Abstract
Biochemical and genetic studies have implicated α-gustducin as a key component in the transduction of both bitter or sweet taste. Yet, α-gustducin-null mice are not completely unresponsive to bitter or sweet compounds. To gain insights into how gustducin mediates responses to bitter and sweet compounds, and to elicit the nature of the gustducin-independent pathways, we generated a dominant-negative form of α-gustducin and expressed it as a transgene from the α-gustducin promoter in both wild-type and α-gustducin-null mice. A single mutation, G352P, introduced into the C-terminal region of α-gustducin critical for receptor interaction rendered the mutant protein unresponsive to activation by taste receptor, but left its other functions intact. In control experiments, expression of wild-type α-gustducin as a transgene in α-gustducin-null mice fully restored responsiveness to bitter and sweet compounds, formally proving that the targeted deletion of the α-gustducin gene caused the taste deficits of the null mice. In contrast, transgenic expression of the G352P mutant did not restore responsiveness of the null mice to either bitter or sweet compounds. Furthermore, in the wild-type background, the mutant transgene inhibited endogenous α-gustducin's interactions with taste receptors, i.e., it acted as a dominant-negative. That the mutant transgene further diminished the residual bitter and sweet taste responsiveness of the α-gustducin-null mice suggests that other guanine nucleotide-binding regulatory proteins expressed in the α-gustducin lineage of taste cells mediate these responses.
Gustducin is a transducin-like heterotrimeric guanine nucleotide-binding protein (G-protein) expressed in taste receptor cells (TRCs; ref. 1). Biochemical assays with bovine taste membranes showed that native bitter-responsive taste receptors selectively couple to gustducin (2, 3). Recently, a multigene family of G-protein-coupled receptors (GPCRs), the T2R/TRB receptors, were shown to be expressed selectively in α-gustducin-positive taste cells (4, 5). One T2R/TRB receptor, mT2R5, was shown to respond to physiologic concentrations of cycloheximide, a compound that is aversive to mice and bitter to humans. mT2R5 specifically coupled to gustducin's α-subunit (α-gustducin) in preference to other G-protein α-subunits (6), consistent with the proposal that gustducin is activated by the T2R receptors in vivo to mediate TRC responses to bitter compounds.
Based on the striking similarity of α-gustducin to α-transducin (1, 7), the presence of both of these α-subunits in TRCs (2), and the high levels of phosphodiesterase (PDE) in taste tissue (8, 9), it was proposed that bitter transduction is mediated by way of α-gustducin activation of taste PDE. Consistent with this proposal, PDE from bovine taste tissue is activated in vitro by α-gustducin and α-transducin (2, 10). Furthermore, several bitter compounds elicit a decrease in cyclic nucleotide monophosphate levels in taste tissue that can be blocked by neutralizing antibodies directed against α-gustducin (11). The taste PDE responsive to activation by α-gustducin and/or α-transducin has been shown recently to be PDE1A (10).
A previously puzzling observation was that many bitter compounds thought to be transduced by gustducin led to pertussis toxin-sensitive inositol triphosphate (IP3) generation (12, 13), but neutralizing antibodies directed against α-gustducin did not block IP3 generation (11). The resolution comes from two recent studies. First, it was shown that rat TRCs express phospholipase (PLC) β2, and that activation of this PLC isoform by bitter denatonium benzoate-stimulated taste receptors is required for taste tissue generation of IP3 (14). Second, a newly discovered G-protein γ-subunit, Gγ13, was shown to be expressed with Gβ3 in all α-gustducin-positive TRCs (15). Gγ13 interacts with α-gustducin in vitro and α-gustducin/Gβ1/Gγ13 heterotrimers are activated in the presence of denatonium benzoate by native taste receptors. Quench-flow studies using neutralizing antibodies have shown that Gγ13 (15), Gβ3 (16), and PLCβ2 (14) are each required to mediate the IP3 response to denatonium benzoate. Thus, heterotrimeric gustducin (α-gustducin/Gβ3/Gγ13) mediates two responses: a decrease in cyclic nucleotide monophosphates via activation of PDE1A by α-gustducin and a rise in IP3 via activation of PLCβ2 by released β3γ13.
Consistent with the above biochemical studies, in vivo analysis of α-gustducin-null mice demonstrated that these mice have markedly reduced behavioral and electrophysiological responses to bitter compounds (17), implicating α-gustducin as a key element in their transduction. However, α-gustducin-null mice also were less responsive to sweet compounds suggesting that gustducin has a role in their transduction. The α-gustducin-null mice are not completely unresponsive to bitter and sweet compounds, suggesting the presence of α-gustducin-independent pathways that function alongside, or independently of, gustducin.
To determine if the gustducin-independent pathways are gustatory in nature and if they occur in those TRCs that normally express α-gustducin, we transgenically expressed in the α-gustducin-positive lineage of TRCs a C-terminally mutated form of α-gustducin incapable of being activated by GPCRs. This mutant acted as a dominant-negative in wild-type (WT) mice to block heterotrimeric gustducin's interactions with taste receptors and disrupted responses to bitter and sweet compounds in WT as well as α-gustducin-null mice. These results suggest that gustducin interacts directly with taste receptors to transduce responses to both bitter and sweet compounds, and that the gustducin-independent pathways depend on taste-receptor interactions with other G-proteins expressed in the same TRCs that normally express α-gustducin.
Materials and Methods
Mutagenesis and in Vitro Translation.
