Abstract
Indoor residual spraying (IRS) with insecticide is now recommended for malaria control in high-transmission settings. However, concerns about insecticide resistance have increased. We conducted a cross-sectional household survey in high-transmission northern Uganda in two districts previously sprayed with pyrethroids before documentation of pyrethroid resistance and at least one round of carbamates and in one contiguous district that was not sprayed. Parasitemia prevalence among children < 5 years of age was lower in the two IRS districts compared with the non-sprayed district: 37.0% and 16.7% versus 49.8%, P < 0.001. Anemia prevalence was also significantly lower in the two IRS districts: 38.8% and 36.8% versus 53.0%, P < 0.001. Multivariable Poisson regression models indicated that a child living in a sprayed district had a 46% and 32% lower risk of parasitemia and anemia, respectively, than a child in a non-sprayed district (P < 0.001). Carefully managed IRS can significantly reduce malaria burden in high-transmission settings.
Background
Despite substantial progress in scaling up malaria control interventions in many countries, malaria still accounts for significant morbidity and mortality, with an estimated 216 million cases and 655,000 deaths each year, primarily in sub-Saharan Africa.1 Indoor residual spraying (IRS) with insecticide is the most frequent vector control intervention for malaria prevention after insecticide-treated bed nets (ITNs) in sub-Saharan Africa. Multiple studies have shown the effectiveness of IRS for reducing vector densities and malaria transmission,2 and extensive historical experience with IRS in southern Africa has shown that large-scale IRS programs have led to substantial reductions in transmission over time.3 Although previously recommended primarily for epidemic-prone settings, IRS is now recommended in areas of intense and perennial malaria transmission.4 However, recent evidence on the effectiveness of IRS in highly endemic settings with widespread ITN use is limited, particularly regarding its impact on health measures of malaria burden.2,5,6 In sub-Saharan Africa, data using clinically relevant outcomes remain relatively scarce, especially at a time when resistance to dichlorodiphenyltrichloroethane (DDT) and pyrethroids has become a significant concern.
Although synthetic pyrethroids are the only class of insecticides approved for use on ITNs, four classes of insecticides—organochlorines (namely, DDT), pyrethroids, carbamates, and organophosphates—are approved for IRS.7 Of concern is emerging evidence of mosquito resistance to selected insecticides, resulting in diminished effectiveness of IRS and ITNs.8 This resistance has been shown in experimental hut trials,9 although limited epidemiological evidence exists on the effect of resistance.10 The Global Plan for Insecticide Resistance Management in malaria vectors has recently recommended rotating insecticide classes used for IRS as one strategy to manage the threat of insecticide resistance and suggested that IRS programs in areas where ITNs are widely used avoid including the pyrethroid class of insecticides for IRS.11
After spraying only in the epidemic-prone western highlands in 2006, Uganda began IRS in 2007 in selected districts of the north, which is highly endemic for malaria. In 2009, entomological monitoring in the north showed resistance to DDT and pyrethroids, which had been used for 2 years. As a result, the IRS program switched to spraying with carbamate insecticides. In addition, there was concern among policymakers and malaria researchers that the IRS program in northern Uganda was not having the intended epidemiological impact, because the slide positivity rate at a sentinel site health facility in the area had not substantially declined after several years of spraying.6 We conducted a cross-sectional survey in two districts in the north that had been sprayed and one comparison district that had not been sprayed to assess the effect of IRS on anemia and parasite prevalence in children under 5 years of age.
Methods
Description of study sites.
