Abstract
The Government of Tanzania introduced indoor residual spraying (IRS) in Muleba district in north-western Tanzania after frequent malaria epidemics. Malaria parasitological baseline and two cross-sectional follow-up surveys were conducted in villages under the IRS program and those not under IRS to assess the impact of IRS intervention. After two rounds of IRS intervention there was a significant reduction of malaria parasitological indices in both two villages. In IRS villages overall, parasitemia prevalence was reduced by 67.2%, splenomegaly was reduced by 75.8%, whereas anemia was reduced by 50.5%. There was also a decline of malaria parasite density from 896.4 at baseline to 128.8 at second follow-up survey. Similarly, there was also a reduction of malaria parasitological indices in non-IRS villages; however, parasitological indices in IRS villages remained far below the levels in non-IRS villages. The reduction of malaria parasitological indices in non-IRS villages might have been contributed by interventions other than IRS.
Introduction
Malaria is the number one public health problem in Tanzania. According to hospital records, malaria remains a major cause of hospital admissions and deaths, especially in children < 5 years of age. For instance, malaria accounts for 50% of all deaths in children < 5 years of age, 38–40% of outpatients, and 50–54% of inpatients of all age groups.1 Until recently, malaria has been a common disease in low altitude rural areas of Tanzania.2 However, because of changes in socio-economic, environmental and vector-related factors, the disease is now common in previously malaria-free areas, predisposing affected communities to devastating epidemics.3,4 The first documented malaria epidemic in Tanzania occurred in 1915 at Bungu in the western Usambara Mountains.5 It is estimated that over 8.6% of the 34.5 million people in Tanzania live in malaria unstable areas prone to epidemics. During the past six decades marked malaria epidemics in Tanzania have occurred in about 10% of the districts.2,3
Muleba district is known to be a malaria epidemic-prone district with unstable transmission of varying seasonality. The highest peak of malaria transmission usually occurs between May and July and November and January, which results from preceding rain seasons. Recent data indicate a mean prevalence of malaria infection of 49–53.3%,6 showing a marked increase in prevalence from 40% to 44% recorded 8 years earlier.7 The district experienced the most devastating epidemic of malaria in 1997/98, which resulted from the El-Nino rains phenomenon. This epidemic caused high morbidity and mortality in children particularly under the age of 5 years, with Nshamba division being the most affected.7
In 2006, the World Health Organization (WHO) recommended that, where appropriate, indoor residual spraying (IRS) with DDT or other insecticides should be a central part of national malaria control strategies. Synthetic pyrethroids are safe alternatives to DDT, especially in modern housing surfaces and in low and seasonal transmission areas. Where malaria vectors are resistant to DDT or pyrethroids, more expensive, shorter duration insecticides have to be used (carbamates and organophosphates). In Africa, sustained IRS has historically been used mainly in the southern part of the continent, where it has been successful in controlling malaria and reducing transmission. With current efforts to scale-up malaria control in Africa, IRS has been introduced in a number of countries with initially high levels of transmission.8 However, IRS requires more complex and costly operational delivery systems than insecticide-treated nets/long-lasting insecticidal nets (ITNs/LLINs) and claims of sustained high coverage often remain unproven.9 Recently, IRS has been shown to be effective in areas of high malaria transmission or to malaria epidemic-prone areas.10 The government of United Republic of Tanzania through Ministry of Health and Social Welfare (MoH&SW) with the support of the President's Malaria Initiative (PMI) started an IRS intervention program in Muleba district, after the 2006 malaria epidemic using lambda-cyhalothrin capsule suspension (ICON 10 CS), targeting villages prone to malaria epidemics.
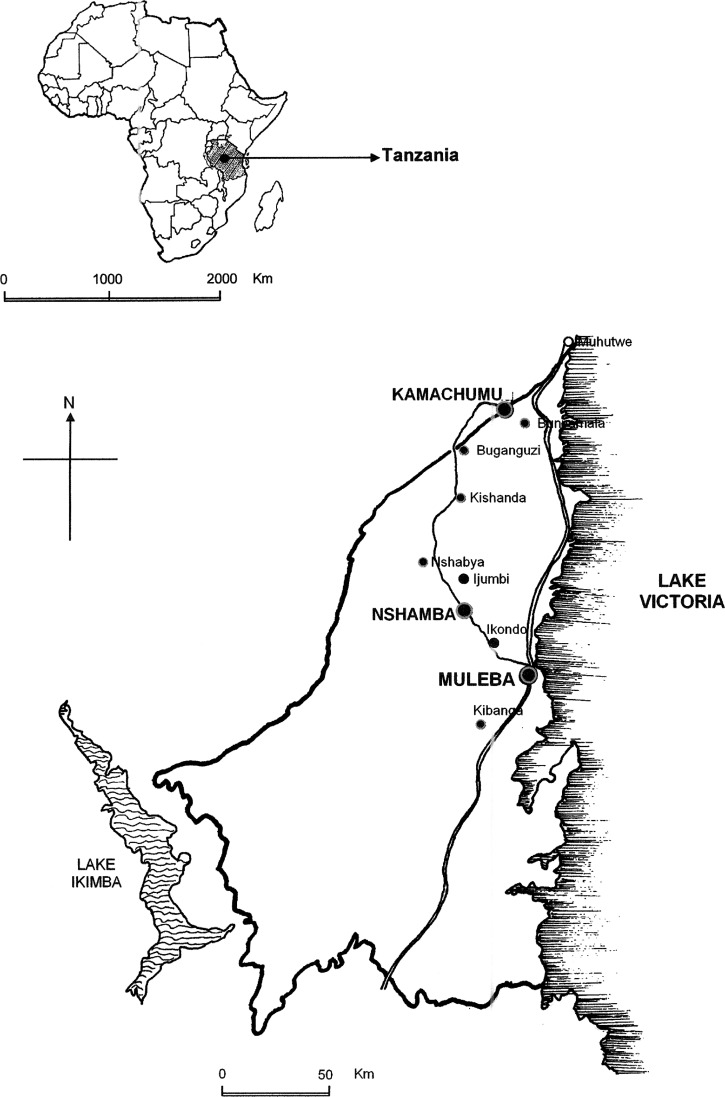
As part of a larger study into the epidemiology of malaria epidemics and the monitoring and evaluation of IRS intervention in Muleba district, north-western Tanzania, a parasitological baseline survey and two serial cross-sectional follow-up surveys were carried out in four randomly selected villages that were previously affected by the 2006 malaria epidemics and earmarked for IRS intervention, and in two randomly selected villages that were not affected by the 2006 epidemic and were not under the IRS program (control). Intervention villages surveyed were Ijumbi, Nshambya, Bushemba from Nshamba division, and Ikondo from Muleba division, whereas control villages surveyed were Kibanga from Muleba division and Bunywambele from Kamachumu division (Figure 1).
Figure 1.
Map showing location of the selected villages for the parasitological surveys carried out pre- and post-IRS intervention in Muleba district north-western Tanzania.
The parasitological baseline survey was conducted before the start of the IRS intervention in May 2007. Monitoring of the impact of the first IRS intervention was done in December 2007, 6 months post IRS intervention, and the monitoring of the impact of the second IRS intervention was evaluated in August 2008, 6 months post second IRS intervention. This work reports a comparison of malaria parasitological indices during the baseline, and two follow-up surveys post IRS intervention to assess the impact of IRS intervention on malaria parasitological indices.