PCR was used to eliminate the 5′ untranslated region of the full-length rat α-gustducin cDNA (1). A PCR product was generated that spanned nucleotides 160–230 and introduced an EcoRI site immediately upstream of the ATG start codon. This product was subcloned with a HindIII-NotI fragment, which encompasses the rest of the α-gustducin cDNA (including the 3′ untranslated region), into the EcoRI and NotI sites of the pBluescript II KS(+) (Stratagene) polylinker. This full-length gustducin clone is referred to as plasmid KSα3. This plasmid was used as a template for mutagenesis and for in vitro transcription/translation in the presence of limiting [35S]methionine by using T3 RNA polymerase and the T3-coupled (TnT) reticulocyte system (Promega). To generate the mutant G352P α-gustducin, we used a kit (CLONTECH) based on the method described (18). The G352P mutant was generated by using the mutagenic primer CTCAAAGACTGTCCGCTATTCTGAGCAACC, which destroys an EarI site in KSα3. The mutagenized regions of the G352P plasmid were sequenced to rule out adventitious mutations. Three independent isolates of the mutant plasmid were analyzed in pilot experiments to control for secondary mutations.
Biochemistry.
Details of the biochemical characterization by means of trypsin sensitivity assay and the activation of in vitro-translated WT and G352P-gustducin by rhodopsin and taste membranes were as described for transducin (2), with modifications for in vitro-translated products (3, 19). After digestion with trypsin, active (GTPγS-bound) α-gustducin yields a 37-kDa fragment, whereas inactive (GDP-bound) α-gustducin yields a 23/25-kDa doublet. By measuring the fraction of 37-kDa fragments among the total (23 + 25 + 37 kDa), we can generate an activation index indicative of the level of activation of the G-protein α-subunit. In vitro-translated α-gustducin was incubated for 15 min at room temperature with βγ-subunits from bovine retina [10 μl at 1 mg/ml in 10 mM Tris (pH 8.0)/10 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS)]. All subsequent reactions were performed on ice. The α-gustducin/βγ mixtures were diluted 1:10 in incubation buffer [25 mM Tris (pH 7.5)/2 mM MgCl2/5 mM DTT/100 mM NaCl/100 μM GDP/0.1 μM GTPγS] containing 0.25–1 mg/ml of bovine taste membrane protein or bovine rhodopsin (0–1 μM). Reaction mixtures were incubated for 1–3 h on ice and then analyzed by the trypsin-digestion assay. For trypsin assays, l-1-tosylamido-2-phenylethyl chloromethyl ketone (and variants) (TPCK)-treated trypsin (1:25 wt/wt trypsin to total protein) was added after the incubation with tastant/taste membrane or rhodopsin. Trypsin digestions were performed at room temperature (15 min) and were stopped by adding soybean trypsin inhibitor (6:1 mol/mol of inhibitor to trypsin). A GDP to GTPγS ratio of 1,000 was necessary to block GTPγS-induced activation of the in vitro-translated products. The reaction mixtures were diluted 1:3 in 2× Laemmli buffer, and the fragments protected from proteolytic cleavage were resolved by using SDS/PAGE in precast 4–20% gels (NOVEX, San Diego). The gels were dried and exposed to a phosphor imager screen. All experiments included independent duplicates, and all the quantifications were performed twice for each band.
Construction of Transgenes.
The rabbit βII intron from pSG5 (Stratagene) was cloned into pBluescript II KS(+) to make pIntron. The SV40 polyadenylation site from pSG5 was cloned into a pBluescript II KS(+) vector containing the 3′ untranslated region of rat α-gustducin to make p3′-poly(A). An ≈1,150-bp HindIII-EcoRV fragment of the KSα3 or G352P plasmids described above was cloned into p3′-poly(A) to generate pWT-3′ and pG352P-3′. A 220-bp EcoRI-HindIII fragment generated by PCR was cloned 3′ of the βII globin intron in pIntron to generate pIntron-5′. A SnaBI-PstI fragment containing the gustducin 8.4-kb promoter (20, 21), a PstI-HindIII fragment from pIntron-5′, and a HindIII-SacI fragment from pWT-3′ or pG352P-3′ were cloned in a single step into the SmaI-SacI sites of the pNEB193 plasmid (New England Biolabs). Six hundred base pairs around each of the joining sites were sequenced to control for ligation errors. An internal SacI site in the 8.4-kb promoter was destroyed by T4 exonuclease. The linear constructs were excised as 10.7-kb unique fragments by using AscI and SacI.
Generation of Transgenic Mice.
Homozygous α-gustducin-null male mice in the 129/SvEmsJ genetic background were bred to superovulated B6CBAF1/J females to generate zygotes for pronuclear injection of the transgenic constructs. Injections were performed as described (22). Acquisition of the transgene was determined as described (23), using PCR with oligonucleotide SC-5′ (CCGGGCCCCTCTGCTAACC from the βII globin intron) and SC-3′ (GCAGTTGTTGGTCCTCTCTACTTCTCGG from the 5′ region of the gustducin-coding sequence). The presence of the gus (knockout) allele was determined as described (17). Founder animals were screened by genomic Southern analysis, using an α-gustducin probe to determine the integrity of the transgene array and to confirm the identity of each transgenic line. Selected founders were backcrossed to 129/SvEmsJ gus/gus (gustducin-null) mice to generate stable transgenic lines. Heterozygous (GUS/gus) mice carrying either transgene were used as parents in a second backcross to the 129/SvEmsJ gus/gus background so that the litters would contain mice of all four possible genotypes in a homogeneous genetic background. A total of 6 lines were generated for the WT transgene, and 12 for the G352P transgene. Three lines of mice for each transgene (nos. 28, 47, and 63 for WT; nos. 81, 82, and 89 for the G352 transgene) were tested in pilot experiments for protein expression and behavioral responses to bitter and sweet compounds. Qualitatively similar results were obtained with the three WT transgenic lines (restoration of responses to bitter and sweet compounds). Likewise, similar results were obtained with the three G352P transgenic lines (no restoration of responses to bitter and sweet compounds). Lines 28 and 82 were chosen for further analysis because the pattern of α-gustducin expression in their TRCs matched or exceeded that of WT.
