriasiform phenotype [36]. Thus, current research has suggested interplay between keratinocytes and immune cells, with the resulting crosstalk leading to hyperproliferation of keratinocytes in psoriasis [32]. Nevertheless, the mechanism underlying uncontrolled proliferation and abnormal differentiation of keratinocytes in psoriasis, as well as the etiology of the disease itself, is still unclear.
Aquaporin-3 (AQP3), an aquaglyceroporin, has recently been ascribed a potential role in skin function (reviewed in [16]). AQP3 is found in the basal cell layer of the epidermis, as well as suprabasal layers [21], and is an efficient transporter of glycerol and water [34]. AQP3 null mice exhibit reduced epidermal glycerol content, impaired skin elasticity, delayed barrier recovery following stratum corneum removal, and delayed wound healing [13]. These phenotypes suggest that AQP3 plays a role in differentiation and proliferation of keratinocytes. Interestingly, topical or oral application of glycerol has been shown to correct defects present in AQP3 null mice including skin hydration, elasticity, and barrier function [14]. Likewise, in the cosmetic industry, glycerol has been used in moisturizers and other topical skin therapies to promote skin repair ([7] and reviewed in [8]). Our laboratory has demonstrated that glycerol, a physiologically relevant primary alcohol, can be utilized by phospholipase D2 (PLD2) to form phosphatidylglycerol both in vitro and in intact keratinocytes [38]. In addition, PLD2 and AQP3 are colocalized in lipid rafts and co-precipitate from these rafts in a protein-mediated manner [37], and we have proposed that together they comprise a signaling module in which AQP3 transports glycerol to PLD2 to synthesize phosphatidylglycerol (reviewed in [4]). Phosphatidylglycerol production is increased by elevated extracellular calcium concentrations that promote keratinocyte differentiation (and inhibit proliferation), and manipulation of this signaling module, either by increasing exogenous glycerol, co-overexpression of AQP3 or direct provision of phosphatidylglycerol, induces differentiation and inhibits proliferation in rapidly dividing cells [38,5].
On the other hand, in a recent study, Verkman and colleagues [17] demonstrated that AQP3 null mice exhibit resistance to skin tumorigenesis, although the described experiments did not determine whether the effect is cell autonomous (i.e., due to the lack of AQP3 in keratinocytes) or non-cell autonomous (for instance, related to changes in the inflammatory response in these mice with a global deletion of AQP3). These authors also reported that RNA interference-mediated knockdown of AQP3 inhibits proliferation in human keratinocytes [15], suggesting that AQP3 promotes proliferation and is pro-tumorigenic. In support of this idea these authors demonstrated that AQP3 protein is strongly expressed in SCC in regions characterized by keratin 14 expression. However, although keratin 14 expression is considered a marker of basal, proliferating keratinocytes, Fuchs and colleagues demonstrated that in normal epidermis keratin 14 protein is found in both basal and suprabasal (spinous) layers [30]. Furthermore, keratin 14 staining is observed in SCCs of all stages of differentiation (from poorly differentiated to well differentiated tumors) [30]. This latter result is consistent with the findings of Perkins et al. [25], who reported increased keratin 14 staining in the more differentiated areas of SCCs, and suggests that keratin 14 protein expression cannot be used as a marker of proliferation in SCC.
The potential involvement of AQP3 in other skin diseases is controversial. For example, Olsson et al. [24] and Nakahigashi et al. [23] have reported increased AQP3 expression in atopic eczema (dermatitis) whereas Boury-Jamot et al. [6] demonstrated a down-regulation of AQP3 in eczema (reviewed in [26]). In keratinocytes from depigmented vitiligo lesions down-regulation of AQP3 has also been observed, and in conjunction with reductions in the levels of E-cadherin, β- and γ-catenins and phosphorylated (active) phosphoinositide 3-kinase, this diminished AQP3 protein expression was suggested to decrease keratinocyte survival. The loss of keratinocytes and keratinocyte-derived growth factors presumably underlies the passive cell death of melanocytes resulting in the depigmentation seen in vitiligo lesions [20]. On the other hand, to our knowledge there is no information in the literature concerning the possible role of PLD2 or the AQP3/PLD2 signaling module in skin diseases.
In contrast, our data concerning the differentiation-promoting role of the AQP3/PLD2 signaling module [5] in keratinocytes would predict that in hyperproliferative skin diseases AQP3 levels might be decreased. Alternatively, the ratio of AQP3 to PLD2 and/or the localization of these two proteins might be critical for their functional interaction and effects on proliferation versus differentiation. Therefore, the goal of this study was to examine the protein expression and localization of AQP3 and PLD2 in SCC, BCC and psoriasis relative to normal epidermis.
MATERIALS AND METHODS
Tissue Samples
The retrospective characterization of de-identified patient specimens was approved by the Medical College of Georgia institutional review board. Paraffin-embedded tissue sections of skin with a diagnosis of SCC, BCC, or plaque psoriasis, as determined and verified by a dermatopathologist (DJS), and normal epidermis were obtained from the tissue archives of the Medical College of Georgia Department of Pathology. The majority of the BCC samples were of the nodular type. Normal epidermis was obtained from samples removed during breast reduction surgery or from traumatic amputation specimens. Four micron thick sections were cut and mounted on slides by conventional histologic techniques.
AQP3 and PLD2 Immunohistochemistry
Slides for AQP3 staining were deparaffinized, washed twice for 5 minutes in phosphate-buffered saline (PBS), incubated 30 minutes in 3% hydrogen peroxide, washed twice for 5 minutes in PBS, incubated 1 hour in 0.3% goat serum, and then incubated overnight with anti-AQP3 (Alomone Labs, Jerusalem, Israel; 1:1000 dilution) in a humidified chamber at 4°C. Following primary antibody incubation, an ABC staining kit (Santa Cruz Biotechnology, Santa Cruz, CA) was used to visualize immunoreactivity by incubation with an appropriate secondary antibody and development with the chromogen 3,3’-diaminobenzidine (DAB) for 3 minutes. After deparaffinization, slides used for PLD2 staining were incubated three times for 10 min in boiling sodium citrate buffer, then stained with anti-PLD2 (Abcam, Cambridge, MA; 1:50 dilution) and visualized (DAB for 10 minutes) using the same methods as for AQP3. All slides were dehydrated, counterstained with hematoxylin, and coverslipped by the Medical College of Georgia Histology Core Facility.
Ki67 Immunohistochemistry
Ki67 (catalog # M7240, DakoUSA, Carpinteria, CA) immunostaining was performed according to the supplier's protocol. AQP3 and Ki67 staining were performed on sequential serial SCC sections.
Statistical Analysis
In the BCC slides, staining intensity in the normal-appearing overlying epidermis and in the lesion were estimated by three independent observers, with 0 representing an absence of staining and 3 representing intense staining. The individual scores were averaged and the difference analyzed for statistical significance with an unpaired Student's t-test using the program InStat (Graphpad, La Jolla, CA).