Materials and Methods
Study area and population.
Muleba district (1°45′ N, 31°40′ E) is north-west of Tanzania with an area of 10,739 km2, of which 62% consists of Lake Victoria. Most parts of the district lie at 1,200–1,500 m above sea level. Administratively, the district has five divisions, namely Izigo, Kamachumu, Kimwani, Muleba, and Nshamba, 31 wards, and 134 registered villages (Figure 1). It has a population of 425,172 people11 with 85,035 (20%) being children < 5 years of age. The district has 36 health facilities, 3 of them being hospitals (Rubya, Kagondo, and Ndolage). Others are health centers (4) and dispensaries (29). Muleba district is characterized by highland villages with unstable malaria transmission prone to malaria epidemics and lowland villages with stable malaria transmission and act as reservoirs for the highland villages. Muleba district experience an equatorial climate with two annual rainy seasons. The short rains between October and December and the long rains between March and May.12,13 The other months are relatively dry with sporadic conventional rains. Malaria transmission intensity in Muleba district follows this seasonal cycle with peaks in malaria incidence occurring 1–2 months following the month of highest rainfall.14 More than 90% of malaria infections in Muleba district are caused by Plasmodium falciparum, whereas Plasmodium malariae and Plasmodium ovale infections makes the rest of the infections.
The IRS intervention in Muleba district was spatially and temporally targeted to villages prone to malaria epidemics to enhance the feasibility and lower cost. The distance from selected IRS households to the nearest non-IRS households was > 10 km. Two rounds of IRS per year were organized, the first IRS round was carried out in May 2007 and the second round was carried out in January–February 2008 before the season of high transmission, which is May–July and November–January.15
Sample size calculations and sampling procedures.
In the parasitological cross-sectional surveys, data were collected for malaria parasitemia, hemoglobin (Hb) levels, and splenomegaly. A malaria prevalence of 50% was assumed to give the largest sample size required for the examination of all events of interest. Using a precision of 0.05 at 95% confidence interval (CI), a sample size of 462 study participants were estimated. This sample was increased by 10% to allow for incomplete information and refusals, thus giving a total of 509 participants. To represent all age groups in the parasitological surveys, members of each village were stratified into three strata of age groups, i.e., < 5 years, 5–14 years, and those ≥ 15 years. Because by design the study involved multi-stage sampling techniques, the sample size was doubled (509 × 2), taking into consideration the design effect of 2, thus giving an overall sample size of 1,018 or 170 study participants per village.
Villages were then stratified into two clusters; namely IRS (intervention) and non-IRS (control) villages. Using a two stage multi-sampling procedures, four IRS and two non-IRS villages were randomly selected. At the second stage of sampling, two sub-villages were randomly selected from each of the selected six villages, making a total of 12 sub-villages (8 IRS and 4 non-IRS sub-villages). Census of the household members from all selected sub-villages were updated by visiting and registering demographic characteristics of all people who slept in the household a previous night before the survey. In each selected sub-village, Village Executive Officers and Village Health Workers were given names of selected participants for the survey. In every survey individuals were randomly selected.
Participants were contacted to explain the purpose of the study, to obtain informed consent, and arrange time, date, and place for the survey. The field team arrived at a prearranged site for malaria parasitological examination.
Malaria parasitological survey.
In every survey, finger prick blood was collected from all consented selected participants for malaria parasites and Hb levels examination. Hemoglobin levels were examined using a digital hemoglobinometer (Hemo-cue EKF-diagnostic GmbH, Magdeburg, Germany). Another drop of blood was used to prepare thick and thin blood smears. Giemsa-stained blood smears were examined microscopically for malaria parasites by an experienced microscopist. If a slide was found to be positive, malaria species were then identified by examining a thin blood smear. Quality control for thick and thin blood smears were carried out by an experienced independent technician re-examining 10% of all slides. The malaria parasite species in positive smears were identified, and parasite densities were recorded as the number of parasites per 200 white blood cells. Parasite densities were converted to the number of parasites per microliter of blood assuming 8,000 white blood cells/μL.
Anemia was defined as Hb levels < 8 g/dL. All study participants were palpated in a horizontal position for the presence of spleen enlargement by same clinical officers during the entire surveys to minimize inter-observer variation. Spleen was classified according to the Hackett scale.16 To represent all age groups in the surveys, selected participants from each sub-village were stratified into three strata of age groups, children < 5 years, children 5–14 years, and adults ≥ 15 years.
Insecticide susceptibility test.
Indoor resting unfed adult female Anopheles funestus mosquitoes were collected using mouth aspirators during the baseline survey. Collected An. funestus were exposed to diagnostic doses of commonly used insecticides for a susceptibility test using insecticide-impregnated papers, as described by the WHO testing protocol.17,18 The following insecticides were tested: Permethrin (0.75%), deltamethrin (0.05%), lambda-cyhalothrin (0.05%), and DDT (4%). Twenty mosquitoes were introduced into each tube. The mosquitoes were exposed to insecticides for 60 minutes and the knockdown time mortality in minutes at 50% and 90% (KTM50 and KTM90) were recorded. Mortalities were recorded after 24 hours and corrected by using the Abbott's formula.17
Quality control of IRS implementation.
Susceptible female Anopheles gambiae s.s. Kisumu strains aged 2–5 days were used for quality control of IRS implementation. After 4 weeks of IRS, five houses were randomly selected from each of the four sprayed villages. The other five unsprayed houses were randomly selected as control from each of the two unsprayed village. The selected houses were of four different sprayed surfaces such as cement, mud, white/painted, and wood surfaces. Contact cone bioassay was conducted according to WHO standards.17 The mosquitoes were exposed to insecticides for 30 minutes and the percentages knockdown after 1 hour (KD60) and 24 hours mortality scores were recorded.
Data analysis.
Data entry was done using Dbase V (Borland International, Scotts Valley, CA). A double entry system was used to ensure quality and consistency of data. The clean Dbase V data files were transferred into STATA format and analyses were done using STATA version 9.0 (STATA Corporation, College Station, TX).
Logistic regression was used to compare proportions of malaria parasitemia, splenomegaly, and anemia at baseline, first and second follow-up surveys between IRS and non-IRS villages. Geometric mean parasite density (GMPD) of positive samples was calculated using the equation GMPD = logn (p × 40 + 1) where n = is the number of positive slides, p = number of parasites. Parasite counts were converted to asexual forms (trophozoites) per microliter of blood assuming an average concentration of 8,000 leukocytes/μL of blood. All statistics were considered to be significant at the level of P ≤ 0.05 CI: 95%.
Ethical consideration.
This study was reviewed and approved by the Medical Research-Coordinating Committee of the National Institute for Medical Research. Permission to conduct the study was obtained from local government officials in Muleba district. Informed consent of the study participants and their children to participate were sought before examination after they had been clearly informed about the study objectives, methodology, anticipated benefits, and discomforts. Where children were involved, consent was obtained from their parents or guardians. All study participants with signs/symptoms of malaria were treated free with artemether lumefantrine (Coartem Novartis, Basel, Switzerland), according to the National Guidelines on Malaria Treatment.19
Results
Parasitemia, splenomegaly, and anemia prevalence.
