Abstract
We classified homogenous river types across Europe and searched for fish metrics qualified to show responses to specific pressures (hydromorphological pressures or water quality pressures) vs. multiple pressures in these river types. We analysed fish taxa lists from 3105 sites in 16 ecoregions and 14 countries. Sites were pre-classified for 15 selected pressures to separate unimpacted from impacted sites. Hierarchical cluster analysis was used to split unimpacted sites into four homogenous river types based on species composition and geographical location. Classification trees were employed to predict associated river types for impacted sites with four environmental variables. We defined a set of 129 candidate fish metrics to select the best reacting metrics for each river type. The candidate metrics represented tolerances/intolerances of species associated with six metric types: habitat, migration, water quality sensitivity, reproduction, trophic level and biodiversity. The results showed that 17 uncorrelated metrics reacted to pressures in the four river types. Metrics responded specifically to water quality pressures and hydromorphological pressures in three river types and to multiple pressures in all river types. Four metrics associated with water quality sensitivity showed a significant reaction in up to three river types, whereas 13 metrics were specific to individual river types. Our results contribute to the better understanding of fish assemblage response to human pressures at a pan-European scale. The results are especially important for European river management and restoration, as it is necessary to uncover underlying processes and effects of human pressures on aquatic communities.
Keywords: Fish metrics, Water Framework Directive, Multiple pressures, River types
Introduction
The development of fish-based methods for the assessment of human pressures on the aquatic ecosystem has a long history. There has been considerable scientific effort to define appropriate fish metrics and fish indices for the assessment of the ecological status of different types of running waters in the United States (Fausch et al., 1990, Lyons, 1992, McCormick et al., 2001, Mebane et al., 2003, Hughes et al., 2004, Whittier et al., 2007, Pont et al., 2009). Most of the work has been within the framework of the “Clean Water Act”, based on the “Index of Biotic Integrity” (IBI) and the related findings of Karr (1981).
In Europe, the EU Water Framework Directive (WFD, European Commission, 2000) has been a major driver in the development of standardised fish based assessment methods and metrics to determine the ecological status of European rivers and the classification of human degradation (Oberdorff et al., 2001, Oberdorff et al., 2002, Pont et al., 2005, Pont et al., 2007, Roset et al., 2007, Segurado et al., 2011, Logez and Pont, 2011).
Subsequent, EU-funded projects such as FAME (FAME Consortium, 2004) and “European Fish Index Plus (EFI+)” (EFI+ Consortium, 2009), have developed multi-metric indices based on fish assemblages and analysed relationships between fishes and human pressures. Additional studies by Noble et al. (2007b), Melcher et al. (2007), Schmutz et al. (2007b), Virbickas and Kesminas (2007), Grenouillet et al. (2007) and Ferreira et al. (2007) aimed to find appropriate metrics that showed different reactions under unimpacted/impacted conditions for various regions in Europe.
Numerous studies have analysed fish metrics to detect pressures by differentiating between reference and degraded sites (Bailey et al., 1998, Hughes et al., 1998, Karr and Chu, 2000, Hering et al., 2006, Pont et al., 2006, Pont et al., 2009, Stoddard et al., 2008, Southerland et al., 2007, Logez and Pont, 2011). Low quality data and information gaps regarding pressures have produced errors and bias in fish metric responses to different types of pressures. Consequently, although providing reliable results at the large scale, pan-European fish metrics were unable to differentiate between unimpacted and impacted conditions in specific areas, river types or pressure situations (Melcher et al., 2007, Schmutz et al., 2007a, Schmutz et al., 2007b, Pont et al., 2007). The EFI+ project (EFI+ Consortium, 2009, http://efi-plus.boku.ac.at/), tried to overcome these problems by identifying and collecting important pressures across Europe on a more accurate and standardised basis. Based on these data, Schinegger et al. (2012) showed that (1) degradation of European rivers is widespread, (2) single water quality pressures (W) are not dominant, but (3) many European rivers are affected by hydromorphological pressures (HMC) or a combination of pressure types (W + HMC). Furthermore, Schinegger et al. (2012) found that hydromorphological pressures (HMC) are the key pressures in alpine regions and headwaters and water quality pressures (W) and multiple pressures (W + HMC) prevail in lowlands.
According to Hering et al. (2006) and Logez and Pont (2011), the signal reflected by metrics should only display the variability of pressures between sites and not the environmental differences between them. Furthermore, Hughes and Oberdorff (1999), Roset et al. (2007) and Pont et al. (2009) stated that the creation of new IBIs and IBI scoring criteria to suit natural regional and local differences might be unsuitable when applied to areas outside those for which they were developed. Subsequently many studies have focused on a predefined ecoregion approach. The Illies ecoregion system (Illies, 1978) is the only widely used pan-European classification and was adopted by the WFD. However, Schmutz et al. (2007a) argue that the Illies system has never been evaluated for its ability to discriminate among fish assemblages at a continental scale. Schmutz et al. (2007a) also stated that two spatial dimensions structure fish assemblages at the large scale: the zoogeography across Europe and the longitudinal pattern within each river. Schmutz et al. (2007b) and Melcher et al. (2007) then developed the Fish Assemblage Types (FATs) as an underlying concept for a “Spatially Based Method (SBM)” of classification, which divides rivers into units with homogenous fish assemblages (i.e. a river type specific approach). The SBM approach was initially applied to individual ecoregions (Ferreira et al., 2007, Grenouillet et al., 2007, Noble et al., 2007b, Virbickas and Kesminas, 2007), and then simultaneously to all ecoregions (Melcher et al., 2007, Schmutz et al., 2007a). However, as the SBM approach only applies to rivers belonging to FATs defined in previous studies, it is necessary to extend the geographic range of the SBM.
Based on these previous findings, our study represents a pan-European approach to test the response of fish assemblages to pressures in different river types. Our intent was (1) to define homogenous river types across Europe and (2) to find appropriate fish metrics for these types, showing a response to specific and multiple human pressures.
Methods and data
Allocation and pre-classification of sites
All data were extracted from an extensive database (EFI+ Consortium, 2007) containing fish surveys conducted by several academic institutions and environmental agencies across Europe. Sites were sampled by electrofishing (wading) during low flow periods using European standards (CEN, 2003). We included only sites with fished areas greater than 100 m2 and having more than 50 caught individuals to minimise the risk of false absences.
Due to multiple sampling sites located in one river, we applied another selection step to compensate for possible spatial autocorrelation. Dispersed distribution of sampling sites was defined in three classes based on upstream catchment size and three thresholds for distance along the stream network between sampling sites. Threshold for (1) small catchments (<1000 km2) was >5 km distance, (2) for medium catchments (1000–10,000 km2) >10 km, and (3) for large catchments (>=10,000 km2) >50 km. The dataset comes for sites from 2079 rivers of which 1553 (74.6%) rivers are associated with only one sampling site, 307 (14.8%) rivers are associated with two sampling sites, and 218 (10.5%) rivers are associated with three or more sampling sites within the entire river. Median catchment size is 82 km2 and 90% of the sites have a catchment size below 1000 km2.
After this first step, 3105 sites in 16 ecoregions and 14 countries were available for our analyses. Pre-classification of sites was done for 15 selected pressure variables (Table 1) in order to separate unimpacted sites (no or very slight pressure) from strongly impacted sites. Pressure variables were selected by Schinegger et al. (2012) according to known effects on aquatic habitats and organisms.
Table 1.
Selected pressures for unimpacted/impacted site differentiation. Type indicates if the pressure is considered as water quality pressure (W) or hydromorphological pressure including connectivity (HMC, for details see Schinegger et al. 2012).
| Pressure variable | Type | Explanation; short description of classes |
|---|---|---|
| Impoundment | HMC | Natural flow velocity reduction on site due to impoundment; 1 = no (no impoundment), 3 = weak, 5 = strong; |
| Hydropeaking | HMC | Site affected by hydropeaking; 1 = no (no hydropeaking), 3 = partial, 3 = yes; |
| Water abstraction | HMC | Site affected by water flow alteration/minimum flow; 1 = no (no water abstraction), 3 = weak to medium (less than half of the mean annual flow), 5 = strong (more than half of mean annual flow); |
| Reservoir flushing | HMC | Fish fauna affected by flushing of reservoirs upstream of site; 1 = no, 3 = yes; |
| Hydrograph modification | HMC | Seasonal hydrograph modification due to hydrological alteration (water storage for irrigation, hydropower etc.); 1 = no, 3 = yes; |
| Channelisation | HMC | Alteration of natural morphological channel plan form; 1 = no, 3 = intermediate, 5 = straightened; |
| Cross section alteration | HMC | Alteration of cross section; 1 = no, 3 = intermediate, 5 = technical cross section./U-profile |
| Instream habitat alteration | HMC | Alteration of instream habitat conditions; 1 = no, 3 = intermediate, 5 = high; |
| Embankment | HMC | Artificial embankment; 1 = no (natural shoreline), 2 = slight (local presence of artificial material for embankment), 3 = intermediate (continuous embankment but permeable), 5 = high (continuous, no permeability); |
| Flood protection | HMC | Presence of dykes for flood protection; 1 = no, 3 = yes; |
| Barriers segment upstream | HMC | Barriers on segment level upstream; 1 = no, 3 = partial, 3 = yesa; |
| Barriers segment downstream | HMC | Barriers on segment level downstream; 1 = no, 4 = partial, 4 = yesa; |
| Acidification | W | Acidification; 1 = no, 3 = yes; |
| Eutrophication | W | Artificial eutrophication; 1 = no, 3 = low, 4 = intermediate (occurrence of green algae), 5 = extreme (oxygen depletion); |
| Organic pollution | W | Is organic pollution observed; 1 = no, 3 = weak, 5 = strong; |
Partial barriers and yes are considered to have the same impact.