Despite continued investment in malaria control, malaria remains highly endemic in over 95% of Uganda, and the malaria burden is especially high in the north. Coverage of key malaria control interventions has increased in recent years, and household ownership of at least one ITN was reported to be 81.8% in three northern districts in 2010, several months after a national campaign targeting pregnant women and children under 5 years with ITNs.12 Although selected northern districts have received IRS since 2007 and have disproportionately benefited from malaria interventions (including bed net distribution and home-based management of fever), parasite prevalence in children remains high. Parasitemia among children less than 5 years of age in nine districts comprising the northern region in 2009 was 62.5% in 2009 according to a household survey, compared with 42.4% nationally.13 This household survey found that anemia was also very common in the northern region, with 72.8% of children having any anemia (hemoglobin [Hb] level < 11.0 g/dL) and 15.9% of children having severe anemia (Hb level < 8.0 g/dL) compared with national estimates of 62.3% and 9.7%, respectively.13
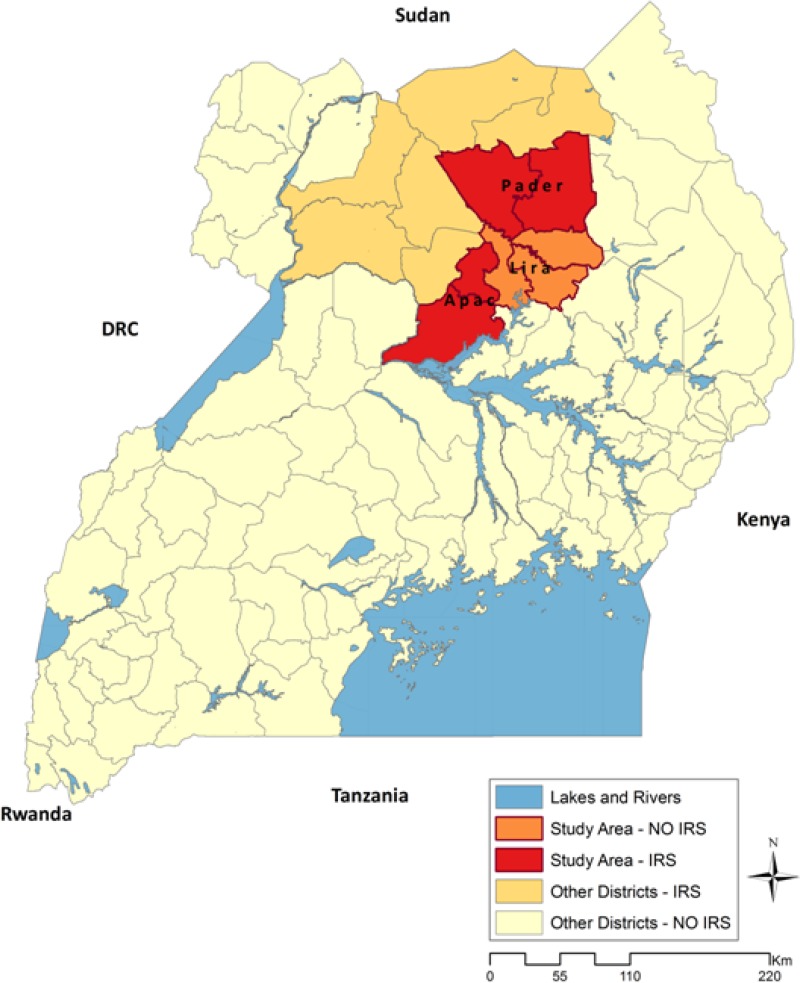
In northern Uganda, Anopheles funestus is the dominant mosquito vector for malaria.14 An. funestus mosquitoes are highly endophagic (feeding indoors) and endophilic (resting indoors), making vector control strategies such as IRS and ITNs promising.15 Plasmodium falciparum is, by far, the most common malaria parasite species, accounting for 99.1% of all malaria infections in children less than 5 years of age.13 Extremely high rates of malaria transmission have been recorded in northern Uganda: one of the highest annual entomological inoculation rates (EIRs) in the world—1,586 infective bites per person per year—was recorded in 2001–2002 in Apac (Figure 1). Parasite prevalence was measured to be 79.8% among children 1–9 years of age in 199914 and 55.8% among children under 5 years old in 2009.16
Figure 1.
Map of Uganda with sprayed districts (red) in the north and the three study districts.
The US President's Malaria Initiative (PMI) began supporting IRS in northern Uganda in 2007, starting with four highly endemic districts (Kitgum, Pader, Gulu, and Amuru) and further expanding to two more districts in 2008 (Apac and Oyam). The current program continues to cover those 6 districts (now 10 districts after administrative reorganization in 2010) and has shifted from use of DDT and pyrethroids to carbamates as a result of resistance data from entomological monitoring. Insecticide resistance monitoring conducted in 2009 showed vector resistance on bioassays to both DDT and pyrethroids, specifically λ-cyhalothrin but not α-cypermethrin17,18; WHO tube bioassays conducted in the Apac district in the fall of 2009 indicated only 52% An. gambiae mortality to DDT after 24 hours and 68%, 72%, and 71% mortality to λ-cyhalothrin 0.05%, etofenprox 0.5%, and permethrin 0.75%, respectively.18 Entomologists have speculated that the resistance mechanism in Uganda is likely caused by the kdr mutation because of the cross-resistance between pyrethroids and DDT.18 As a result, the National Malaria Control Programme of Uganda (NMCP) decided to switch to carbamates—specifically bendiocarb—to support the rotation of different classes of insecticides as a strategy to manage insecticide resistance. Use of carbamates began in 2010 (Table 1). Between 2008 and 2011, PMI spent $45.5 million US on IRS in northern Uganda.
Table 1.
Dates of spraying and types of insecticides used in Apac and Pader
| District | Period of operation | Total houses sprayed (% coverage)* | Insecticide used |
|---|---|---|---|
| 2007 | |||
| Pader (round 1) | June to July | 138,458 (97%) | Pyrethroid 1 |
| 2008 | |||
| Apac (round 1) | March to May | 103,025 (92%) | DDT |
| Pader (round 2) | July to August | 162,281 (95) | Pyrethroid 2 |
| 2009 | |||
| Pader (round 3) | January to February | 141,928 (95%) | Pyrethroid 2 |
| Pader (round 4) | November to December | 172,681 (99%) | Pyrethroid 2 |
| 2010 | |||
| Apac (round 2) | March to April | 151,179 (99.5%) | Pyrethroid 2 |
| Pader (round 5) | June to July | 181,202 (98.8%) | Carbamate |
| Apac (round 3) | August | 163,486 (99.6%) | Carbamate |
| Pader (round 6) | December | 207,498 (99.8%) | Carbamate |
Carbamate = bendiocarb; pyrethroid 1 = λ-cyhalothrin; pyrethroid 2 = α-cypermethrin.