Table 1 summarizes parasitemia, splenomegaly, and anemia prevalence in randomly surveyed IRS and non-IRS villages at baseline, 6 months post first IRS intervention and 2 years post second IRS intervention. During the baseline survey, a total of 1,161 participants were examined of whom 818 (70.5%) were from IRS villages and 343 (29.5%) from non-IRS villages. In the first follow-up survey, a total of 1,159 participants were examined of whom 754 (65.1%) and 405 (34.9%) participants were from IRS and non-IRS villages, respectively. For the second follow-up survey a total of 1,035 participants were examined of whom 719 (69.5%) and 316 (30.5%) were from IRS and non-IRS villages, respectively (Table 1). Plasmodium falciparum was the most dominant malaria species accounting for (99.1%) of malaria infection in the district. Other species were P. malariae (0.6%) and P. ovale (0.3%). The results were compared with those at baseline and follow-up surveys in both age groups. Two rounds of IRS intervention significantly reduced the malaria parasitological indices in both age groups. In IRS villages the overall parasitemia prevalence was 20.1% (95% CI: 17.3–22.8; N = 818) at baseline was reduced to 6.6% (95% CI: 4.8–8.4; N = 719) at second follow-up survey an overall reduction of 67.2%. Children < 5 years of age had the highest parasitemia reduction from 24.1% (95% CI: 18.2–30.2; N = 194) at baseline to 4.3% (95% CI: 0.6–7.9; N = 117) at second follow-up survey an overall reduction 82.2%. The overall splenomegaly prevalence at baseline was 3.3% (95% CI: 2.0–4.5; N = 789). After two rounds of IRS intervention splenomegaly was reduced to 0.8% (95% CI: 0.1–1.4; N = 719) an overall reduction of 75.8%. Children < 5 years of age had an overall splenomegaly reduction of 100% at second follow-up survey. The overall anemia prevalence at baseline was 31.7% (95% CI: 28.5–34.9; N = 804) was reduced to 15.7% (95% CI: 13.0–18.3; N = 718) at second follow-up survey an overall reduction of 50.5%. Furthermore, children 5–14 years of age had the highest reduction of anemia prevalence from 28.5% (95% CI: 23.1–33.9; N = 270) at baseline to 12.8% (95% CI: 9.1–16.5; N = 312) at second follow-up an overall reduction of 55.1%.
Table 1.
Prevalence of parasitemia, splenomegaly, and anemia in the randomly surveyed IRS and non-IRS villages pre- and post-IRS intervention in Muleba district north-western, Tanzania
| Variable | IRS villages (N = 4) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | First follow-up | Sec. follow-up | Overall | ||||
| May 2007 (95% CI) | Dec 2007 (95% CI) | Aug 2008 (95% CI) | Reduction in % | ||||
| Parasitemia prev in % | |||||||
| Overall prevalence | 20.1 (17.3–22.8) | 9.1 (7.0–11.1) | 6.6 (4.8–8.4) | 67.2 | |||
| (N = 818) | (N = 754) | (N = 719) | |||||
| By age groups | |||||||
| Children < 5 yrs | 24.2 (18.2–30.2) | 4.6 (0.7–8.5) | 4.3 (0.6–7.9) | 82.2 | |||
| (N = 194) | (N = 108) | (N = 117) | |||||
| Children 5–14 yrs | 27.2 (21.9–32.5) | 14.9 (11.1–18.6) | 11.2 (7.7–14.7) | 58.8 | |||
| (N = 273) | (N = 343) | (N = 313) | |||||
| Adults ≥ 15 yrs | 15.7 (11.9–19.5) | 5.9 (3.3–8.6) | 3.1 (1.1–5.1) | 80.3 | |||
| (N = 351) | (N = 303) | (N = 289) | |||||
| Splenomegaly prev in % | |||||||
| Overall prevalence | 3.3 (2.0–4.5) | 2.9 (1.7–4.1) | 0.8 (0.1–1.4) | 75.8 | |||
| (N = 789) | (N = 754) | (N = 719) | |||||
| Children < 5 yrs | 3.6 (0.1–6.2) | 0.9 (0.9–2.7) | 0.0 (−) | 100 | |||
| (N = 193) | (N = 108) | (N = 117) | |||||
| Children 5–14 yrs | 3.4 (1.2–5.6) | 3.5 (1.6–5.6) | 1.3 (0.04–2.6) | 61.8 | |||
| (N = 267) | (N = 343) | (N = 313) | |||||
| Adults ≥ 15 yrs | 3.0 (1.2–4.8) | 3.0 (1.1–4.9) | 0.7 (−0.2–1.7) | 76.7 | |||
| (N = 329) | (N = 303) | (N = 289) | |||||
| Anemia prev in % | |||||||
| Overall prevalence | 31.7 (28.5–34.9) | 8.9 (6.9–10.3) | 15.7 (13.0–18.3) | 50.5 | |||
| (N = 804) | (N = 753) | (N = 718) | |||||
| Children < 5 yrs | 59.0 (52.1–65.9) | 25.0 (16.3–33.2) | 37.5 (28.7–46.3) | 36.4 | |||
| (N = 195) | (N = 108) | (N = 117) | |||||
| Children 5–14 yrs | 28.5 (23.1–33.9) | 7.9 (5.0–10.7) | 12.8 (9.1–16.5) | 55.1 | |||
| (N = 270) | (N = 343) | (N = 312) | |||||
| Adults ≥ 15 yrs | 18.5 (14.5–22.6) | 4.0 (1.8–6.2) | 9.7 (6.3–13.1) | 47.6 | |||
| (N = 339) | (N = 302) | (N = 289) | |||||
| Non-IRS villages (N = 2) | |||||||
| Parasitemia prev in % | |||||||
| Overall prevalence | 34.8 (29.8–39.8) | 16.3 (12.7–19.9) | 9.3 (6.1–12.3) | 73.3 | |||
| (N = 348) | (N = 405) | (N = 316) | |||||
| By age groups | |||||||
| Children < 5 yrs | 38.5 (27.7–49.2) | 24.3 (14.2–34.3) | 9.1 (0.7–18.9) | 76.4 | |||
| (N = 78) | (N = 70) | (N = 33) | |||||
| Children 5–14 yrs | 46.9 (37.7–56.1) | 24.7 (18.1–31.3) | 11.2 (6.6–15.8) | 76.1 | |||
| (N = 113) | (N = 166) | (N = 179) | |||||
| Adults ≥ 15 yrs | 24.2 (17.5–30.9) | 4.7 (1.5–7.9) | 6.7 (N = 1.9–11.5) | 72.3 | |||
| (N = 157) | (N = 169) | (N = 104) | |||||
| Splenomegaly prev in % | |||||||
| Overall prevalence | 11.6 (8.2–15.0) | 10.7 (7.7–13.8) | 0.9 (−0.1–1.9) | 92.2 | |||
| (N = 337) | (N = 405) | (N = 316) | |||||
| Children < 5 yrs | 9.0 (2.6–15.3) | 17.1 (8.3–26.0) | 0.0 (−) | 100 | |||
| (N = 78) | (N = 70) | (N = 33) | |||||
| Children 5–14 yrs | 20.4 (12.8–28.0) | 9.6 (5.1–14.1) | 1.7 (0.2–3.5) | 77.3 | |||
| (N = 108) | (N = 166) | (N = 179) | |||||
| Adults ≥ 15 yrs | 6.6 (2.6–10.6) | 9.5 (5.1–13.9) | 0.0 (−) | 100 | |||
| (N = 151) | (N = 169) | (N = 104) | |||||
| Anemia prev in % | |||||||
| Overall prevalence | 38.2 (32.7–43.7) | 19.3 (15.4–23.1) | 9.8 (6.5–13.1) | 74.3 | |||
| (N = 303) | (N = 405) | (N = 316) | |||||
| Children < 5 yrs | 73.1 (63.3–82.9) | 53.7 (41.9–65.3) | 30.3 (14.6–46.0) | 58.6 | |||
| (N = 78) | (N = 70) | (N = 33) | |||||
| Children 5–14 yrs | 40.9 (31.7–50.1) | 15.1 (9.6–20.5) | 11.2 (6.6–15.8) | 72.6 | |||
| (N = 110) | (N = 166) | (N = 179) | |||||