Immunofluorescence.
Sectioning, fixation, and immunofluorescence were as described (2, 21). The rabbit antiserum GD1 (24), elicited against an α-gustducin-specific peptide, was used at 1:500 dilution in blocking buffer for 1 h at room temperature. After washes in PBS, the sections were incubated with a Cy3 fluorescent anti-rabbit IgG conjugate for 30 min at room temperature, washed in PBS, mounted, and photographed by using a fluorescent microscope.
Behavioral Analysis.
The mice were grouped according to genotype by PCR to score for endogenous α-gustducin and neo (to determine whether they were GUS/gus or gus/gus) and for the transgenes. Young adult males were used in all experiments. The genetic background was 87.5% 129/SvEmsJ, 6% C57BL/6J, and 6% CBA/J. Heterozygous GUS/gus mice were used as WT controls; previous experiments indicated no gross anatomical or behavioral differences between GUS/GUS and GUS/gus animals (data not shown). Two-bottle preference testing was as described (17).
Results
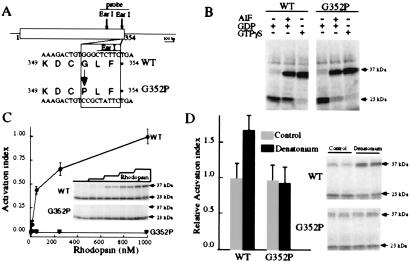
Several biochemical studies suggest that the interaction of gustducin with its cognate taste receptors is similar to that of transducin with rhodopsin (2, 3, 7). A key result from these studies is that the C terminus of α-gustducin is a critical determinant for its interaction with taste receptors. Based on inferences derived from structural studies of transducin (25, 26), and on peptide and mutagenic studies of the interactions of transducin and other G-proteins with GPCRs (27–30), we generated an α-gustducin mutant with a single glycine to proline substitution at position 352 near its C terminus (G352P; Fig. 1A). The G352P-α-gustducin mutant was anticipated to be defective in its interaction with taste receptors, because the equivalent glycine of rod-transducin (G348) is a key amino acid in the β-sheet of the C terminus (25, 26), and its substitution by alanine or proline disrupts the structure of the C terminus and interferes with transducin's interaction with rhodopsin (27–30). G348P-α-transducin was shown to have a selective deficit in coupling to rhodopsin while maintaining its ability to bind guanine nucleotides and undergo GTPγS-dependent conformational activation (29).
Figure 1.
Biochemical characterization of the G352P mutant of α-gustducin. (A) Oligonucleotide mutagenesis was used to substitute glycine at position 352 of α-gustducin (WT) with proline (G352P). The substitution destroys an EarI restriction endonuclease site. (B) The conformationally sensitive trypsin assay monitors the activation state of G-protein α-subunits such as α-gustducin. After digestion with trypsin, active (GTPγS-bound) α-gustducin yields a 37-kDa fragment, whereas inactive (GDP-bound) α-gustducin yields a 23/25-kDa doublet. This assay demonstrates that G352P-α-gustducin undergoes guanine nucleotide-dependent shifts in its conformation indistinguishable from those of WT. GDP-bound WT and G352P-gustducin are in the inactive conformation (note diagnostic 23-kDa tryptic fragments) and WT and G352P-α-gustducin bound to 100 μM GTPγS or GDP + 1 μM AlF4− are in the active conformation (note diagnostic 37-kDa fragments). (C) The trypsin-sensitivity assay shows that G352P-α-gustducin cannot be activated by rhodopsin. Rhodopsin (0.01–1 μM) activates WT gustducin (filled circles) with half maximal activation at ≈30 nM rhodopsin. G352P-gustducin (open triangles) is not activated by rhodopsin, even at the saturating 1 μM concentration. The Inset image shows an autoradiogram of a representative trypsin assay monitoring rhodopsin activation of WT and G352P-α-gustducin (the concentration of rhodopsin in the adjacent duplicate lanes was 0, 10, 50, 300, and 1,000 nM). The graph is the quantitation of three independent assays. The activation index was derived by dividing the intensity of the 37-kDa (“active”) band by the total intensities of the 37 + 23/25-kDa (“inactive”) bands. At concentrations of rhodopsin = 1 μM, maximal activation of WT-α-gustducin was achieved, hence, this point was defined as an activation index of 1.0. (D) WT-α-gustducin, but not G352P-α-gustducin, is activated by bovine taste membranes plus bitter denatonium. The image shows a representative autoradiogram with duplicate independent samples; note the activation of WT gustducin, but not G352P-α-gustducin, on the addition of denatonium (evidenced by the increased intensity of the 37-kDa band). The relative activation index displayed in the bar graph was calculated from the ratio of the activation indices in the presence and absence of denatonium (from three independent experiments).
We used the conformationally sensitive trypsin assay to monitor the activation state of G352P-α-gustducin vs. WT-α-gustducin. Like G348P-α-transducin, we found G352P-α-gustducin to be intact grossly because it could be activated in a receptor-independent fashion by AlF4− or GTPγS (Fig. 1B). The G352P-α-gustducin mutant had the same affinity as WT for GDP and GTPγS and interacted normally with G-protein βγ-subunits (data not shown). However, G352P-α-gustducin could not be activated by rhodopsin (Fig. 1C) or by denatonium-responsive receptors present in taste membranes (Fig. 1D), suggesting that, as expected, G352P-α-gustducin maintained all features of the WT G-protein except the ability to be activated by seven-transmembrane helix receptors. This result, together with peptide competition studies (2), and the G-protein βγ dependence of α-gustducin activation by denatonium benzoate plus taste receptors (3), supports the idea that the observed activation of WT-α-gustducin and α-transducin by denatonium-stimulated taste membranes is mediated indeed by GPCRs.
