Opinion statement
Varicella zoster virus (VZV) is an exclusively human neurotropic alphaherpesvirus. Primary infection causes varicella (chickenpox), after which virus becomes latent in ganglionic neurons along the entire neuraxis. With advancing age or immunosuppression, cell-mediated immunity to VZV declines and virus reactivates to cause zoster (shingles), which can occur anywhere on the body. Skin lesions resolve within 1-2 weeks, while complete cessation of pain usually takes 4-6 weeks. Zoster can be followed by chronic pain (postherpetic neuralgia), cranial nerve palsies, zoster paresis, meningoencephalitis, cerebellitis, myelopathy, multiple ocular disorders and vasculopathy that can mimic giant cell arteritis. All of the neurological and ocular disorders listed above may also develop without rash. Diagnosis of VZV-induced neurological disease may require examination of CSF, serum and/ or ocular fluids. In the absence of rash in a patient with neurological disease potentially due to VZV, CSF should be examined for VZV DNA by PCR and for anti-VZV IgG and IGM. Detection of VZV IgG antibody in CSF is superior to detection of VZV DNA in CSF to diagnose vasculopathy, recurrent myelopathy, and brainstem encephalitis.
Oral antiviral drugs speed healing of rash and shorten acute pain. Immunocompromised patients require intravenous acyclovir. First-line treatments for post-herpetic neuralgia include tricyclic antidepressants gabapentin, pregabalin, and topical lidocaine patches. VZV vasculopathy, meningoencephalitis, and myelitis are all treated with intravenous acyclovir.
Keywords: Varicella zoster virus, Complications, Herpes zoster, Cranial nerves, Zoster paresis, Pathology, Treatment, Postherpetic neuralgia, Vasculopathy, Temporal artery infection, Myelopathy, Meningoencephalitis, Cerebellitis, Ocular disorders, Zoster sine herpete, Diagnostic tests
Introduction
Varicella zoster virus (VZV) is an exclusively human neurotropic alphaherpesvirus. Primary infection causes varicella (chickenpox), after which virus becomes latent in ganglionic neurons along the entire neuraxis. With advancing age or immunosuppression, cell-mediated immunity to VZV declines and virus reactivates to cause herpes zoster (shingles) which is often complicated by chronic pain (postherpetic neuralgia), cranial nerve palsies, zoster paresis, vasculopathy, meningoencephalitis, cerebellitis, myelopathy and multiple ocular disorders (Table 1). VZV reactivation also produces chronic radicular pain without rash (zoster sine herpete).
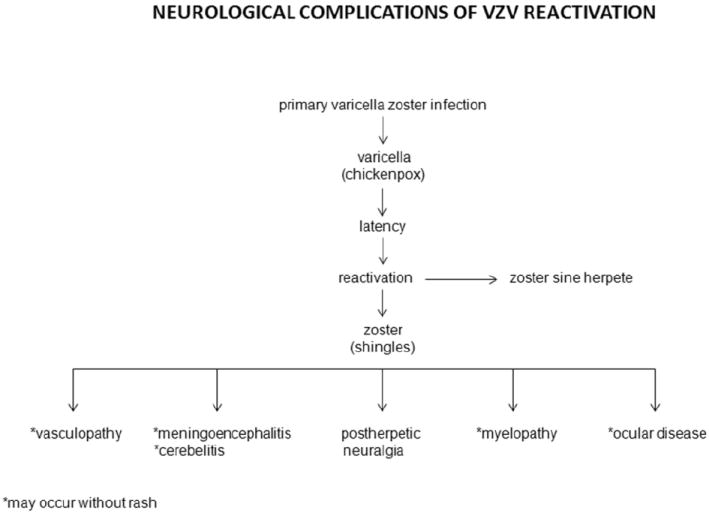
Table 1.
Neurological complications of VZV reactivation. (Reproduced from Gilden et al. [106]; copyright 2011, John Wiley & Sons; with permission.)
|
NEUROLOGICAL COMPLICATIONS OF VZV REACTIVATION
Herpes zoster
Herpes zoster is the most common manifestation of VZV reactivation. Zoster is characterized by a vesicular eruption on an erythematous base in one to three dermatomes, accompanied by severe, lancinating radicular pain; itching and unpleasant sensations (dysesthesias) produced by touch (allodynia), as well as decreased sensation in the affected area. Rash and pain usually develop within a few days of each other, although pain can precede rash by weeks to months [1]. After reactivation from cranial nerve, dorsal root or autonomic ganglia, VZV can travel peripherally to the corresponding dermatome; thus, zoster can affect any level of the neuraxis. The thoracic region is most commonly affected (>50%), followed by the face, cervical and lumbosacral regions.
The annual rate of zoster in the U.S. is 3.2 per 1,000 person-years [2]. Zoster most frequently occurs in the elderly [3] as cell-mediated immunity to VZV declines. Other groups at risk for zoster are patients taking immunosuppressive drugs (such as cancer patients, organ transplant recipients, and patients with autoimmune diseases and AIDS) [4]. Zoster in an otherwise healthy young person may be the first manifestation of HIV infection [5, 6]. Varicella in infancy also predisposes to zoster in early adulthood [7].
Zoster with cranial nerve involvement
In conjunction with dermatological manifestations of VZV reactivation, VZV can reactivate from one or more cranial nerve ganglia to cause disease. Zoster may be followed by optic neuritis [8, 9] or ophthalmoplegia [9]. Herpes zoster ophthalmicus (HZO) is often accompanied by keratitis, which can lead to blindness. Patients with HZO and visual symptoms should have an immediate slit-lamp examination by an ophthalmologist, particularly if skin lesions extend to the nose (Hutchinson sign). Involvement of the maxillary and mandibular distribution of the trigeminal nerve can produce osteonecrosis and spontaneous tooth exfoliation [10].
Involvement of the geniculate ganglion (cranial nerve VII) causes weakness or paralysis of ipsilateral facial muscles. The combination of facial palsy and vesicles in the external auditory canal or on the tympanic membrane (zoster oticus) or on the ipsilateral anterior two-thirds of the tongue or hard palate [11] constitutes the Ramsay Hunt syndrome (RHS) which is often associated with tinnitus, hearing loss, nausea, vomiting, vertigo and nystagmus, indicating involvement of cranial nerve VIII within the bony facial canal. Facial paralysis in RHS is often more severe than in Bell’s palsy, and patients are less likely to recover completely [12]. Zoster can also followed by involvement of cranial nerves IX, X, XI and XII [13, 14].
Cranial neuropathies frequently occur weeks after zoster, raising the possibility that disease is due to micro-infarction of cranial nerves. Virus particles can potentially spread transaxonally along trigeminal and other ganglionic afferent fibers to cause occlusion of small vessels supplying cranial nerves in the same manner that produces VZV vasculopathy in larger arteries (see VZV vasculopathy below). The blood supply of cranial nerves III, IV, V1 and VI comes from the carotid circulation, while V2, V3 and VII, IX, X, XI and XII are supplied by the external carotid circulation [15]. It is important to recognize that multiple forms of trigeminal- [16, 17] and facial- [18] distribution zoster as well as polyneuritis cranialis due to VZV [19, 20] may occur in the absence of rash.
Zoster paresis
VZV reactivation from ganglia in the cervical, thoracic or lumbosacral region can also cause weakness (zoster paresis). Arm weakness or diaphragmatic paralysis [21, 22] occurs after cervical distribution zoster, abdominal muscle weakness and hernia after thoracic distribution zoster [23, 24], leg weakness after lumbar or sacral distribution zoster and urinary retention after sacral distribution zoster [25, 26]. Magnetic resonance imaging (MRI) of patients with zoster paresis reveals involvement of both anterior and posterior roots at the spinal level that corresponds to the patient’s clinical deficit [27]. Rarely, clinical deficit in cervical zoster paresis extends to the brachial plexus, confirmed by both electrodiagnostic testing and MRI [28]. In 45 patients with zoster paresis, 67% had near-complete recovery [29], and in another 61 cases, 55% had complete functional recovery [30].
