Abstract
Objective
The burden of Charcot Marie Tooth type 1A, the most common inherited peripheral neuropathy, including impact on patient quality of life is not well understood. This study aims to qualitatively describe the range of symptoms associated with Charcot Marie Tooth type 1A and impact on quality of life.
Methods
We performed qualitative interviews with 16 adult Charcot Marie Tooth type 1A patients. Each interview was analyzed using a qualitative framework technique to identify and index symptoms by theme.
Results
Sixteen patients provided 656 quotes. One hundred and forty-five symptoms of importance were identified representing 20 symptomatic themes. Symptoms associated with difficulty with mobility and ambulation, specific activity impairment, and emotional distress were the most frequently mentioned.
Conclusions
Multiple symptoms contribute to Charcot Marie Tooth type 1A disease burden, some previously under-recognized. Improved recognition of under-recognized symptoms will optimize patient care and quality of life.
Keywords: Charcot Marie Tooth disease type 1A, quality of life, symptoms, inherited neuropathies
Introduction
Charcot Marie Tooth neuropathy type 1A (CMT1A) is a demyelinating polyneuropathy that typically presents in childhood and causes distal weakness, length-dependent sensory loss, and foot deformities.1 CMT1A is the most common inherited neuropathy, affecting 1:5000 individuals, and is caused by a 1.4 Mb duplication in the peripheral myelin protein gene.2,3
Prior studies examining adults with CMT1A have utilized generic instruments to assess the impact of the disease on certain aspects of quality of life (QOL). Use of the Short-form (SF-36) has identified a lower quality of life in CMT patients compared to Italian normative data.4,5 The Multidimensional Fatigue Inventory, Epworth Sleepiness scale, fatigue subscale of the Checklist Inventory strength, and the Pittsburgh Sleep Quality Index have been used to describe increased fatigue, a higher prevalence of daytime sleepiness, and worse sleep quality associated with CMT.6,7 In pediatric CMT patients, the Child Health Questionnaire associated decreased QOL with leg cramps and poor endurance.8 Notably, the QOL scores did not correlate with standard functional measures.9 To date, while several survey-based measures have evaluated aspects of quality of life, there has not been a comprehensive analysis of all symptoms that may impact disease related quality of life.
As such, the identification of symptoms that have the greatest impact on CMT1A-related QOL in adults remains limited. The current study utilizes a comprehensive, qualitative interview method to broadly identify all symptoms and themes associated with CMT1A-related QOL.
Materials and Methods
Subjects over the age of 21 with genetically confirmed CMT1A or clinically and electrophysiologically defined CMT1 with an affected 1st degree relative with genetically confirmed CMT1A were selected for participation in this study. A purposive sampling strategy was utilized to identify subjects with a wide range of ages and Charcot Marie Tooth Neuropathy Scores (a measure of CMT severity).10 In this process, subjects were enrolled sequentially to ensure a wide distribution, providing for an accurate representation of symptoms experienced by those mildly affected and those severely affected. This study was performed with institutional review board approval.
Interviews were completed by a study coordinator or investigator with experience in qualitative interviews. Interviews were completed in person or by telephone. Interviews with subjects utilized a semi-structured approach to elicit details regarding all aspects of their health and how CMT affects it. Interviews were initiated with open ended questions (supplemental methods) and subjects were subsequently allowed to expand on the issues that they felt had the greatest importance in their lives. Interviews were transcribed verbatim for analysis.
A framework technique was utilized to qualitatively analyze the interviews.11,12 Quotes from interview transcripts were indexed and interpreted using a team consensus approach.11,12 Quotes repeated across participants were recorded and a quote frequency for each symptom was determined. Symptoms were further classified by investigators into themes of CMT health and each theme was categorized into a model of health using physical, mental, social, or disease specific aspect of health. The themes were compared to the Charcot Marie Tooth Neuropathy Score (CMTNS) using a Kruskal-Wallis statistical test with a two-tailed p-value of <0.05 considered significant. A model and frequency table was created to identify areas of patient-identified CMT health. Patient interviews were continued until saturation was obtained. Saturation is defined as absence of significantly novel symptoms divergent from prior interview analyses.