Administrative coverage rates reported by spray operators.
To assess the effect of IRS on malaria parasite prevalence and anemia, we conducted a cross-sectional survey based on district-wide representative samples of households in two districts receiving IRS (Pader and Apac) and one comparison district not receiving IRS (Lira) according to district boundaries in place before 2010. Lira was originally slated to receive IRS; however, funds for the program ran short before it was sprayed. The three surveyed districts are contiguous (Figure 1). All three districts benefitted from routine ITN distribution through antenatal care since 2007. Lira also received ITNs from a mass campaign conducted by the United Nations Children's Fund (UNICEF) in 2008 in 14 of its 19 subdistricts. In May of 2010, Apac, Pader, and the five subcounties of Lira not covered by the 2008 UNICEF campaign received ITNs from the Global Fund to Fights AIDS, Tuberculosis, and Malaria in a campaign targeting pregnant women and children under 5 years of age.
Sampling frame and data collection.
Sampling of households was based on a two-stage cluster sampling procedure within each district. The primary sampling units were the enumeration areas (EAs) of the 2002 National Housing and Population Census. Thirty EAs were randomly selected in each of the three districts using probability proportionate to size (PPS) sampling. Within each sampled EA, all households were listed by teams from the Uganda Bureau of Statistics, and 20 households were randomly sampled from the listing for inclusion in the survey; 600 households were surveyed in each district. At each sampled household, the head of the household or designate was interviewed using a structured questionnaire on household assets, usual household members, IRS during the last 12 months, and bed net ownership and use after informed consent was given. A woman's questionnaire was used to interview all women ages 15–49 years about malaria knowledge, education, and care-seeking for children with fever. To facilitate comparison with data from the 2009 malaria indicator survey (MIS), the questionnaires were modeled on the standard MIS instruments developed by the Roll Back Malaria Monitoring and Evaluation Reference Group.19 Questionnaires were translated into Luo (Acholi and Lango), the major local language commonly spoken in northern Uganda. Data were double-entered into CSPro software (Washington, DC) in Kampala.
All children ages 0–59 months in sampled households were eligible for anemia and parasitemia testing. After verbal consent from parents was given, laboratory technicians on each team performed a finger or heel stick to collect blood using a portable spectrophotometer (HemoCue; Quest Diagnostics, Madison, NJ) for tests for anemia and preparation of thick and thin blood slides. In addition, Paracheck Pf HRP2 rapid diagnostic tests (RDTs; Orchid Biomedical Systems, Goa, India) were performed on all children to guide treatment of parasitemic children during the survey. Children who tested positive for malaria using the RDT were evaluated by the team nurse and provided with an age-appropriate dose of artemether-lumefantrine, the nationally recommended treatment for uncomplicated malaria in Uganda. Slides were double-read by expert microscopists at the Uganda Malaria Surveillance Project Molecular Research Laboratory at Mulago Hospital in Kampala to determine malaria parasitemia and parasite species. Discrepant readings were resolved by a third microscopist.
The survey was conducted between mid-December of 2010 and mid-January of 2011 to coincide with the peak malaria transmission season; the 2009 MIS was done during this same season 1 year earlier.
Analytical approach.
The key outcomes of parasite prevalence (presence of any asexual parasites by microscopy) and anemia prevalence (Hb < 11.0 g/dL) were compared at a district level between the three districts (the two sprayed and one non-sprayed district). Household sample size was based on the ability to detect a 25% difference in parasite prevalence between each intervention district and the control district (a prevalence ratio of 1.25 or greater) with 80% power, assuming a design effect of 1.7. District-level parasitemia and anemia prevalence were compared using χ2 statistics adjusted for the complex survey design using the svy commands in Stata 11.0 (Statcorp, College Station, TX). In addition, the two primary outcomes were analyzed using random-effects Poisson regression models with robust standard errors using gllamm,20 with each child as the unit of analysis and a random effect at the cluster level. Prevalence ratios from Poisson regression with robust standard errors have been shown to more accurately reflect risk when the outcome is common.21 In addition to the primary predictor of interest (whether the child lived in a district that had received IRS or not), covariates were included in the model if they were known to be related to the outcome from previous research (e.g., ITN use, wealth, and age). Household wealth was estimated using principle component analysis on a set of variables, including ownership of 17 household assets and various housing characteristics.22
Plausible interaction terms were assessed and kept in the model if they were found to be significant. In addition to individual-level ITN use, we attempted to control for the effect of community-level ITN ownership23 using the proportion of households in the cluster owning at least one ITN using an approach used previously in modeling the impact of malaria control interventions.24 Finally, potential confounders were assessed and kept in the model if they changed the odds ratio of the predictor of interest (IRS) by 20% or more. All tests and confidence intervals use the 5% level of significance.