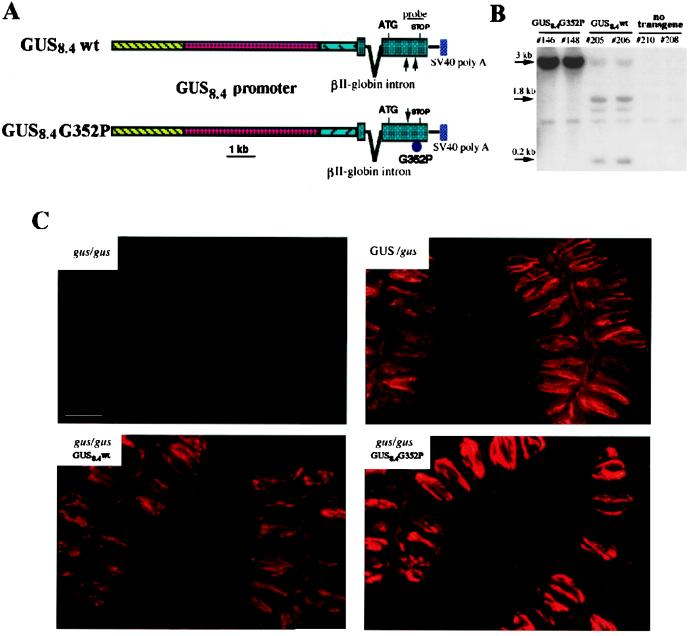
To determine if transgenic expression of G352P-α-gustducin in the α-gustducin lineage of TRCs would competitively inhibit WT gustducin and/or other G-proteins expressed in these TRCs, we used the murine α-gustducin promoter (15, 20, 21) to drive expression of G352P- and WT-α-gustducin transgenes. To analyze the effects of transgenic expression of G352P and WT forms of α-gustducin on bitter and sweet transduction in vivo, we generated appropriate recombinant constructs driven by the GUS8.4 promoter (diagrammed in Fig. 2A). The G352P mutation destroys an EarI restriction endonuclease site (Fig. 1A) that can be used to differentiate G352P-gustducin from WT by Southern blot (Fig. 2B). Southern analysis with a C-terminal α-gustducin probe (Fig. 2A) labeled, in all animals, a 1-kb EarI fragment corresponding to the endogenous α-gustducin gene; diagnostic 1.8- and 0.2-kb fragments were present in GUS8.4WT transgenic animals, but absent from GUS8.4G352P mice (Fig. 2B). The relative intensity of the labeled bands was related directly to the copy number of the transgenes (10–50 copies for GUS8.4G352P; 2–5 copies for GUS8.4WT). Gustducin-specific immunofluorescence demonstrated TRC expression of G352P-α-gustducin and WT transgenes (Fig. 2C). α-Gustducin-null mice (gus/gus) were completely devoid of gustducin protein, whereas null mice carrying the WT α-gustducin transgene (gus/gus-GUS8.4WT) expressed protein levels comparable to those of heterozygous nontransgenic (Gus/gus) siblings. Null mice carrying the mutant G352P transgene (gus/gus-GUS8.4G352P), however, overexpressed the mutant protein at about 3–5 times the level of WT α-gustducin in the heterozygous/nontransgenic siblings.
Figure 2.
Generation of GUS8.4WT- and GUS8.4G352P-α-gustducin transgenic lines. (A) Schematic maps of the constructs used for pronuclear injection. An 8.4-kb fragment of the mouse α-gustducin gene (GUS8.4 promoter) that drives TRC-specific expression was cloned 5′ to the rat WT or G352P-α-gustducin cDNAs. The position and relative size of the α-gustducin probe used for screening is shown. (B) Genomic Southern analysis of transgenic lines. EarI-digested genomic DNAs from two GUS8.4G352P, two GUS8.4WT, and two nontransgenic GUS/gus mice were electrophoresed, transferred, and probed. The endogenous GUS gene yields a 1.0-kb EarI fragment present in all the mice analyzed. The WT transgene yields 1.8- and 0.2-kb bands; the G352P transgene yields a 3.0-kb band. Differences in the intensities of the bands across transgenic lines reflect variation in the copy number of the transgenes. (C) Expression of WT and G352P-gustducin transgenes. Indirect immunofluorescence staining of circumvallate papillae from homozygous null (gus/gus), heterozygous WT/null (GUS/gus), and transgenic animals expressing WT or G352P-α-gustducin in the gus/gus background. No α-gustducin is detected in TRCs of gus/gus mice; gus/gus mice transgenic for GUS8.4WT-α-gustducin (gus/gus-GUS8.4WT) express the protein at levels comparable to GUS/gus littermates; gus/gus mice transgenic for the G352P-α-gustducin (gus/gus-GUS8.4G352P) have ≈3–5-fold more gustducin in their TRCs in comparison to their GUS/gus nontransgenic siblings.