Results
Sixteen subjects participated in this study (table 1).
Table 1.
Participant Demographics
| Characteristic | Mean | Range |
|---|---|---|
| Age | 52 years old | 26–73 years old |
| Gender | 62.5% female | |
| CMTNS | 14.6 | 4–23 |
| Employment Status | 40% working, 26.6% medically disabled, 33.3% retired |
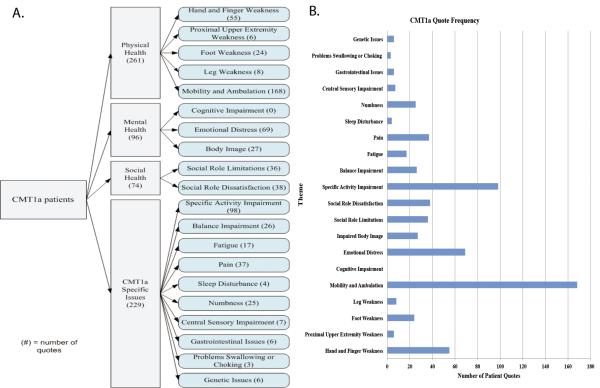
From the 16 CMT1A participants, 656 direct quotes were coded, identifying 145 symptoms of importance. These symptoms represent 20 symptomatic themes and 4 major domains (figure 1, supplemental table 1). The CMT1A themes mentioned most frequently by patients included difficulty with mobility and ambulation (168 quotes), specific activity impairment (98 quotes), and emotional distress (69 quotes). To illustrate the difficulties with mobility and ambulation, one patient reports “…well since I was little I couldn't do gym or I remember I couldn't do gym, I couldn't run as well as anybody else and now in my daily life, I'm weak, I drop things, I can't, I can't run or walk.”
Figure 1.
Charcot Marie Tooth Disease Type 1A Symptom Domain Model (A) and Quote Frequency (B).
The most frequently noted individual symptoms were impaired walking (26 quotes), falls (18 quotes), and tripping (16 quotes). One patient noted that “I go down like a sack, I mean I cannot catch myself, so um I've hurt myself a couple of times really pretty badly on my ankles, uh because I kind of go down a certain way all of the time and I come down across my leg onto my ankle and so that can be very uh, in fact I think that's how I ended up breaking my ankle uh, after it had already been fused once by coming down like that on it, so I'm very, very aware of that and I have to be extremely careful.”
Fifteen participants had a Charcot Marie Tooth Neuropathy Score (CMTNS). Using a CMTNS score of 16 to separate participants into two groups of disease severity, 7 participants had a CMTNS score of less than 16, and 8 participants had a CMTNS score of more than 16. When comparing the average number of quotes by a participant per theme, 16 themes did not differ by more than one quote per theme and no theme showed a statistical difference in quote frequency between groups.
A full list of symptoms, quote frequency, and theme classification is provided (supplemental table 1). During analysis of the 15th and 16th interviews, no novel symptoms were identified; saturation was subsequently declared.
Discussion
This study used qualitative interviews to identify symptoms and themes that have a high impact on the QOL of adult patients with CMT1A. In some instances, our interviews identified areas of impact that have been previously described. 4,6,13 This study not only confirms prior findings, but synthesizes the patient's point of view in describing the physical, social, and mental burdens of CMT1A patients. Perhaps more importantly, this work highlights some symptoms that are potentially treatable in the clinical setting.
Mobility and ambulation dysfunction was the most represented interview theme. The great importance of this theme to CMT1A has been previously supported in prior work by Vinci and Padua among others.4,13 In a clinical setting, problems with mobility and ambulation may be addressed through a multidisciplinary approach, including physical therapists, occupational therapists, and orthotists.