The protocol was reviewed and approved by the Centers for Disease Control and Prevention in Atlanta, GA. Ethical approval for the survey was also obtained from the Makerere University College of Health Sciences Research and Ethics Committee and the Uganda National Council of Science and Technology. The protocol was also approved by the Committee for Human Research of the University of California San Francisco.
Results
Household response rates for the survey were uniformly high across the three districts, with 97.8% of occupied households successfully interviewed. Response rates among eligible children were similarly high in all districts (at 95.8% on average), yielding 608 children with complete anemia and parasitemia results in Apac, 602 children in Pader, and 560 children in Lira (Table 2).
Table 2.
Demographic and clinical characteristics of children less than 5 years of age with anemia and parasitemia data by district
| Characteristic | Sprayed | Not sprayed (Lira; N = 560) | P value* | |
|---|---|---|---|---|
| Apac (N = 608) | Pader (N = 602) | |||
| Number unique households with children < 5 years | 370 | 366 | 352 | – |
| Mean age in months (range) | 30.6 | 31.9 | 31.1 | 0.239 |
| Female (%) | 47.4 | 51.3 | 46.4 | 0.365 |
| Urban location (%) | 3.4 | 5.4 | 7.1 | 0.815 |
| Mother never attended school (%)† | 16.3 | 35.9 | 24.6 | 0.001 |
| Wealth quintile‡ | ||||
| 1 (poorest) | 11.0 | 41.5 | 18.2 | < 0.001 |
| 2 | 23.0 | 20.7 | 21.7 | |
| 3 | 30.0 | 14.2 | 19.8 | |
| 4 | 23.5 | 14.8 | 22.0 | |
| 5 (least poor) | 12.6 | 8.8 | 18.4 | |
| Food insecure (often or always has trouble satisfying food needs; %) | 56.6 | 59.1 | 53.8 | 0.629 |
| Household owns at least one ITN (%) | 97.0 | 91.9 | 75.1 | < 0.001 |
| Slept under ITN the previous night (%) | 63.7 | 64.1 | 49.9 | 0.002 |
| House sprayed in last 12 months (%) | 87.6 | 89.8 | 0.2 | < 0.001 |
| Malaria parasitemia (%) | 37.0 | 16.7 | 49.8 | < 0.001 |
| Geometric mean parasite density§ | 3,528.3 | 2,448.8 | 4,224.1 | N/A |
| Gametocytemia (%) | 22.5 | 9.2 | 24.0 | < 0.001 |
| Anemia (%) | ||||
| Any (Hb < 11.0 g/dL) | 38.8 | 36.8 | 53.0 | < 0.001 |
| Mild (Hb = 10.0–10.9 g/dL) | 21.0 | 17.4 | 23.5 | 0.112 |
| Moderate (Hb = 8.0–9.9 g/dL) | 15.2 | 18.1 | 25.0 | 0.004 |
| Severe (Hb < 8.0 g/dL) | 2.6 | 1.3 | 4.5 | 0.039 |
| Mean Hb level (g/dL) | 11.3 | 11.3 | 10.8 | 0.002 |
Statistical tests account for cluster sampling using the survey commands in Stata. P values were from χ2 tests and linear regressions as appropriate.
Data missing for 393 children for this variable.
Created by running principle component analysis on children from all three districts together.
Per microliter among children with parasitemia.
A higher proportion of mothers in Pader had no education (35.9%) compared with Lira and Apac (24.6% and 16.3%, respectively, P = 0.001). In addition, surveyed children in Pader were significantly poorer than children in Apac and Lira (41.5% in poorest wealth quintile versus 11.0% and 18.2%, respectively, P ≤ 0.001). However, self-reported food insecurity, measured by the percentage of household respondents saying they always or often have trouble satisfying the household's food needs, was similarly high at over 50% across all three districts (Table 2).
ITN ownership and use were significantly higher in the two IRS districts. The proportion of children less than 5 years of age with anemia and parasitemia data who were reported to have slept under an ITN the previous night was 64% in the two sprayed districts compared with 50% in Lira (P = 0.002). Nearly 90% of children in Apac and Pader lived in households that reported being sprayed in the last 12 months with IRS. Over 90% of households in these districts reported being approached for spraying, and less than 3% refused (data not shown). Only 0.2% of children in Lira lived in households reported to have been sprayed.
Parasite prevalence was highest in Lira (49.8%), which was not sprayed; significantly lower in Apac (37.0%), which had received one round of DDT, one round of pyrethroids, and one round of carbamates 4 months before the survey; and lowest in Pader (16.7%), which had received four rounds of pyrethroids and two rounds of carbamates, the second round less than 1 month before the survey (P < 0.001). Geometric mean parasite density followed a similar trend in the three districts. Gametocytemia was similar in Apac and Lira (at 22.5% and 24.0%, respectively) but significantly lower in Pader (at 9.2%, P < 0.001) (Table 2).