Pathology
The cardinal pathological features of zoster are characterized by inflammation and hemorrhagic necrosis with associated neuritis, localized leptomeningitis, unilateral segmental poliomyelitis and degeneration of related motor and sensory roots [31, 32]. Demyelination is seen in areas with mononuclear cell (MNC) infiltration and microglial proliferation. Intranuclear inclusions, viral antigen and herpesvirus particles have been found in acutely infected ganglia [33-35].
Treatment
Antiviral drugs, such as oral valacyclovir (1 gm three times daily for 7-10 days) or acyclovir (800 mg 5 times daily for 7-10 days) speed healing of rash and shorten the duration of acute pain. Immunocompromised patients require intravenous acyclovir (10-15 mg/kg every 8 hours for 10-14 days). Because zoster pain may be associated with inflammation, many clinicians administer a short course of corticosteroids, e.g., oral prednisone, 1 mg/kg for 5-7 days, in addition to antiviral therapy.
Postherpetic neuralgia (PHN)
The most common neurological complication of zoster is PHN, defined as dermatomal-distribution pain that persists for more than 3 months after zoster. Age is the most important factor in predicting the development of PHN. More than 40% of zoster patients > 60 years of age experience chronic pain. Except for its longevity, the pain of PHN and associated allodynia are the same as in zoster. The incidence of PHN is slightly greater in women [36] and after trigeminal distribution zoster [36-38].
The cause and pathogenesis of PHN are unknown. Two non-mutually exclusive theories are that: (1) excitability of ganglionic or even spinal cord neurons is altered; and (2) persistent productive virus infection exists in ganglia. Analysis of ganglia from an early case of PHN of 2.5 months’ duration revealed diffuse and focal infiltration by chronic inflammatory cells [39], an observation confirmed by Watson et al. [40] who found prominent collections of lymphocytes in ganglia from a patient with PHN of 2 years’ duration. The inflammatory response in ganglia of these subjects raised the possibility of prolonged viral infection. Further evidence that PHN may be produced by low-level ganglionitis has come from the detection of VZV DNA and proteins in blood MNCs of many patients with PHN [41-43] and from the favorable response of some PHN patients to antiviral treatment [4, 44].
PHN is difficult to manage and no universal treatment exists. First-line therapies include tricyclic antidepressants (TCAs), gabapentin and pregabalin, and topical lidocaine patches. Opioids, tramadol, capsaicin cream and the capsaicin 8% patch are recommended as second- or third-line therapies. TCAs such as amitriptyline are usually started at a dose of 10-25 mg orally at bedtime with a maximum dose of 150-200 mg/day. Secondary amine TCAs, such as nortriptyline and despramine, can also be used due to a superior safety profile compared to the tertiary amine amitryptyline [45]. The calcium channel alpha (2)-delta ligands gabapentin and pregabalin are also used, with pregabalin providing equivalent efficacy to that of gabapentin but at much lower doses due to its higher bioavailability and rapid absorption. Pregabalin is given at 75 to 150 mg orally twice daily or 50 to 100 mg orally three times daily (150 to 300 mg/day). If minimal relief is obtained at 300 mg daily for 2 weeks, the dose can be increased to a maximum of 600 mg/day in two or three divided doses. Opioids such as extended-release oxycodone, morphine and methadone have shown efficacy in patients with PHN. Tramadol is better tolerated but less effective than these stronger opioids. The lidocaine 5% patch has significant analgesic efficacy in patients with PHN [46]. Capsaicin 0.075% cream is sometimes prescribed, but the American Academy of Neurology (AAN) guidelines state that the analgesia provided is below the threshold for a clinically important effect [47]. The new capsaicin 8% patch [48,49], which delivers a high concentration of capsaicin in a single 60-minute application after application of local anesthetic, is promising for the treatment of PHN but its use awaits long-term safety data. Combination therapy such as gabapentin and nortriptyline [50], morphine and gabapentin [51] or pregabalin and the lidocaine 5% patch [52] may provide greater analgesic effects.
In patients who are refractory to non-invasive pharmacological intervention, botulinum toxin has successfully decreased PHN in several cases [53-55]. Epidural injection of steroids produced modest effects but relief was short-lived [56]. Spinal cord stimulation has limited short-term success [57].
A newer potentially promising treatment for PHN is percutaneous peripheral nerve field stimulation. Rare reports indicate its effectiveness for refractory PHN. Subjects became pain-free with minimal to no medication needed after ophthalmic- [58], cervical- [59] and thoracic- [60, 61] distribution PHN.
VZV vasculopathy
After VZV reactivation from ganglia, virus can also travel centrally to infect cerebral arteries and cause ischemic and hemorrhagic stroke (VZV vasculopathy). The exact incidence of VZV vasculopathy is unknown although it is a significant stroke risk factor. In adults with zoster, the risk of stroke is increased by 30% within the following year [62] and by 4.5-fold when zoster is in the ophthalmic distribution of the trigeminal nerve [63]. Furthermore, up to one-third of pediatric ischemic arteriopathies are associated with varicella [64]. VZV vasculopathy affects both immunocompromised and immunocompetent individuals and can present as headache, mental status changes and focal neurological deficits. In a study of 30 virologically verified cases of VZV vasculopathy [65], lesions at grey-white matter junctions were frequently seen on MRI (Fig. 1A), and magnetic resonance angiography (MRA) revealed focal arterial stenosis and occlusion in more than two-thirds of patients. Both large and small arteries were involved in 50% patients, small arteries in 37%, and large arteries alone in only 13% in the 30 subjects. Importantly: (1) up to one-third of patients did not have preceding zoster rash; (2) up to one-third did not have a CSF pleocytosis; (3) detection of anti-VZV IgG antibody was superior to detection of VZV DNA in CSF for diagnosis; and (4) symptoms and signs often occurred months after zoster [65].
Fig. 1. MRI scans of patients with varicella zoster virus (VZV) multifocal vasculopathy and myelopathy.
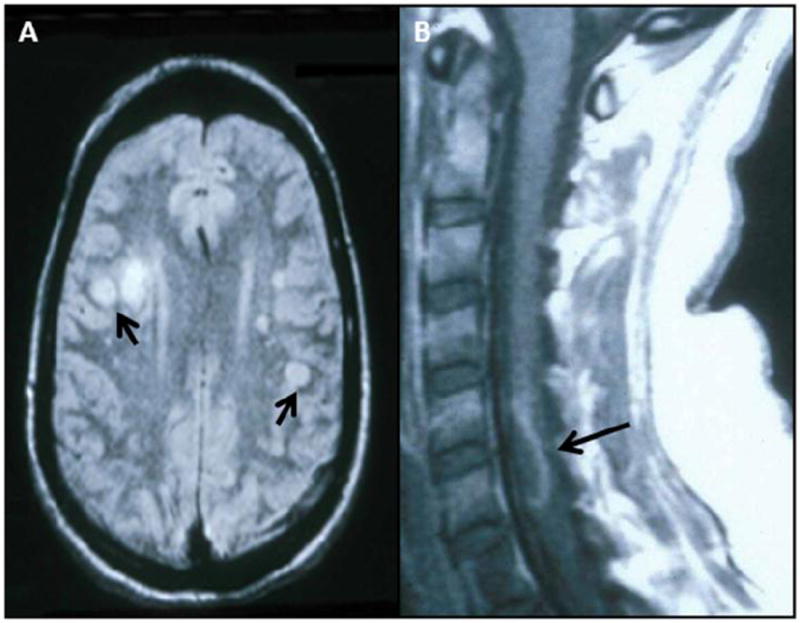
(A) Proton-density brain MRI scan shows multiple areas of infarction in both hemispheres, particularly involving white matter. Arrows point to gray-white matter junction lesions. (Reproduced from Gilden et al. [107]; copyright 2002, Springer; with permission.) (B) Note cervical, longitudinal, serpiginous enhancing lesions (arrows). (Reproduced from Gilden et al. [81]; copyright 1994, Wolters Kluwer Health; with permission.)