| Adults ≥ 15 yrs | 18.7 (11.6–25.8) | 8.9 (4.6–13.2) | 1.0 (−0.9–2.9) | 94.7 | |||
| (N = 115) | (N = 169) | (N = 104) | |||||
In non-IRS villages the results were also compared with those at baseline and follow-up surveys in both age groups. Overall parasitemia prevalence in non-IRS villages was 34.8% (95% CI: 29.8–39.8; N = 348) at baseline was reduced to 9.3% (95% CI: 6.1–12.3; N = 316) at second follow-up survey an overall reduction of 73.3%. Children < 5 years had the highest parasitemia reduction of 76.4%, which was from 38.5% (95% CI: 27.7–49.2 N = 78) at baseline to 9.1% (95% CI: 0.7–18.9; N = 33) at second follow-up survey. The overall splenomegaly prevalence in non-IRS villages was 11.6% (95% CI: 8.2–15.0; N = 337) at baseline was reduced to 0.9% (95% CI: −0.1–1.9; N = 316) at second follow-up survey an overall reduction of 92.2%. Children < 5 years of age and adults ≥ 15 years of age had an overall splenomegaly reduction of 100% at second follow-up survey. For anemia in non-IRS villages overall prevalence was 38.2% (95% CI: 32.7–43.7; N = 303) at baseline was reduced to 9.8% (95% CI: 6.5–13.1; N = 316) at second follow-up survey an overall reduction of 74.3%. At baseline children < 5 years of age had a higher anemia prevalence of 73.1% (95% CI: 63.3–82.9; N = 78) when compared with other age groups. Adults ≥ 15 years of age had anemia prevalence of 18.7% (95% CI: 11.6–25.8; N = 115) at baseline and was reduced to 1.0% (95% CI: −0.9–2.9; N = 104) at second follow-up survey an overall reduction of 94.7% (Table 1). Significant reduction of the prevalence of malaria parasitological indices were shown in both IRS and non-IRS villages. A more important level of malaria parasitological indices prevalence across age groups post IRS intervention in both IRS and non-IRS villages remained far below the original levels pre-IRS intervention. However, participants from non-IRS villages showed slightly higher prevalence of malaria parasitological indices than their counterparts in IRS villages.
Table 2 shows age distribution of parasitemia in IRS and non-IRS villages. Malaria parasitemia prevalence varied significantly across age groups. The results show that there was a significant reduction of parasitemia prevalence in all age groups during the first follow-up survey in IRS villages compared with non-IRS villages. During the baseline survey parasitemia prevalence in children < 5 years of age was 24.3% and 38.5% (P = 0.02) in IRS and non-IRS villages, respectively (Table 2). In children 5–14 years of age prevalence was 22.7% and 46.9% (P < 0.0001) in IRS and non-IRS villages, respectively, whereas for adults ≥ 15 years of age it was recorded as 15.7% and 24.2% (P = 0.02) in IRS and non-IRS villages, respectively. In the first follow-up survey malaria parasitemia prevalence in children < 5 years of age was 4.6% and 24.3% (P = 0.0004) in IRS and non-IRS villages, respectively, in children 5–14 years of age was 14.9% and 24.7% (P = 0.007) in IRS and non-IRS villages, respectively. Although for the second follow-up survey malaria parasitemia prevalence did not statistically differ across age groups between IRS and non-IRS villages (Table 2). Children 5–14 years of age had consistently higher malaria parasitemia prevalence than other age groups in all cross sectional surveys.
Table 2.
Age distributions of prevalence of parasitemia observed by random surveys in IRS and non-IRS villages pre- and post-IRS intervention in Muleba district
| Surveys | Children < 5 years | Children 5–14 years | Adults ≥ 15 years | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Prevalence (N) | OR (95% CI) | P = value | Prevalence (N) | OR (95% CI) | P = value | Prevalence (N) | OR (95% CI) | P = value | |
| Baseline | |||||||||
| IRS villages | 24.3% (194) | 2.0 | 0.02* | 22.7% (273) | 3.0 | < 0.0001* | 15.7% (351) | 1.7 | 0.02* |
| Non-IRS villages | 38.5% (78) | 46.9% (113) | 24.2% (157) | ||||||
| 1st follow-up | |||||||||
| IRS villages | 4.6% (108) | 6.6 | 0.0004* | 14.9% (343) | 1.9 | 0.007* | 5.9% (303) | 0.8 | 0.58 |
| Non-IRS villages | 24.3% (70) | 24.7% (166) | 4.7% (169) | ||||||
| 2nd follow-up | |||||||||
| IRS villages | 4.3% (117) | 2.2 | 0.29 | 11.2% (313) | 1.0 | 1 | 3.1% (289) | 2.2 | 0.11 |
| Non-IRS villages | 9.1% (33) | 11.2% (179) | 6.7% (104) | ||||||
Statistically significant.
OR = odds ratio; CI = confidence interval.
Malaria parasite density.
The GMPD as a function of age groups was examined. Children < 5 years and children 5–14 years of age had higher malaria parasite density in both IRS and non-IRS villages pre-IRS intervention (Table 3). The GMPD in children < 5 years of age at baseline survey was 846.3 (95% CI: 498.1–1437.9) and 1229.1 (95% CI: 683.5–2210.2) in IRS and non-IRS villages, respectively. However, the observed malaria parasite density difference in children < 5 years of age between IRS and non-IRS villages was not statistically significant with t test = 0.20 (P = 0.84). During the first follow-up survey geometric mean parasite density in children < 5 years of age was reduced to 64.5 (95% CI: 2.5–1633.3) in IRS villages but remained consistently higher in non-IRS villages at 682.4 (95% CI: 265.4–1754.6). The observed malaria parasite density difference in children < 5 years of age between IRS and non-IRS villages was not statistically significant with t test = 1.18 (P = 0.25) (Table 3). During the second follow-up survey children 5–14 years of age in IRS villages had higher malaria parasite density than other age groups 136.9 (95 CI: 90.4–207.0), whereas in non-IRS villages children < 5 years of age had higher malaria parasite density than other age groups 805.6 (95% CI: 5.1–125400.7). Adults ≥ 15 years of age had the lowest malaria parasite density than other age groups in both IRS and non-IRS villages at baseline and the subsequent follow-up surveys.