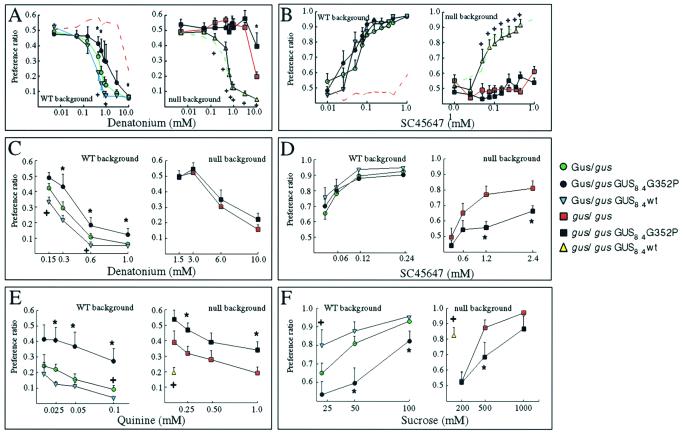
To confirm that transgenes expressed from the α-gustducin promoter function as well as the endogenous α-gustducin gene, we tested behavioral responses of null mice carrying the WT transgene (gus/gus-GUS8.4WT) to compounds that are perceived by humans as bitter (denatonium and quinine) or sweet (sucrose and SC45647, a high-potency artificial sweetener). Transgenic expression of WT rat α-gustducin in α-gustducin-null mice led to the recovery of responsiveness to both denatonium (bitter) and SC45647 (sweet) at levels no different from that of heterozygous WT (GUS/gus) siblings (Fig. 3 A and B and Table 1). These results and immunohistochemical analysis of mice expressing β-galactosidase or green fluorescent protein as transgenes driven by the GUS8.4 promoter (15, 20, 21) demonstrate that this promoter drives appropriate and functional expression of transgenes to the α-gustducin lineage of TRCs.
Figure 3.
Mean preference ratios from 48-h two-bottle preference tests. Males of six different genotypes, heterozygous WT (GUS/gus; green circles), homozygous null (gus/gus; red squares), GUS8.4G352P transgenic in the heterozygous WT background (GUS/gus-GUS8.4G352P; filled circles), GUS8.4G352P transgenic in the homozygous null background (gus/gus-GUS8.4G352P; filled squares), GUS8.4WT transgenic in the heterozygous WT background (GUS/gus-GUS8.4WT; inverted blue triangles), and GUS8.4WT transgenics in the homozygous null background (gus/gus-GUS8.4WT; yellow triangles), were compared. To aid comparison, the dashed lines in A and B show the gus/gus (Left) and the GUS/gus (Right) groups. The experiments of A and B were performed with one set of animals, experiments in C–F with another set, and a third set of animals was used in a denatonium single presentation experiment (Table 1). Presentation of tastants was in the following ascending orders. (A) Denatonium benzoate (0.005, 0.05, 0.15, 0.45, 0.62, 0.85, 1.0, 3.0, and 10.0 mM). (B) SC45647 (0.01, 0.025, 0.05, 0.075, 0.1, 0.15, 0.23, 0.35, 0.45, and 1.0 mM). (C) Denatonium benzoate (0.15, 0.3, 0.6, and 1 mM for groups in the heterozygous WT background and 1.5, 3.0, 6.0, and 10.0 mM for groups in the homozygous null background). (D) SC45647 (0.03, 0.06, 0.12, and 0.24 mM for groups in the WT background and 0.3, 0.6, 1.2, and 2.4 mM for groups in the null background). (E) Quinine hydrochloride (0.01, 0.025, 0.05, and 0.1 mM for groups in the WT background and 0.1, 0.25, 0.5, and 1 mM for groups in the null background). (F) Sucrose (20, 50, and 100 mM for groups in the WT background and 200, 500, and 1,000 mM for groups in the null background). Two-way ANOVA (between factor, genotype; within factor, concentration of tastant) was used for statistical comparison of mean preference ratios between the groups. The exact P values and F statistics for each comparison, as well as the t tests for pairwise mean comparisons between strains (collapsed across concentration), are shown (Table 1). For individual concentrations of the different tastants, the figure indicates the significant (P < 0.05, two-tailed t test) pairwise comparisons of GUS8.4G352P transgenic animals vs. nontransgenic littermates (*) or of GUS8.4WT transgenic animals vs. nontransgenic littermates (+).
Table 1.
Statistical analysis of two-bottle preference test
| Tastant | Concentration range, C | Genetic background | Transgene (strain: S) | n | Preference ratio (overall mean ± SD) | ANOVA, S × C* | Strain comparison t test† |
|---|---|---|---|---|---|---|---|
| Denatonium | 5 μM–10 mM | GUS/gus | no transgene (A) | 13 | 0.26 ± 0.19 | F = 5.3 | C > A P < 0.01 |
| GUS8.4WT (B) | 8 | 0.23 ± 0.19 | P < 0.013 | C > B P < 0.001 | |||
| GUS8.4 G352P (C) | 9 | 0.33 ± 0.21 | A > B P < 0.07 | ||||
| Denatonium | 5 μM–10 mM | gus/gus | no transgene (D) | 10 | 0.50 ± 0.13 | F = 42.4 | D > E P < 0.0001 |
| GUS8.4WT (E) | 11 | 0.27 ± 0.19 | P < 0.0001 | F > E P < 0.0001 | |||
| GUS8.4 G352P (F) | 9 | 0.51 ± 0.15 | |||||
| Denatonium | 0.1–1 mM | GUS/gus | no transgene (A) | 19 | 0.22 ± 0.19 | F = 4.9 | C > A P < 0.02 |
| GUS8.4WT (B) | 10 | 0.16 ± 0.14 | P < 0.013 | C > B P < 0.003 | |||
| GUS8.4 G352P (C) | 12 | 0.31 ± 0.25 | A > B P < 0.09 | ||||
| Denatonium | 1–10 mM | gus/gus | no transgene (D) | 11 | 0.37 ± 0.21 | F = 0.49 | |
| GUS8.4 G352P (F) | 11 | 0.40 ± 0.19 | P < 0.5 | ||||
| Denatonium | 0.5 mM | GUS/gus | no transgene (A) | 10 | 0.13 ± 0.13 | C > A P < 0.01 | |
| GUS8.4 G352P (C) | 12 | 0.29 ± 0.16 | |||||
| Denatonium | 5 mM | gus/gus | no transgene (D) | 12 | 0.14 ± 0.11 | F > D P < 0.01 | |