Infarctions are mostly bland but can also be hemorrhagic. Deep white-matter lesions often predominate and are ischemic or demyelinative, depending on the size of blood vessels involved. Infected cerebral arteries contain multinucleated giant cells, Cowdry A inclusion bodies, herpesvirus particles detected by electron microscopy, as well as VZV DNA and VZV antigen (Fig. 2). An analysis of 63 arteries from 45 normal subjects revealed no VZV DNA or VZV antigen, thus supporting the significance of VZV in arteries of stroke patients [66]. A variety of vascular pathology has been reported, ranging from neointimal proliferation to necrosis with and without inflammation [67]. A recent study that examined the histological and immunohistochemical features of VZV-infected cerebral arteries from subjects with VZV vasculopathy revealed several distinct features: (1) the presence of VZV antigen in the arterial adventitia from early VZV vasculopathy and in the media and intima of protracted cases of VZV vasculopathy, supporting the notion of virus persistence in the artery and spread from the “outside-in”; (2) a thickened arterial intima composed of myofibroblasts and cells most likely of medial smooth muscle origin; (3) a disrupted internal elastic lamina; and (4) a disrupted medial layer with a significant loss of normal smooth muscle cells [68]. The morphological changes help to explain why there is arterial occlusion and loss of vascular contractility, contributing to stroke. A follow-up study revealed that in VZV-infected arteries: (1) inflammatory cells, predominantly composed of T cells and macrophages, are present in adventitia and intima; (2) neutrophils are present in adventitia in early but not late VZV vasculopathy; and (3) adventitial inflammation was associated with an overlying thickened intima [69], supporting previous reports that inflammation contributes to vascular remodeling, as seen in models of coronary and pulmonary vascular disease.
Fig. 2. Pathological and virological findings in arteries of patients who died from varicella zoster virus (VZV) vasculopathy.
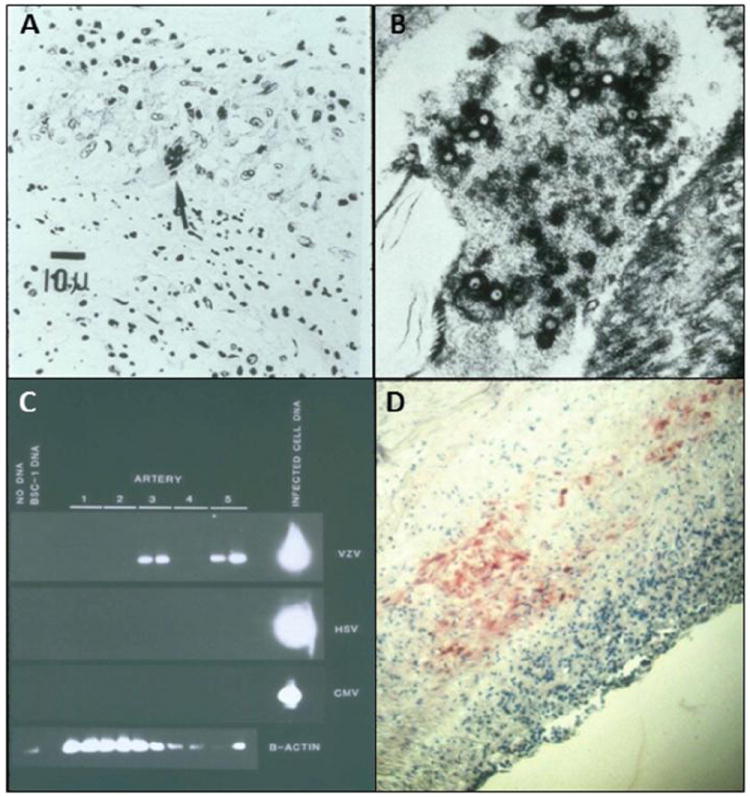
(A) Cerebral artery with multinucleated giant cells (arrow). (B) Multiple herpes virions within a cerebral artery. (Panels A and Bare reproduced from Gilden et al. [108]; copyright 2009, The Lancet Neurology/Elsevier; with permission.) (C) VZV DNA in the posterior cerebral artery (lane 3) and basilar artery (lane 5). (D) VZV antigen (red) in the media of a cerebral artery. (Panels C and D reproduced from Gilden et al. [109]; copyright 1996, Wolters Kluwer Health; with permission.) CMV=cytomegalovirus. HSV=herpes zoster virus-1. VZV=varicella zoster virus.
After zoster, the increased risk of stroke from intracranial artery involvement rather than coronary, pulmonary or other systemic vascular complications most likely reflects inherent differences in the cellular and structural composition of intracranial versus systemic arteries. For example, intracranial cerebral arteries, unlike systemic arteries, contain neither an external elastic lamina that may affect transmural migration of virus and cells nor a vaso vasorum in the adventitia above the level of the medulla unless pathological angiogenesis occurs [70]. Like horseradish peroxidase, which travels along trigeminal ganglionic afferent fibers to trigeminal ganglia after application to the external surface of cerebral arteries [71, 72], reactivated VZV in ganglia may also travel along ganglionic afferent fibers to the adventitia of cerebral arteries, a notion consisted with the presence of viral antigen predominantly in the adventitia of cerebral arteries in early VZV vasculopathy [73] and in the media and intima of protracted cases [69].
Multifocal VZV vasculopathy with temporal artery infection
We have identified multifocal VZV vasculopathy in three patients with clinical and laboratory features mimicking giant cell arteritis (GCA). The first patient was an 80-year-old man with left ophthalmic-distribution zoster who developed painless left-sided loss of vision with an elevated ESR and CRP; he was diagnosed with possible GCA and underwent temporal artery biopsy while being treated with steroids without improvement of vision. Biopsy was GCA negative. Virological analysis revealed VZV antigen in his temporal artery, after which he was treated with intravenous acyclovir and his vision improved [73]. A second even more remarkable case was a 75-year-old woman without a history of zoster, who developed left periorbital pain and loss of vision with an elevated ESR and normal CRP; she was treated with steroids for presumed GCA and vision worsened; temporal artery biopsy revealed VZV antigen, and CSF analysis revealed the presence of anti-VZV IgG antibody with reduced serum/CSF ratios of anti-VZV IgG antibody compared to ratios for albumin and total IgG, indicative of intrathecal synthesis of anti-VZV IgG antibody; vision improved after antiviral treatment [74]. A third case was a 54-year-old diabetic woman with no history of zoster rash who presented with ischemic optic neuropathy and an elevated CRP [75]. The ischemic optic neuropathy was followed by acute retinal necrosis with extensive venous beading characteristic of vasculopathy; PCR analysis of vitreous fluid in the same eye was positive for VZV DNA. The patient later developed jaw claudication and intermittent scalp pain; while temporal artery biopsy was GCA-negative, further study of the artery revealed VZV antigen (Fig. 3). These findings demonstrate that in patients with clinically suspect GCA, but whose temporal arteries are GCA-negative; VZV produces a multifocal vasculopathy affecting the ophthalmic and/or retinal arteries to cause vision loss, in addition to ipsilateral temporal artery infection. Treatment of these patients (with and without a history of zoster), with steroids for presumed GCA led to no improvement or to actual worsening of vision, while antiviral treatment improved vision.