One previously under-identified area of importance is the effect of CMT1A on body image. Specifically, participants noted concerns related to gait (3 participants), braces (5 participants), and loss of muscle bulk in hands, feet, and legs (7 participants). As advances in orthotic technology produce less noticeable devices, the effects on patient body image may lessen. In addition, surgical techniques such as hand rejuvenation may be considered in patients with significant muscle atrophy and impaired QOL related to impaired body image.14
Participants identified significant emotional distress related to CMT1A. Specifically, participants commonly identified depression, anxiety, frustration, among other emotional symptoms. Future studies and care guidelines should increase practitioner awareness of the range of emotional issues related to CMT1A, and consider the optimal methods (counseling, pharmacotherapy) to address these in the clinic.
The current study provides an important supplement to prior CMT1Aresearch. Our qualitative approach allowed patients to openly describe and prioritize symptoms affecting their quality of life. Prior studies utilized generic survey instruments developed for other populations.4–6,15 These instruments may not capture all possible symptoms and may inappropriately prioritize symptoms within the CMT1A population. To the authors' knowledge, this is the first study using a patient centered qualitative approach to describe the symptoms affecting adult CMT1A patients.
CMT1A participants identified a unique pattern of disease burden. Compared to myotonic dystrophy type-1 interviews, and fascioscapulohumeral muscular dystrophy interviews, CMT1A participants' comments focused more on issues related to distal weakness.16,17 CMT1A participants also mentioned issues related to balance and numbness, more often than participants with either DM1 or FSHD and CMT1A participants were less likely to highlight symptoms related to truncal or proximal weakness.16,17 Although cognitive issues occur frequently in many neuromuscular populations, CMT1A participants did not frequently mention this as an area of importance. The diverse disease perspective among participants with different neuromuscular disorders emphasizes the desirability of development of a disease specific QOL instrument for adult CMT1A patients.
Interviews were limited to a small number of participants. Although these participants were selected using a purposive sampling strategy (involving a variety of patient ages, and disease severities), the potential for sampling error exists. This study utilized an interview strategy and did not collect detailed objective strength or functional measurements. For this reason, the items identified are solely the participants' perspectives and without functional correlates. Given the format of this qualitative study, the overall quote frequency did not correlate with the CMTNS score. Future research will be needed to objectively correlate the items identified with specific objective measurements.
In conclusion, this study utilized a qualitative patient-centered approach to identify symptoms affecting the QOL of CMT1A patients. Many of the issues identified were previously under-described and represent important and immediate therapeutic targets. Additional research will be needed to further categorize and prioritize these symptoms in the larger CMT1A population.
This open-ended symptom description however provides a platform for development of a disease-specific quality of life instrument, which is needed for future therapeutic trials in CMT1A.18
Supplementary Material
Acknowledgements
DNH is supported in part by the Inherited Neuropathy Consortium Rare Disease Clinical Research Network, 1U54NS0657 (NINDS). CRH is supported by the Muscular Dystrophy Association and 1K23AR055947 (NIAMS/NIH). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the supporting agencies.