RESULTS
AQP3 Expression
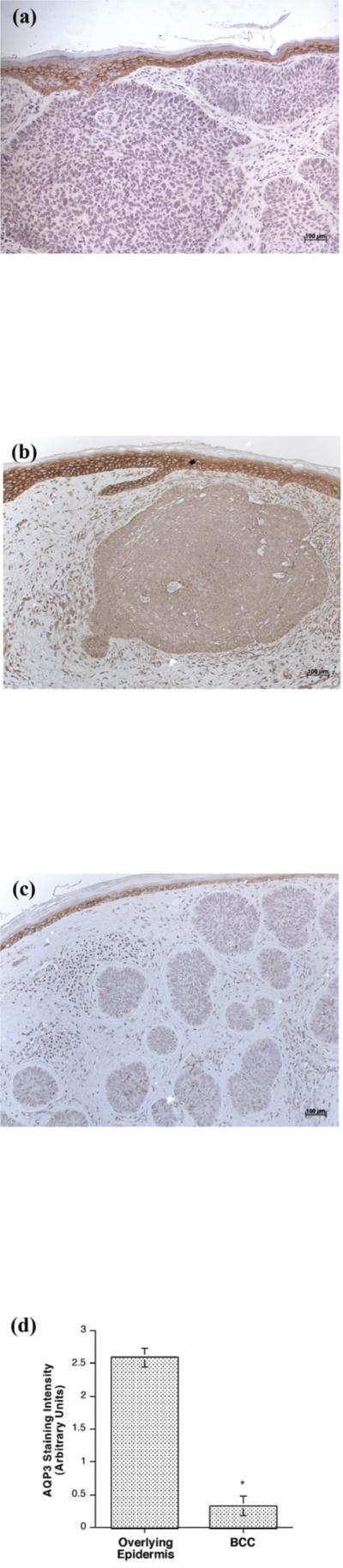
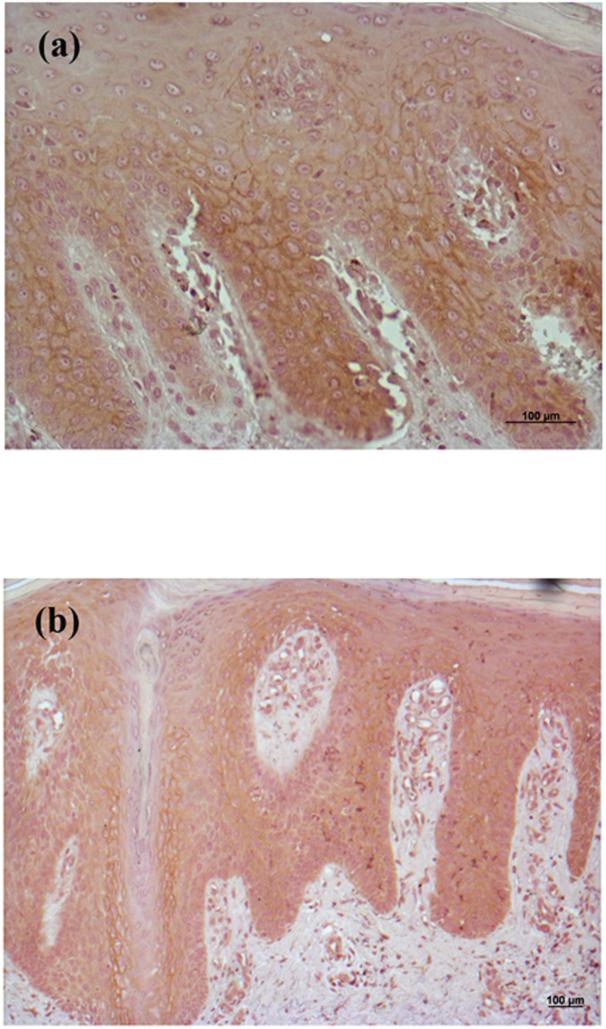
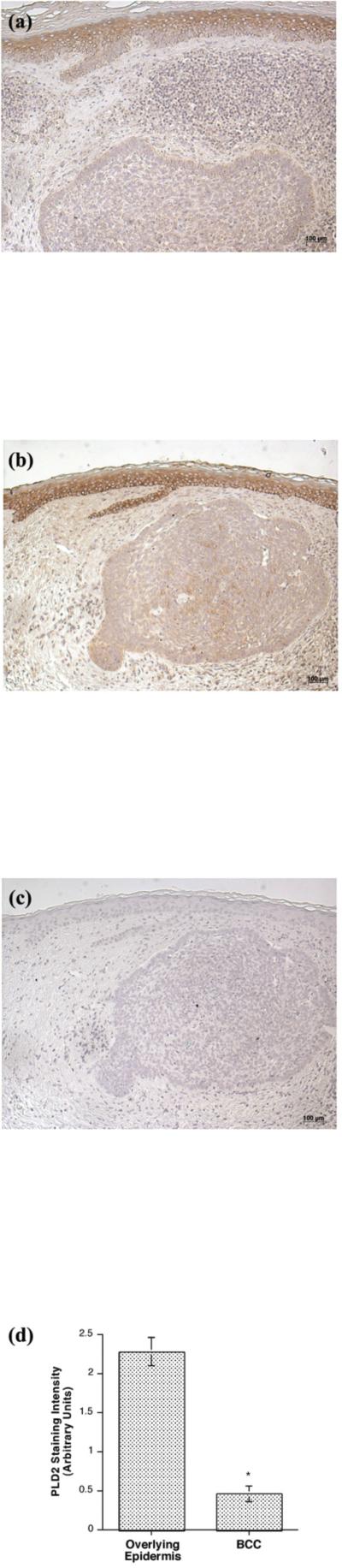
Whereas we have hypothesized that the PLD2/AQP3 signaling module is anti-proliferative and pro-differentiative [5], Verkman and colleagues have proposed instead that AQP3 is pro-proliferative [15,17]. These authors also report that SCC express high levels of AQP3 and suggest that this result also supports the idea that AQP3 promotes proliferation [17]. In an attempt to resolve this issue, we examined the expression of AQP3 in the non-melanoma skin cancers BCC and SCC by immunohistochemistry. This analysis showed predominant AQP3 expression in the plasma membrane (essentially outlining the cells) of basal keratinocytes in the normal-appearing overlying epidermis observed in many BCC sections (Figure 1), consistent with the fact that AQP3 is an integral membrane protein. In BCC, AQP3 was down-regulated and in some cases was completely absent despite readily apparent staining in the normal-appearing overlying epidermis. We observed reduced or absent AQP3 staining in all 13 of the basal cell carcinomas examined (13/13), with an average decrease of approximately 8-fold (Figure 1D) [normal: 2.59 ± 0.14 versus lesion: 0.33 ± 0.15 (p<0.001).] T
Figure 1. AQP3 Immunoreactivity was Reduced or Absent in Basal Cell Carcinoma (BCC).