Fig. 3. Varicella zoster virus (VZV) in the temporal artery of a patient with VZV multifocal vasculopathy.
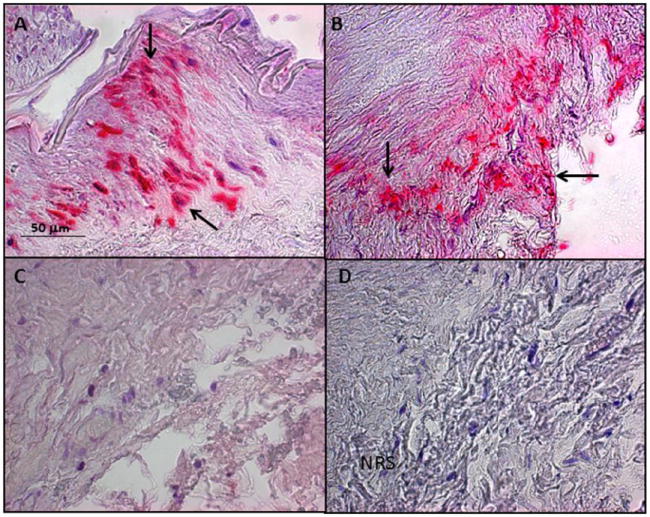
(A) Positive control cadaveric cerebral artery 14 days after VZV infection in vitro (pink color, arrows). (B) Note VZV antigen in the adventitia of the temporal artery after staining with anti-VZV antibody (pink color, arrows), but not after staining adjacent sections with anti-HSV-1 antibody (C) or normal rabbit serum (D). Magnification 200X. (Reproduced from Mathias et al. [110]; copyright 2013, Elsevier; with permission.)
Since VZV vasculopathy with temporal artery infection mimics GCA, we examined formalin-fixed, paraffin-embedded temporal arteries obtained from patients with clinically suspect GCA but whose temporal arteries were pathologically negative. Immunohistochemical analysis revealed VZV antigen, but not HSV-1 antigen in five (21%) of the 24 temporal arteries in multiple regions of each artery [76]; in contrast, none of 13 control temporal arteries obtained postmortem from subjects without symptoms or signs of GCA contained VZV antigen. Overall, these findings indicate that in patients presenting with clinical symptoms (particularly early visual disturbances), signs and laboratory abnormalities (particularly elevated CRP) consistent with GCA, the diagnosis of multifocal VZV vasculopathy is an important consideration and warrants examination of the temporal artery not only for histopathological features of GCA, but also for immunohistochemical evidence of VZV antigen. Larger studies are needed to determine the full spectrum of symptoms, signs and laboratory abnormalities, as well as the characteristic histopathological features in temporal arteries of patients with multifocal VZV vasculopathy and temporal artery infection.
VZV meningitis, meningoencephalitis, meningoradiculitis and cerebellitis
Like VZV vasculopathy, these neurological complications of VZV reactivation can occur in the absence of zoster rash, as demonstrated by recent reports of VZV meningitis [77], meningoradiculitis [78] and cerebellitis [79, 80] in which diagnosis was confirmed by the detection of VZV DNA and anti-VZV antibody in CSF.
VZV myelopathy
VZV myelopathy can present as a self-limiting, monophasic spastic paraparesis, with or without sensory features and sphincter problems. This so-called post-infectious myelitis usually occurs in immunocompetent patients days to weeks after acute varicella or zoster. Its pathogenesis is unknown. The CSF usually contains a mild mononuclear pleocytosis, with a normal or slightly elevated protein. Steroids are used to treat these patients, although some improve spontaneously.
VZV may directly invade the spinal cord or affect the spinal arteries to produce myelopathy. In such instances, VZV myelopathy may present as an insidious, progressive and sometimes fatal myelitis, mostly in immunocompromised individuals, such as patients with AIDS. MRI reveals longitudinal serpiginous enhancing lesions (Fig. 1B). Diagnosis is confirmed by the presence of VZV DNA or anti-VZV IgG or both in CSF [81]. Pathological and virological analyses of the spinal cord from fatal cases have revealed frank invasion of VZV in the parenchyma [67] and, in some instances, spread of virus to adjacent nerve roots [82]. Early diagnosis and aggressive treatment with intravenous acyclovir have been helpful, even in immunocompromised patients [83]. The benefit of steroids in addition to antiviral agents is unknown. Rarely, VZV myelitis recurs, even in immunocompetent patients [81]. VZV myelitis may also occur in the absence of zoster rash. VZV can also produce spinal cord infarction identified by diffusion-weighted MRI and confirmed virologically [84]. Thus, VZV vasculopathy can cause stroke in the spinal cord as well as in the brain.
Treatment
VZV vasculopathy, meningoencephalitis and myelitis are all treated with intravenous acyclovir, 10-15 mg/kg for 10-14 days. In immunocompromised patients, continued oral valacyclovir for months is sometimes necessary to prevent recurrence.
Ocular disease
VZV infection produces acute retinal necrosis (ARN) or progressive outer retinal necrosis (PORN). ARN in both immunocompetent and immunocompromised individuals presents with periorbital pain and floaters with hazy vision and loss of peripheral vision. Treatment is typically intravenous acyclovir, steroids and aspirin followed by oral acyclovir [85]. Intravitreal injections of foscarnet and oral acyclovir have also been effective. PORN presents with painless loss of vision, floaters and constricted visual fields with resultant retinal detachment. Multifocal, discrete opacified lesions begin in the outer retinal layers peripherally and/or posterior pole; only late in disease are inner retinal layers involved. Diffuse retinal hemorrhages and whitening with macular involvement bilaterally are characteristic findings. VZV is the most common cause of PORN, although HSV and cytomegalovirus can also cause this disease. Most cases are seen in AIDS patients with CD4+ T cell counts less than 10 cells/mm3 of blood [86] as well as in other immunosuppressed individuals [87]. PORN may be preceded by retrobulbar optic neuritis and aseptic meningitis [88], central retinal artery occlusion or ophthalmic-distribution zoster [89] and may occur together with multifocal vasculopathy or myelitis. Treatment with intravenous acyclovir has given poor or inconsistent results [90] and even when acyclovir helped, VZV retinopathy recurred when drug was tapered or stopped. PORN patients treated with ganciclovir alone or in combination with foscarnet had a better final visual acuity than those treated with acyclovir or foscarnet [91]. The best treatment for PORN in AIDS patients may be prevention with HAART [92].
Like other neurological disorders caused by VZV, ocular disease caused by VZV can also occur in the absence of rash. Multiple cases of PORN [93, 94] and a case of severe unremitting eye pain without rash were shown to be caused by VZV based on detection of VZV DNA in nasal and conjunctival samples [95]. In addition, third cranial nerve palsies [96], retinal periphlebitis [97], uveitis [96], iridocyclitis [98] and disciform keratitis [99] that occurred without rash were confirmed virologically to be caused by VZV.
Zoster sine herpete (radicular pain in the absence of rash)
Zoster sine herpete is recognized by clinicians as chronic radicular pain without rash caused by VZV. Zoster sine herpete was first described in a report of multiple patients with dermatomal distribution radicular pain in areas distinct from pain with rash in zoster [100]. The first two virologically confirmed cases of zoster sine herpete were verified by detection of VZV DNA in CSF [101]. A third case of thoracic-distribution zoster sine herpete, in which electromyography of paraspinal muscles demonstrated frequent fibrillation potentials restricted to chronically painful thoracic root segments was confirmed by detection of VZV DNA in blood MNCs and anti-VZV IgG antibody in CSF [102]. Blumenthal et al. [103] recently described a patient with zoster sine herpete whose CSF did not contain amplifiable VZV DNA but did contain anti-VZV IgG with reduced serum/CSF ratios of anti-VZV IgG indicative of intrathecal synthesis. Perhaps the most compelling evidence that persistent radicular pain without rash can be caused by chronic active VZV ganglionitis came from analysis of a trigeminal ganglionic mass removed from an immunocompetent adult who had experienced relentless trigeminal-distribution pain for more than a year; pathological and virological analyses of the ganglionic mass revealed active VZV ganglionitis [17]. The detection of VZV DNA and anti-VZV IgG and IgM antibody has expanded the spectrum of neurological disease produced by VZV in the absence of rash to include VZV meningoencephalitis, vasculopathy, myelitis, cerebellar ataxia and polyneuritis cranialis.