Table 3.
Geometric mean parasite density of malaria infection among infected participants in IRS and non-IRS villages pre- and post-IRS intervention in Muleba district north-western Tanzania
| Age group (years) | IRS villages (N = 4) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline May 2007 | First follow-up December 2007 | Second follow-up August 2008 | |||||||
| Number | GM | 95% CI | Number | GM | 95% CI | Number | GM | 95% CI | |
| Children < 5 | 47 | 846.3 | (498.1–1437.9) | 5 | 64.5 | (2.5–1633.3) | 5 | 131.2 | (43.1–399.0) |
| Children 5–14 | 62 | 973.1 | (605.1–1564.7) | 51 | 100.9 | (56.2–181.1) | 35 | 136.8 | (90.4–207.0) |
| Adults ≥ 15 | 55 | 858.5 | (560.6–1314.6) | 18 | 21.4 | (10.5–43.5) | 9 | 100.8 | (38.6–262.9) |
| Total | 164 | 896.4 | (684.6–1173.8) | 74 | 67.2 | (41.7–108.2) | 49 | 128.8 | (92.0–180.3) |
| Non-IRS villages (N = 2) | |||||||||
| Children < 5 | 30 | 1229.1 | (683.5–2210.2) | 17 | 682.4 | (265.4–1754.6) | 3 | 805.7 | (5.2–125400.7) |
| Children 5–14 | 53 | 686.1 | (463.9–1014.5) | 41 | 179.5 | (89.3–360.8) | 20 | 429.7 | (244–754.2) |
| Adults ≥ 15 | 38 | 256.9 | (187.5–352.0) | 8 | 41.3 | (6.9–248.5) | 7 | 245.6 | (118.6–508.4) |
| Total | 121 | 582.3 | (448.2–756.6) | 66 | 211.9 | (122.0–368.0) | 30 | 401.6 | (255.3–631.6) |
Resistance status.
A total of 320 adult female An. funestus were tested for susceptibility to four commonly used insecticides, permethrin, deltamethrin, lambda-cyhalothrin, and DDT. After the exposure of adult female An. funestus to the WHO impregnated papers, all tested female An. funestus were fully susceptible to all four insecticides. Susceptibility for permethrin at KTM 50 and 90 were 19.3 (95% CI: 17.7–20.9; N = 80) and 28.97 (95% CI: 26.01–33.7; N = 80), respectively, deltamethrin at KTM 50 and 90 were 17.98 (95% CI: 15.06–21.01; N = 80) and 29.5 (95% CI: 24.6–41.1; N = 80), respectively, lambda-cyhalothrin at KTM 50 and 90 were 28.6 (95% CI: 24.3–33.8; N = 80) and 46.9 (95% CI: 39.1–65.3; N = 80), respectively, whereas DDT at KTM50 and 90 were 73.1 (95% CI: 70.2–76.8; N = 80) and 113.8 (95% CI: 104.6–126.9; N = 80) respectively. Mortality scores after 24 hours of exposure were 100% to all four insecticides (Table 4).
Table 4.
Susceptibility test of An. funestus to selected insecticides collected in Muleba district during baseline survey (N = 320)*
| Insecticide | No. tested | KTM 50 (95% CI) | KTM 90 (95% CI) | % 24 hours corrected mortality |
|---|---|---|---|---|
| Permethrin 0.75% | 80 | 19.3 (17.7–20.9) | 28.97 (26.01–33.7) | 100 |
| Deltamethrin 0.05% | 80 | 17.98 (15.06–21.01) | 29.5 (24.6–41.1) | 100 |
| Lambda-cyhalothrin 0.05% | 80 | 28.6 (24.3–33.8) | 46.9 (39.1–65.3) | 100 |
| DDT 4% | 80 | 73.1 (70.2–76.8) | 113.8 (104.6–126.9) | 100 |
KTM = knockdown time mortality; CI = confidence interval.
Contact cone bioassay.
The percentage KD60 and 24 hours mortality scores were above 95% and 80%, respectively, in all tested treated surfaces using a known susceptible strain of An. gambiae s.s. (results not shown). These results indicate that IRS was properly done in different treated surfaces in the district.
Discussion
Indoor residual spraying intervention contributed to reducing the prevalence of parasitemia, splenomegaly, and anemia in IRS villages and kept the prevalence low. After two rounds of IRS intervention prevalence of malaria parasitological indices across age groups was still lower than at baseline and more importantly malaria parasite density also decreased across age groups after two rounds of IRS intervention.
The results of malaria parasitological baseline survey and first cross-sectional follow-up survey 6 months post first IRS intervention showed that the overall malaria parasitemia prevalence in IRS villages was reduced by 2-fold, in the same period there was also a reduction of malaria parasitemia prevalence in non-IRS villages by 2-fold. However, malaria parasitemia prevalence in non-IRS villages remained slightly higher than IRS villages. This suggests that individuals living in IRS villages had greater protection against malaria than non-IRS individuals. The IRS intervention with lambda-cyhalothrin has already been shown to have effectively lowered the malaria parasitemia and anemia prevalence and achieved a rapid reduction of malaria prevalence in Muleba district. Recently, similar success was shown in Zanzibar Archipelago, with the IRS using lambda-cyhalothrin.20,21 Successful use of IRS against malaria vectors has also been reported from Sri Lanka.22 Our results are in accordance with these studies, suggesting that targeted IRS intervention as implemented in Muleba district is highly cost-effective in reducing malaria infections in a malaria epidemic-prone district. Presently, IRS with lambda-cyhalothrin is still considered as one of the most effective intervention measure of malaria in epidemic prone rural areas.23 Malaria vectors control with IRS of lambda-cyhalothrin is the first large-scale intervention in mainland Tanzania in recent years, and the initial malaria parasitological results are encouraging and are consistent with other IRS interventions in sub-Saharan countries.24
During the first cross-sectional follow-up survey the observed overall parasitemia prevalence reduction between IRS and non-IRS villages was statistically significant. However, during the second cross-sectional follow-up survey the observed overall parasitemia prevalence reduction between IRS and non-IRS villages was not statistically significant, though parasitemia prevalence remained slightly higher in non-IRS villages than IRS villages. The low prevalence of malaria parasitological indices in non-IRS villages might have been contributed by other interventions introduced to the district after the 2006 malaria epidemic. The interventions likely to have contributed to lower malaria parasitological indices prevalence in non-IRS villages during the second follow-up survey might be the distribution of LLINs in the district after the 2006 malaria epidemic25 and the introduction of artemisinin-based combination therapy (ACT) as the first-line treatment of uncomplicated malaria after the 2006 malaria epidemic in the district.26–28 These two important interventions LLINs and ACT should therefore be considered when implementing integrated malaria control programs in malaria epidemic-prone areas.