Archived paraffin-embedded basal cell carcinoma samples were sectioned (4 μm), deparaffinized, stained with antibodies recognizing AQP3 and visualized with an ABC kit and 3,3’-diaminobenzidine (DAB). Sections were counterstained with hematoxylin. Results illustrate three BCCs from different patients and are representative of 13 of 13 BCCs. The results were quantified as 3+ (intense staining), 2+ (moderate staining), + (weak staining) or 0 (no staining) by three independent observers and the values averaged. Shown in panel D is the quantitation (means ± SEM) of the 13 BCCs relative to normal-appearing overlying epidermis with the data analyzed using a student's t-test; *p<0.05. A negative control in which the primary antibody was omitted showed no AQP3 staining (data not shown).
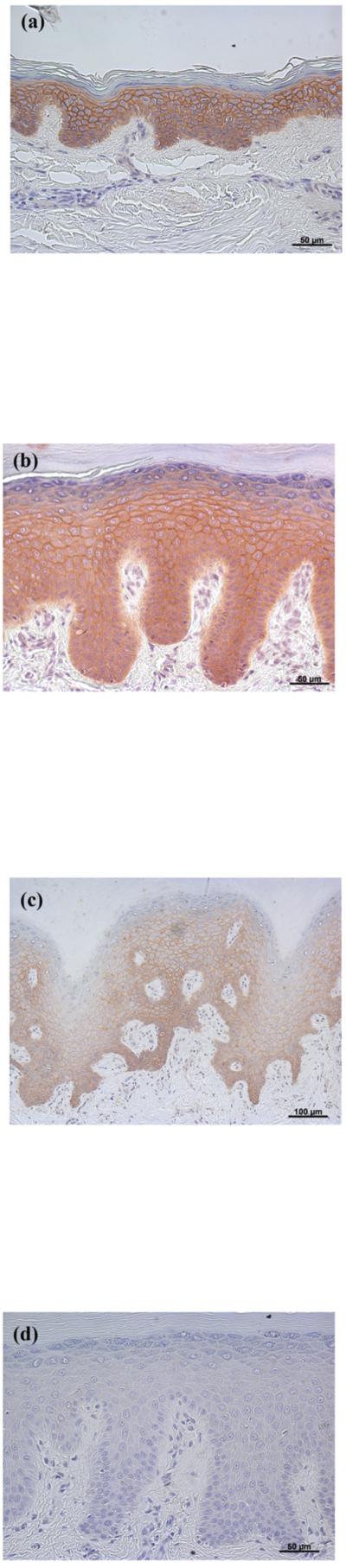
Next, the staining pattern observed in the normal-appearing overlying epidermis was compared with that found in normal epidermis obtained from breast reduction surgery or traumatic amputation. The normal-appearing overlying epidermis and normal epidermis (Figure 2) were shown to have similar AQP3 localization, with well-defined plasma membrane staining outlining individual keratinocytes in the majority of the normal epidermal specimens.
Figure 2. AQP3 Immunoreactivity was Localized Largely to the Plasma Membrane in Normal Epidermis (as in Normal-appearing Epidermis Overlying Basal Cell Carcinoma).
Archived paraffin-embedded normal human epidermal samples were sectioned (4 μm) and deparaffinized. Sections were then stained with an antibody recognizing AQP3 and visualized with an ABC kit and DAB. Note that AQP3 staining localizes to the plasma membrane, essentially outlining the cells, as observed also in normal-appearing overlying epidermis in basal cell carcinoma (Figure 1). AQP3 staining of three patients (representative of 10) is illustrated in panels A through C and panel D shows a negative control in which the primary antibody was omitted.
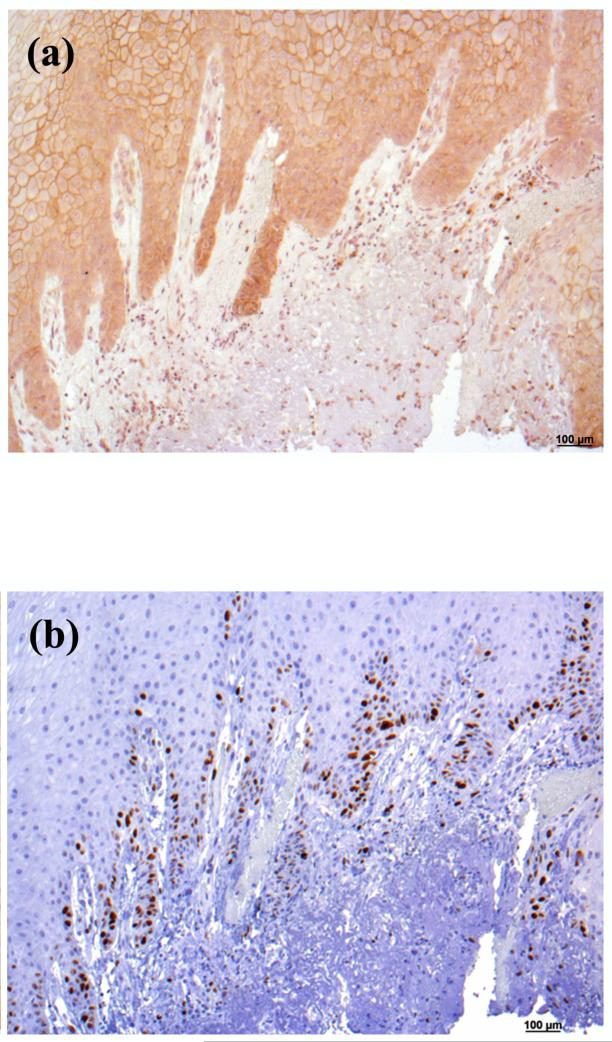
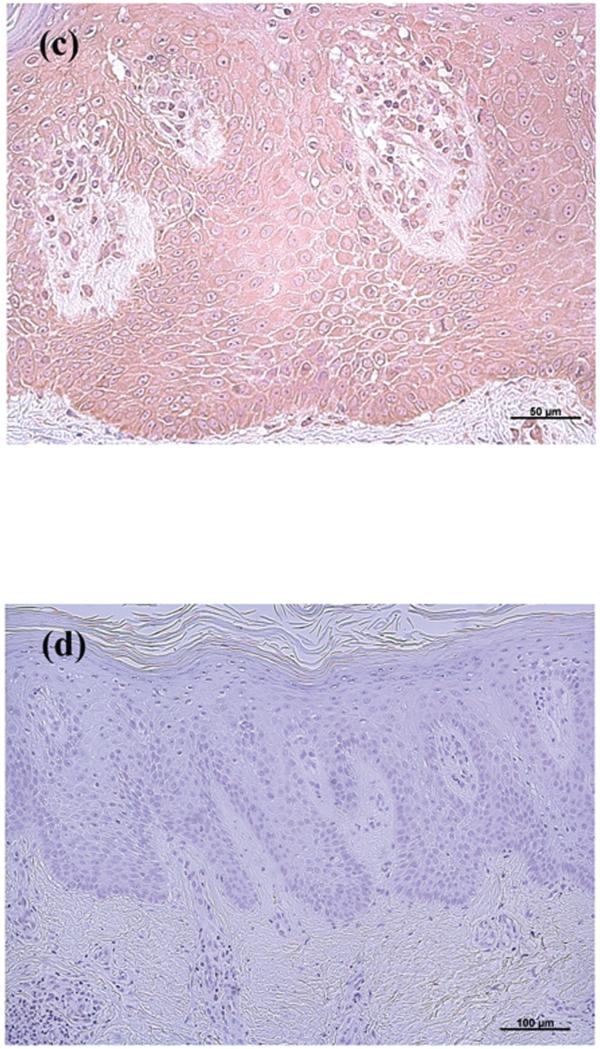