| GUS8.4 G352P (F) | 13 | 0.27 ± 0.15 | |||||
| Quinine | 60 μM–0.5 mM | GUS/gus | no transgene (A) | 19 | 0.20 ± 0.23 | F = 3.2 | C > A P < 0.02 |
| GUS8.4WT (B) | 10 | 0.17 ± 0.23 | P < 0.05 | C > B P < 0.02 | |||
| GUS8.4 G352P (C) | 12 | 0.37 ± 0.31 | A > B P < 0.12 | ||||
| Quinine | 0.6–5 mM | gus/gus | no transgene (D) | 11 | 0.29 ± 0.19 | F = 4.7 | |
| GUS8.4 G352P (F) | 11 | 0.42 ± 0.16 | P < 0.04 | ||||
| SC45647 | 10 μM–1 mM | GUS/gus | no transgene (A) | 13 | 0.78 ± 0.20 | F = 1.2 | |
| GUS8.4WT (B) | 8 | 0.71 ± 0.18 | P < 0.32 | ||||
| GUS8.4 G352P (C) | 9 | 0.82 ± 0.24 | |||||
| SC45647 | 10 μM–1 mM | gus/gus | no transgene (D) | 10 | 0.48 ± 0.11 | F = 39.1 | D < E P < 0.0001 |
| GUS8.4WT (E) | 11 | 0.73 ± 0.23 | P < 0.0001 | F < E P < 0.0001 | |||
| GUS8.4 G352P (F) | 9 | 0.49 ± 0.10 | |||||
| SC45647 | 30 μM–0.3 mM | GUS/gus | no transgene (A) | 19 | 0.82 ± 0.20 | F = 0.59 | |
| GUS8.4WT (B) | 10 | 0.82 ± 0.17 | P < 0.56 | ||||
| GUS8.4 G352P (C) | 12 | 0.87 ± 0.16 | |||||
| SC45647 | 0.3 μM–3 mM | gus/gus | no transgene (D) | 11 | 0.68 ± 0.22 | F = 5.17 | |
| GUS8.4 G352P (F) | 11 | 0.56 ± 0.16 | P < 0.03 | ||||
| Sucrose | 20–100 mM | GUS/gus | no transgene (A) | 19 | 0.80 ± 0.21 | F = 6.23 | C < A P < 0.0001 |
| GUS8.4WT (B) | 10 | 0.87 ± 0.18 | P < 0.005 | C < B P < 0.0001 | |||
| GUS8.4 G352P (C) | 12 | 0.66 ± 0.26 | A < B P < 0.09 | ||||
| Sucrose | 0.2–1 M | gus/gus | no transgene (D) | 11 | 0.79 ± 0.22 | F = 1.48 | |
| GUS8.4 G352P (F) | 11 | 0.69 ± 0.16 | P < 0.24 |
Nonsignificant differences are indicated by bold type.
Strain comparison t tests were collapsed across concentration.
Expression of the WT transgene in the heterozygous background led to a slight but significantly increased sensitivity to denatonium (Fig. 3 A and C and Table 1), quinine (Fig. 3E and Table 1), and sucrose (Fig. 3F and Table 1), suggesting that limiting amounts of α-gustducin in heterozygous nontransgenics may affect their sensitivity to these compounds; alternatively, the rat transgenes may differ functionally from murine α-gustducin despite their sequence similarity. Responses to SC45647 did not differ significantly in nontransgenic heterozygotes vs. heterozygotes expressing the WT transgene (Fig. 3D and Table 1). It is unclear why transgenic overexpression of WT-α-gustducin affects responses to quinine, denatonium, and sucrose but not to SC45647.
Given that the α-gustducin promoter provided appropriate expression of the WT α-gustducin transgene, we set out to determine if overexpression of the G352P-α-gustducin transgene would act as a “dominant-negative” to inhibit the functioning of WT α-gustducin and any other G-proteins present in the α-gustducin lineage of TRCs. Expression of the G352P-α-gustducin transgene in the WT-α-gustducin background (GUS/gus-GUS8.4G352P) diminished responses to both sweet and bitter compounds (Fig. 3 WT background). In contrast to the nontransgenic GUS/gus siblings, the GUS/gus-GUS8.4-G352P transgenic mice showed a marked reduction in their responsiveness to denatonium (Fig. 3 A and C and Table 1), quinine (Fig. 3E and Table 1), and sucrose (Fig. 3F and Table 1), although curiously no such effect was observed with SC45647 (Fig. 3D and Table 1). As expected, expression of the G352P transgene in the α-gustducin-null background did not restore responsiveness to either sweet or bitter compounds (Fig. 3). In the range of concentrations of denatonium (0.1–1.0 mM) and SC45647 (0.1–1.0 mM) at which heterozygous nontransgenic (GUS/gus) siblings demonstrated maximal behavioral responses, no statistically significant differences were observed between null (gus/gus) mice and null littermates carrying the GUS8.4G352P transgene (Fig. 3 A and B null background). However, at the higher concentrations of denatonium (6.0–10.0 mM), SC45647 (1.2–1.5 mM), quinine (0.5–1 mM), and sucrose (0.5–1.0 M) required to elicit responses from gus/gus mice, the gus/gus-GUS8.4G352P transgenic mice showed significantly decreased responses in comparison to their gus/gus-nontransgenic littermates (Fig. 3 C–F null background and Table 1). At these higher concentrations of tastants, the G352P-α-gustducin protein acted as a dominant negative to inhibit the gustducin-independent mechanisms operating in α-gustducin-null mice. These results suggest that these “backup” mechanisms use another G-protein α-subunit expressed in the same TRCs that normally express gustducin; possible candidates include α-transducin, αi2, αi3, α14, α15, or αS, which have been shown to be present in TRCs (1, 31–33).