Diagnostic tests
The diagnosis of VZV-induced neurological disease is straightforward when the characteristic dermatomal distribution rash of zoster is present. When zoster rash is not present in a patient with neurological disease that can be caused by VZV (e.g., zoster sine herpete, vasculopathy, meningoencephalitis, myelopathy or retinal necrosis), examination of CSF and serum and ocular fluids is necessary. The routine CSF cell count can be helpful, since a mild lymphocytic pleocytosis is characteristically found in VZV vasculopathy, myelitis and meningoencephalitis. Furthermore, increased red blood cells and polymorphonuclear leukocytes are may also be seen when VZV infects the nervous system.
In the absence of rash, the CSF should be examined virologically for VZV DNA by PCR and for anti-VZV IgG and IgM. Detection of VZV DNA in CSF or anti-VZV IgM in serum or CSF is strong presumptive evidence of recent VZV infection. If anti-VZV IgG antibody is present in CSF, the antibody index should be calculated to determine whether anti-VZV antibody is being produced intrathecally. For molecules such as albumin and total IgG, the serum/CSF ratio is usually more than 100:1. A reduced ratio of anti-VZV IgG antibody compared to ratios for albumin or total IgG is seen in many neurological diseases produced by VZV. Importantly, many cases of VZV vasculopathy are protracted and VZV DNA is only found ~30% of the time [65]. The detection of anti-VZV IgG antibody in CSF with intrathecal synthesis is superior to detection of VZV DNA in CSF to diagnose VZV vasculopathy [104], recurrent myelopathy and brainstem encephalitis produced by VZV [105].
Acknowledgments
This work was supported in part by National Institutes of Health grants AG006127 and AG032958 to DG and NS067070 to MAN. The authors wish to thank Marina Hoffman for editorial assistance and Lori DePriest for manuscript preparation.
Footnotes
Conflict of Interest
Maria A. Nagel and Don Gilden declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Maria A. Nagel, Email: maria.nagel@ucdenver.edu.
Don Gilden, Email: don.gilden@ucdenver.edu.
References
-
*
Importance
-
**
Major importance
- 1.Gilden DH, Dueland AN, Cohrs R, et al. Preherpetic neuralgia. Neurology. 1991;41:1215–1218. doi: 10.1212/wnl.41.8.1215. [DOI] [PubMed] [Google Scholar]
- 2.Insinga RP, Itzler RF, Pellissier JM, et al. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20:748–753. doi: 10.1111/j.1525-1497.2005.0150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harnisch JP. Zoster in the elderly: clinical, immunologic and therapeutic considerations. J Am Geriatr Soc. 1984;32:789–793. doi: 10.1111/j.1532-5415.1984.tb06298.x. [DOI] [PubMed] [Google Scholar]
- 4.Gilden DH, Cohrs RJ, Mahalingam R. Clinical and molecular pathogenesis of varicella virus infection. Viral Immunol. 2003;16:243–258. doi: 10.1089/088282403322396073. [DOI] [PubMed] [Google Scholar]
- 5.Leppard B, Naburi AE. Herpes zoster: an early manifestation of HIV infection. Afr Health. 1998;21:5–6. [PubMed] [Google Scholar]
- 6.Tyndall MW, Nasio J, Agoki E, et al. Herpes zoster as the initial presentation of human immunodeficiency virus type 1 infection in Kenya. Clin Infect Dis. 1995;21:1035–1037. doi: 10.1093/clinids/21.4.1035. [DOI] [PubMed] [Google Scholar]
- 7.Kakourou T, Theodoridou M, Mostrou G, et al. Herpes zoster in children. J Am Acad Dermatol. 1998;39:207–210. doi: 10.1016/s0190-9622(98)70076-3. [DOI] [PubMed] [Google Scholar]
- 8.Selbst RG, Selhorst JB, Harbison JW, et al. Parainfectious optic neuritis report and review following varicella. Arch Neurol. 1983;40:347–350. doi: 10.1001/archneur.1983.04050060047007. [DOI] [PubMed] [Google Scholar]
- 9.Kurimoto T, Tonari M, Ishizaki N, et al. Orbital apex syndrome associated with herpes zoster ophthalmicus. Clin Ophthalmo. 2011;5:1603–1608. doi: 10.2147/OPTH.S25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambade P, Lambade D, Saha TK, et al. Maxillary osteonecrosis and spontaneous teeth exfoliation following herpes zoster. Oral Maxillofac Surg. 2012;16:369–372. doi: 10.1007/s10006-011-0303-8. [DOI] [PubMed] [Google Scholar]
- 11.Payten RJ, Dawes JDK. Herpes zoster of the head and neck. J Laryngol Otol. 1972;86:1031–1055. doi: 10.1017/s0022215100076179. [DOI] [PubMed] [Google Scholar]
- 12.Robillard RB, Hilsinger RL, Jr, Adour KK. Ramsay Hunt facial paralysis: clinical analyses of 185 patients. Otolaryngol Head Neck Surg. 1986;95:292–297. doi: 10.1177/01945998860953P105. [DOI] [PubMed] [Google Scholar]
- 13.Steffen R, Selby G. ‘Atypical’ Ramsay Hunt syndrome. Med J Aust. 1972;1:227–230. doi: 10.5694/j.1326-5377.1972.tb46771.x. [DOI] [PubMed] [Google Scholar]
- 14.Asnis DS, Micic L, Giaccio D. Ramsay Hunt syndrome presenting as a cranial polyneuropathy. Cutis. 1996;57:421–424. [PubMed] [Google Scholar]
- 15.Lapresle J, Lasjaunias P. Cranial nerve ischaemic arterial syndromes. Brain. 1986;109:207–215. doi: 10.1093/brain/109.1.207. [DOI] [PubMed] [Google Scholar]
- 16.Easton HG. Zoster sine herpete causing acute trigeminal neuralgia. Lancet. 1970;2:1065–1066. doi: 10.1016/s0140-6736(70)90291-6. [DOI] [PubMed] [Google Scholar]
- 17.Hevner R, Vilela M, Rostomily R, et al. An unusual cause of trigeminal-distribution pain and tumour. Lancet Neurol. 2003;2:567–572. doi: 10.1016/s1474-4422(03)00506-4. [DOI] [PubMed] [Google Scholar]
- 18.Murakami S, Honda N, Mizobuchi M, et al. Rapid diagnosis of varicella zoster virus infection in acute facial palsy. Neurology. 1998;51:1202–1205. doi: 10.1212/wnl.51.4.1202. [DOI] [PubMed] [Google Scholar]
- 19.Osaki Y, Matsubayashi K, Okumiya K, et al. Polyneuritis cranialis due to varicella-zoster virus in the absence of rash. Neurology. 1995;45:2293–2294. doi: 10.1212/wnl.45.12.2293. [DOI] [PubMed] [Google Scholar]
- 20.Murata K, Miwa H, Kondo T. Polyneuritis cranialis caused by varicella zoster virus in the absence of rash. Neurology. 2010;74:85–86. doi: 10.1212/WNL.0b013e3181c7da35. [DOI] [PubMed] [Google Scholar]
- 21.Brostoff J. Diaphragmatic paralysis after herpes zoster. Br Med J. 1966;2:1571–1572. doi: 10.1136/bmj.2.5529.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stowasser M, Cameron J, Oliver WA. Diaphragmatic paralysis following cervical herpes zoster. Med J Aust. 1990;153:555–556. doi: 10.5694/j.1326-5377.1990.tb126199.x. [DOI] [PubMed] [Google Scholar]
- 23.Tjandra J, Mansel RE. Segmental abdominal herpes zoster paresis. Aust N Z J Surg. 1986;56:807–808. doi: 10.1111/j.1445-2197.1986.tb02331.x. [DOI] [PubMed] [Google Scholar]