Over the three malaria parasitological cross-sectional surveys conducted, parasitemia prevalence was higher among children 5–14 years of age in both IRS and non-IRS villages than other age groups. The finding of this study corroborates with the finding of Odongo-Aginya and others,29 which observed that the prevalence of malaria parasitemia and malaria parasite density decreases with the increasing of age or age group. The higher peaks of parasitemia prevalence observed in children 5–14 years of age might be caused by a less developed immune system, which is incapable of clearing parasite more effectively compared with adults ≥ 15 years of age.30 A study conducted in Tanzania by Imperato31 also reported a higher prevalence of parasitemia among the youngest age groups and lowest in those 35 years of age and older.
The age groups distribution of malaria intensity of infections provides suggestive information about the level of naturally acquired immunity to malaria and indirectly, about the long-term intensity and stability of malaria transmission.32 Malaria parasite density among infected individuals was higher in children < 5 years of age in non-IRS villages. However, in IRS-villages malaria parasite density was higher in children 5–14 years of age pre- and post-IRS intervention. Adults ≥ 15 years of age had the lowest malaria parasite density when compared with other age groups in both IRS and non-IRS villages pre- and post-IRS intervention. In general, the higher the malaria transmission intensity the more rapidly immunity is acquired and the younger the peak age groups for malaria parasite density.33 In these cross-sectional surveys malaria parasitemia prevalence was higher in children < 5 years and children 5–14 years of age than adults ≥ 15 years of age in both IRS and non-IRS villages, and in non-IRS villages children < 5 years of age had consistently higher malaria parasite density, whereas in IRS villages children 5–14 years of age had higher malaria parasite density than adults ≥ 15 years of age pre-IRS intervention. After IRS intervention malaria parasite density was significantly reduced in IRS villages in all age groups, however remained slightly higher in children < 5 years of age in non-IRS villages.
The parasitemia prevalence was higher in children < 5 years and children 5–14 years of age and decreased in adults ≥ 15 years of age. The proportion of anemia in each age group mirrored the malaria parasite density in non-IRS villages, suggesting that high malaria parasite density and young age groups may predispose children to anemia and those children in non-IRS villages are most at risk of developing anemia and spleen enlargement, a symptom and sign of prolonged exposure to malaria infection. The higher the parasitemia prevalence in children aged 5–14 years is therefore more likely to be related to a difference in infection risk or health-seeking behavior. This is in agreement with the observations of Snow and others,33 and Modiano and others,34 who found that malaria in high transmission areas affects much younger children compared with adults and those children are vulnerable to malaria infection and are therefore at risk of developing severe anemia. A higher prevalence of severe anemia in young age groups has also been reported by a study conducted in Kenya by Akhwale and others.35
The proportion of individuals with anemia and enlarged spleens was similarly high in non-IRS villages compared with IRS villages. There was a paradoxical relationship between spleen enlargement and malaria parasitemia prevalence. Malaria prevalence was higher in non-IRS than IRS villages before IRS intervention; however, those villages did not experience malaria epidemics indicating that malaria transmission in non-IRS villages was stable.15 The baseline data confirmed the significant malaria burden in children in non-IRS villages. Children in non-IRS villages had therefore continuous exposure to malaria infection and quickly might have developed protective immunity against severe malaria, therefore during subsequent malaria episodes they suffer less severe forms or uncomplicated malaria.33,36 The low reduction seen on spleen enlargement prevalence after the first cross-sectional follow-up survey in IRS villages could be because the first follow-up survey was conducted only 6 months after the first round of IRS intervention, which could be too short an interval to show significant change on morbidity. In contrast, during the second cross-sectional follow-up survey there was a 4-fold and a 12-fold reduction of spleen enlargement in IRS and non-IRS villages, respectively. This means that prolonged exposure to malaria infection in both villages has been significantly reduced because of the IRS and other interventions introduced to the district after the 2006 malaria epidemic.
Malaria epidemics are a serious and increasing public health problem in the population living in malaria unstable areas in Tanzania. To protect that population and the economy from the potentially devastating effect of malaria epidemics, a concerted effort is needed. As a result of the experience from Zanzibar Archipelago,20 reintroduction of vector control by IRS of lambda-cyhalothrin should be scaled up to all epidemic prone districts of mainland Tanzania. Indoor residual spraying is likely to be more effective in reducing malaria prevalence in a very unstable transmission setting as shown in the highlands areas of Kenya37 and Tanzania.38 In the context of vector control, identifying and targeting malaria vector hot spots is very crucial when implementing IRS programs. With the current effort to scale-up malaria control in Africa, IRS has been introduced into a number of countries that initially had high levels of malaria transmission.8,24,39 A recent review of the evidence of cost and consequences of large-scale vector control for malaria40 concluded that both IRS and ITNs are highly cost-effective vector control strategies.9 Similar to our study, malaria vector controls based on IRS of lambda-cyhalothrin have been shown to be effective tools in reducing malaria transmission to a very low levels in the epidemics prone areas.30,37 Malaria transmission in Africa prone to epidemics is usually focal41 and mosquito breeding sites are usually more common in the lowlands as seen in the highlands of Kenya,42 Tanzania,43 Rwanda, and Burundi.24 To improve the cost-effectiveness of such methods, IRS intervention should be targeted to malaria high risk areas prone to epidemics in a resources-limited setting.
Our study has limitations, we did not conduct surveys on ITNs/LLINs coverage and ACTs access in the district. After the 2006 malaria epidemics there were ITNs/LLINs distribution campaigns in the district. Furthermore, the Tanzania government introduced ACTs as the first-line treatment of uncomplicated malaria. These two combined interventions might have played important role in malaria reduction in both IRS and non-IRS villages. The survey on ITNs/LLINs ownership and usage would have provided additional information about whether the effects of IRS and ITNs/LLINs act synergistically in the intervention villages. Without information on ITNs/LLINs coverage and ACTs access it is not possible to draw a firm conclusion that reduction of parasitological indices in IRS villages was only because of IRS intervention. However, the results show that individuals living in IRS villages have an additional benefit of IRS intervention because malaria parasitological indices in IRS villages remain significantly lower than non-IRS villages.
A susceptibility test of adult female An. funestus to the four insecticides was conducted during the baseline survey in the district. The mosquitoes used for the susceptibility test were unfed female An. funestus species because of the major malaria vector species collected in the district. Moreover, An. funestus has been implicated as a major malaria vector in sub-Saharan Africa where pyrethroid insecticides are widely used in agriculture and public health.44 All adult female An. funestus species tested were fully susceptible to all four insecticides. Elsewhere in Africa there have been reports of emergence of resistances to some of the tested insecticides.45 Although information was not sought on the use of insecticides for agricultural purposes in the district, we suspect that the higher susceptibility of An. funestus species in the district is linked to lesser use of insecticides for agricultural and other economic activities. Furthermore, the higher susceptibility of the An. funestus to all four insecticides commonly used in Tanzania suggest that malaria vector control by IRS of lambda-cyhalothrin is feasible, effective, and may be an appropriate malaria intervention strategy for the control of future malaria epidemics in the district.