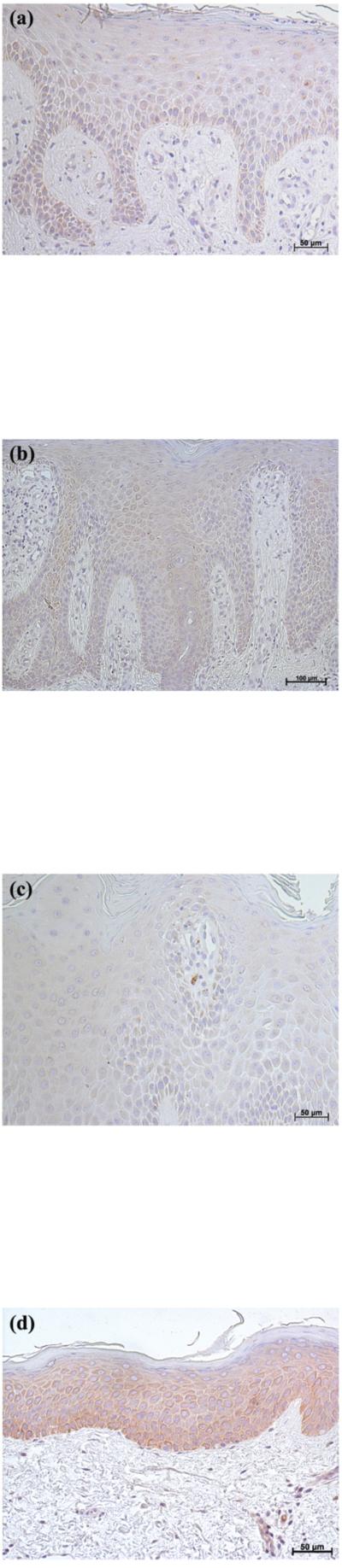
In contrast to the clear down-regulation observed in BCC, the results in SCC were less definitive, in that AQP3 immunoreactivity was “patchy,” with some regions of the lesion staining intensely for AQP3 and others showing little or no staining (Figure 3). These findings were consistent in 5 of 5 lesions examined. AQP3 staining seemed reduced particularly at the SCC borders, which appeared to be invading the dermis. Interestingly, in sequential serial sections the regions showing little AQP3 immunoreactivity stained positive for the proliferation marker, Ki67 (Figure 4A and B); this suggested that proliferation correlates with down-regulation of AQP3 in both BCC and SCC.
Figure 3. AQP3 Immunoreactivity was “Patchy” in Squamous Cell Carcinoma (SCC).
Archived paraffin-embedded squamous cell carcinoma (SCC) samples were sectioned (4 μm) and deparaffinized. Sections were then stained with antibodies recognizing AQP3 and visualized with an ABC kit and DAB. Sections were counterstained with hematoxylin. Illustrated in panels A through C is AQP3 staining in 3 patients with results representative of 5 of 5 SCCs. A negative control in which the primary antibody was omitted showed no AQP3 staining (panel D).
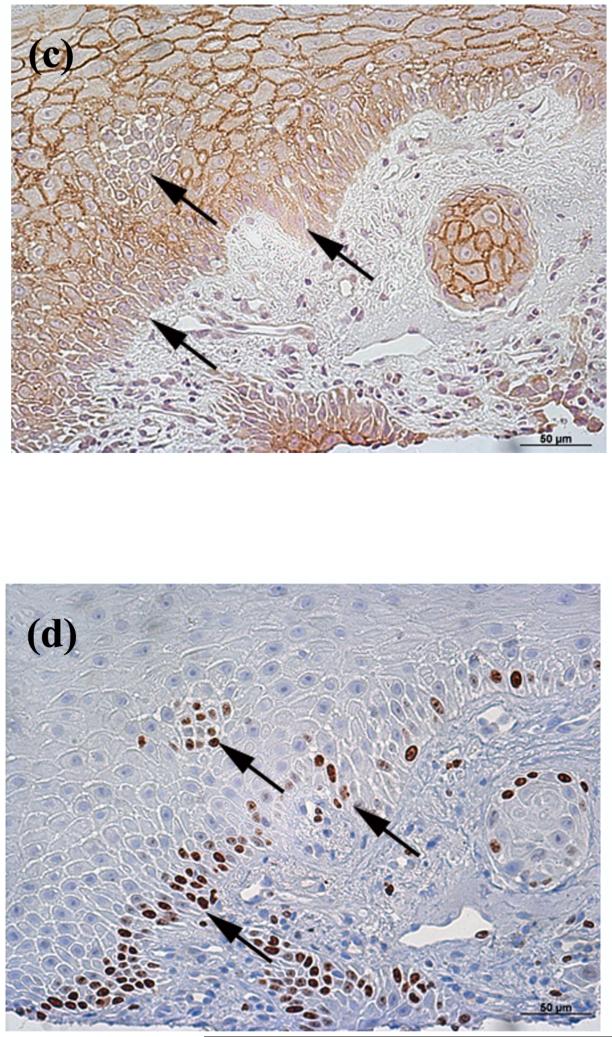
Figure 4. AQP3 Staining was Reduced in Ki67-Positive Cells in Squamous Cell Carcinoma.
Archived paraffin-embedded squamous cell carcinoma (SCC) samples were sectioned (4 μm) and deparaffinized. Sequential serial sections were then stained with antibodies recognizing (A, C) AQP3 or (B, D) Ki67 [an antigen expressed in proliferating cells (Dako, Carpinteria, CA)] and visualized with an ABC kit and DAB. Sections were counterstained with hematoxylin. Note that cells that are Ki67 positive exhibit reduced or absent AQP3 staining (arrows in panels C and D). The inverse correlation of Ki67 positivity with AQP3 immunoreactivity is representative of 4 of 4 SCCs. (E) Human tonsil stained with Ki67 as a positive control. (F) Primary antibody was omitted as a negative control.