- 24.Molinero J, Nagore E, Obón L, et al. Metameric motor paresis following abdominal herpes zoster. Cutis. 2002;69:143–144. [PubMed] [Google Scholar]
- 25.Izumi AK, Edwards J., Jr Herpes zoster and neurogenic bladder dysfunction. JAMA. 1973;224:1748–1749. [PubMed] [Google Scholar]
- 26.Jellinek EH, Tulloch WS. Herpes zoster with dysfunction of bladder and anus. Lancet. 1976;2:1219–1222. doi: 10.1016/s0140-6736(76)91144-2. [DOI] [PubMed] [Google Scholar]
- 27.Umehara T, Sengoku R, Mitsumura H, et al. Findings of segmental zoster paresis on MRI. J Neurol Neurosurg Psychiatry. 2011;82:694. doi: 10.1136/jnnp.2010.235127. [DOI] [PubMed] [Google Scholar]
- 28.Choi JY, Kang CH, Kim BJ, et al. Brachial plexopathy following herpes zoster infection: two cases with MRI findings. J Neurol Sci. 2009;285:224–226. doi: 10.1016/j.jns.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Gupta SK, Helal BH, Kiely P. The prognosis in zoster paralysis. J Bone Joint Surg Br. 1969;51:593–603. [PubMed] [Google Scholar]
- 30.Thomas EJ, Howard FM., Jr Segmental zoster paresis – a disease profile. Neurology. 1972;22:459–466. doi: 10.1212/wnl.22.5.459. [DOI] [PubMed] [Google Scholar]
- 31.Head H, Campbell AW. The pathology of herpes zoster and its bearing on sensory localization. Brain. 1900;23:353–523. doi: 10.1002/(sici)1099-1654(199709)7:3<131::aid-rmv198>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Denny-Brown D, Adams RD, Fitzgerald PJ. Pathologic features of herpes zoster: A note on “geniculate herpes”. Arch Neurol Psychiatry. 1944;51:216–231. [Google Scholar]
- 33.Cheatham WJ, Dolan TF, Jr, Dower JC, et al. Varicella: report on two fatal cases with necropsy, virus isolation, and serologic studies. Am J Pathol. 1956;32:1015–1035. [PMC free article] [PubMed] [Google Scholar]
- 34.Esiri MM, Tomlinson AH. Herpes zoster: demonstration of virus in trigeminal nerve and ganglion by immunofluorescence and electron microscopy. J Neurol Sci. 1972;15:35–48. doi: 10.1016/0022-510x(72)90120-7. [DOI] [PubMed] [Google Scholar]
- 35.Ghatak NR, Zimmerman HM. Spinal ganglion in herpes zoster. Arch Pathol. 1973;95:411–415. [PubMed] [Google Scholar]
- 36.Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract. 1975;25:571–575. [PMC free article] [PubMed] [Google Scholar]
- 37.de Moragas JM, Kierland RR. The outcome of patients with herpes zoster. Arch Dermatol. 1957;75:193–196. doi: 10.1001/archderm.1957.01550140037006. [DOI] [PubMed] [Google Scholar]
- 38.Rogers RS, III, Tindall JP. Herpes zoster in the elderly. Postgrad Med. 1971;50:153–157. doi: 10.1080/00325481.1971.11697705. [DOI] [PubMed] [Google Scholar]
- 39.Smith FP. Pathological studies of spinal nerve ganglia in relation to intractable intercostal pain. Surg Neurol. 1978;10:50–53. [PubMed] [Google Scholar]
- 40.Watson CPN, Deck JH, Morshead C, et al. Postherpetic neuralgia: further post-mortem studies of cases with and without pain. Pain. 1991;44:105–117. doi: 10.1016/0304-3959(91)90124-G. [DOI] [PubMed] [Google Scholar]
- 41.Vafai A, Wellish M, Gilden DH. Expression of varicella-zoster virus in blood mononuclear cells of patients with postherpetic neuralgia. Proc Natl Acad Sci USA. 1988;85:2767–2770. doi: 10.1073/pnas.85.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devlin ME, Gilden DH, Mahalingam R, et al. Peripheral blood mononuclear cells of the elderly contain varicella-zoster virus DNA. J Infect Dis. 1992;165:619–622. doi: 10.1093/infdis/165.4.619. [DOI] [PubMed] [Google Scholar]
- 43.Mahalingam R, Wellish M, Brucklier J, et al. Persistence of varicella-zoster virus DNA in elderly patients with postherpetic neuralgia. J NeuroVirol. 1995;1:130–133. doi: 10.3109/13550289509111018. [DOI] [PubMed] [Google Scholar]
- 44.Terada K, Niizuma T, Kawano S, et al. Detection of varicella-zoster virus DNA in peripheral mononuclear cells from patients with Ramsay Hunt syndrome or zoster sine herpete. J Med Virol. 1998;56:359–363. [PubMed] [Google Scholar]
- 45.Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain. Eur J Neurol. 2010;17:1113–1188. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 46.Hans G, Sabatowski R, Binder A, et al. Efficacy and tolerability of a 5% lidocaine medicated plaster for the topical treatment of post-herpetic neuralgia: results of a long-term study. Curr Med Res Opin. 2009;25:1295–1305. doi: 10.1185/03007990902901368. [DOI] [PubMed] [Google Scholar]
- 47.Dubinsky RM, Kabbani H, El-Chami Z, et al. Practice parameter: treatment of postherpetic neuralgia: an evidence-based report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2004;63:959–965. doi: 10.1212/01.wnl.0000140708.62856.72. [DOI] [PubMed] [Google Scholar]
- 48.Backonja M, Wallace MS, Blonsky ER, et al. NGX-4010 C116 Study Group. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurol. 2008;7:1106–1112. doi: 10.1016/S1474-4422(08)70228-X. [DOI] [PubMed] [Google Scholar]
- 49.Backonja MM, Malan TP, Vanhove GF, et al. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomized, double-blind, controlled study with an open-label extension. Pain Med. 2010;11:600–608. doi: 10.1111/j.1526-4637.2009.00793.x. [DOI] [PubMed] [Google Scholar]
- 50.Gilron I, Bailey JM, Tu D. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet. 2009;374:1252–1261. doi: 10.1016/S0140-6736(09)61081-3. [DOI] [PubMed] [Google Scholar]
- 51.Gilron I, Bailey JM, Tu D, et al. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352:132–1334. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- 52.Rehm S, Binder A, Baron R. Post-herpetic neuralgia: 5% lidocaine medicated plaster, pregabalin, or a combination of both? A randomized, open, clinical effectiveness study. Curr Med Res Opin. 2010;26:1607–1619. doi: 10.1185/03007995.2010.483675. [DOI] [PubMed] [Google Scholar]
- 53.Ruiz Huete C, Bermejo PE. Botulinum toxin type A in the treatment of neuropathic pain in a case of postherpetic neuralgia [in Spanish] Neurologia. 2008;23:259–262. [PubMed] [Google Scholar]
- 54.Sotiriou E, Apalla Z, Panagiotidou D, et al. Severe post-herpetic neuralgia successfully treated with botulinum toxin A: three case reports. Acta Derm Venereol. 2009;89:214–215. doi: 10.2340/00015555-0609. [DOI] [PubMed] [Google Scholar]
- 55.Xiao L, Mackey S, Hui H, et al. Subcutaneous injection of botulinum toxin a is beneficial in postherpetic neuralgia. Pain Med. 2010;11:1827–1833. doi: 10.1111/j.1526-4637.2010.01003.x. [DOI] [PubMed] [Google Scholar]