Discussion
We have characterized biochemically the G352P-α-gustducin mutant in vitro and expressed transgenically WT- and G352P-α-gustducin in TRCs in vivo to gain insight into α-gustducin-dependent and α-gustducin-independent taste transduction pathways. The WT transgene restored bitter and sweet responsiveness of α-gustducin-null mice to WT levels, whereas the G352P mutant, which cannot be activated by GPCRs, did not restore these responses. Rather, the mutant transgene acted as a dominant negative to further reduce the residual taste responses of the null mice.
In α-gustducin-null mice, the gustducin heterotrimer does not form, and downstream signals carried by the βγ substituents of the heterotrimer are lost along with those signals mediated by α-gustducin. In the G352P-α-gustducin transgenic mice, the gustducin heterotrimer forms, but because the G352P mutant cannot be activated by its receptor, blocking receptor-mediated activation of gustducin's α- and βγ-subunits, it acts as a “βγ sink” to bind all available βγ-subunits and remove them from the pool of receptor-activatable heterotrimers. That there is no recovery of responsiveness to bitter or sweet compounds in null mice expressing the G352P-α-gustducin transgene suggests that both bitter and sweet transduction involve activation of the gustducin heterotrimer. Furthermore, by its action as a βγ sink, the G352P-α-gustducin mutant may block receptor activation of other heterotrimers expressed in the gustducin lineage of TRCs.
We conclude that the gustducin-independent responses of α-gustducin-null mice to quinine, denatonium, sucrose, SC45647, and presumably other bitter and sweet compounds, are mediated by other heterotrimeric G-proteins expressed in the gustducin lineage of TRCs. α-Transducin seems the most likely “backup” for α-gustducin because it is expressed in TRCs (2) and is indistinguishable biochemically from gustducin (7). Consistent with this proposal is the finding that GUS8.4-promoter expression of the α-rod-transducin transgene in the gustducin lineage of TRCs partially restored behavioral responses to bitter and sweet compounds of α-gustducin-null mice (W. He and R.F.M., unpublished data).
How gustducin can transduce the tastes of both bitter and sweet compounds is presently unclear. The interrelationship of bitter and sweet and possible crosstalk between these pathways have been noted (34–39). Psychophysical studies describe the phenomenon of “mixture suppression,” where mixtures of sweet and bitter compounds are neither as sweet nor as bitter as the individual compounds alone (40, 41); nerve recording studies suggest that some of the mixture suppression may occur peripherally (42, 43). It may be that bitter and sweet receptors are segregated physically in bitter- or sweet-specific TRCs, and that both types of TRCs use gustducin in taste transduction. In support of this notion is the striking pattern of T2R/TRB coexpression with α-gustducin; at the rear of the tongue, two-thirds of the α-gustducin-positive TRCs are also positive for T2R/TRB, whereas at the front of the tongue, most of the α-gustducin-positive TRCs are negative for T2R/TRB. Perhaps the α-gustducin-positive/T2R-TRB-negative TRCs are sweet-responsive cells.
Acknowledgments
We thank S. Zou for immunohistochemistry. R.F.M. is an Associate Investigator of the Howard Hughes Medical Institute. This research was supported by National Institutes of Health Grants RO1DC03055 and RO1DC03155 (to R.F.M.), F32DC00142-02 (G.T.W.), and RO3DC04766 (to S.D.) and by a grant from Comisio Interdepartamental de Recerca i Innovacio Tecnologica (to L.R.-A.).
Abbreviations
- G-protein
guanine nucleotide-binding regulatory protein
- GPCR
G-protein coupled receptor
- IP3
inositol triphosphate
- PDE
phosphodiesterase
- TRC
taste receptor cells
- WT
wild type
References
- 1.McLaughlin S K, McKinnon P J, Margolskee R F. Nature (London) 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Avila L, McLaughlin S K, Wildman D, McKinnon P J, Robichon A, Spickofsky N, Margolskee R F. Nature (London) 1995;376:80–85. doi: 10.1038/376080a0. [DOI] [PubMed] [Google Scholar]
- 3.Ming D, Ruiz-Avila L, Margolskee R F. Proc Natl Acad Sci USA. 1998;95:8933–8938. doi: 10.1073/pnas.95.15.8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adler E, Hoon M A, Mueller K L, Chandrashekar J, Ryba N J P, Zuker C S. Cell. 2000;10:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 5.Matsunami H, Montmayeur J-P, Buck L B. Nature (London) 2000;404:601–604. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]
- 6.Chandrashekar J, Mueller K L, Hoon M A, Adler E, Feng L, Guo W, Zuker C S, Ryba N J P. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 7.Hoon M A, Northup J K, Margolskee R F, Ryba N J P. Biochem J. 1995;309:629–636. doi: 10.1042/bj3090629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price S. Nature (London) 1973;241:54–55. doi: 10.1038/241054a0. [DOI] [PubMed] [Google Scholar]
- 9.Kurihara K. FEBS Lett. 1972;27:279–281. doi: 10.1016/0014-5793(72)80640-9. [DOI] [PubMed] [Google Scholar]
- 10.Bakre, M. M., Glick, J. G., Rybalkin, S., Max, M., Beavo J. & Margolskee, R. F. (2001), Proc. Natl. Acad. Sci. USA, in press.