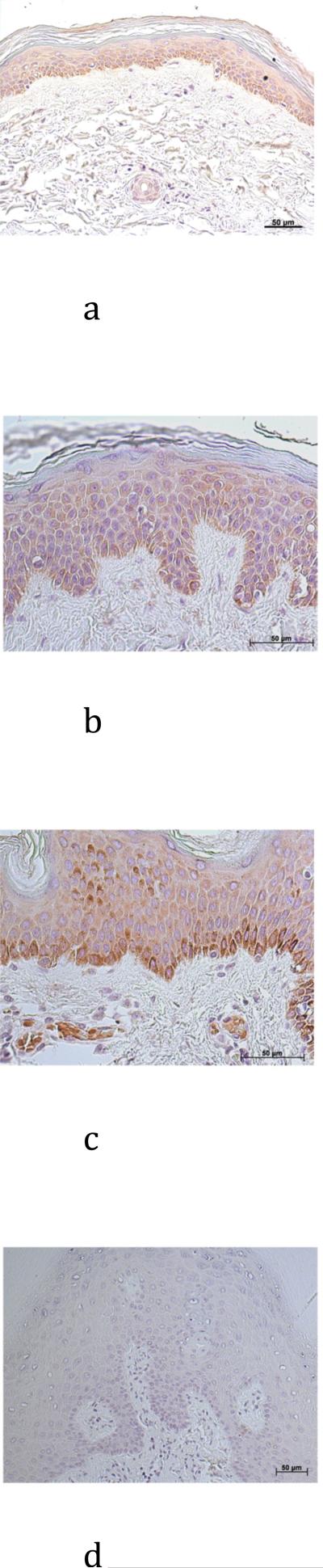
We next obtained archival, paraffin-embedded tissue blocks with a diagnosis of plaque psoriasis and compared AQP3 immunoreactivity in these sections with that in normal epidermis (Figure 2). We found that, in contrast to normal-appearing epidermis overlying BCC (Figure 1) and the majority of normal epidermis (Figure 2), AQP3 staining in most psoriatic lesions was localized intracellularly in most of the lesion rather than to the plasma membrane, although some membrane localization could be observed in occasional suprabasal regions of the hyperproliferative epidermis (Figure 5). We observed intracellular AQP3 localization in 8 out of 10 psoriatic patients. In contrast, AQP3 stained the plasma membrane of follicular keratinocytes as it does in normal epidermis in many of the psoriasis samples. In the remaining 2 of 10 psoriatic samples, AQP3 staining was localized to the cell membrane as in normal epidermis with no apparent appreciable expression deficit (data not shown).
Figure 5. AQP3 Immunoreactivity was Mislocalized in a Majority of Psoriatic Lesions.
(A through C) Archived paraffin-embedded psoriasis samples were sectioned (4 μm) and deparaffinized. Sections were then stained with an antibody recognizing AQP3 and visualized with an ABC kit and DAB. Results from three patients are shown and the staining pattern is representative of 8 of 10 psoriatic samples examined. (D) A negative control was performed by omitting primary antibody. Note that AQP3 is localized to the cytoplasm rather than to the plasma membrane in a majority of the psoriatic epidermis. All sections were counterstained with hematoxylin.
PLD2 Expression
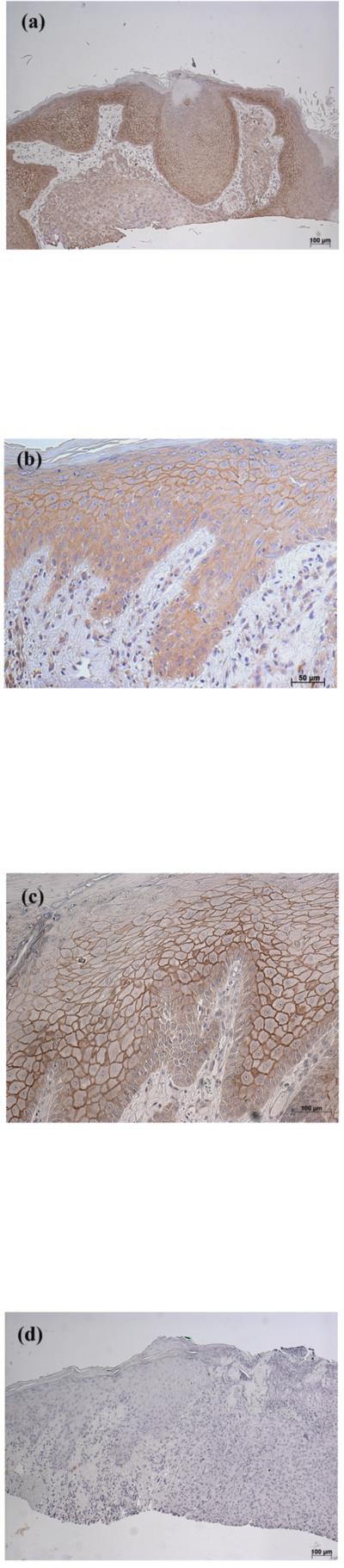
Consistent with the fact that the enzyme is not a transmembrane protein but is instead membrane associated, PLD2 expression was predominantly localized to the perinuclear region and the cell periphery in normal epidermis (Figure 6) and in the normal-appearing epidermis overlying BCC (Figure 7). PLD2 immunoreactivity was distributed throughout the basal and suprabasal layers in normal epidermis (Figure 6) as well as in the normal-appearing epidermis overlying BCC (Figure 7) but was reduced in the BCC. This decrease was again observed in all 13 of the BCCs (13/13), with an approximate 5–fold reduction in BCC relative to the normal-appearing overlying epidermis (Figure 7D) [normal: 2.28 ± 0.18 versus BCC: 0.46 ± 0.10 (p<0.001)]. This result suggests that decreased AQP3 and PLD2 immunoreactivity is associated with the hyperproliferation observed in BCC. On the other hand, there was little or no change in PLD2 in SCC as compared to normal epidermis (data not shown).
Figure 6. PLD2 Immunoreactivity was Localized Largely to the Cell Periphery in Normal Epidermis (as in Normal-appearing Epidermis Overlying Basal Cell Carcinoma).
Archived paraffin-embedded normal human epidermal samples were sectioned (4 μm) and deparaffinized. Sections were then stained with an antibody recognizing PLD2 and visualized with an ABC kit and DAB. Note that PLD2 staining localizes to the cell periphery, as observed also in normal-appearing overlying epidermis in basal cell carcinoma (Figure 8). PLD2 staining of three patients is illustrated in panels A through C, and panel D shows a negative control in which the primary antibody was omitted.