- 56.van Wijck AJ, Opstelten W, Moons KG, et al. The PINE study of epidural steroids and local anaesthetics to prevent postherpetic neuralgia: a randomized controlled trial. Lancet. 2006;367:219–224. doi: 10.1016/S0140-6736(06)68032-X. [DOI] [PubMed] [Google Scholar]
- 57.Harke H, Gretenkort P, Ladleif HU, et al. Spinal cord stimulation in postherpetic neuralgia and in acute herpes zoster pain. Anesth Analg. 2002;94:694–700. doi: 10.1097/00000539-200203000-00040. [DOI] [PubMed] [Google Scholar]
- 58.Surjya PU, Shiv PR, Mishra S, et al. Successful treatment of an intractable postherpetic neuralgia (PHN) using peripheral nerve field stimulation (PNFS) Am J Hosp Palliat Care. 2010;27:59–62. doi: 10.1177/1049909109342089. [DOI] [PubMed] [Google Scholar]
- 59.Lynch PJ, McJunkin T, Eross E, et al. Case report: successful epiradicular peripheral nerve stimulation of the C2 dorsal root ganglion for postherpetic neuralgia. Neuromodulation. 2011;14:58–61. doi: 10.1111/j.1525-1403.2010.00307.x. [DOI] [PubMed] [Google Scholar]
- 60.Yakovlev AE, Peterson AT. Peripheral nerve stimulation in treatment of intractable postherpetic neuralgia. Neuromodulation. 2007:373–375. doi: 10.1111/j.1525-1403.2007.00126.x. [DOI] [PubMed] [Google Scholar]
- 61.Kouroukli I, Neofytos D, Panaretou V, et al. Peripheral subcutaneous stimulation for the treatment of intractable postherpetic neuralgia: two case reports and literature review. Pain Prac. 2009;9:225–229. doi: 10.1111/j.1533-2500.2009.00263.x. [DOI] [PubMed] [Google Scholar]
- 62.Kang JH, Ho JD, Chen YH, et al. Increased risk of stroke after a herpes zoster attack: a population-based follow-up study. Stroke. 2009;40:3443–3448. doi: 10.1161/STROKEAHA.109.562017. [DOI] [PubMed] [Google Scholar]
- 63.Lin HC, Chien CW, Ho JD. Herpes zoster ophthalmicus and the risk of stroke: a population-based follow-up study. Neurology. 2010;74:792–797. doi: 10.1212/WNL.0b013e3181d31e5c. [DOI] [PubMed] [Google Scholar]
- 64.Amlie-Lefond C, Bernard TJ, Sébire G, et al. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the International Pediatric Stroke Study. Circulation. 2009;119:1417–1423. doi: 10.1161/CIRCULATIONAHA.108.806307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagel MA, Cohrs RJ, Mahalingam R, et al. The varicella zoster vasculopathies: clinical, CSF, imaging, and virologic features. Neurology. 2008;70:853–860. doi: 10.1212/01.wnl.0000304747.38502.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagel MA, Choe A, Khmeleva N, et al. Search for varicella zoster virus and herpes simplex virus-1 in normal human cerebral arteries. J NeuroVirol. 2013 doi: 10.1007/s13365-013-0155-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kleinschmidt-DeMasters BK, Gilden DH. Varicella-zoster virus infections of the nervous system: clinical and pathologic correlates. Arch Pathol Lab Med. 2001;125:770–780. doi: 10.5858/2001-125-0770-VZVIOT. [DOI] [PubMed] [Google Scholar]
- 68*.Nagel MA, Traktinskiy I, Azarkh Y, et al. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology. 2011;77:364–370. doi: 10.1212/WNL.0b013e3182267bfa. Correlative analysis of viral and muscle cell markers in cerebral arteries from patients with VZV vasculopathy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69*.Nagel MA, Traktinskiy I, Stenmark KR, et al. Varicella-zoster virus vasculopathy: immune characteristics of virus-infected arteries. Neurology. 2013;80:62–68. doi: 10.1212/WNL.0b013e31827b1ab9. Analysis of the immune repertoire in arteries of patients with VZV vasculopathy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee RM. Morphology of cerebral arteries. Pharmacol Ther. 1995;66:149–173. doi: 10.1016/0163-7258(94)00071-a. [DOI] [PubMed] [Google Scholar]
- 71.Mayberg MR, Langer RS, Zervas NT, et al. Perivascular meningeal projections from cat trigeminal ganglia: possible pathway for vascular headaches in man. Science. 1981;213:228–230. doi: 10.1126/science.6166046. [DOI] [PubMed] [Google Scholar]
- 72.Mayberg MR, Zervas NT, Moscowitz MA. Trigeminal projections to supratentorial pial and dural blood vessels in cats demonstrated by horseradish peroxidase histochemistry. J Comp Neurol. 1984;223:46–56. doi: 10.1002/cne.902230105. [DOI] [PubMed] [Google Scholar]
- 73.Salazar R, Russman AN, Nagel MA, et al. VZV ischemic optic neuropathy and subclinical temporal artery involvement. Arch Neurol. 2011;68:517–520. doi: 10.1001/archneurol.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nagel MA, Russman AN, Feit DO, et al. VZV ischemic optic neuropathy andsubclinical temporal artery infection without rash. Neurology. 2013;80:220–222. doi: 10.1212/WNL.0b013e31827b92d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mathias M, Nagel MA, Khmeleva N, et al. VZV multifocal vasculopathy withischemic optic neuropathy, acute retinal necrosis and temporal artery infection in the absence of zoster rash. J Neurol Sci. 2013;325:180–182. doi: 10.1016/j.jns.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76**.Nagel MA, Bennett JL, Khmeleva N, et al. Multifocal VZV vasculopathy withtemporal artery infection mimics giant cell arteritis. Neurology. 2013 doi: 10.1212/WNL.0b013e318294b477. in press. Exciting new study indicating that the clinical features of multifocal VZV vasculopathy with temporal artery infection can be the same as seen in classic giant cell arteritis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Habib AA, Gilden D, Schmid DS, et al. Varicella zoster virus meningitis with hypoglycorrhachia in the absence of rash and in an immunocompetent woman. J Neurovirol. 2009;15:206–208. doi: 10.1080/13550280902725550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gunson RN, Aitken C, Gilden D. A woman with acute headache and sacral dermatomal numbness. J Clin Virol. 2011;50:191–193. doi: 10.1016/j.jcv.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moses H, Nagel MA, Gilden DH. Acute cerebellar ataxia in a 41 year old woman. Lancet Neurol. 2006;5:984–988. doi: 10.1016/S1474-4422(06)70601-9. [DOI] [PubMed] [Google Scholar]
- 80.Ratzka P, Schlachetzki JC, Bähr M, et al. Varicella zoster virus cerebellitis ina 66- year-old patient without herpes zoster. Lancet. 2006;367:182. doi: 10.1016/S0140-6736(06)67967-1. [DOI] [PubMed] [Google Scholar]
- 81.Gilden DH, Beinlich BR, Rubinstien EM, et al. Varicella-zoster virus myelitis: an expanding spectrum. Neurology. 1994a;44:1818–1823. doi: 10.1212/wnl.44.10.1818. [DOI] [PubMed] [Google Scholar]