This study further suggests that monitoring and evaluation is a crucial component of any large-scale IRS intervention program to evaluate its effectiveness. The effectiveness of the IRS of lambda-cyhalothrin program in Muleba district is shown by the significant reduction of malaria parasitological indices post IRS intervention in IRS villages and remains far below the original levels before IRS intervention. Findings supported the significant reduction of parasite density in IRS villages across age groups after the first round of IRS intervention, whereas in non-IRS villages parasite density remained consistently higher in children < 5 years of age. This observation also shows that children < 5 years of age in IRS villages have additional benefits by IRS intervention as opposed to their counterparts in non-IRS villages.
ACKNOWLEDGMENTS
We thank the villagers of Muleba district for their participation in this study. We also thank the logistic support provided to the study by the National Institute for Medical Research, Mwanza Center. Last but not least, we express our sincere gratitude to the Muleba District Medical Officer, the Ward and Village Executive Officers of the selected sites for their field assistance.
Footnotes
Financial support: This study was supported by the U.S. President's Malaria Initiative through Research Triangle Institute - International and the Ministry of Health and Social Welfare, of the United Republic of Tanzania. The sponsor had no role in the study design, data collection, data analysis, data interpretation, or writing the manuscript.
Authors' addresses: Fabian M. Mashauri, Safari M. Kinung'hi, Godfrey M. Kaatano, Coleman Kishamawe, Joseph R. Mwanga, Soori E. Nnko, and Chacha N. Mero, National Institute for Medical Research, Mwanza Research Centre, Mwanza, Tanzania, E-mails: mashauri@hotmail.com, kinunghi_csm@hotmail.com, gmkaatano@yahoo.com, kishamawe@yahoo.com, jrmwanga@yahoo.co.uk, soseremi@yahoo.com, and meroncn@yahoo.com. Stephen M. Magesa, Research Triangle International, Malaria Initiative, Nairobi, Kenya, E-mail: smagesa@hotmail.com. Robert C. Malima, National Institute for Medical Research, Amani Research Centre, Tanzania, E-mail: r_malima@hotmail.com. Leonard E. G. Mboera, National Institute for Medical Research, Headquarters, Dar es Salaam, Tanzania, E-mail: lmboera@nimr.or.tz.
References
- 1.Kitua AY. Malaria control in the context of integrated management of childhood illness in Tanzania: the challenges ahead. Tanzan Health Res Bull. 2003;5:1–4. [Google Scholar]
- 2.Mboera LE, Kitua AY. Malaria epidemics in Tanzania: an overview. Afr J Health Sci. 2001;8:14–18. [PubMed] [Google Scholar]
- 3.Mboera LE. Environmental and socio-economic determinants of malaria epidemics in the highlands of Tanzania. Tanzan Health Res Bull. 2004;6:11–17. [Google Scholar]
- 4.Maegga BT, Kalinga AK, Chacha SW, Kibona M, Mwayawale J, Jangson K. Malaria in Bulambya, Ileje district, south-west Tanzania. Tanzan Health Res Bull. 2006;8:17–21. doi: 10.4314/thrb.v8i1.14265. [DOI] [PubMed] [Google Scholar]
- 5.Clyde DF. Malaria in Tanzania. London, UK: Oxford University Press; 1967. [Google Scholar]
- 6.Mboera LE, Fanello CI, Malima RC, Talbert A, Fogliati P, Bobbio F, Molteni F. Comparison of the Paracheck-Pf tests to microscopy for confirmation of Plasmodium falciparum malaria in Tanzania. Ann Trop Med Parasitol. 2006;100:115–122. doi: 10.1179/136485906X78571. [DOI] [PubMed] [Google Scholar]
- 7.Garay J. Epidemiological Survey and Situation Analysis: Malaria Epidemic in Nshamba Division, Muleba District, Tanzania. Spain: Medecins sans Frontieres (MSF); 1998. [Google Scholar]
- 8.Kleinschmidt I, Sharp B, Benavente LE, Schwabe C, Torrez M, Kuklinski J, Morris N, Raman J, Carter J. Reduction in infection with Plasmodium falciparum one year after the introduction of malaria control interventions on Bioko Island, Equatorial Guinea. Am J Trop Med Hyg. 2006;74:972–978. [PubMed] [Google Scholar]
- 9.Kleinschmidt I, Schwabe C, Shiva M, Segura JL, Sima V, Mabunda SJ, Coleman M. Combining indoor residual spraying and insecticide-treated net interventions. Am J Trop Med Hyg. 2009;81:519–524. [PMC free article] [PubMed] [Google Scholar]
- 10.Hamel MJ, Otieno P, Bayoh N, Kariuki S, Were V, Marwanga D, Laserson KF, Williamson J, Slutsker L, Gimnig J. The combination of indoor residual spraying and insecticide-treated nets provides added protection against malaria compared with insecticide-treated nets alone. Am J Trop Med Hyg. 2011;85:1080–1086. doi: 10.4269/ajtmh.2011.10-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.URT . 2002 Population and Housing Census General Report. Dar es Salaam, Tanzania: Government Printers; 2003. Central Census Office National Bureau of Statistics, President's Office, Planning and Privatization. [Google Scholar]
- 12.Oesterholt MJ, Bousema JT, Mwerinde OK, Harris C, Lushino P, Masokoto A, Mwerinde H, Mosha FW, Drakeley CJ. Spatial and temporal variation in malaria transmission in a low endemicity area in northern Tanzania. Malar J. 2006;5:98. doi: 10.1186/1475-2875-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pohl B, Camberlin P. Influence of the Madden-Julian oscillation on east African rainfall. I: intraseasonal variability and regional dependency. Q J R Meteorol Soc. 2006;132:2521–2539. [Google Scholar]
- 14.Wort UU, Hastings IM, Carlstedt A, Mutabingwa TK, Brabin BJ. Impact of El Nino and malaria on birthweight in two areas of Tanzania with different malaria transmission patterns. Int J Epidemiol. 2004;33:1311–1319. doi: 10.1093/ije/dyh256. [DOI] [PubMed] [Google Scholar]
- 15.Jones AE, Wort UU, Morse AP, Hastings IM, Gagnon AS. Climate prediction of El Niño malaria epidemics in north-west Tanzania. Malar J. 2007;6:162. doi: 10.1186/1475-2875-6-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilles H. Bruce-Chwantt's Essential Malariology. Boston: Little, Brown and Co; 1993. Epidemiology of malaria. [Google Scholar]
- 17.WHO . Manual on Practical Entomology in Malaria, Part II. Methods and Technique. Geneva: World Health Organization; 1975. p. 191. WHO Offset Publications No. 13. [Google Scholar]
- 18.Service MW. Mosquito Ecology: Field Sampling Methods. London, UK: Applied Science Publishers Ltd; 1976. [Google Scholar]
- 19.Ministry of Health . Summary Report of the Task Force on Antimalarial Drug Policy. United Republic of Tanzania: Ministry of Health; 1999. [Google Scholar]
- 20.IRIN, Tanzania Zanzibar Winning Its Fight against Malaria. 2006. http://www.irinnews.org/report.aspx?reported=61287 Available at. Accessed August 17, 2011.