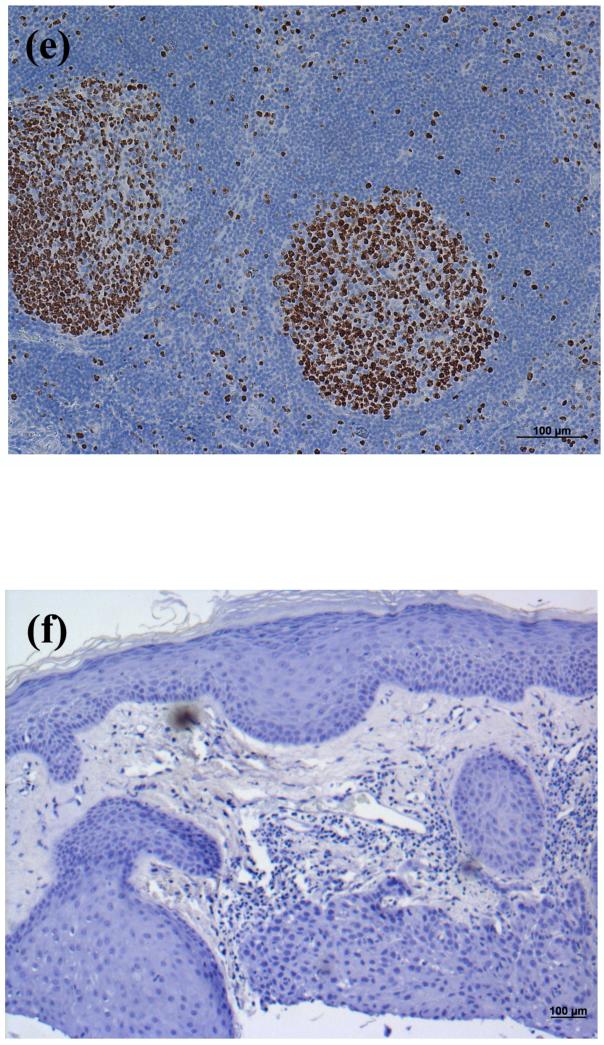
Figure 7. PLD2 Immunoreactivity was Reduced or Absent in Basal Cell Carcinoma (BCC).
(A and B) Archived paraffin-embedded basal cell carcinoma samples were sectioned (4 μm), deparaffinized, stained with an antibody recognizing PLD2 (Santa Cruz Biotechnology, Santa Cruz, CA) and visualized with an ABC kit and DAB. Sections were counterstained with hematoxylin. Results are representative of 13 of 13 BCCs. (C) A negative control in which the primary antibody was omitted was performed and showed no PLD2 staining. (D) Shown in panel D is the quantitation (means ± SEM) of the PLD2 immunoreactivity in 13 BCCs relative to normal-appearing overlying epidermis. The results were quantified as 3+ (intense staining), 2+ (moderate staining), + (weak staining) or 0 (no staining) by three independent observers, the values averaged and the data analyzed by an unpaired Student's t-test as described in Methods; *p<0.05.
In the majority of psoriatic samples (8 of 10), PLD2 staining seemed aberrant, with weak staining (Figure 8A, C) or largely abnormal localization (Figure 8B, C). In the remaining 2 psoriatic samples, PLD2 staining was essentially indistinguishable from that in normal epidermis (Figure 8D). Interestingly, the 2 samples showing normal PLD2 immunoreactivity also exhibited typical staining for AQP3.
Figure 8. PLD2 Immunoreactivity Seemed Aberrant in Psoriatic Lesions.
(A through C) Archived paraffin-embedded human epidermal samples from psoriatic patients were sectioned (4 μm) and deparaffinized. Sections were then stained with an antibody recognizing PLD2 and visualized with an ABC kit and DAB. Note that PLD2 staining was aberrant (extremely weak) in 8 of 10 psoriasis samples. (D) Immunoreactivity in 1 of the 2 psoriatic samples with essentially normal PLD2 staining is illustrated. All sections were counterstained with hematoxylin (blue staining).
DISCUSSION
Our results suggest that down-regulation of AQP3 in the epidermis may be a marker of tumorigenic potential, since expression of this glycerol channel is decreased in BCC and in the areas of SCC that are most rapidly proliferating (i.e., Ki67 positive). In the study of Verkman and colleagues examining AQP3 expression in human SCC, AQP3 was found to be strongly expressed and co-localized with the basal marker keratin 14; the presence of AQP3 in SCC was confirmed by immunohistochemistry in our study. However, we found that AQP3 protein expression within the epidermis of SCC appeared to be excluded from the atypical keratinocytes corresponded to cells on the inner edge of the epidermis. More importantly, these atypical keratinocytes were proliferating actively, as marked by Ki67 positivity. Since keratin 14 protein expression has been observed even in well-differentiated SCC [30,25], keratin 14 may not be the ideal marker for proliferation, and colocalization of AQP3 and keratin 14 does not preclude a correspondence with differentiation rather than proliferation. Thus, our results showing that Ki67 immunoreactivity correlated with reduced AQP3 protein expression suggest that AQP3 may be associated with the differentiation program. Indeed, this idea is supported by a recent study [20] demonstrating that RNA interference-mediated silencing of AQP3 in human keratinocytes results in reduced expression of keratin 10, a differentiation marker, in response to an elevated extracellular calcium level, a known inducer of differentiation.
However, the possibility remains that AQP3 (and PLD2) could be pro-proliferative and down-regulated in hyperproliferative disorders in an attempt to compensate for the excessive growth. Indeed, using RNA-interference-mediated knock down of AQP3 levels, Verkman and colleagues have shown that loss of AQP3 inhibits human keratinocyte proliferation, and adenovirus-mediated AQP3 re-expression returns proliferation values to normal [15]. On the other hand, whether or not AQP3 mediates proliferation or differentiation may depend on whether or not the protein is associated with PLD2 (and vice versa). Thus, the relative overexpression of AQP3 or PLD2 alone may allow proliferation (Qin and Bollag, manuscript in preparation), whereas when regulated in tandem, the interaction of the two proteins should allow phosphatidylglycerol production and early differentiation. Alternatively, the localization (intracellularly or at the plasma membrane) of this signaling module may determine the cellular response, proliferation or differentiation. Indeed, our previous immunocytochemistry data suggest a partial colocalization of AQP3 and PLD2, with both plasma membrane and intracellular staining [18]. Similarly, down-regulation of PLD2 in BCC suggests that this enzyme also may play a role as an anti-proliferative signal, although the same caveat concerning the possibility that the cells down-regulate PLD2 in an attempt to compensate for the hyperproliferation also applies. Thus, the decreased AQP3 in BCC and proliferating regions of SCC provides evidence for the possibility that the AQP3/PLD2 signaling module may act as a tumor suppressor, although obviously additional studies to clarify this issue are warranted. Further studies in the epidermis and other epithelial cancers, such as those of the lung and colon, which also express AQP3 and PLD2 [21,27,35,31,28,18,19,33], might elucidate this mechanism and further our understanding of the role of AQP3 and PLD2 in epithelial cells.