- 82.Devinsky O, Cho ES, Petito CK, et al. Herpes zoster myelitis. Brain. 1991;114:1181–1196. doi: 10.1093/brain/114.3.1181. [DOI] [PubMed] [Google Scholar]
- 83.de Silva SM, Mark AS, Gilden DH, et al. Zoster myelitis: improvement with antiviral therapy in two cases. Neurology. 1996;47:929–931. doi: 10.1212/wnl.47.4.929. [DOI] [PubMed] [Google Scholar]
- 84.Orme HT, Smith G, Nagel MA, et al. VZV spinal cord infarction identified bydiffusion-weighted magnetic resonance imaging (DWI) Neurology. 2007;69:398–400. doi: 10.1212/01.wnl.0000266390.27177.7b. [DOI] [PubMed] [Google Scholar]
- 85.Bonfioli AA, Eller AW. Acute retinal necrosis. Semin Ophthalmol. 2005;20:155–160. doi: 10.1080/08820530500232027. [DOI] [PubMed] [Google Scholar]
- 86.Guex-Crosier Y, Rochat C, Herbort CP. Necrotizing herpetic retinopathies. A spectrum of herpes virus-induced diseases determined by the immune state of the host. Ocul Immunol Inflamm. 1997;5:259–265. doi: 10.3109/09273949709085066. [DOI] [PubMed] [Google Scholar]
- 87.Lewis JM, Nagae Y, Tano Y. Progressive outer retinal necrosis after bone marrow transplantation. Am J Ophthalmol. 1996;122:892–895. doi: 10.1016/s0002-9394(14)70391-5. [DOI] [PubMed] [Google Scholar]
- 88.Franco-Paredes C, Bellehemeur T, Merchant A, et al. Aseptic meningitis andoptic neuritis preceding varicella-zoster progressive outer retinal necrosis in a patient with AIDS. AIDS. 2002;16:1045–1049. doi: 10.1097/00002030-200205030-00011. [DOI] [PubMed] [Google Scholar]
- 89.Menerath JM, Gerard M, Laurichesse H, et al. Bilateral acute retinal necrosis in a patient with acquired immunodeficiency syndrome. J Fr Ophtalmol. 1995;18:625–633. [PubMed] [Google Scholar]
- 90.Johnston WH, Holland GN, Engstrom RE, Jr, et al. Recurrence ofpresumed varicella-zoster virus retinopathy in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1993;116:42–50. doi: 10.1016/s0002-9394(14)71742-8. [DOI] [PubMed] [Google Scholar]
- 91.Moorthy RS, Weinberg DV, Teich SA, et al. Management of varicella zoster virus retinitis in AIDS. Br J Ophthalmol. 1997;81:189–194. doi: 10.1136/bjo.81.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Austin RB. Progressive outer retinal necrosis syndrome: a comprehensivereview of its clinical presentation, relationship to immune system status, andmanagement. Clin Eye Vis Care. 2000;12:119–129. doi: 10.1016/s0953-4431(00)00052-7. [DOI] [PubMed] [Google Scholar]
- 93.Friedman SM, Mames RN, Sleasman JW, et al. Acute retinal necrosis after chickenpox in a patient with acquired immunodeficiency syndrome. Arch Ophthalmol. 1993;111:1607–1608. doi: 10.1001/archopht.1993.01090120029011. [DOI] [PubMed] [Google Scholar]
- 94.Galindez OA, Sabates NR, Whitacre MM, et al. Rapidly progressive outer retinal necrosis caused by varicella zoster virus in a patient infected with humanimmunodeficiency virus. Clin Infect Dis. 1996;22:149–151. doi: 10.1093/clinids/22.1.149. [DOI] [PubMed] [Google Scholar]
- 95.Goon P, Wright M, Fink C. Ophthalmic zoster sine herpete. J R Soc Med. 2000;93:191–192. doi: 10.1177/014107680009300409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hon C, Au WY, Cheng VC. Ophthalmic zoster sine herpete presenting as oculomotor palsy after marrow transplantation for acute myeloid leukemia. Haematologica. 2005;90:12 EIM04. [PubMed] [Google Scholar]
- 97.Noda Y, Nakazawa M, Takahashi D, et al. Retinal periphlebitis as zostersine herpete. Arch Ophthalmol. 2001;119:1550–1552. [PubMed] [Google Scholar]
- 98.Yamamoto S, Tada R, Shimomura Y, et al. Detecting varicella-zoster virus DNA in iridocyclitis using polymerase chain reaction: a case of zoster sine herpete. Arch Ophthalmol. 1995;113:1358–1359. doi: 10.1001/archopht.1995.01100110018009. [DOI] [PubMed] [Google Scholar]
- 99.Silverstein BE, Chandler D, Neger R, et al. Disciform keratitis: a case of herpes zoster sine herpete. Am J Ophthalmol. 1997;123:254–255. doi: 10.1016/s0002-9394(14)71044-x. [DOI] [PubMed] [Google Scholar]
- 100.Lewis GW. Zoster sine herpete. Br Med. 1958;J 2:418–442. doi: 10.1136/bmj.2.5093.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gilden DH, Wright RR, Schneck SA, et al. Zoster sine herpete, a clinical variant. Ann Neurol. 1994b;35:530–533. doi: 10.1002/ana.410350505. [DOI] [PubMed] [Google Scholar]
- 102.Amlie-Lefond C, Mackin GA, Ferguson M, et al. Another case of virologically confirmed zoster sine herpete with electrophysiologic correlation. J Neurovirol. 1996;2:136–138. doi: 10.3109/13550289609146547. [DOI] [PubMed] [Google Scholar]
- 103.Blumenthal DT, Shacham-Shmueli E, Bokstein F, et al. Zoster sine herpete: virological verification by detection of anti-VZV IgG antibody in CSF. Neurology. 2011;76:484–485. doi: 10.1212/WNL.0b013e31820a0d28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nagel MA, Forghani B, Mahalingam R, et al. The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology. 2007;68:1069–1073. doi: 10.1212/01.wnl.0000258549.13334.16. [DOI] [PubMed] [Google Scholar]
- 105.Haug A, Mahalingam R, Cohrs RJ, et al. Recurrent polymorphonuclear pleocytosis with increased red blood cells caused by varicella zoster virus infection of the central nervous system. J Neurol Sci. 2010;292:85–88. doi: 10.1016/j.jns.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gilden D, Mahalingam R, Nagel MA, Pugazhenthi S, Cohrs RJ. Review: The neurobiology of varicella zoster virus infection. Neuropathol Appl Neurobiol. 2011;37:441–463. doi: 10.1111/j.1365-2990.2011.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gilden DH, Mahalingam R, Cohrs RJ, et al. The protean manifestations of varicella-zoster virus vasculopathy. J NeuroVirol. 2002;8:75–79. doi: 10.1080/13550280290167902. [DOI] [PubMed] [Google Scholar]
- 108.Gilden D, Cohrs RJ, Mahalingam R, et al. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8:731–740. doi: 10.1016/S1474-4422(09)70134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gilden DH, Kleinschmidt-DeMasters BK, Wellish M, et al. Varicella zoster virus, a cause of waxing and waning vasculitis: the NEJM case 5-1995 revisited. Neurology. 1996;47:1441–1446. doi: 10.1212/wnl.47.6.1441. [DOI] [PubMed] [Google Scholar]
- 110.Mathias M, Nagel MA, Khmeleva N, et al. VZV multifocal vasculopathy with ischemic optic neuropathy, acute retinal necrosis and temporal artery infection in the absence of zoster rash. J Neurol Sci. 2013;325:180–182. doi: 10.1016/j.jns.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