- 21.Jaenisch T, Sullivan DJ, Dutta A, Deb S, Ramsan M, Othman MK, Gaczkowski R, Tielsch J, Sazawal S. Malaria incidence and prevalence on Pemba Island before the onset of the successful control intervention on the Zanzibar Archipelago. Malar J. 2010;9:32. doi: 10.1186/1475-2875-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yapabandara AM, Curtis CF, Wickramasinghe MB, Fernando WP. Control of malaria vectors with the insect growth regulator pyriproxyfen in a gem-mining area in Sri Lanka. Acta Trop. 2001;80:265–276. doi: 10.1016/s0001-706x(01)00178-4. [DOI] [PubMed] [Google Scholar]
- 23.Mabaso ML, Sharp B, Lengeler C. Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying. Trop Med Int Health. 2004;9:846–856. doi: 10.1111/j.1365-3156.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 24.Protopopoff N, Van Bortel W, Marcotty T, Van Herp M, Maes P, Baza D, D'Alessandro U, Coosemans M. Spatial targeted vector control in the highlands of Burundi and its impact on malaria transmission. Malar J. 2007;6:158. doi: 10.1186/1475-2875-6-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widmar M, Nagel CJ, Ho DY, Benziger PW, Hennig N. Determining and addressing obstacles to the effective use of long-lasting insecticide-impregnated nets in rural Tanzania. Malar J. 2009;8:315. doi: 10.1186/1475-2875-8-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Njau JD, Goodman CA, Kachur SP, Mulligan J, Munkondya JS, McHomvu N, Abdulla S, Bloland P, Mills A. The costs of introducing artemisinin-based combination therapy: evidence from district-wide implementation in rural Tanzania. Malar J. 2008;7:4. doi: 10.1186/1475-2875-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO/RBM . Changing Malaria Treatment Policy to Artemisinin-Based Combinations: An implementation Guide. Arlington, VA: WHO/RBM; 2005. [Google Scholar]
- 28.Kabanywanyi AM, Mwita A, Sumari D, Mandike R, Mugittu K, Abdulla S. Efficacy and safety of artemisinin-based antimalarial in the treatment of uncomplicated malaria in children in southern Tanzania. Malar J. 2007;6:146. doi: 10.1186/1475-2875-6-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odongo-Aginya E, Ssegwanyi G, Kategere P, Vuzi PC. Relationship between malaria infection intensity and rainfall pattern in Entebbe peninsula, Uganda. Afr Health Sci. 2005;5:238–245. doi: 10.5555/afhs.2005.5.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jambou R, Ranaivo L, Raharimalala L, Randrianaivo J, Rakotomanana F, Modiano D, Pietra V, Boisier P, Rabarijaona L, Rabe T, Raveloson N, De Giorgi F. Malaria in the highlands of Madagascar after five years of indoor house spraying of DDT. Trans R Soc Trop Med Hyg. 2001;95:14–18. doi: 10.1016/s0035-9203(01)90317-7. [DOI] [PubMed] [Google Scholar]
- 31.Imperato PJ. Malaria parasitemia in healthy Africans in North Mara, Tanzania. J Community Health. 1986;11:92–97. doi: 10.1007/BF01321510. [DOI] [PubMed] [Google Scholar]
- 32.Syafruddin D, Krisin K, Asih P, Sekartuti S, Dewi RM, Coutrier F, Rozy IE, Susanti AI, Elyazar IR, Sutamihardja A, Rahmat A, Kinzer M, Rogers WO. Seasonal prevalence of malaria in West Sumba district, Indonesia. Malar J. 2009;8:8. doi: 10.1186/1475-2875-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snow RW, Marsh K. New insights into the epidemiology of malaria relevant for disease control. Br Med Bull. 1998;54:293–309. doi: 10.1093/oxfordjournals.bmb.a011689. [DOI] [PubMed] [Google Scholar]
- 34.Modiano D, Chiucchiuini A, Petrarca V, Sirima BS, Luoni G, Roggero MA, Corradin G, Coluzzi M, Esposito F. Interethnic differences in the humoral response to non-repetitive regions of the Plasmodium falciparum circumsporozoite protein. Am J Trop Med Hyg. 1999;61:663–667. doi: 10.4269/ajtmh.1999.61.663. [DOI] [PubMed] [Google Scholar]
- 35.Akhwale WS, Lum JK, Kaneko A, Eto H, Obonyo C, Bjorkman A, Kobayakawa T. Anemia and malaria at different altitudes in the western highlands of Kenya. Acta Trop. 2004;91:167–175. doi: 10.1016/j.actatropica.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med. 1999;5:340–343. doi: 10.1038/6560. [DOI] [PubMed] [Google Scholar]
- 37.Guyatt HL, Corlett SK, Robinson TP, Ochola SA, Snow RW. Malaria prevention in highland Kenya: indoor residual house-spraying vs. insecticides-treated bednets. Trop Med Int Health. 2002;7:298–303. doi: 10.1046/j.1365-3156.2002.00874.x. [DOI] [PubMed] [Google Scholar]
- 38.Maxwell CA, Chambo W, Mwaimu M, Magogo F, Carneiro IA, Curtis CF. Variation of malaria transmission and morbidity with altitude in Tanzania and with introduction of alphacypermethrin treated nets. Malar J. 2003;2:28. doi: 10.1186/1475-2875-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolaczinski K, Kolaczinski J, Kilian A, Meek S. Extension of indoor residual spraying for malaria control into high transmission settings in Africa. Trans R Soc Trop Med Hyg. 2007;101:852–853. doi: 10.1016/j.trstmh.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Yukich JO, Lengeler C, Tediosi F, Brown N, Mulligan JA, Chavasse D, Stevens W, Justino J, Conteh L, Maharaj R, Erskine M, Mueller DH, Wiseman V, Ghebremeskel T, Zerom M, Goodman C, McGuire D, Urrutia JM, Sakho F, Hanson K, Sharp B. Costs and consequences of large-scale vector control for malaria. Malar J. 2008;7:258. doi: 10.1186/1475-2875-7-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ernst KC, Adoka SO, Kowuor DO, Wilson ML, John CC. Malaria hotspot areas in a highland Kenya site are consistent in epidemic and non-epidemic years and are associated with ecological factors. Malar J. 2006;5:78. doi: 10.1186/1475-2875-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Githeko AK, Ayisi JM, Odada PK, Atieli FK, Ndenga BA, Githure JI, Yan G. Topography and malaria transmission heterogeneity in western Kenya highlands: prospects for focal vector control. Malar J. 2006;5:107. doi: 10.1186/1475-2875-5-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith A. Effect of residual house spraying in the plains of anopheline densities in huts on the Pare Mountains. Nature. 1959;183:198–199. doi: 10.1038/183198a0. [DOI] [PubMed] [Google Scholar]
- 44.Vezenegho SB, Brooke BD, Hunt RH, Coetzee M, Koekemoer LL. Malaria vector composition and insecticide susceptibility status in Guinea Conakry, West Africa. Med Vet Entomol. 2009;23:326–334. doi: 10.1111/j.1365-2915.2009.00840.x. [DOI] [PubMed] [Google Scholar]
- 45.Mouatcho JC, Hargreaves K, Koekemoer LL, Brooke BD, Oliver SV, Hunt RH, Coetzee M. Indoor collections of the Anopheles funestus group (Diptera: Culicidae) in sprayed houses in northern KwaZulu-Natal, South Africa. Malar J. 2007;6:30. doi: 10.1186/1475-2875-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]