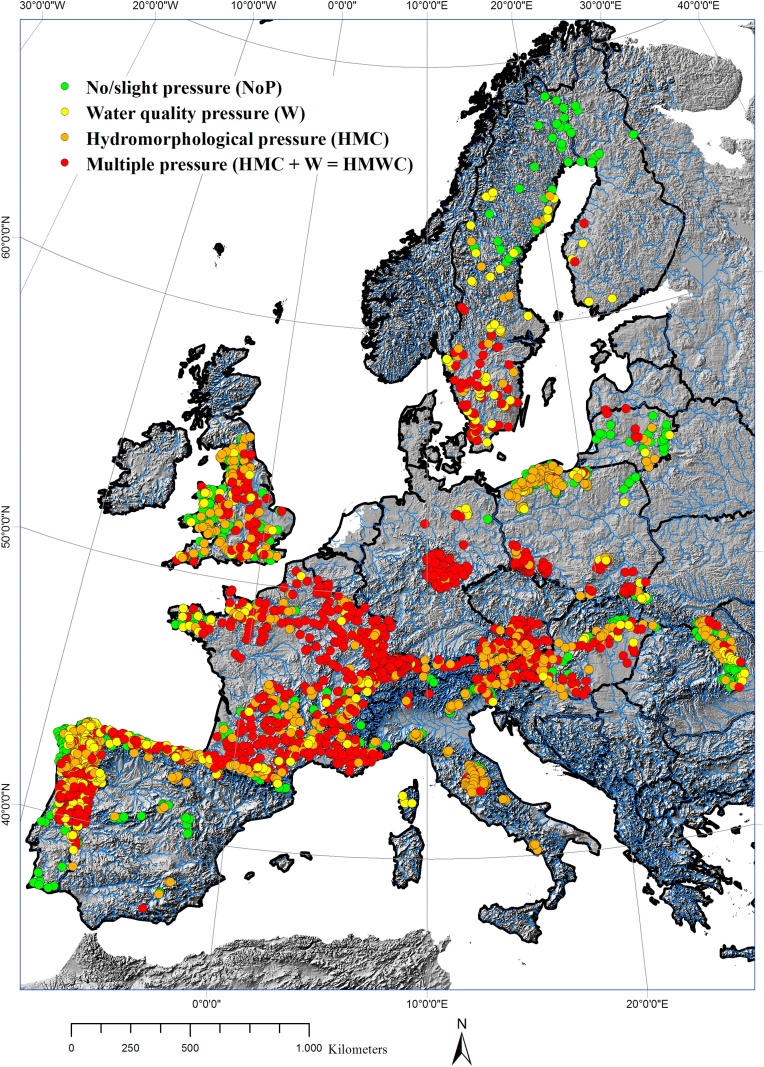
In total, 716 sites were classified as unimpacted (classes 1 and 2) and 2389 sites as impacted (classes 3, 4 and 5). Furthermore, impacted sites were associated with specific pressures and pressure combinations according to Schinegger et al. (2012), (see “Group” in Table 1 for details). In this context, 390 sites were impacted only by water quality pressures (W), 771 sites only by hydromorphological pressures (HMC) and 1228 sites by multiple pressures (HMWC), i.e. a combination of water quality and hydromorphological pressures (Schinegger et al., 2012). Fig. 1 shows the spatial location and pressure status of sites.
Fig. 1.
Spatial location and pressure status of sites [n = 3105].
Fish metrics description
As suggested the EFI+ Consortium (2009), six structural and functional types of metrics were considered for candidate metrics: biodiversity, habitat, migration, reproduction, trophic level and water quality sensitivity. In the dataset, 116 fish species were assigned to tolerances related to these attributes according to the EFI+ classification, based on previous literature and completed by expert judgement (Holzer, 2008, EFI+ Consortium, 2009; Annex Table 1).
In total, 129 candidate metrics were pre-selected for further analyses (Table 2). The selected metrics included six variants: number of species, density (number of individuals per ha) and biomass (kg per ha) per metric as well as relative information on number of species, density and biomass (as percentage of total species). According to Noble et al. (2007a) and Virbickas and Kesminas (2007), these variants reflect most of the important ecological aspects of metrics. Associated references and reactions can be found in Table 2. As information on fish length was not available for a large part of our dataset, we decided not to consider metrics based on size classes/life stages.
Table 2.
Name and definition of candidate metrics for further analyses. Type: biodiv = biodiversity, hab = habitat, mig = migration, repro = reproduction, troph = trophic level, wq = water quality; Variants: nsp = number of species, dens = density [Ind/ha], biom = biomass [kg/ha], perc_nsp: number of species of guild in relation to all species, perc_dens = density of guild in relation to all guilds, perc_biom = biomass of guild in relation to all guilds, all = all six variants are included; Direction: incr = metrics that increases with increasing human pressure, decr = metric that decreases with increasing human pressure; Reaction according to reference in text.
| Metric name | Definition | Type | Variants | Direction | Reaction |
|---|---|---|---|---|---|
| Nsp_all | Total number of fish species, including native and alien species. | biodiv | nsp, dens, biom | decr/incr | Generally inclines along the longitudinal course of a river, increase in species poor river types and decrease in lowland rivers considered as pressure. |
| Nsp_native | Number of native species. | biodiv | nsp, dens, biom, perc_nsp | decr | Native species. |
| Nsp_alien | Number of alien species. | biodiv | dens, biom | incr | Replace native species. |
| HTOL_HINTOL | Habitat degradation intolerance. | hab | all | decr | Reaction of species with narrow habitat flexibility. |
| HTOL_HTOL | Habitat degradation tolerance. | hab | all | incr | Reaction of species having a large flexibility in terms of habitat degradation. |
| Hab_EURY | Degree of rheophily. Fish that exhibit a wide tolerance of flow conditions, although generally not considered to be rheophilic. | hab | nsp, dens, perc_nsp, perc_dens | incr | Degradation of lotic and lentic habitats. |
| Hab_LIMNO | Degree of rheophily. Fish prefer to live, feed and reproduce in a habitat with slow flowing to stagnant conditions. | hab | all | incr | Degradation of lentic habitats. |
| Hab_RH | Degree of rheophily. Fish prefer to live in a habitat with high flow conditions and clear water. | hab | all | decr | Degradation of lotic habitats. |
| HabSp_LIPAR | Preference to spawn in stagnant waters. | hab | all | incr | Degradation of lentic spawning habitats. |
| HabSp_RHPAR | Preference to spawn in running waters. | hab | all | decr | Degradation of lotic spawning habitats. |
| Mig_POTAD | Species migrate between river zones or more than 5–10 km. | mig | all | decr | Sensitive to ecologicial connectivity of river systems. |
| Repro_PELA | Fish spawn into the pelagic zone. | repro | all | incr | Degradation of pelagic spawning habitats. |
| Repro_PHYT | Fish deposit eggs in clear water habitats on submerged plants. | repro | all | decr | Degradation of plant-related spawning habitats. |
| Repro_POLY | Non-specialised spawners. | repro | all | incr | Degradation of spawning habitats. |
| Repro_LITH | Fish spawn exclusively on gravel, rocks, stones, rubbles or pebbles, hatchlings are photophobic. | repro | all | decr | Degradation of gravel spawning habitats, sensitive to siltation. |
| Atroph_INSV | Insectivorous species. | troph | all | decr | Surrogate for evaluating the degree that the invertebrate assemblage is degraded by human pressures. |
| Atroph_PISC | Piscivorous species. | troph | all | decr | Top predator, surrogate for prey fishes. |
| Atroph_PLAN | Planktivorous species. | troph | all | decr | Surrogate for plankton. |
| Atroph_OMNI | Food of adult consists of more than 25% plant material and more than 25% animal material. Generalists. | troph | all | incr | Degree that the food base is altered to favour species that can digest both plant and animal foods. |
| WQgen_INTOL | In general intolerant to usual water quality parameters. | wq | all | decr | Reaction of species with narrow flexibility in terms of water quality degradation. |
| WQgen_TOL | In general tolerant to usual water quality parameters. | wq | all | incr | Reaction of species having a wide flexibility in terms of water quality degradation. |
| WQO2_O2INTOL | Tolerant to low Oxygen concentration. More than 6 mg/l in water. | wq | all | decr | Reaction of species with narrow flexibility in terms of oxygen concentration problems. |
| WQO2_O2TOL | Tolerant to low Oxygen concentration: 3 mg/l or less. | wq | all | incr | Reaction of species having a wide flexibility in terms of oxygen concentration problems. |
River type modelling
To classify fish data in similar groups across Europe, homogenous river types (river types) based on fish assemblage data were modelled using only unimpacted sites. We conducted a hierarchical cluster analysis (agnes, R Core Development Team, 2011) after Ward's method, with Euclidean distance as similarity measure including four fish metrics: percentage of lithophilic species (Repro_LITH_perc_nsp), percentage of omnivorous species (Atroph_OMNI_perc_nsp), percentage of potamodromous species (Mig_POTAD_perc_nsp) and percentage of rheophilic species (Hab_RH_perc_nsp) as well as geographic position to include regionalisation. The threshold for identifying distinct river types was set by eye in the cluster dendrogram to find a feasible number of strong and well-separated river types.
According to Hughes et al. (2004), Whittier et al. (2006) and Pont et al. (2009), lotic fish assemblages are limited by many natural variables as elevation, temperature, precipitation, flow regime, and channel slope etc. Therefore, to describe the local environmental characteristics of the sampling sites, we conducted classification tree analysis (rpart, R Core Development Team, 2011) with river type as dependent variable and seven environmental variables as possible descriptors: altitude, river slope, mean annual precipitation, mean annual air temperature, mean air temperature in January, latitude and longitude. These variables were chosen because they describe both the regional position in the hydrographic network and the organisation of sites along the longitudinal continuum of rivers. River slope is the drop of altitude divided by river segment length [m/km], where segment length is 1 km for small streams (<100 km2), 5 km for intermediate rivers (100–1000 km2) and 10 km for large rivers (>1000 km2). River slope was measured in maps with scale 1:50,000 or 1:100,000.