Prevalence of any anemia was similar in the two sprayed districts (at 38.8% and 36.8%) but significantly higher in non-sprayed Lira (at 53.0%, P < 0.001) (Table 2). Moderate and severe anemia rates were also significantly higher in Lira compared with the two IRS districts. Mean Hb level was also significantly lower in Lira (10.8 g/dL compared with 11.3 g/dL in Apac and Pader, P = 0.0022.)
At the individual child level, living in a sprayed district was associated with significantly lower risk of malaria parasitemia (Table 3). Adjusted for other factors, a child living in a sprayed district was estimated to have a 46% reduction in risk of parasitemia compared with a child living in a non-sprayed district (P < 0.001). Reported use of an ITN the night before the survey was not significantly associated with either parasitemia or anemia. To assess the additional benefit of ITN usage in a sprayed household, we included an interaction term between sprayed district and ITN use in the model, but the term was not significant (data not shown). Wealth was inversely related to parasitemia, with a child in the wealthiest quintile having only 41% the risk of a child in the poorest quintile of being parasitemic (P < 0.001). Parasitemia increased with age, and children ages 24–59 months had more than 6.2 times the risk of parasitemia than children less than 6 months of age, ceteris paribus.
Table 3.
Bivariate and multivariate prevalence ratios of selected variables and malaria parasitemia
| Variable | Bivariate prevalence ratio | 95% CI | P value | Multivariate prevalence ratio | 95% CI | P value |
|---|---|---|---|---|---|---|
| Child lives in sprayed district | 0.568 | 0.465, 0.694 | < 0.001 | 0.541 | 0.445, 0.658 | < 0.001 |
| Child slept under ITN previous night | 0.877 | 0.742, 1.035 | 0.120 | 0.977 | 0.859, 1.110 | 0.717 |
| Child's age (months) | ||||||
| < 6 | Referent | Referent | – | Referent | Referent | – |
| 6–11 | 2.072 | 1.126, 3.812 | 0.019 | 2.040 | 1.112, 3.740 | 0.021 |
| 12–23 | 3.899 | 2.382, 6.384 | < 0.001 | 3.968 | 2.435, 6.468 | < 0.001 |
| 24–59 | 6.239 | 3.810, 10.216 | < 0.001 | 6.200 | 3.795, 10.129 | < 0.001 |
| Wealth category | ||||||
| 1 (poorest) | Referent | Referent | Referent | Referent | ||
| 2 | 0.939 | 0.791, 1.115 | 0.472 | 0.944 | 0.802, 1.112 | 0.490 |
| 3 | 0.721 | 0.580, 0.897 | 0.003 | 0.767 | 0.626, 0.939 | 0.010 |
| 4 | 0.706 | 0.586, 0.851 | < 0.001 | 0.731 | 0.610, 0.875 | 0.001 |
| 5 (least poor) | 0.621 | 0.483, 0.799 | < 0.001 | 0.594 | 0.459, 0.769 | < 0.001 |
Prevalence ratios and confidence intervals (CIs) from random effects Poisson regression models with robust variance.
For a child less than 5 years of age, living in a sprayed district was also associated with a 32% reduced risk of anemia adjusted for other factors (P < 0.001) (Table 4). Reported ITN use was not associated with anemia. A child living in a wealthier household tended to have a lower rate of anemia, although this relationship was less strong than the relationship between wealth and parasitemia. The risk of a child being anemic increased between 0 and 6 months (P < 0.001) but then decreased significantly with each month of age for a child 6–59 months (P < 0.001).
Table 4.
Bivariate and multivariate odds ratios of selected variables and any anemia (Hb < 11.0 g/dL)
| Variable | Bivariate prevalence ratio | 95% CI | P value | Multivariate prevalence ratio | 95% CI | P value |
|---|---|---|---|---|---|---|
| Household sprayed in last 12 months | 0.692 | 0.602, 0.794 | < 0.001 | 0.683 | 0.591, 0.788 | < 0.001 |
| Child slept under ITN previous night | 0.945 | 0.835, 1.069 | 0.369 | 0.939 | 0.827, 1.067 | 0.335 |
| Child's age in months, 0–6 months* | 1.278 | 1.145, 1.427 | < 0.001 | 1.241 | 1.151, 1.337 | < 0.001 |
| Child's age in months, 6–59 months* | 0.986 | 0.983, 0.990 | < 0.001 | 0.986 | 0.983, 0.989 | < 0.001 |
| Wealth category | ||||||
| 1 (poorest) | Referent | Referent | Referent | Referent | ||
| 2 | 0.973 | 0.791, 1.197 | 0.794 | 0.961 | 0.822, 1.123 | 0.614 |
| 3 | 0.847 | 0.791, 1.197 | 0.794 | 0.840 | 0.716, 0.986 | 0.033 |
| 4 | 0.787 | 0.627, 0.988 | 0.039 | 0.768 | 0.652, 0.904 | 0.002 |
| 5 (least poor) | 0.926 | 0.724, 1.183 | 0.537 | 0.825 | 0.688, 0.990 | 0.038 |
Prevalence ratios and confidence intervals (CIs) from random effects Poisson regression models with robust variance.