In the majority of the epidermal samples of plaque psoriasis, another hyperproliferative disorder of keratinocytes, AQP3 expression was localized diffusely to the cytoplasm as opposed to its plasma membrane localization in normal epidermis. It should be noted that in normal epidermal samples, such intracellular staining was also sometimes observed in basal keratinocytes, and Sougrat et al. [29] have also reported cytoplasmic staining in basal keratinocytes of human skin and reconstructed human epidermis. Since AQP3 must be in the plasma membrane in order to transport extracellular glycerol into cells, this result suggests that AQP3 transport activity is compromised in keratinocytes in psoriatic lesions and that phosphatidylglycerol levels should be reduced in this hyperproliferative skin disease. Indeed, it seems possible that localization of AQP3 to an intracellular compartment or the plasma membrane, as well as colocalization with PLD2, could determine whether this aquaglyceroporin promotes proliferation or differentiation. On the other hand, a minority of the psoriatic samples (2 of 10) exhibited apparently normal AQP3 localization. This result may not be entirely unexpected, given the complexity and multiple subphenotypes of this disease [11], although in this study plaque-type psoriasis was not classified by subphenotypes [10]. Additional studies to examine AQP3 and PLD2 immunoreactivity in these subtypes of plaque psoriasis, as well as other subtypes of psoriasis, seem warranted.
In conclusion, our results are consistent with the idea that the AQP3/PLD2 signaling module is abnormal in human skin diseases, with down-regulated levels of both proteins in BCC, decreased AQP3 immunoreactivity in proliferating regions of SCC and anomalous distribution and/or decreased levels of both proteins in psoriasis compared to normal human epidermis. Although our results do not prove either a pro-proliferative or a pro-differentiative role for AQP3 and/or PLD2, our data do demonstrate that both AQP3 and PLD2 are abnormally expressed and/or localized in human skin diseases and thus may play a role in or serve as surrogate markers for the pathogenesis of these diseases.
ACKNOWLEDGEMENTS
This project was supported in part by a grant from the National Institutes of Health/ National Institute of Arthritis, Musculoskeletal and Skin Diseases #AR45212. The authors thank Kimberly Smith and Doris Cawley in the Georgia Research Pathology Services for preparing the sections and performing the Ki67 immunohistochemistry and the Medical College of Georgia Histology Core Facility for assistance with immunohistochemistry.
Footnotes
CONFLICT OF INTEREST:
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Bikle DD, Pillai S. Vitamin D, calcium and epidermal differentiation. Endocrine Rev. 1993;14:3–19. doi: 10.1210/edrv-14-1-3. [DOI] [PubMed] [Google Scholar]
- 2.Bikle DD. Vitamin D and skin cancer. J Nutr. 2004;134:3472S–3478S. doi: 10.1093/jn/134.12.3472S. [DOI] [PubMed] [Google Scholar]
- 3.Bollag WB, Dodd ME, Shapiro BA. Protein kinase D and keratinocyte proliferation. Drug News Persp. 2004;17:117–126. doi: 10.1358/dnp.2004.17.2.829045. [DOI] [PubMed] [Google Scholar]
- 4.Bollag WB, Zheng X. Phospholipase D and keratinocyte biology. In: Robinson JW, editor. Trends in Protein Research. Nova Science Publishers, Inc.; New York: 2005. pp. 79–118. [Google Scholar]
- 5.Bollag WB, Xie D, Zhong X, Zheng X. A potential role for the phospholipase D2-aquaporin-3 signaling module in early keratinocyte differentiation: Production of a novel phosphatidylglycerol lipid signal. J Invest Dermatol. 2007;127:2823–2831. doi: 10.1038/sj.jid.5700921. [DOI] [PubMed] [Google Scholar]
- 6.Boury-Jamot M, Sougrat R, Tailhardat M, Le Varlet B, Bonte F, Dumas M, Verbavatz J-M. Expression and function of aquaporins in human skin: Is aquaporin-3 just a glycerol transporter? Biochim Biophys Acta. 2006;1758:1034–1042. doi: 10.1016/j.bbamem.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Fluhr JW, Gloor M, Lehmann L, Lazzerinin S, Distante F, Berardesca E. Glycerol accelerates recovery of barrier function in vivo. Acta Derm Venereol. 1999;79:418–421. doi: 10.1080/000155599750009825. [DOI] [PubMed] [Google Scholar]
- 8.Fluhr JW, Darlenski R, Surber C. Glycerol and the skin: holistic approach to its origin and function. Br J Dermatol. 2008;159:23–34. doi: 10.1111/j.1365-2133.2008.08643.x. [DOI] [PubMed] [Google Scholar]
- 9.Ghoreschi K, Weigert C, Röcken M. Immunopathogenesis and role of T cells in psoriasis. Clin Dermatol. 2007;25:574–580. doi: 10.1016/j.clindermatol.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths CEM, Christophers E, Barker JNWN, Chalmers RJG, Chimenti S, Krueger GG, Leonardi C, Menter A, Ortonne J-P, Fry L. A classification of psoriasis vulgaris according to phenotype. Br J Dermatol. 2007;156:258–262. doi: 10.1111/j.1365-2133.2006.07675.x. [DOI] [PubMed] [Google Scholar]
- 11.Gudjonsson JE, Johnston A, Sigmundsdottir H, Valdimarsson H. Immunopathogenic mechanisms in psoriasis. Clin Exp Immunol. 2004;135:1–8. doi: 10.1111/j.1365-2249.2004.02310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gudjonsson JE, Johnston A, Dyson M, Valdimarsson H, Elder JT. Mouse models of psoriasis. J Invest Dermatol. 2007;127:1292–1308. doi: 10.1038/sj.jid.5700807. [DOI] [PubMed] [Google Scholar]
- 13.Hara M, Ma T, Verkman AS. Selectively reduced glycerol in skin of aquaporin-3 deficient mice may account for impaired skin hydration, elasticity and barrier recovery. J Biol Chem. 2002;277:46616–46621. doi: 10.1074/jbc.M209003200. [DOI] [PubMed] [Google Scholar]
- 14.Hara M, Verkman AS. Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc Natl Acad Sci USA. 2003;100:7360–7365. doi: 10.1073/pnas.1230416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hara-Chikuma M, Verkman AS. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J Mol Med. 2008;86:221–231. doi: 10.1007/s00109-007-0272-4. [DOI] [PubMed] [Google Scholar]