The chosen model fitting algorithm ‘rpart’ uses a 10-fold cross-validation. The training set is split into 10 roughly equally sized parts and the tree is grown on nine parts while using the tenth for testing (Venables and Ripley, 2003). The results are averaged and expressed as xerror, which is the cross-validated error estimation of the model as mean square error of the predictions at each split in the tree. Only four of the seven environmental variables (altitude, mean annual air temperature, mean annual precipitation and latitude) were finally used by the algorithm for tree construction.
Next, a prognosis of river types for impacted sampling sites based on the ‘rpart’ model was conducted, i.e. river type affiliation for impacted sites was modelled based on the four environmental variables. By comparing the mean metric values of unimpacted with impacted sites within each river type we can define the river type specific sensitivity and intensity of the alteration of fish assemblages as a reaction to human pressures. To avoid extrapolation in the prediction, impacted sites outside the range of the environmental characteristics of the unimpacted sites (between 5% and the 95% percentile) were eliminated from the dataset.
Response of metrics to pressures
As some fish metrics decrease in response to increasing human pressures (less fish of a guild leading to reduced density and biomass, disappearance of species) but in contrast, several others tend to increase (e.g. metrics associated with generalist and tolerant species), their testing for sensitivity and intensity is in reverse direction. Therefore, we set the direction of a metric's response to human pressures from literature (Oberdorff, 1996, Oberdorff et al., 2002, Karr, 1981, Verneaux, 1981, Grandmottet, 1983, Pont et al., 2006, Melcher et al., 2007, Noble et al., 2007a, Logez and Pont, 2011) and later used this classification for the direction of statistical tests (Table 2). Out of a total of 129 metrics, we defined 79 metrics as decreasing with human pressure, 49 as increasing and one metric as both, increasing and decreasing (Nsp_all, including both native and non-native species, see Table 2).
A one-sided Welch two sample t-test (Bonferroni-correction, p = 0.083) was used to test the differences between mean metric values of unimpacted and impacted sites (sensitivity), estimating p-values with the alternative hypothesis that the true difference in means (unimpacted–impacted) is greater than zero for those metrics supposed to decrease with increasing human pressure. Metrics classified as increasing are tested with the alternative hypothesis that the true difference in means is less than zero.
Furthermore, we used the ecological quality ratio (EQR) to identify the intensity of metric response between unimpacted and impacted conditions.
For metrics classified as decreasing with increasing pressure, the EQR was calculated as follows:
where i = 1…4, x is the arithmetic mean of fish metric values and RT the river type.
The EQR is calculated inverse for metrics classified as increasing with increasing pressure:
where i = 1…4, x is the arithmetic mean of fish metric values and RT the river type.
This is to ensure an EQR scale from 0 (impacted) to 1 (unimpacted condition). A metric was classified as qualified if it showed a significant difference between unimpacted vs. impacted sites (p < 0.05) and if the EQR was less than 0.7 – i.e. if the difference between unimpacted and impacted condition was greater than 30%. Furthermore, we tried to avoid biased results due to high frequencies of zero values for specific metrics in certain river types. Frequent true zero values can occur for metrics related to rare species (e.g. piscivorous guild) if absent in reference conditions and, hence, cannot decrease in impacted conditions. Therefore, we defined that at least 50% of sites must have a valid metric value (unequal to zero, i.e. for metrics classified as decreasing >50% of unimpacted sites, for sites classified as increasing >50% of impacted sites). The metric response tests for sensitivity and intensity were conducted within each river type separately.
To avoid redundancy, Pearson correlation analysis was conducted for the overall dataset and for each river type separately for the final selection, i.e. for metrics with a correlation coefficient higher or equal 0.7, only one (the first) metric was retained for the final metric list.
To prove if the selected metrics respond to specific pressures or pressure combinations (multiple pressures), paired t-tests were then repeated to show the response between unimpacted sites (NoP), sites impacted only by hydromorphological pressures (HMC), sites impacted only by water quality pressure (W), or by multiple pressures (HMCW). In the results section, this difference is figured out with notched boxplots: if the notches do not overlap, this is strong evidence that their medians differ (Chambers et al., 1983, p. 62). All statistical analyses were performed in R version 2.13.1 (R Core Development Team, 2011).
Results
Metrics selection
Restrictions for the occurrence of zero-values in the data led to the exclusion of 54 of the 129 candidate metrics. In total, 31 metrics showed a significant response to human pressures (unimpacted vs. impacted) and a high EQR (> than 30% change). Furthermore, 14 metrics were removed in the next step due to redundancy based on numerous correlations with other metrics (correlation coefficient >0.7; see Annex Table 2, Annex Table 3, Annex Table 4, Annex Table 5 for details). Finally, 17 metrics were selected for final testing of pressure specific and multiple pressure responses.
River types
The river types were defined based on fish community; the environmental characteristics were associated lately with the classification tree. This resulted in four river types. The classification tree model could classify 75% of the 716 overall reference sites correctly. The correct ratio for HWS was 63%, for MGR 81%, for LLR 77% and for MES 80%. The validation of the model supports a quite stable model with an estimated error of 0.43 rising to 0.52 in tenfold cross-validation. The river types can be classified as follows: head water streams (HWS), medium gradient rivers (MGR), lowland rivers (LLR) and Mediterranean streams (MES, a special type in the Peri-Mediterranean area of Europe according to Reyjol et al., 2007 in which there are many basin-endemic taxa according to Segurado et al., 2011).
HWS are inhabited by 86% lithophilic species, 70% potamodromous, 78% rheophilic, and 6% omnivorous species (Table 3). MGR and MES showed similar means in lithophilic, omnivorous, and potamodromous species but differed in rheophilic species (MGR: 84%, MES: 51%). LLR bore the highest mean of omnivorous (32%) and the smallest of lithophilic species (42%). Furthermore, species composition (based on the total number of individuals caught per species) also showed clear differences (Table 3): HWS were highly dominated by brown trout (Salmo trutta), MGR were dominated by European minnow (Phoxinus phoxinus) and brown trout. LLR were associated with assemblages dominated by roach (Rutilus rutilus) and gudgeon (Gobio gobio) and MES were dominated by brown trout, minnow and dace (Leuciscus souffia). The environmental characteristics of river types are shown in Table 4.
Table 3.
Association with percentage of species metrics used for river type modelling and distribution of fish species (based on the total number of individuals caught per species) in modelled river types (HWS = head water streams, MGR = medium gradient rivers, LLR = lowland rivers, MES = Mediterranean streams).
| HWS |
MGR |
LLR |
MES |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metric for RT modelling | % Total | Fish species | % Total catch | Metric for RT modelling | % Total | Fish species | % Total catch | Metric for RT modelling | % Total | Fish species | % Total catch | Metric for RT modelling | % total | Fish species | % total catch |
| Repro_LITH_perc_nsp | 86 | Salmo trutta | 70.3% | Repro_LITH_perc_nsp | 69 | Phoxinus phoxinus | 25.6% | Repro_LITH_perc_nsp | 42 | Rutilus rutilus | 16.0% | Repro_LITH_perc_nsp | 65 | Salmo trutta | 33.6% |
| Atroph_OMNI_perc_nsp | 6 | Pseudochondrostoma duriense | 7.6% | Atroph_OMNI_perc_nsp | 13 | Salmo trutta | 20.0% | Atroph_OMNI_perc_nsp | 32 | Gobio gobio | 13.7% | Atroph_OMNI_perc_nsp | 17 | Phoxinus phoxinus | 11.8% |
| Mig_POTAD_perc_nsp | 70 | Squalius pyrenaicus | 5.6% | Mig_POTAD_perc_nsp | 47 | Cottus gobio | 9.6% | Mig_POTAD_perc_nsp | 33 | Salmo trutta | 12.1% | Mig_POTAD_perc_nsp | 43 | Leuciscus souffia | 7.0% |
| Hab_RH_perc_nsp | 78 | Others | 16.5% | Hab_RH_perc_nsp | 84 | Barbatula barbatula | 9.3% | Hab_RH_perc_nsp | 58 | Phoxinus phoxinus | 11.0% | Hab_RH_perc_nsp | 50 | Rutilus rubilio | 6.4% |
| Salmo salar | 7.5% | Alburnoides bipunctatus | 8.4% | Anguilla anguilla | 6.4% | ||||||||||
| Others | 27.9% | Cottus gobio | 5.9% | Others | 34.8% | ||||||||||
| Total number individuals | 21,506 | Others | 33.0% | ||||||||||||
| Mean # species | 1.66 | 51,503 | 24,757 | 12,538 | |||||||||||
| SD # species | 0.92 | 4.4 | 7.23 | 3.47 | |||||||||||
| 2.6 | 3.23 | 1.71 | |||||||||||||
Table 4.