Bivariate regressions run separately for children < 6 months (N = 191) and children 6–59 months (N = 1,582). Linear spline (knot at 6 month) used in multivariate regression.
Discussion
Our results from a highly malaria-endemic region of Uganda indicate that a child living in one of two districts with IRS had significantly lower anemia and parasite prevalence than a child in a neighboring district that had not been sprayed. These findings add to the growing body of evidence that IRS can be effective in reducing malaria burden in high-transmission settings. Despite the presence of resistance to DDT and certain pyrethroids in the spray areas, the IRS program in northern Uganda seems to be effective in reducing the malaria burden.
The large and significant reduction in parasitemia in this high-transmission setting is consistent with a recent meta-analysis of the impact of IRS in studies conducted since 2000, when insecticide resistance began to be a potential problem in many places.25 According to the meta-analysis results, IRS can significantly reduce malaria prevalence by 62% on average, and reductions are highest in areas with higher initial prevalence and multiple rounds of spraying.25 Pader district, which was sprayed with a total of six rounds, had significantly lower parasite prevalence than Apac, which had received only three rounds of IRS at the time that the survey was conducted. Apac, in turn, had significantly lower parasite prevalence than non-sprayed Lira, and its transmission seems to have declined in the last few years. Although true baseline data are lacking, a 2009 study in Apac indicated a 55.8% parasite prevalence rate in children < 5 years.16 The sampling methods are different between this survey and our survey (testing of community members at health centers versus testing during household interviews), but this prevalence is substantially higher than the 37.0% parasite rate that we found in our survey nearly 2 years later after two additional rounds of IRS had been conducted.
Our study indicated that ITNs were not associated with a significant reduction in anemia or parasite prevalence, a finding that held even when we conducted separate analyses in the IRS and non-IRS areas (data not shown). This result is in line with findings from a recent evaluation of IRS in a high-transmission setting in Malawi, which showed no significant protective effect of ITNs.5 Previous studies on the combined benefits of IRS and ITNs have found mixed results.26–28 One of the only prospective cohort studies to assess this combination found that, compared with ITNs alone, ITNs combined with IRS had a protective efficacy of 62% against incidence of P. falciparum parasitemia in western Kenya.29 A recent study in Ethiopia using cross-sectional surveys found that IRS with DDT combined with ITNs was associated with significantly lower vector density and parasite prevalence compared with ITNs alone.30 Although previous studies and the results of the current study point to the benefits of IRS over ITNs, it should be noted that bed net ownership and use were relatively high in these settings: for example, in our study, one-half of the children under 5 years slept under an ITN the previous night in the control district, and bed net usage in the IRS districts was even higher. Therefore, the individual benefit of sleeping under an ITN in these settings may be obscured by the relatively high overall use and the community effect of ITNs. What these findings do suggest is that IRS provides a significant added benefit, even in settings where ITN ownership and use is high.
Adding to the plausibility argument that the effect of IRS measured in this survey reflects its true impact, we found that wealth was higher in the comparison district, Lira, versus the IRS districts. Because wealth is inversely related to parasitemia, any residual confounding from failing to fully control for this difference would have diminished the effect size of IRS, which we still found to be significant. Additionally, given that Lira is geographically between Apac and Pader, any spillover effect of IRS from the two sprayed districts into Lira would have underestimated the impact that we found of IRS, thereby strengthening our findings.
Limitations.
This study has several notable limitations. Most importantly, our findings come from a cross-sectional survey without baseline data. In addition, bed net ownership and use were higher in the IRS districts, which benefited fully from the 2010 Global Fund bed net distribution campaign, versus Lira, where only 5 of 19 subdistricts were part of the 2010 campaign. Therefore, any community effect of ITNs on reducing parasitemia would have been stronger in the two sprayed districts.
We were also unable to assess the relative effectiveness of different insecticide classes and spraying intensity on parasite prevalence and anemia. Pader, which had received four rounds of spraying with pyrethroids and two rounds of spraying with carbamates, had significantly lower parasitemia than Apac, which had received one round of DDT, one round of pyrethroids, and only one round of carbamates. However, spraying with carbamates in Pader took place less than 1 month before the survey, whereas spraying in Apac took place 4 months before the survey. Bendiocarb, the carbamate used in both districts, may only be effective for 3 months,24 and duration of time since spraying has been shown to be related to parasitemia.31 However, recent analysis of facility-based data from a malaria sentinel site facility in Apac indicates that bendiocarb may have had a greater effect on malaria morbidity than DDT or pyrethroids.6 Variations in the spray timing within the two sprayed districts were not large enough to permit analysis of the impact of the time since spraying on anemia and parasitemia.
Lastly, we attempted to control for the effect of community-level ITN ownership (using the proportion of households in the cluster owning at least one ITN) but were unable to do so, because this variable was collinear with household-level ITN use.