- 16.Hara-Chikuma M, Verkman AS. Roles of aquaporin-3 in the epidermis. J Invest Dermatol. 2008;128:2145–2151. doi: 10.1038/jid.2008.70. [DOI] [PubMed] [Google Scholar]
- 17.Hara-Chikuma M, Verkman AS. Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol Cell Biol. 2008;28:326–332. doi: 10.1128/MCB.01482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang DW, Park MH, Lee YJ, Kim HS, Kwon TK, Park W-S, Min DS. Phorbol ester up-regulates phospholipase D1 but not phospholipase D2 expression through a PKC/Ras/ERK/NFkB-dependent pathway and enhances matrix metalloproteinase-9 secretion in colon cancer cells. J Biol Chem. 2008;283:4094–4104. doi: 10.1074/jbc.M707416200. [DOI] [PubMed] [Google Scholar]
- 19.Kim H, Lee J, Kim S, Shin MK, Mindo S, Shin T. Differential expression of phospholipases D1 and D2 in mouse tissues. Cell Biol Int. 2007;31:148–155. doi: 10.1016/j.cellbi.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Kim N-H, Lee A-Y. Reduced aquaporin3 expression and survival of keratinocytes in the depigmented epidermis of vitiligo. J Invest Dermatol. 2010;130:2231–2239. doi: 10.1038/jid.2010.99. [DOI] [PubMed] [Google Scholar]
- 21.Matsuzaki T, Suzuki T, Koyama H, Tanaka S, Takata K. Water channel protein AQP3 is present in epithelia exposed to the environment of possible water loss. J Histochem Cytochem. 1999;47:1275–1286. doi: 10.1177/002215549904701007. [DOI] [PubMed] [Google Scholar]
- 22.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Amer Acad Dermatol. 1994;30:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 23.Nakahigashi K, Kabashima K, Ikoma A, Verkman AS, Miyachi Y, Hara-Chikuma M. Upregulation of aquaporin-3 is involved in keratinocyte proliferation and epidermal hyperplasia. J Invest Dermatol. 2010 Dec 30; doi: 10.1038/jid.2010.395. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Olsson M, Broberg A, Jernas M, Carlsson L, Rudemo M, Suurküla M, Svensson P-A, Benson M. Increased expression of aquaporin 3 in atopic eczema. Allergy. 2006;61:1132–1137. doi: 10.1111/j.1398-9995.2006.01151.x. [DOI] [PubMed] [Google Scholar]
- 25.Perkins W, Campbell I, Leigh IM, MaKie RM. Keratin expression in normal skin and epidermal neoplasms demonstrated by a panel of monoclonal antibodies. J Cutan Pathol. 1992;19:476–482. doi: 10.1111/j.1600-0560.1992.tb01600.x. [DOI] [PubMed] [Google Scholar]
- 26.Qin H, Zheng X, Zhong X, Satyaprakash A, Elias PM, Bollag WB. Aquaporin-3 in keratinocytes and skin: Its role and interaction with phospholipase D2. Arch Biochem Biophys. 2011 Jan 25; doi: 10.1016/j.abb.2011.01.014. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato K, Kobayashi K, Aida S, Tamai S. Bronchiolar expression of aquaporin-3 (AQP3) in rat lung and its dynamics in pulmonary oedema. Pflugers Arch. 2004;449:106–114. doi: 10.1007/s00424-004-1310-5. [DOI] [PubMed] [Google Scholar]
- 28.Silberstein C, Kierbel A, Amodeo G, Zotta E, Bigi F, Berkowski D, Inbarram C. Functional characterization and localization of AQP3 in the human colon. Braz J Med Biol Res. 1999;32:1303–1313. doi: 10.1590/s0100-879x1999001000018. [DOI] [PubMed] [Google Scholar]
- 29.Sougrat R, Morand M, Gondran C, Barre P, Gobin R, Bonte F, Dumas M, Verbavatz JM. Functional expression of AQP3 in human skin epidermis and reconstructed epidermis. J Invest Dermatol. 2002;118:678–685. doi: 10.1046/j.1523-1747.2002.01710.x. [DOI] [PubMed] [Google Scholar]
- 30.Stoler A, Kopan R, Duvic M, Fuchs E. Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J Cell Biol. 1988;107:427–446. doi: 10.1083/jcb.107.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka M, Inase N, Fushimi K, Ishibashi K, Ichioka M, Sasaki S, Marumo F. Induction of aquaporin 3 by corticosteroid in a human airway epithelial cell line. Am J Physiol. 1997;273:L1090–L1095. doi: 10.1152/ajplung.1997.273.5.L1090. [DOI] [PubMed] [Google Scholar]
- 32.Tonel G, Conrad C. Interplay between keratinocytes and immune cells – recent insights into psoriasis pathogenesis. Int J Biochem Cell Biol. 2008;41:963–968. doi: 10.1016/j.biocel.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Cummings R, Zhao Y, Kazlauskas A, Sham JK, Morris A, Georas S, Brindley DN, Natarajan V. Involvement of phospholipase D2 in lysophosphatidate-induced transactivation of platelet-derived growth factor receptor-beta in human bronchial epithelial cells. J Biol Chem. 2003;278:39931–39940. doi: 10.1074/jbc.M302896200. [DOI] [PubMed] [Google Scholar]
- 34.Yang B, Verkman AS. Water and glycerol permeabilities of aquaporins 1-5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J Biol Chem. 1997;272:16140–16146. doi: 10.1074/jbc.272.26.16140. [DOI] [PubMed] [Google Scholar]
- 35.Zelenina M, Bondar AA, Zelenin S, Aperia A. Nickel and extracellular acidification inhibit the water permeability of human aquaporin-3 in lung epithelial cells. J Biol Chem. 2003;278:30037–30043. doi: 10.1074/jbc.M302206200. [DOI] [PubMed] [Google Scholar]
- 36.Zenz R, Efer R, Kenner L, Florin L, Hummerich L, Mehic D, Scheuch H, Angel P, Tschachler E, Wagner EF. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437:369–375. doi: 10.1038/nature03963. [DOI] [PubMed] [Google Scholar]
- 37.Zheng X, Bollag WB. Aquaporin 3 colocates with phospholipase D2 in caveolin-rich membrane microdomains and is regulated by keratinocyte differentiation. J Invest Dermatol. 2003;121:1487–1495. doi: 10.1111/j.1523-1747.2003.12614.x. [DOI] [PubMed] [Google Scholar]
- 38.Zheng X, Ray S, Bollag WB. Modulation of phospholipase D-mediated phosphatidylglycerol formation by differentiating agents in primary mouse epidermal keratinocytes. Biochim Biophys Acta. 2003;1643:25–36. doi: 10.1016/j.bbamcr.2003.08.006. [DOI] [PubMed] [Google Scholar]