Median, range and standard deviation (SD) of environmental characteristics for four river types (HWS = head water streams, MGR = medium gradient rivers, LLR = lowland rivers, MES = Mediterranean streams).
| River type | Altitude [m.a.s.l.]a | Mean annual air temperature [°C]a | Mean annual precipitation [mm]a | Latitudea | Longitude | Mean january air temperature [°C] | Slope [‰] | Catchment size [km2] | Distance from source [m] | |
|---|---|---|---|---|---|---|---|---|---|---|
| HWS | Median | 478.0 | 12.3 | 1189.6 | 43.03943 | −7.327000 | 6.0 | 15.9 | 21.0 | 8.0 |
| Range | 0.0–2043.0 | 1.1–14.6 | 557.8–1564.5 | 37.84787–58.87025 | −9.090744–26.547563 | −6.4–9.7 | 0.9–294.6 | 1.0–681.0 | 1.0–62.0 | |
| SD | 363.5 | 2.6 | 254.4 | 2.909860 | 8.563482 | 4.1 | 38 | 79.1 | 9.4 | |
| MGR | Median | 210 | 8.1 | 763.9 | 48.43017 | 15.961294 | −3.5 | 7.1 | 47.0 | 13.0 |
| Range | 1.0–1595.0 | −2.3–14.7 | 474.3–1623.3 | 40.22691–68.49354 | −8.552902–29.509454 | −15.8–9.7 | 0–194.5 | 1.0–40157.0 | 1.0–521.0 | |
| SD | 291.1 | 3.4 | 251.7 | 6.771172 | 11.789414 | 5.5 | 21.1 | 4158.7 | 71.8 | |
| LLR | Median | 75.0 | 7.7 | 659.1 | 53.85842 | 17.456455 | −3.5 | 1.8 | 91.0 | 17.0 |
| Range | 0.0–470.0 | 2.4–15.9 | 562.4–1277.9 | 39.57362–63.66892 | −8.995163 27.042127 | −10.3–11.2 | 0–28.8 | 2.0–6855.0 | 1.0–240.0 | |
| SD | 82.2 | 2.1 | 108.7 | 3.799002 | 8.992162 | 3.9 | 4.4 | 1159.6 | 47.7 | |
| MES | Median | 208.0 | 13.7 | 1101.5 | 42.66090 | −7.744205 | 7.0 | 12.4 | 27.0 | 10.0 |
| Range | 2.0–1275.0 | 8.9–17.0 | 522.5 – 1562.0 | 37.18996–53.99423 | −9.245325–13.390564 | −10.3–11.2 | 0.01–97.2 | 2.0–1163.0 | 2.0–89.0 | |
| SD | 294.0 | 1.7 | 280.3 | 2.142648 | 6.236943 | 2.8 | 18.4 | 131.2 | 11.5 | |
Indicates variables used for river type modelling.
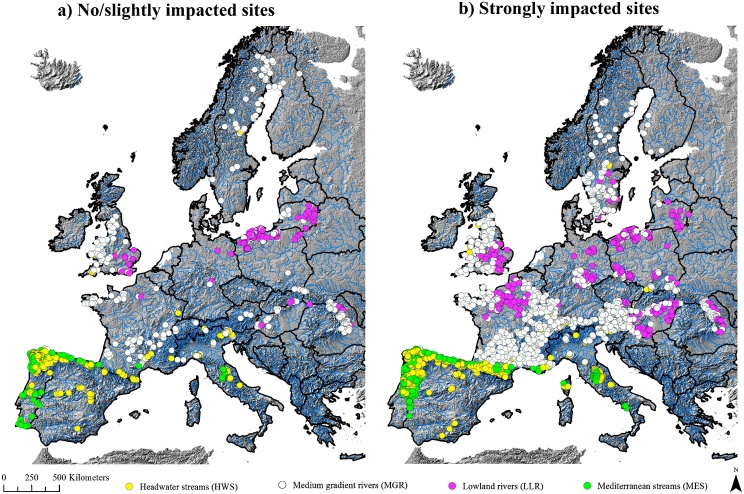
In total, 22% of sites (unimpacted and impacted) were located in HWS, 48% in MGR, 15% in LLR and 15% in MES (Fig. 2). Table 5 shows the association of these sites with specific and multiple human pressure status.
Fig. 2.
(a) assignment of unimpacted/slightly impacted sites (n = 716) to four modelled river types; (b) geographical distribution of strongly impacted sites (n = 2389) and modelled association with river types.
Table 5.
Number (N) and percentage (PERC) of sites associated with specific (W, HMC) and multiple (HMWC) human pressures in modelled river types (HWS = head water streams, MGR = medium gradient rivers, LLR = lowland rivers, MES = Mediterranean streams).
| River type |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pressure type | HWS |
MGR |
LLR |
MES |
All sites |
|||||
| N | PERC | N | PERC | N | PERC | N | PERC | N | PERC | |
| NoP | 195 | 28.6% | 296 | 19.7% | 128 | 27.3% | 97 | 21.5% | 716 | 23.1% |
| HMC | 209 | 30.6% | 351 | 23.4% | 102 | 21.7% | 109 | 24.1% | 771 | 24.8% |
| W | 120 | 17.6% | 151 | 10.1% | 37 | 7.9% | 82 | 18.1% | 390 | 12.6% |
| HMWC | 159 | 23.3% | 703 | 46.8% | 202 | 43.1% | 164 | 36.3% | 1228 | 39.5% |
| Total | 683 | 100.0% | 1501 | 100.0% | 469 | 100.0% | 452 | 100.0% | 3105 | 100.0% |
Pressure specific reaction
One metric responded in river types HWS, LLR and MES: Density of species intolerant to water quality degradation (WQgen_INTOL_dens). Another four metrics responded in these river types: Density of species intolerant to O2 depletion (WQO2_O2INTOL_dens) in HWS and MES; number of species tolerant to water quality degradation (WQgen_TOL_nsp) in MGR and MES; percent density of species intolerant to water quality degradation (WQgen_INTOL_perc_dens) in LLR and MES and percent density of species tolerant to water quality degradation (WQgen_TOL_perc_dens) in MGR and MES. The remaining 12 metrics were specific to individual river types (Table 6).
Table 6.
Final selection of metrics per river type (HWS = head water streams, MGR = medium gradient rivers, LLR = lowland rivers, MES = Mediterranean streams): Associated metric type (Type) bio = biodiversity, hab = habitat, troph = trophic level, wq = water quality; expected reaction under pressure (Reaction) incr = increasing, decr = decreasing; ** indicates a significant difference between unimpacted and impacted conditions in general (p.gen); significant pressure specific responses (p.spec) related to W = water quality pressure, HMC = hydromorphological pressure including connectivity, HMWC = combination of W + HMC. In bold: metrics reacting either to W or to HMC.
| Metric | Type | Reaction | River type |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HWS |
MGR |
LLR |
MES |
|||||||
| P.gen | P.spec | P.gen | P.spec | P.gen | P.spec | P.gen | P.spec | |||
| WQgen_INTOL_dens | wq | decr | ** | HMC, W, HMWC | ** | W | ** | HMC, W, HMWC | ||
| WQO2_O2INTOL_biom | wq | decr | ** | W, HMWC | ||||||
| HabSp_RHPAR_dens | hab | decr | ** | HMC, W, HMWC | ||||||
| WQO2_O2INTOL_dens | wq | decr | ** | HMC, W | ** | xxx | ||||
| WQgen_INTOL_perc_nsp | wq | decr | ** | HMC, W, HMWC | ||||||
| Nsp_all | bio | incr | ** | HMC, W, HMWC | ||||||
| WQgen_TOL_biom | wq | incr | ** | HMWC | ||||||
| HTOL_HTOL_perc_biom | hab | incr | ** | HMWC | ||||||
| WQgen_INTOL_perc_dens | wq | decr | ** | W, HMWC | ** | HMC, W, HMWC | ||||
| Atroph_PISC_perc_nsp | troph | decr | ** | HMC, HMWC | ||||||
| WQO2_O2TOL_perc_nsp | wq | incr | ** | HMWC | ||||||
| Atroph_OMNI_perc_biom | troph | incr | ** | HMWC | ||||||
| WQgen_TOL_perc_dens | wq | incr | ** | HMWC | ** | HMC, HMWC | ||||
| WQgen_INTOL_biom | wq | decr | ** | HMWC | ||||||
| WQgen_TOL_nsp | wq | incr | ** | HMWC | ** | HMC, HMWC | ||||
| WQO2_O2INTOL_perc_nsp | wq | decr | ** | HMWC | ||||||
| Atroph_OMNI_perc_nsp | troph | incr | ** | HMC, HMWC | ||||||
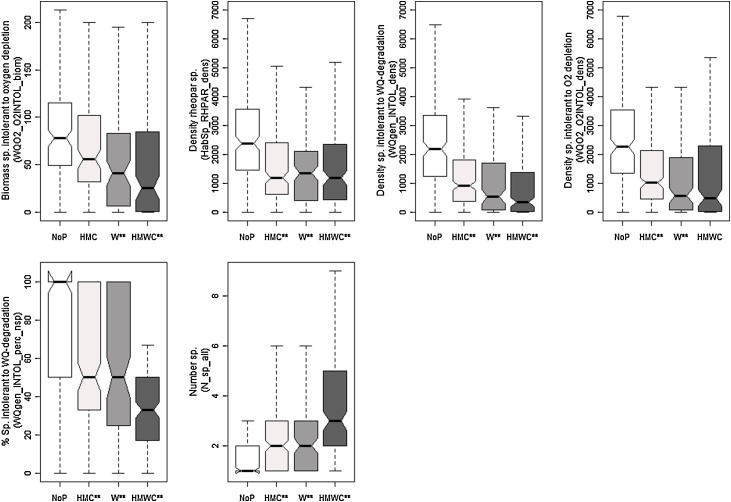
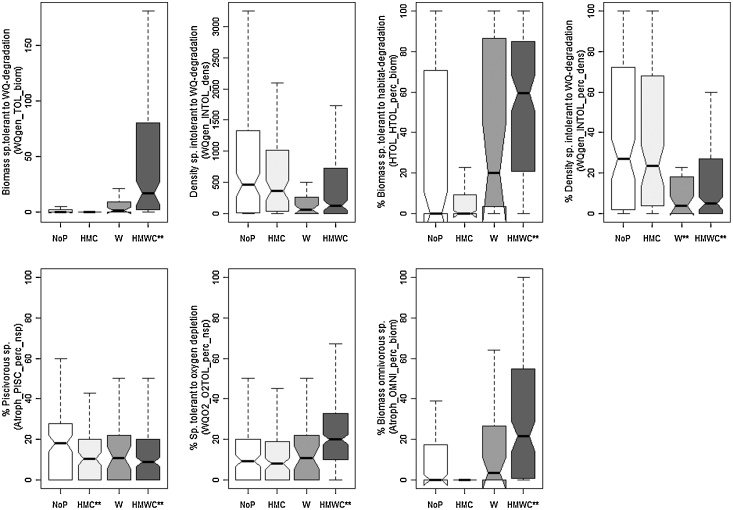
For HWS, six metrics finally were selected. All showed significant responses to water quality pressures and five metrics responded to hydromorphological pressure and multiple pressures (Fig. 3). They are associated with water quality sensitivity type (4 metrics), and habitat and biodiversity type (one metric each) (Table 6). For MGR, two metrics finally were selected; both only showed a significant reaction between unimpacted vs. multiple pressures (Fig. 4) and both are associated with water quality sensitivity metric type (Table 6).