Conclusions.
Our findings add to the growing body of recent evidence about the effectiveness of IRS in high-transmission settings.5,6 Despite measured resistance to certain insecticides that were previously used before a change to the IRS program in northern Uganda, we found that only 16.7% and 37.0% of children in two sprayed districts had parasitemia compared with nearly one-half of the children in a neighboring non-sprayed district.
However, our findings raise several key questions that should be explored by additional research and programmatic activities. Additional research should explore the optimal timing and frequency of IRS with carbamates, questions that we were not able to address with the current study. In addition, studies explicitly designed to evaluate the effect of insecticide rotation in settings where resistance is present would help provide empirical evidence in support of recommended resistance management strategies. Finally, careful entomological monitoring should be continued in northern Uganda to determine when warning signs of resistance warrant consideration of changing insecticides.
ACKNOWLEDGMENTS
The authors thank the data collectors and supervisors who carried out this survey along with the many Ugandan families in the north who participated in this research. The authors thank the staff at the Uganda Bureau of Statistics who assisted with the sampling, training, and data entry. We also extend special thanks to Ruth Kigozi and Moses Kiggundu among many others at the Uganda Malaria Surveillance Project who assisted with this research.
Footnotes
Financial support: This study was funded by the President's Malaria Initiative (PMI).
Authors' addresses: Laura C. Steinhardt and Ryan E. Wiegand, Centers for Disease Control and Prevention (CDC), Atlanta, GA, E-mails: LSteinhardt@cdc.gov and RWiegand@cdc.gov. Adoke Yeka, Asadu Sserwanga, Humphrey Wanzira, Geoff Lavoy, and Moses Kamya, Uganda Malaria Surveillance Project (UMSP), Kampala, Uganda, E-mails: yadoke@muucsf.org, asserwanga@muucsf.org, wanzirah@yahoo.com, glavoy@muucsf.org, and mkamya@infocom.co.ug. Sussann Nasr, Centers for Disease Control and Prevention—Angola, Luanda, Angola, E-mail: Snasr@usaid.gov. Dennis Rubahika, Uganda National Malaria Control Programme, Kamapala, Uganda, E-mail: drubahika@yahoo.com. Grant Dorsey, University of California, San Francisco, CA, E-mail: gdorsey@medsfgh.ucsf.edu. Scott Filler, The Global Fund to Fight AIDS, Tuberculosis, and Malaria. Geneva, Switzerland, E-mail: Scott.Filler@theglobalfund.org.
References
- 1.The World Health Organization . World Malaria Report. Geneva: World Health Organization; 2011. [Google Scholar]
- 2.Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev. 2010;4:CD006657. doi: 10.1002/14651858.CD006657.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mabaso ML, Sharp B, Lengeler C. Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying. Trop Med Int Health. 2004;9:846–856. doi: 10.1111/j.1365-3156.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 4.The World Health Organization . Use of Indoor Residual Spraying for Scaling Up Global Malaria Control and Elimination. Geneva: World Health Organization; 2006. [Google Scholar]
- 5.Skarbinski J, Mwandama D, Wolkon A, Luka M, Jafali J, Smith A, Mzilahowa T, Gimnig J, Campbell C, Chiphwanya J, Ali D, Mathanga DP. Impact of indoor residual spraying with lambda-cyhalothrin on malaria parasitemia and anemia prevalence among children less than five years of age in an area of intense, year-round transmission in Malawi. Am J Trop Med Hyg. 2012;86:997–1004. doi: 10.4269/ajtmh.2012.11-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kigozi R, Baxi SM, Gasasira A, Sserwanga A, Kakeeto S, Nasr S, Rubahika D, Dissanayake G, Kamya MR, Filler S, Dorsey G. Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PLoS One. 2012;7:e42857. doi: 10.1371/journal.pone.0042857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The World Health Organization . The Technical Basis for Coordinated Action Against Insecticide Resistance: Preserving the Effectiveness of Modern Malaria Vector Control: Meeting Report. Geneva: The World Health Organization; 2011. [Google Scholar]
- 8.Baleta A. Insecticide resistance threatens malaria control in Africa. Lancet. 2009;374:1581–1582. doi: 10.1016/s0140-6736(09)61933-4. [DOI] [PubMed] [Google Scholar]
- 9.N'Guessan R, Corbel V, Akogbeto M, Rowland M. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerg Infect Dis. 2007;13:199–206. doi: 10.3201/eid1302.060631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranson H, N'Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 11.WHO Global Malaria Programme . Global Plan for Insecticide Resistance Management in Malaria Vectors. Geneva: World Health Organization; 2012. [Google Scholar]