References
- 1.Reilly MM, Shy ME. Diagnosis and new treatments in genetic neuropathies. J Neurol Neurosurg Psychiatry. 2009;80:1304–14. doi: 10.1136/jnnp.2008.158295. [DOI] [PubMed] [Google Scholar]
- 2.Lupski JR, de Oca-Luna RM, Slaugenhaupt S, et al. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991;66:219–32. doi: 10.1016/0092-8674(91)90613-4. [DOI] [PubMed] [Google Scholar]
- 3.Raeymaekers P, Timmerman V, Nelis E, et al. Duplication in chromosome 17p11.2 in Charcot-Marie-Tooth neuropathy type 1a (CMT 1a). The HMSN Collaborative Research Group. Neuromuscul Disord. 1991;1:93–7. doi: 10.1016/0960-8966(91)90055-w. [DOI] [PubMed] [Google Scholar]
- 4.Vinci P, Serrao M, Millul A, et al. Quality of life in patients with Charcot-Marie-Tooth disease. Neurology. 2005;65:922–4. doi: 10.1212/01.wnl.0000176062.44360.49. [DOI] [PubMed] [Google Scholar]
- 5.Padua L, Pareyson D, Aprile I, et al. Natural history of CMT1A including QoL: a 2-year prospective study. Neuromuscul Disord. 2008;18:199–203. doi: 10.1016/j.nmd.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Boentert M, Dziewas R, Heidbreder A, et al. Fatigue, reduced sleep quality and restless legs syndrome in Charcot-Marie-Tooth disease: a web-based survey. J Neurol. 2010;257:646–52. doi: 10.1007/s00415-009-5390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalkman JS, Zwarts MJ, Schillings ML, van Engelen BG, Bleijenberg G. Different types of fatigue in patients with facioscapulohumeral dystrophy, myotonic dystrophy and HMSN-I. Experienced fatigue and physiological fatigue. Neurol Sci. 2008;29(Suppl 2):S238–40. doi: 10.1007/s10072-008-0949-7. [DOI] [PubMed] [Google Scholar]
- 8.Burns J, Ryan MM, Ouvrier RA. Quality of life in children with Charcot-Marie-Tooth disease. J Child Neurol. 2010;25:343–7. doi: 10.1177/0883073809339877. [DOI] [PubMed] [Google Scholar]
- 9.Burns J, Ramchandren S, Ryan MM, Shy M, Ouvrier RA. Determinants of reduced health-related quality of life in pediatric inherited neuropathies. Neurology. 2010;75:726–31. doi: 10.1212/WNL.0b013e3181eee496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shy ME, Chen L, Swan ER, et al. Neuropathy progression in Charcot-Marie-Tooth disease type 1A. Neurology. 2008;70:378–83. doi: 10.1212/01.wnl.0000297553.36441.ce. [DOI] [PubMed] [Google Scholar]
- 11.McColl E. Developing questionnaires. In: Fayers P, Hays R, editors. Assessing Quality of Life in Clinical Trials. 2nd ed Oxford Press; Oxford: 2005. pp. 9–25. [Google Scholar]
- 12.Ritchie J, Spencer L. Anonymous Analyzing Qualitative Data. Routledge; 1994. Qualitative data analysis for applied research; pp. 173–194. [Google Scholar]
- 13.Padua L, Shy ME, Aprile I, et al. Correlation between clinical/neurophysiological findings and quality of life in Charcot-Marie-Tooth type 1A. J Peripher Nerv Syst. 2008;13:64–70. doi: 10.1111/j.1529-8027.2008.00159.x. [DOI] [PubMed] [Google Scholar]
- 14.Puwanant A, Evangelisti SM, Griggs RC. Treating the chief complaint: hand rejuvenation for Hirayama disease. Neurology. 2011;77:190–1. doi: 10.1212/WNL.0b013e3182242da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalkman JS, Schillings ML, Zwarts MJ, van Engelen BG, Bleijenberg G. The development of a model of fatigue in neuromuscular disorders: a longitudinal study. J Psychosom Res. 2007;62:571–9. doi: 10.1016/j.jpsychores.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Heatwole C, Bode R, Johnson N, et al. Patient-reported impact of symptoms in myotonic dystrophy type 1 (PRISM-1) Neurology. 2012;79:348–57. doi: 10.1212/WNL.0b013e318260cbe6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson NE, Quinn C, Chin N, Eastwood E, Tawil R, Heatwole CR. Patient identified disease burden in Facioscapulohumeral Muscular Dystrophy. Muscle and Nerve. 2012;46(6):948–50. doi: 10.1002/mus.23529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns TM, Graham CD, Rose MR, Simmons Z. Quality of life and measures of quality of life in patients with neuromuscular disease. Muscle and Nerve. 2012;46(1):9–25. doi: 10.1002/mus.23245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.