Fig. 3.
Response of final metric selection for HWS to different pressure types. ** indicates significant difference according to t-tests.
Fig. 4.
Response of final metric selection for MGR to different pressure types. ** indicates significant difference according to t-tests.
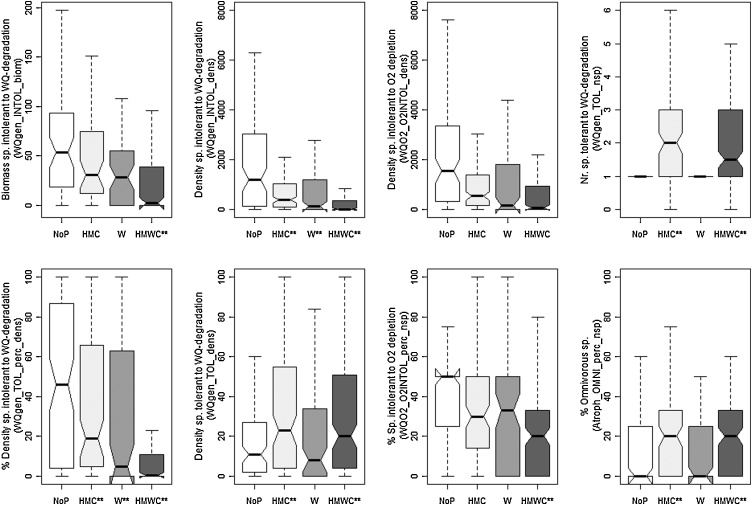
For LLR, seven metrics finally were selected. Three metrics showed a response to specific pressures (two metrics to water quality pressures and one to hydromorphological pressures). Six metrics showed a significant response only to multiple pressures (Fig. 5). One metric is associated with habitat-, two with trophic, and four with water quality sensitivity metric group (Table 6).
Fig. 5.
Response of final metric selection for LLR to different pressure types. ** indicates significant difference according to t-tests.
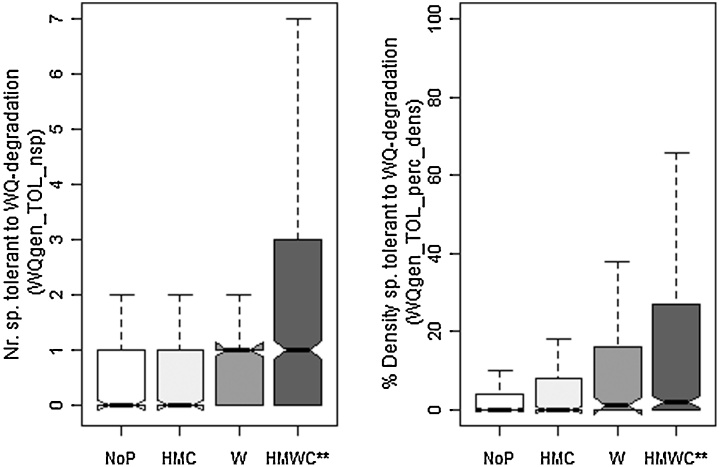
For MES, eight metrics finally were selected. Five metrics showed a significant response to hydromorphological pressures, two metrics to water quality pressures and seven metrics to multiple pressures. One metric (WQO2_O2INTOL_dens) showed no significant pressure specific and multiple pressure response (Fig. 6). Seven metrics are associated with water quality sensitivity metric type, only one with trophic level metric type (Table 6).
Fig. 6.
Response of selected metric selection for MES to different pressure types. ** indicates significant difference according to t-tests.
Overall, eight of the 17 finally selected metrics were “absolute number” metrics (three “biomass” metrics, three “density metrics” and two “number of species” metrics). The other nine metrics were “relative” metrics (five “percentage of species”, two “percentage of density” and two “percentage of biomass”).
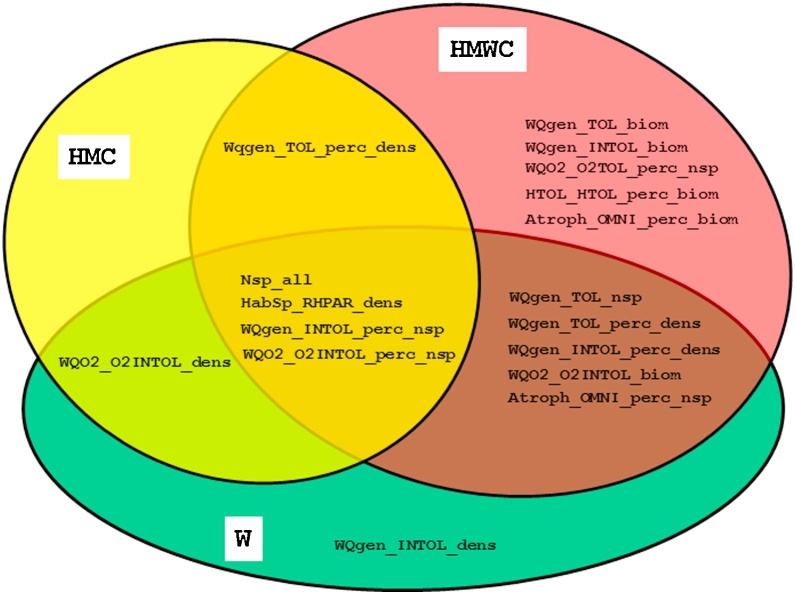
Overall, eight metrics were sensitive only to multiple pressures (HMCW), however we found seven “diagnostic metrics”, that reacted exclusively to water quality pressures (Table 6, in bold). Further three metrics showed a response to both specific pressures (W and HMC) and multiple pressures (Table 6) and one metric only to specific pressures.
Discussion
Many attempts were already made to identify metrics that show a response to human pressures and to generate multi-metric (fish) indices for the evaluation of the ecological status of running waters all over the globe (e.g. Karr, 1981, Fausch et al., 1990, Hughes et al., 2004, Oberdorff and Hughes, 1992, Pont et al., 2009). Moreover, the FAME Consortium (2004), Pont et al. (2007) and the EFI+ Consortium (2009) had already developed fish-based assessment methods derived from very large datasets across Europe. However, for the development of these methods the response of metrics to specific human pressures, i.e. hydromorphological-, water quality- and multiple pressures in different river types were not tested before.
River types
We classified four homogenous river types across Europe based on the percentage composition of rheophilic, lithophilic, omnivorous and potamodromous species. These metrics were selected based on the assumption that they gave a representative overview of the dominating fish assemblages in our dataset. Fish Assemblage Types (FATs) were already developed at the European scale by Melcher et al. (2007) and Schmutz et al. (2007b). However, their approach applied only to rivers belonging to FATs defined in their studies and there was need to extend the geographic range. Moreover, they used stepwise discriminant analysis to predict the fish types for impacted conditions, but according to Schmutz et al. (2007a), a disadvantage of discriminant function analysis is that the contributions of individual environmental variables are hidden in the discriminant functions of the model because of its multi-dimensional nature. In contrast, we searched for environmental variables that were able to predict the modelled river types for impacted conditions in a more traceable way.
Metrics selection and pressure specific reaction
Finally, seventeen out of our 129 candidate metrics showed a significant response to specific and multiple pressures in four river types. Pont et al. (2007) defined 10 metrics that showed the best response to human pressures (slight vs. strong impact, but not pressure specific) for the European Fish Index (EFI): These were two metrics related to trophic structure (density of omnivorous species and density of insectivorous species), two metrics related to reproduction guilds (density of phytophilic species and relative abundance of lithophilic species) and two metrics related to physical habitat (number of benthic species and number of rheophilic species). Furthermore, relative number of tolerant and intolerant species reflected the capacity of fish assemblages to support disturbance in general, and two metrics reflected migratory species richness. The EFI+ project was another attempt to evaluate the ecological status of European rivers by one index (EFI+ Consortium 2009). The EFI+ consists of four final fish metrics, wherein two of the following metrics are selected, depending on a fish zone (salmonid or cyprinid): Rheophilic reproduction habitat species richness, oxygen depletion intolerant species abundance, lithophilic reproduction habitat species abundance and abundance of individuals <15 cm of habitat intolerant species. And finally, in their exercise to develop a predictive index of biotic integrity for aquatic-vertebrate assemblages of western U.S. streams, Pont et al. (2009) retained 5 metrics (2 vertebrate and bentic metrics, one index on assemblages’ tolerances and proportion of invertivore–piscivore species as well as proportion of lithophilic-reproducing species).
In contrast, our final selection did not contain migratory, insectivorous, benthivorous, lithophilic and rheophilic metrics, as we applied strict and standardised rules through the whole metric selection process to avoid redundancy (correlations >0.7) – many metrics therefore were removed in a stepwise procedure (see Annex Table 2, Annex Table 3, Annex Table 4, Annex Table 5). However, our selection also contained two habitat metrics responding to water quality-, hydromorphological- and to multiple pressures in HWS and LLR: density of rheopar species (HabSp_RHPAR_dens) and percent biomass of species tolerant to habitat degradation (HTOL_HTOL_perc_biom). For MES and LLR, our selection also contained three trophic level metrics that responded to hydromorphological- and multiple pressures: Percent piscivorous species (Atroph_PISC_perc_nsp), percent omnivorous species (Atroph_OMNI_perc_nsp) and percent biomass omnivorous species (Atroph_OMNI_perc_biom). One biodiversity metric (Nsp_all) responded to water quality-, hydromorphological- and to multiple pressures in HWS. This metric is generally expected to increase along the longitudinal course of a river but also to decrease with increasing environmental degradation in naturally poor species river types. In HWS, an increase of this metric therefore mainly shows a response that is described as a potamalisation-effect (Jungwirth et al., 1995, Schmutz et al., 2000).