- 11.Yan W, Sunavala G, Rosenzweig S, Dasso M, Brand J G, Spielman A I. Am J Physiol. 2001;280:C742–C751. doi: 10.1152/ajpcell.2001.280.4.C742. [DOI] [PubMed] [Google Scholar]
- 12.Spielman A I, Huque T, Nagai H, Whitney G, Brand J. Physiol Behav. 1994;56:1149–1155. doi: 10.1016/0031-9384(94)90359-x. [DOI] [PubMed] [Google Scholar]
- 13.Spielman A I, Nagai H, Sunavala G, Dasso M, Breer H, Boekhoff I, Huque T, Whitney G, Brand J. Am J Physiol. 1996;270:C926–C931. doi: 10.1152/ajpcell.1996.270.3.C926. [DOI] [PubMed] [Google Scholar]
- 14.Rossler P, Kroner C, Freitag J, Noe J, Breer H. Eur J Cell Biol. 1998;77:253–261. doi: 10.1016/s0171-9335(98)80114-3. [DOI] [PubMed] [Google Scholar]
- 15.Huang L, Shanker Y G, Dubauskaite J, Zheng J Z, Yan W, Rosenzweig S, Spielman A I, Max M, Margolskee R F. Nat Neurosci. 1999;2:1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- 16.Rossler P, Boekhoff I, Tareilus E, Beck S, Breer H, Freitag J. Chem Senses. 2000;25:413–421. doi: 10.1093/chemse/25.4.413. [DOI] [PubMed] [Google Scholar]
- 17.Wong G T, Gannon K S, Margolskee R F. Nature (London) 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- 18.Deng W P, Nickoloff J A. Anal Biochem. 1992;200:81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- 19.Neer E J, Denker B M, Thomas T C, Schmidt C J. Methods Enzymol. 1994;237:226–239. doi: 10.1016/s0076-6879(94)37065-6. [DOI] [PubMed] [Google Scholar]
- 20.Wong G T, Ruiz-Avila L, Ming D, Gannon K S, Margolskee R F. Cold Spring Harbor Symp Quant Biol. 1996;61:173–184. [PubMed] [Google Scholar]
- 21.Wong G T, Ruiz-Avila L, Margolskee R F. J Neurosci. 1999;19:5802–5809. doi: 10.1523/JNEUROSCI.19-14-05802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. [Google Scholar]
- 23.Laird P W, Zijderveld A, Linders K, Rudnicki M A, Jaenisch R, Berns A. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takami S, Getchell M L, McLaughlin S K, Margolskee R F, Getchell T V. Mol Brain Res. 1994;22:193–203. doi: 10.1016/0169-328x(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 25.Conklin B R, Bourne H R. Cell. 1993;73:631–641. doi: 10.1016/0092-8674(93)90245-l. [DOI] [PubMed] [Google Scholar]
- 26.Noel J P, Hamm H E, Sigler P B. Nature (London) 1993;366:654–663. doi: 10.1038/366654a0. [DOI] [PubMed] [Google Scholar]
- 27.Hamm H E, Rarick H M. Methods Enzymol. 1994;237:423–436. doi: 10.1016/s0076-6879(94)37079-6. [DOI] [PubMed] [Google Scholar]
- 28.Garcia P D, Onrust R, Bell S M, Sakmar T P, Bourne H R. EMBO J. 1995;14:4460–4469. doi: 10.1002/j.1460-2075.1995.tb00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osawa S, Weiss E R. J Biol Chem. 1995;270:31052–31058. doi: 10.1074/jbc.270.52.31052. [DOI] [PubMed] [Google Scholar]
- 30.Onrust R, Herzmark P, Chi P, Garcia P D, Lichtarge O, Kingsley C, Bourne H R. Science. 1997;275:381–384. doi: 10.1126/science.275.5298.381. [DOI] [PubMed] [Google Scholar]
- 31.McLaughlin S K, McKinnon P J, Spickofsky N, Danho W, Margolskee R F. Physiol Behav. 1994;56:1157–1164. doi: 10.1016/0031-9384(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 32.Kusakabe Y, Yamaguchi E, Tanemura K, Kameyama K, Chiba N, Arai S, Emori Y, Abe K. Biochim Biophys Acta. 1998;1403:265–272. doi: 10.1016/s0167-4889(98)00062-7. [DOI] [PubMed] [Google Scholar]
- 33.Kusakabe Y, Yasuoka A, Asano-Miyoshi M, Iwabuchi K, Matsumoto I, Arai S, Emori Y, Abe K. Chem Senses. 2000;25:525–531. doi: 10.1093/chemse/25.5.525. [DOI] [PubMed] [Google Scholar]
- 34.Shallenberger R S, Acree T E. Nature (London) 1967;216:480–482. doi: 10.1038/216480a0. [DOI] [PubMed] [Google Scholar]
- 35.Belitz H-D, Chen W, Jugel H, Treleano R, Weiser H, Gasteiger J, Marsili M. Am Chem Soc Symp Ser. 1979;115:93–131. [Google Scholar]
- 36.Birch G G, Mylvaganam A R. Nature (London) 1976;260:6332–6334. [Google Scholar]
- 37.Lee C K. Adv Carbohydr Chem Biochem. 1987;45:199–351. doi: 10.1016/s0065-2318(08)60140-7. [DOI] [PubMed] [Google Scholar]
- 38.Kinnamon S C, Margolskee R F. Curr Opin Neurobiol. 1996;6:506–513. doi: 10.1016/s0959-4388(96)80057-2. [DOI] [PubMed] [Google Scholar]
- 39.Lindemann B. Curr Biol. 1996;10:1234–1237. doi: 10.1016/s0960-9822(96)00704-x. [DOI] [PubMed] [Google Scholar]
- 40.Bartoshuk L M. Physiol Behav. 1975;14:643–649. doi: 10.1016/0031-9384(75)90193-6. [DOI] [PubMed] [Google Scholar]
- 41.Lawless H. Physiol Behav. 1982;29:149–152. doi: 10.1016/0031-9384(82)90379-1. [DOI] [PubMed] [Google Scholar]
- 42.Formaker B K, Frank M E. Brain Res. 1996;727:79–90. doi: 10.1016/0006-8993(96)00356-3. [DOI] [PubMed] [Google Scholar]
- 43.Formaker B K, MacKinnon B I, Hettinger T P, Frank M E. Brain Res. 1997;772:239–242. doi: 10.1016/s0006-8993(97)00845-7. [DOI] [PubMed] [Google Scholar]