- 12.Uganda Malaria Surveillance Project . Malaria Intervention Coverage and Associated Morbidity Survey in Children Under Five Years: Indoor Residual Spraying in Northern Uganda and LLIN Coverage in Central Uganda. Kampala, Uganda: 2012. [Google Scholar]
- 13.Uganda Bureau of Statistics . Uganda Malaria Surveillance Project Molecular Laboratory, National Malaria Control Programme, ICF Macro, 2010. Uganda Malaria Indicator Survey 2009. Kampala, Uganda: 2010. [Google Scholar]
- 14.Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, D'Alessandro U, Coosemans M. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–225. [PubMed] [Google Scholar]
- 15.Yeka A, Gasasira A, Mpimbaza A, Achan J, Nankabirwa J, Nsobya S, Staedke SG, Donnelly MJ, Wabwire-Mangen F, Talisuna A, Dorsey G, Kamya MR, Rosenthal PJ. Malaria in Uganda: challenges to control on the long road to elimination: I. Epidemiology and current control efforts. Acta Trop. 2012;121:184–195. doi: 10.1016/j.actatropica.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proietti C, Pettinato DD, Kanoi BN, Ntege E, Crisanti A, Riley EM, Egwang TG, Drakeley C, Bousema T. Continuing intense malaria transmission in northern Uganda. Am J Trop Med Hyg. 2011;84:830–837. doi: 10.4269/ajtmh.2011.10-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okia M. Preliminary Results of Insecticide Susceptibility Tests Conducted Using Alphacypermethrin 0.1% WHO Test Papers in Apac and Gulu Districts, January 2010. Kampala, Uganda: 2010. [Google Scholar]
- 18.Okia M, Protopopoff N. Report on Malaria Vector Susceptibility to Public Health Insecticides in Uganda September to October 2009. Kampala, Uganda: Stop Malaria Uganda Malaria Consortium; 2009. [Google Scholar]
- 19.Roll Back Malaria Monitoring and Evaluation Reference Group . Malaria Indicator Survey: Basic Documentation for Survey Design and Implementation. Geneva: The World Health Organization; 2005. [Google Scholar]
- 20.Rabe-Hesketh S, Skrondal A, Pickles A. Generalized multilevel structural equation modeling. Psychometrika. 2004;69:167–190. [Google Scholar]
- 21.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data–or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 23.Hawley WA, Phillips-Howard PA, ter Kuile FO, Terlouw DJ, Vulule JM, Ombok M, Nahlen BL, Gimnig JE, Kariuki SK, Kolczak MS, Hightower AW. Community-wide effects of permethrin-treated bed nets on child mortality and malaria morbidity in western Kenya. Am J Trop Med Hyg. 2003;68:121–127. [PubMed] [Google Scholar]
- 24.Larsen D, Angelwicz P, Eisele T. Examining the community effect of insecticide-treated bed nets using survival analysis. Proceedings of the 60th Annual Meeting of the American Society of Tropical Medicine and Hygiene; Philadelphia, PA. 2011. [Google Scholar]
- 25.Kim D, Fedak K, Kramer R. Reduction of malaria prevalence by indoor residual spraying: a meta-regression analysis. Am J Trop Med Hyg. 2012;87:117–124. doi: 10.4269/ajtmh.2012.11-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyarango PM, Gebremeskel T, Mebrahtu G, Mufunda J, Abdulmumini U, Ogbamariam A, Kosia A, Gebremichael A, Gunawardena D, Ghebrat Y, Okbaldet Y. A steep decline of malaria morbidity and mortality trends in Eritrea between 2000 and 2004: the effect of combination of control methods. Malar J. 2006;5:33. doi: 10.1186/1475-2875-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Protopopoff N, Van Bortel W, Marcotty T, Van Herp M, Maes P, Baza D, D'Alessandro U, Coosemans M. Spatial targeted vector control in the highlands of Burundi and its impact on malaria transmission. Malar J. 2007;6:158. doi: 10.1186/1475-2875-6-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleinschmidt I, Schwabe C, Shiva M, Segura JL, Sima V, Mabunda SJ, Coleman M. Combining indoor residual spraying and insecticide-treated net interventions. Am J Trop Med Hyg. 2009;81:519–524. [PMC free article] [PubMed] [Google Scholar]
- 29.Hamel MJ, Otieno P, Bayoh N, Kariuki S, Were V, Marwanga D, Laserson KF, Williamson J, Slutsker L, Gimnig J. The combination of indoor residual spraying and insecticide-treated nets provides added protection against malaria compared with insecticide-treated nets alone. Am J Trop Med Hyg. 2011;85:1080–1086. doi: 10.4269/ajtmh.2011.10-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bekele D, Belyhun Y, Petros B, Deressa W. Assessment of the effect of insecticide-treated nets and indoor residual spraying for malaria control in three rural kebeles of Adami Tulu District, South Central Ethiopia. Malar J. 2012;11:127. doi: 10.1186/1475-2875-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleinschmidt I, Torrez M, Schwabe C, Benavente L, Seocharan I, Jituboh D, Nseng G, Sharp B. Factors influencing the effectiveness of malaria control in Bioko Island, equatorial Guinea. Am J Trop Med Hyg. 2007;76:1027–1032. [PMC free article] [PubMed] [Google Scholar]