Although Logez and Pont (2011) and partly the EFI+ project considered individual fish body size in order to distinguish between different life stages to detect human disturbances in European coldwater streams, we decided not to use metrics based on size classes/life stages, as the information on fish length was not available for most sites of our dataset and coverage of whole countries would have been lost. Hence, we tested this aspect indirectly by considering density- and biomass metrics with the hypothesis that responding biomass metrics tend to represent a reaction of adult- and density metrics of juvenile fish.
Moreover, we found differences between river types in terms of metrics response, i.e. we observed a shift from intolerant metrics in HWS to tolerant metrics in LLR. This is an obvious proof that various metrics are needed to show the response to pressures in different river types – an important aspect to be considered in further attempts for fish-based assessment on a wider geographical range. Furthermore, the seven “diagnostic metrics”, that showed a pressure specific reaction (exclusively to water quality or hydromorphological pressures) are only valid for three river types (HWS, LLR and MES). For MGR, no pressure specific metric was found (Table 6). Overall, metrics more often responded to multiple pressures than to specific pressures. Fig. 7 also shows that although some metrics are similar, they passed the redundancy tests and therefore are able to give additional information on e.g. the response of size-classes to pressures. Moreover, a clear shift from specific pressures to multiple pressures can be detected: The association between density-related metrics and specific pressures (either W or HMC) is almost total (only exception of WQO2_O2Intol_biom). However, in response to multiple pressures, there is a combination of biomass-related metrics and density-related metrics.
Fig. 7.
Venn diagram showing fish metrics with relevant response to specific and multiple pressures.
Weaknesses and uncertainties
Our dataset covered a wide range of different ecoregions across Europe. Although the basis of our work was a common database provided by 14 countries, there were some inhomogeneities and a paucity of data for some areas (EFI+ Consortium, 2007). For example, in terms of characterisation of human pressures, Schinegger et al. (2012) has already shown that there are data gaps for particular regions of Europe (e.g. south-eastern countries) and in certain river types (particularly in large rivers) in the EFI+ dataset. Therefore, our pressure analysis was conducted on a general level–i.e. we focused on two specific pressures and their combinations. We are aware that the collective term “hydromorphological pressures” implies various single pressures that influence the response of certain metrics differently in terms of intensity and direction (see Schinegger et al., 2012 for details). However, to find metrics on a pan-European scale and for various river types, more accurate data are needed on a single pressure level for future attempts. Furthermore, we agree with Hering et al. (2010), who stated that other stressors such as land use, climate change, siltation, new toxic substances and alien species will be important for future work and that diagnostic metrics are currently only available for common types of degradation. Finally, we support the findings of Ormerod et al. (2010), that there are major challenges to understand the nature of multiple-stressor effects on species populations, communities and ecosystems, to identify and prioritise the major management issues and to seek the means to identify, diagnose and tackle multiple-stressors effects.
Acknowledgements
This work was funded by the Austrian Science Fund (FWF, research project LANPREF, contract number P 21735-B16) and partly by the EFI+ project (Contract number 044096, 6th Framework programme). Special thanks are due to all partners and contributing institutions in Europe that provided data for the EFI+ project. Sincere thanks are also given to Elizabeth Ashley Steel and Andreas Melcher for their helpful comments on the analyses and the manuscript. In addition, Erwin Lautsch and Friedrich Leisch provided helpful statistical advice and two anonymous reviewers valuable comments.
Footnotes
This article is part of a Special Issue entitled 9th International Symposium on Ecohydraulics 2012.
Annex.
Annex Table 1.
Association of most frequent species in the dataset with various guilds: WQgen (Water quality general), WQO2 (Water quality O2 concentration), HTOL (Habitat degradation), Hab (Preferred living habitat), Atroph (Trophic situation), Mig (Migration), Repro (Reproduction habitat), HabSp (Spawning habit). Types: INTOL = intolerant, TOL = tolerant, IM = intermediate, RH = rheophilic, EURY = eurytopic, DETR = feeding on detritus, INSV = insectivorous, OMNI = omnivorous, PISC = piscivorous. RESID = resident, POTAD = potamodromous, LONG = long distance migrant. LITH = lithophilic, PELA = pelagophilic, SPEL = speleophilic, PSAM = psamnmophilic, PHLI = phyto-lithophilic.
| Species | Family | WQgen | WQO2 | HTOL | Hab | Atroph | Mig | Repro | HabSp |
|---|---|---|---|---|---|---|---|---|---|
| Alburnoides bipunctatus | Cyprinidae | INTOL | INTOL | INTOL | RH | INSV | RESID | LITH | RHPAR |
| Anguilla anguilla | Anguillidae | TOL | TOL | TOL | EURY | INSV | LONG | PELA | LIPAR |
| Barbatula barbatula | Nemacheilidae | IM | IM | IM | RH | INSV | RESID | LITH | EUPAR |
| Cottus gobio | Cottidae | INTOL | INTOL | INTOL | RH | INSV | RESID | SPEL | RHPAR |
| Gobio gobio | Cyprinidae | IM | INTOL | TOL | RH | INSV | RESID | PSAM | RHPAR |
| Leuciscus souffia | Cyprinidae | INTOL | INTOL | INTOL | RH | INSV | RESID | LITH | RHPAR |
| Phoxinus phoxinus | Cyprinidae | IM | INTOL | INTOL | RH | INSV | RESID | LITH | EUPAR |
| Pseudochondrostoma duriense | Cyprinidae | IM | O2IM | HIM | RH | DETR | POTAD | LITH | RHPAR |
| Rutilus rubilio | Cyprinidae | IM | IM | IM | EURY | INSV | RESID | PHLI | RHPAR |
| Rutilus rutilus | Cyprinidae | TOL | TOL | TOL | EURY | OMNI | POTAD | PHLI | EUPAR |
| Salmo salar | Salmonidae | INTOL | INTOL | INTOL | RH | PISC | LONG | LITH | RHPAR |
| Salmo trutta | Salmonidae | INTOL | INTOL | INTOL | RH | INSV | POTAD | LITH | RHPAR |
| Squalius pyrenaicus | Cyprinidae | IM | IM | IM | EURY | INSV | RESID | LITH | EUPAR |
Annex Table 2.
Pearson's correlation coefficient for metrics in HWS (before final selection), metrics in bold are finally selected. Values in bold indicate a correlation coefficient >0.7.
| Metrics HWS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| WQO2_O2INTOL_biom | HabSp_RHPAR_dens | HTOL_HINTOL_dens | WQgen_INTOL_dens | WQO2_O2INTOL_dens | perc_nha_WQgen_INTOL | WQgen_INTOL_perc_nsp | Mig_POTAD_dens | Nsp_all | |
| WQO2_O2INTOL_biom | 1.00 | 0.49 | 0.43 | 0.65 | 0.44 | 0.43 | 0.30 | 0.52 | −0.21 |
| HabSp_RHPAR_dens | 0.49 | 1.00 | 0.55 | 0.64 | 0.47 | 0.07 | 0.09 | 0.77 | 0.11 |
| HTOL_HINTOL_dens | 0.43 | 0.55 | 1.00 | 0.59 | 0.95 | 0.10 | 0.14 | 0.56 | −0.01 |
| WQgen_INTOL_dens | 0.65 | 0.64 | 0.59 | 1.00 | 0.62 | 0.48 | 0.43 | 0.75 | −0.26 |
| WQO2_O2INTOL_dens | 0.44 | 0.47 | 0.95 | 0.62 | 1.00 | 0.14 | 0.18 | 0.50 | −0.01 |
| perc_nha_WQgen_INTOL | 0.43 | 0.07 | 0.10 | 0.48 | 0.14 | 1.00 | 0.83 | 0.23 | −0.62 |
| WQgen_INTOL_perc_nsp | 0.30 | 0.09 | 0.14 | 0.43 | 0.18 | 0.83 | 1.00 | 0.22 | −0.65 |
| Mig_POTAD_dens | 0.52 | 0.77 | 0.56 | 0.75 | 0.50 | 0.23 | 0.22 | 1.00 | −0.10 |
| Nsp_all | −0.21 | 0.11 | −0.01 | −0.26 | −0.01 | −0.62 | −0.65 | −0.10 | 1.00 |
Annex Table 3.
Pearson's correlation coefficient for metrics in MGR (before final selection), metrics in bold are finally selected. Values in bold indicate a correlation coefficient >0.7.
| Metrics MGR |
||||||
|---|---|---|---|---|---|---|
| HTOL_HTOL_nsp | WQgen_TOL_nsp | HTOL_HTOL_perc_biom | HTOL_HTOL_perc_dens | WQgen_TOL_perc_dens | WQgen_TOL_perc_nsp | |
| HTOL_HTOL_nsp | 1.00 | 0.96 | 0.73 | 0.72 | 0.66 | 0.76 |
| WQgen_TOL_nsp | 0.96 | 1.00 | 0.67 | 0.69 | 0.66 | 0.79 |
| HTOL_HTOL_perc_biom | 0.73 | 0.67 | 1.00 | 0.80 | 0.72 | 0.70 |
| HTOL_HTOL_perc_dens | 0.72 | 0.69 | 0.80 | 1.00 | 0.91 | 0.74 |
| WQgen_TOL_perc_dens | 0.66 | 0.66 | 0.72 | 0.91 | 1.00 | 0.74 |
| WQgen_TOL_perc_nsp | 0.76 | 0.79 | 0.70 | 0.74 | 0.74 | 1.00 |
Annex Table 4.
Pearson's correlation coefficient for metrics in LLR (before final selection), metrics in bold are finally selected. Values in bold indicate a correlation coefficient >0.7.
| Metrics LLR |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| WQgen_TOL_biom | WQO2_O2TOL_nsp | HTOL_HTOL_perc_biom | WQgen_INTOL_perc_biom | WQgen_TOL_perc_biom | WQgen_INTOL_perc_dens | Atroph_PISC_perc_nsp | WQO2_O2TOL_perc_nsp | Atroph_OMNI_perc_biom | |
| WQgen_TOL_biom | 1.00 | 0.53 | 0.41 | −0.34 | 0.57 | −0.22 | 0.01 | 0.36 | 0.46 |
| WQO2_O2TOL_nsp | 0.53 | 1.00 | 0.46 | −0.41 | 0.46 | −0.35 | 0.06 | 0.74 | 0.38 |
| HTOL_HTOL_perc_biom | 0.41 | 0.46 | 1.00 | −0.88 | 0.75 | −0.60 | 0.15 | 0.49 | 0.64 |
| WQgen_INTOL_perc_biom | −0.34 | −0.41 | −0.88 | 1.00 | −0.67 | 0.71 | −0.06 | −0.44 | −0.66 |
| WQgen_TOL_perc_biom | 0.57 | 0.46 | 0.75 | −0.67 | 1.00 | −0.42 | 0.06 | 0.46 | 0.84 |
| WQgen_INTOL_perc_dens | −0.22 | −0.35 | −0.60 | 0.71 | −0.42 | 1.00 | −0.06 | −0.36 | −0.45 |
| Atroph_PISC_perc_nsp | 0.01 | 0.06 | 0.15 | −0.06 | 0.06 | −0.06 | 1.00 | −0.02 | −0.01 |
| WQO2_O2TOL_perc_nsp | 0.36 | 0.74 | 0.49 | −0.44 | 0.46 | −0.36 | −0.02 | 1.00 | 0.35 |
| Atroph_OMNI_perc_biom | 0.46 | 0.38 | 0.64 | −0.66 | 0.84 | −0.45 | −0.01 | 0.35 | 1.00 |
Annex Table 5.
Pearson's correlation coefficient for metrics in MES (before final selection), metrics in bold are finally selected. Values in bold indicate a correlation coefficient >0.7.
| Metrics MES |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atroph_INSV_biom | WQgen_INTOL_biom | WQO2_O2INTOL_biom | WQgen_INTOL_dens | HTOL_HTOL_nsp | WQgen_TOL_nsp | WQgen_INTOL_perc_biom | HTOL_HINTOL_perc_dens | WQgen_INTOL_perc_dens | WQgen_TOL_perc_dens | WQO2_O2INTOL_dens | WQgen_INTOL_perc_nsp | WQO2_O2INTOL_perc_nsp | Atroph_OMNI_nsp | Atroph_OMNI_perc_dens | Atroph_OMNI_perc_nsp | |
| Atroph_INSV_biom | 1.00 | 0.92 | 0.77 | 0.64 | 0.28 | 0.10 | 0.38 | 0.42 | 0.41 | −0.23 | 0.46 | 0.38 | 0.37 | −0.02 | −0.28 | −0.18 |
| WQgen_INTOL_biom | 0.92 | 1.00 | 0.77 | 0.65 | 0.22 | 0.07 | 0.52 | 0.44 | 0.50 | −0.23 | 0.46 | 0.42 | 0.37 | −0.05 | −0.26 | −0.18 |
| WQO2_O2INTOL_biom | 0.77 | 0.77 | 1.00 | 0.76 | −0.04 | −0.17 | 0.54 | 0.56 | 0.58 | −0.36 | 0.62 | 0.53 | 0.51 | −0.20 | −0.32 | −0.26 |
| WQgen_INTOL_dens | 0.64 | 0.65 | 0.76 | 1.00 | 0.00 | −0.10 | 0.50 | 0.56 | 0.67 | −0.30 | 0.60 | 0.52 | 0.46 | −0.16 | −0.28 | −0.27 |
| HTOL_HTOL_nsp | 0.28 | 0.22 | −0.04 | 0.00 | 1.00 | 0.89 | −0.15 | −0.20 | −0.18 | 0.31 | −0.20 | −0.23 | −0.25 | 0.78 | 0.26 | 0.34 |
| WQgen_TOL_nsp | 0.10 | 0.07 | −0.17 | −0.10 | 0.89 | 1.00 | −0.29 | −0.32 | −0.26 | 0.52 | −0.34 | −0.34 | −0.40 | 0.83 | 0.43 | 0.49 |
| WQgen_INTOL_perc_biom | 0.38 | 0.52 | 0.54 | 0.50 | −0.15 | −0.29 | 1.00 | 0.75 | 0.79 | −0.41 | 0.79 | 0.76 | 0.73 | −0.36 | −0.41 | −0.38 |
| HTOL_HINTOL_perc_dens | 0.42 | 0.44 | 0.56 | 0.56 | −0.20 | −0.32 | 0.75 | 1.00 | 0.75 | −0.46 | 0.91 | 0.70 | 0.79 | −0.41 | −0.53 | −0.51 |
| WQgen_INTOL_perc_dens | 0.41 | 0.50 | 0.58 | 0.67 | −0.18 | −0.26 | 0.79 | 0.75 | 1.00 | −0.40 | 0.80 | 0.79 | 0.62 | −0.38 | −0.42 | −0.43 |
| WQgen_TOL_perc_dens | −0.23 | −0.23 | −0.36 | −0.30 | 0.31 | 0.52 | −0.41 | −0.46 | −0.40 | 1.00 | −0.51 | −0.37 | −0.45 | 0.41 | 0.69 | 0.51 |
| WQO2_O2INTOL_dens | 0.46 | 0.46 | 0.62 | 0.60 | −0.20 | −0.34 | 0.79 | 0.91 | 0.80 | −0.51 | 1.00 | 0.77 | 0.83 | −0.42 | −0.52 | −0.50 |
| WQgen_INTOL_perc_nsp | 0.38 | 0.42 | 0.53 | 0.52 | −0.23 | −0.34 | 0.76 | 0.70 | 0.79 | −0.37 | 0.77 | 1.00 | 0.84 | −0.40 | −0.41 | -0.49 |
| WQO2_O2INTOL_perc_nsp | 0.37 | 0.37 | 0.51 | 0.46 | −0.25 | −0.40 | 0.73 | 0.79 | 0.62 | −0.45 | 0.83 | 0.84 | 1.00 | −0.43 | −0.45 | −0.52 |
| Atroph_OMNI_nsp | −0.02 | −0.05 | −0.20 | −0.16 | 0.78 | 0.83 | −0.36 | −0.41 | −0.38 | 0.41 | −0.42 | −0.40 | −0.43 | 1.00 | 0.57 | 0.74 |
| Atroph_OMNI_perc_dens | −0.28 | −0.26 | −0.32 | −0.28 | 0.26 | 0.43 | −0.41 | −0.53 | −0.42 | 0.69 | −0.52 | −0.41 | −0.45 | 0.57 | 1.00 | 0.75 |
| Atroph_OMNI_perc_nsp | −0.18 | −0.18 | −0.26 | −0.27 | 0.34 | 0.49 | −0.38 | −0.51 | −0.43 | 0.51 | −0.50 | −0.49 | −0.52 | 0.74 | 0.75 | 1.00 |
References
- Bailey R.C., Kennedy M.G., Dervish M.Z., Taylor R.M. Biological assessment of freshwater ecosystems using a reference condition approach: comparing predicted and actual benthic invertebrate communities in Yukon streams. Freshw. Biol. 1998;39:765–774. [Google Scholar]
- CEN . European Committee for Standardization; Brussels: 2003. Water Quality – Sampling of Fish with Electricity. European Standard – EN 14011:2003; p. 18. [Google Scholar]
- Chambers J.M., Cleveland W.S., Kleiner B., Tukey P.A. Wadsworth; Boston, MA: 1983. Graphical Methods for Data Analysis. [Google Scholar]
- EFI+ Consortium Improvement and spatial extension of the European Fish Index (EFI+). D1.1-D1.3, Lists and descriptions of sampling methods, type of fish data, environmental variables and pressure variables [online] http://efi-plus.boku.ac.at/downloads/EFI+ 0044096 Deliverable D1_1-1_3.pdf (accessed March 2012)
- EFI+ Consortium Manual for the application of the new European Fish Index – EFI+. Improvement and spatial extension of the European Fish Index [online] http://efi-plus.boku.ac.at/software/doc/EFI+Manual.pdf (accessed March 2012)
- European Commission . 2000. Directive 2000/60/EC of the European Parliament and the Council of 23 October 2000 Establishing A Framework for Community Action in the Field of Water Policy; pp. 1–73. OJEC, L 327. [Google Scholar]
- FAME Consortium Development, Evaluation & Implementation of a Standardised Fish-based Assessment Method for the Ecological Status of European Rivers – A Contribution to the Water Framework Directive (FAME). Final Report. EVK1-CT-2001-00094 [online] http://fame.boku.ac.at/downloads.htm (accessed March 2012)
- Fausch K.D., Lyons J., Karr J.R., Angermeier P.L. Fish communities as indicators of environmental degradation. Am. Fish. Soc. Symp. 1990;8:123–144. [Google Scholar]
- Ferreira T., Caiola N., Casals F., Oliveira J.M., de Sostoa A. Assessing perturbation of river fish communities in the Iberian Ecoregion. Fish. Manage. Ecol. 2007;14:519–530. [Google Scholar]
- Grandmottet J.P. Principales exigences des téléostéens dulcicoles vis-à-vis de l’habitat aquatique. Ann. Sci. Univ. Besançon. 1983;4:3–32. [Google Scholar]
- Grenouillet G., Roset N., Goffaux D., Breine J., Simoens I., de Leeuw J.J., Kestemont P. Fish assemblages in European Western Highlands and Western Plains: a type-specific approach to assess ecological quality of running waters. Fish. Manage. Ecol. 2007;14:509–517. [Google Scholar]
- Hering D., Feld C.K., Moog O., Ofenböck T. Cook book for the development of a multimetric index for biological condition of aquatic ecosystems: experiences from the European AQEM and STAR projects and related initiatives. Hydrobiologia. 2006;566:311–324. [Google Scholar]
- Hering D., Borja A., Carstensen J., Carvalho L., Elliott M., Feld C.K., Heiskanen A.S., Johnson R.K., Moe J., Pont D., Lyche Solheim A., van de Bund W. The European Water Framework Directive at the age of 10: a critical review of the achievements with recommendations for the future. Sci. Total Environ. 2010;408:4007–4019. doi: 10.1016/j.scitotenv.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Holzer S. University of Natural Resources and Life Sciences; Vienna: 2008. European fish species: taxa and guilds classification regarding fish-based assessment methods; p. 195. http://othes.univie.ac.at/2400/1/2008-11-05_9900506.pdf (accessed March 2012) (Master thesis) [Google Scholar]
- Hughes R.M., Kaufmann P.R., Herlihy A.T., Kincaid T.M., Reynolds L., Larsen D.P. A process for developing and evaluating indices of fish assemblage integrity. Can. J. Fish. Aquat. Sci. 1998;55:1618–1631. [Google Scholar]
- Hughes R.M., Oberdorff T. In: Assessing the Sustainability and Biological Integrity of Water Resources Using Fish Communities. Simon T.P., editor. CRC Press; Boca Raton, FL: 1999. Applications of IBI concepts and metrics to waters outside the United States and Canada; pp. 79–93. [Google Scholar]
- Hughes R.M., Howlin S., Kaufmann P.R. A bio-integrity index (IBI) for coldwater streams of Western Oregon and Washington. Trans. Am. Fish. Soc. 2004;133:1497–1515. [Google Scholar]
- Illies J. Gustav Fischer Verlag; New York, Stuttgart: 1978. Limnofauna Europaea. 2. Auflage. [Google Scholar]
- Jungwirth M., Muhar S., Schmutz S. The effects of recreated instream and ecotone structures on the fish fauna of an epipotamal river. Hydrobiologia. 1995;303:195–206. [Google Scholar]
- Karr J.R. Assessment of biotic integrity using fish communities. Fisheries. 1981;6:21–27. [Google Scholar]
- Karr J.R., Chu E.W. Sustaining living rivers. Hydrobiologia. 2000;422:1–14. [Google Scholar]
- Logez M., Pont D. Development of metrics based on fish body size and species traits to assess European coldwater streams. Ecol. Indic. 2011;11:1204–1245. [Google Scholar]
- Lyons J. General Technical Report, NC-149, North Central Forest Experiment Station. U.S. Forest Service; St. Paul, MN: 1992. Using the index of biotic integrity (IBI) to measure environmental quality in warmwater streams of Wisconsin. [Google Scholar]
- McCormick F.H., Hughes R.M., Kaufmann P.R., Peck D.V., Stoddard J.L., Herlihy A.T. Development of an index of biotic integrity for the mid-Atlantic Highlands region. Trans. Am. Fish. Soc. 2001;130:857–877. [Google Scholar]
- Melcher A., Schmutz S., Haidvogl G., Moder K. Spatially based methods to assess the ecological status of European fish assemblage types. Fish. Manage. Ecol. 2007;14:453–463. [Google Scholar]
- Mebane C.A., Maret T.R., Hughes R.M. An index of biological integrity (IBI) for Pacific Northwest rivers. Trans. Am. Fish. Soc. 2003;132:239–261. [Google Scholar]
- Noble R.A.A., Cowx I.G., Goffaux D., Kestemont P. Assessing the health of European rivers using functional ecological guilds of fish communities: standardising species classification and approaches to metric selection. Fish. Manage. Ecol. 2007;14:381–392. [Google Scholar]
- Noble R.A.A., Cowx I.G., Starkie A.S. Development of fish-based methods for the assessment of ecological status in English and Welsh rivers. Fish. Manage. Ecol. 2007;14:495–508. [Google Scholar]
- Oberdorff T., Hughes R.M. Modification of an index of biotic integrity based on fish assemblages to characterize rivers of the Seine Basin. Hydrobiologia. 1992;228:117–130. [Google Scholar]
- Oberdorff T. 1996. Réseau Hydrobiologique et Piscicole (RHP). Bassin Seine-Normandie. Synthèse des données 1995. Conseil supérieur de la pêche, Délégation Régionale no 1 – Agence de l’Eau Seine-Normandie; p. 57. [Google Scholar]
- Oberdorff T., Pont D., Hugueny B., Chessel D. A probabilistic model characterizing fish assemblages of French rivers: a framework for environmental assessment. Freshw. Biol. 2001;46:399–415. [Google Scholar]
- Oberdorff T., Pont D., Hugueny B., Porcher J.P. Development and validation of a fish-based index for the assessment of river health in France. Freshw. Biol. 2002;47:1720–1734. [Google Scholar]
- Ormerod S.J., Dobson M., Hildrew A.G., Townsend C.R. Multiple stressors in freshwater ecosystems. Freshw. Biol. 2010;55(s1):1–4. [Google Scholar]
- Pont D., Hugueny B., Oberdorff T. Modelling habitat requirement of European fishes: do species have similar responses to local and regional environmental constraints? Can. J. Fish. Aquat. Sci. 2005;62:163–173. [Google Scholar]
- Pont D., Hugueny B., Beier U., Goffaux D., Melcher A., Noble R., Rogers C., Roset N., Schmutz S. Assessing river biotic condition at a continental scale: a European approach using functional metrics and fish assemblages. J. Appl. Ecol. 2006;43:70–80. [Google Scholar]
- Pont D., Hugueny B., Rogers C. Development of a fish-based index for the assessment of river health in Europe: the European fish index. Fish. Manage. Ecol. 2007;14:427–439. [Google Scholar]
- Pont D., Hughes R.M., Whittier T.R., Schmutz S. A predictive index of biotic integrity model for aquatic-vertebrate assemblages of western U.S. streams. Trans. Am. Fish. Soc. 2009;138:292–305. [Google Scholar]
- Reyjol Y., Hugueny B., Pont D., Bianco P.G., Beier U., Caiola N., Casals F., Cowx I., Economou A., Ferreira T., Haidvogl G., Noble R., De Sostoa A., Vigneron T., Virbickas T. Patterns in species richness and endemism of European freshwater fish. Global Ecol. Biogeogr. 2007;16:65–75. [Google Scholar]
- Roset N., Grenouillet G., Goffaux D., Pont D., Kestemont P. A review of existing fish assemblage indicators and methodologies. Fish. Manage. Ecol. 2007;14:393–405. [Google Scholar]
- R Development Core Team . 2011. R: A language and environment for statistical computing. Vienna, Austria. [Google Scholar]
- Schinegger R., Trautwein C., Schmutz S., Melcher A. Multiple human pressures and their spatial patterns in European running waters. Water Environ. J. 2012;26:261–273. doi: 10.1111/j.1747-6593.2011.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz S., Kaufmann M., Vogel B., Jungwirth M., Muhar S. A multi-level concept for fish-based, river-type-specific assessment of ecological integrity. Hydrobiologia. 2000;422:279–289. [Google Scholar]
- Schmutz S., Cowx I.G., Haidvogl G., Pont D. Fish-based methods for assessing European running waters: a synthesis. Fish. Manage. Ecol. 2007;14:369–380. [Google Scholar]
- Schmutz S., Melcher A., Frangez C., Haidvogl G., Beier U., Böhmer J., Breine J., Simoens I., Caiola N., de Sostoa A., Ferreira M.T., Oliveira J., Grenouillet G., Goffaux D., de Leeuw J.J., Noble R.A.A., Roset N., Virbickas T. Spatially based methods to assess the ecological status of riverine fish assemblages in European ecoregions. Fish. Manage. Ecol. 2007;14:441–452. [Google Scholar]
- Segurado P., Santos J.M., Pont D., Melcher A.H., Jalon D.G., Hughes R.M., Ferreira M.T. Estimating species tolerance to human perturbation: expert judgment versus empirical approaches. Ecol. Indic. 2011;11(6):1623–1635. [Google Scholar]
- Southerland M.T., Rogers G.M., Kline M.J., Morgan R.P., Boward D.M., Kazyak R., Klauda R.J., Stranko S.A. Improving biological indicators to better assess the condition of streams. Ecol. Indic. 2007;7:751–767. [Google Scholar]
- Stoddard J.L., Herlihy A.T., Peck D.V., Hughes R.M., Whittier T.R., Tarquinio E. A process for creating multimetric indices for large-scale aquatic surveys. J. N. Am. Benthol. Soc. 2008;27:878–891. [Google Scholar]
- Venables W.N., Ripley B.D. 4th ed. 2003. Modern Applied Statistics with S; p. 512. [Google Scholar]
- Verneaux J. Les poissons et la qualité des cours d’eau. Ann. Sci. Univ. Franche Comté. 1981;2:33–41. [Google Scholar]
- Virbickas T., Kesminas V. Development of fish-based assessment method for the ecological status of rivers in the Baltic region. Fish. Manage. Ecol. 2007;14:531–539. [Google Scholar]
- Whittier T.R., Stoddard J.L., Hughes R.M., Lomnicky G. In: Landscape Influences on Stream Habitat and Biological Assemblages. Hughes R.M., Wang L., Seelbach P.M., editors. American Fisheries Society, Symposium 48; Bethesda, Maryland: 2006. Associations among catchment- and site-scale disturbance indicators and biological assemblages at least- and most-disturbed stream and river sites in the western USA; pp. 641–664. [Google Scholar]
- Whittier T.R., Hughes R.M., Stoddard J.L., Lomnicky G.A., Peck D.V., Herlihy A.T. A structured approach for developing indices of biotic integrity: three examples from western USA streams and rivers. Trans. Am. Fish. Soc. 2007;136:718–735. [Google Scholar]